Abstract
Starvation and cell density regulate the developmental expression of Myxococcus xanthus gene 4521. Three classes of mutants allow expression of this developmental gene during growth on nutrient agar, such that colonies of strains containing a Tn5 lac Ω4521 fusion are Lac+. One class of these mutants inactivates SasN, a negative regulator of 4521 expression; another class activates SasS, a sensor kinase-positive regulator of 4521 expression; and a third class blocks lipopolysaccharide (LPS) O-antigen biosynthesis. To identify additional positive regulators of 4521 expression, 11 Lac− TnV.AS transposon insertion mutants were isolated from a screen of 18,000 Lac+ LPS O-antigen mutants containing Tn5 lac Ω4521 (Tcr). Ten mutations identified genes that could encode positive regulators of 4521 developmental expression based on their ability to abolish 4521 expression during development in the absence of LPS O antigen and in an otherwise wild-type background. Eight of these mutations mapped to the sasB locus, which encodes the known 4521 regulators SasS and SasN. One mapped to sasS, whereas seven identified new genes. Three mutations mapped to a gene encoding an NtrC-like response regulator homologue, designated sasR, and four others mapped to a gene designated sasP. One mutation, designated ssp10, specifically suppressed the LPS O-antigen defect; the ssp10 mutation had no effect on 4521 expression in an otherwise wild-type background but reduced 4521 developmental expression in the absence of LPS O antigen to a level close to that of the parent strain. All of the mutations except those in sasP conferred defects during growth and development. These data indicate that a number of elements are required for 4521 developmental expression and that most of these are necessary for normal growth and fruiting body development.
Myxococcus xanthus multicellular development, which culminates in the formation of spores and fruiting bodies, is initiated by nutrient limitation at a high cell density (15, 16). M. xanthus cells must sense, integrate, transduce, and respond to information concerning these two environmental conditions (39). Evidence indicates that the cells sense starvation, at least in part, through the accumulation of guanosine tetra- or pentaphosphate (23, 43). Cell density appears to be sensed through the accumulation of the A signal (34), which is composed of a specific subset of amino acids, at a concentration of greater than 10 μM (33). A-signal generation is asg dependent. The Asg regulators (AsgA [41], AsgB [38], AsgC [13], and AsgD [12]) respond to starvation by releasing proteases that degrade surface proteins to amino acids and peptides (40). At a high cell density, the extracellular A-signal concentration surpasses the minimum threshold and development is initiated (34).
Dissection of the circuitry that connects starvation and high cell density to the behavioral response of fruiting body development has focused on the regulation of a class of developmental genes whose expression is responsive to both conditions (7, 28). The best-studied gene in this class was identified on the basis of the Tn5 lac transcriptional fusion Ω4521 and is designated 4521 (29). Its expression increases between 1.5 and 2 h after starvation at high density (28, 32). Starving wild-type cells at low density do not express 4521 unless any one of the A-signal amino acids is added at a concentration of greater than 10 μM. The expression of 4521 remains at a basal level in starving asg mutants, presumably because the concentrations of A-signal amino acids are significantly below 10 μM (34). The expression of 4521 can be restored to these asg cells by the addition of the A signal (34) or by the presence of suppressor mutations designated sas (28).
The screen which identified the sas mutations was designed to identify bypass suppressor mutants that could express 4521 inappropriately, either when the cells were not starved or when the A signal was not available during development (28). Lac+ colonies of strains containing Tn5 lac Ω4521 were identified on nutrient agar plates from among the colonies of the Lac− asgB480 Tn5 lac Ω4521-containing parents. This mutagenesis identified two types of suppressors. One type bypasses both starvation control and cell density control of 4521 expression, allowing 4521 expression during growth and resulting in high β-galactosidase activities in cultures of these cells. This type contains all of the null mutations of sasN, which encodes the only identified negative regulator of 4521 expression (46). Another of these suppressor mutations is a gain-of-function mutation in sasS (sasS*), which encodes the SasS sensor histidine protein kinase (47). Wild-type SasS has been proposed to activate 4521 expression in response to the A signal (47). The second type of suppressor bypasses only cell density control of 4521 expression. Cells carrying these suppressors express 4521 at low density only when starved; when grown in liquid cultures, these cells express 4521 at a basal level. However, older colonies of these mutants containing Tn5 lac Ω4521 are Lac+, presumably because the cells within these older colonies are starving. Mutations that disrupt lipopolysaccharide (LPS) O-antigen biosynthesis comprise this group (21). Thus, the screen for asg suppressors identified positive (47) and negative (46) regulators of 4521 expression and revealed that the loss of LPS O antigen stimulates 4521 expression by an unknown mechanism (6, 21).
To identify additional positive regulators of 4521 expression, we isolated transposon insertions that abolished or reduced 4521 expression in strains that expressed 4521 on nutrient agar because of the absence of LPS O antigen. At least two classes of mutants were expected, those that reduce 4521 expression because they are missing a required activator and those that specifically interfere with the stimulation of gene expression due to the absence of LPS O antigen. Both classes of mutants were identified by the mutagenesis experiments presented here. From the 11 mutants obtained, at least four different genes required for 4521 expression were identified.
MATERIALS AND METHODS
Bacterial strains, plasmids, phages, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. The M. xanthus recipient strains DK6620, DK6621, and DK6625 contain a Tn5 lac Ω4521 (Tcr) insertion to monitor 4521 expression (28). Strain DK6620 was used as the wild type because the Tn5 lac Ω4521 (Tcr) insertion does not appear to alter any growth or developmental functions. Strain DK6621, the parent strain for mutagenesis, carries asgB480 and sasA1; the latter is an original asgB480 suppressor allele that interferes with LPS O-antigen biosynthesis and restores 4521 expression during development to the DK6600 (asgB480) parent strain, which is deficient in A-signal generation (28). Strain DK6625 carries asgB480 and sasB7, the sasS gain-of-function mutant allele (sasS*), so that it expresses 4521 at a high level during growth and development (47). The sglA1 mutation listed in some of the strain genotypes (Table 1) is an allele of the pilQ gene which results in a defect in social motility (44). Myxophage Mx4 (ts18 ts27 hrm) has been described by Campos et al. (10) and Geisselsoder et al. (19). Phage P4 has been described by Kahn et al. (26).
TABLE 1.
Plasmid and strain list
| Plasmid or strain | Relevant characteristic(s) | Derivationa | Source or reference |
|---|---|---|---|
| Plasmids | |||
| pBluescript KS(+) | Ampr | Stratagene | |
| pTF1 | Kanr | 18 | |
| pTF1.AS | Kanr | This study | |
| P4::Tn5 | Kanr | B. Julien | |
| P4::TnV.AS | Kanr | This study | |
| Strains | |||
| E. coli DH5α | supE44 ΔlacU169 (φ80 lacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA1) | 22 | |
| M. xanthus | |||
| DK1622 | Wild type | 27 | |
| DK6620 | Tn5 lac Ω4521 (Tcr) sglA1 | 28 | |
| DK6621 | Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | 28 | |
| DK6625 | Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7b | 28 | |
| HK1101 | ssp1 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1102 | ssp2 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1103 | ssp3 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1104 | ssp4 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1105 | ssp5 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1106 | ssp6 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1107 | ssp7 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1108 | ssp8 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1109 | ssp9 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1110 | ssp10 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1111 | ssp11 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasA1 | TnV.AS insertion into DK6621 | This study |
| HK1112 | ssp5 Tn5 lac Ω4521 (Tcr) sglA1 | HK1105(Mx4) × DK6620 | This study |
| HK1113 | ssp6 Tn5 lac Ω4521 (Tcr) sglA1 | HK1106(Mx4) × DK6620 | This study |
| HK1114 | ssp7 Tn5 lac Ω4521 (Tcr) sglA1 | HK1107(Mx4) × DK6620 | This study |
| HK1115 | ssp8 Tn5 lac Ω4521 (Tcr) sglA1 | HK1108(Mx4) × DK6620 | This study |
| HK1116 | ssp9 Tn5 lac Ω4521 (Tcr) sglA1 | HK1109(Mx4) × DK6620 | This study |
| HK1117 | ssp10 Tn5 lac Ω4521 (Tcr) sglA1 | HK1110(Mx4) × DK6620 | This study |
| HK1118 | ssp11 Tn5 lac Ω4521 (Tcr) sglA1 | HK1111(Mx4) × DK6620 | This study |
| HK1119 | ssp5 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1105(Mx4) × DK6625 | This study |
| HK1120 | ssp6 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1106(Mx4) × DK6625 | This study |
| HK1121 | ssp7 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1107(Mx4) × DK6625 | This study |
| HK1122 | ssp8 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1108(Mx4) × DK6625 | This study |
| HK1123 | ssp9 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1109(Mx4) × DK6625 | This study |
| HK1124 | ssp10 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1110(Mx4) × DK6625 | This study |
| HK1125 | ssp11 Tn5 lac Ω4521 (Tcr) sglA1 asgB480 sasB7 | HK1111(Mx4) × DK6625 | This study |
| HK1126 | ssp5 | HK1105(Mx4) × DK1622 | This study |
| HK1127 | ssp6 | HK1106(Mx4) × DK1622 | This study |
| HK1128 | ssp7 | HK1107(Mx4) × DK1622 | This study |
| HK1129 | ssp8 | HK1108(Mx4) × DK1622 | This study |
| HK1130 | ssp9 | HK1109(Mx4) × DK1622 | This study |
| HK1131 | ssp10 | HK1110(Mx4) × DK1622 | This study |
| HK1132 | ssp11 | HK1111(Mx4) × DK1622 | This study |
Strain constructions are described in an abbreviated form. For HK1112, a Mx4 phage lysate propagated on HK1105 was used to infect DK6620, and Kanr transductants were selected.
The sasB7 mutation is a gain-of-function mutation designated in the text as sasS*.
M. xanthus was grown at 32°C in CTT liquid medium (1% Casitone [Difco Laboratories], 10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4 [pH 7.7], 8 mM MgSO4; final pH of the mixture, 7.6) with vigorous shaking or on CTT agar (CTT liquid medium with 1.5% Bacto Agar [Difco]). Kanamycin at 40 μg/ml was added when appropriate. The growing cells were used in the mid-exponential phase (80 to 160 Klett units, which is approximately 4 × 108 to 8 × 108 cells per ml). Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) (42) liquid medium or on LB agar (LB liquid medium with 1.5% Bacto Agar), each of which contained ampicillin (100 μg/ml) or kanamycin (40 μg/ml) when appropriate to maintain plasmids.
M. xanthus development and measurement of β-galactosidase specific activity and sporulation efficiency.
M. xanthus cells that developed on TPM starvation agar (10 mM Tris-HCl [pH 7.6], 1 mM K2HPO4-KH2PO4 [pH 7.7], 8 mM MgSO4, 1.5% Bacto Agar) according to the method of Kroos et al. (29) were used for the measurement of developmental β-galactosidase specific activity and analysis of fruiting body morphology. For development on starvation agar, growing cells in mid-exponential phase were harvested by centrifugation, and the cell pellet was resuspended in TPM buffer at a density of 4 × 109 cells/ml. Drops of the cell suspension on dry TPM agar plates were incubated at 32°C and, at the appropriate times, were either photographed or scraped from the agar, suspended in TPM buffer, and stored immediately at −20°C. The β-galactosidase specific activity of the thawed samples was quantitated using the method of Kroos et al. (29) as modified by Gulati et al. (20). Protein concentrations were determined by the Bradford assay (9) using a Bio-Rad reagent and immunoglobulin G as the protein standard.
M. xanthus cells that developed in submerged cultures according to the protocol of Kuner and Kaiser (30) as modified by Kuspa et al. (32) were used for sporulation analysis. For development in submerged cultures, mid-exponential-phase M. xanthus cells were harvested, resuspended in MC7 buffer (10 mM morpholinepropanesulfonic acid [MOPS, pH 7.0], 1 mM CaCl2) to a calculated cell density of 2.5 × 108 cells/ml, and placed in 24-well tissue culture plates. The plates were incubated in a humid chamber at 32°C for up to 7 days. Under these conditions, the wild-type cells settled to form a thin mat on the bottom of the well and developed with timing similar to that seen on TPM agar. The production of heat-resistant and sonication-resistant spores in submerged culture preparations was measured. Briefly, the cells were diluted to 108 cells/ml with MC7 buffer, and the plates were incubated at 50°C for 2.5 h. The suspensions were then sonicated to break the rod-shaped cells, and 10-fold dilutions were plated on CTT nutrient agar. The number of viable spores was measured by counting the number of CFU.
Construction of a modified TnV transposon.
To facilitate the physical mapping of transposon insertions and to improve transposition efficiency, we modified the TnV transposon developed by Furuichi et al. (18) and used an alternate delivery strategy. TnV itself is a modified version of transposon Tn5 to which a pSC101 origin of replication has been added. This original modification allows the one-step cloning of M. xanthus DNA flanking the transposon insertion.
The TnV transposon was modified by the addition of AseI and SpeI restriction enzyme recognition sites, generating a new transposon designated TnV.AS. This modification allows the location of the transposon insertion on the physical map of the M. xanthus chromosome to be easily determined. The M. xanthus physical map is derived from two ordered sets of 16 AseI and 21 SpeI restriction fragments (11, 24). The AseI and SpeI restriction enzyme recognition sites were introduced into TnV by the addition of a DNA linker carrying these sites (5′-GCATTAATCGGCACTAGTCG-3′). This DNA linker was inserted into the unique SmaI site in the TnV transposon on plasmid pTF1 (18), and the resulting plasmid was designated pTF1.AS. As a result of this modification, each transposon insertion adds to the chromosome a new set of restriction enzyme recognition sites that can be identified by pulsed-field gel electrophoresis. One of each of the AseI and SpeI fragments should be replaced by two smaller fragments. Identification of the missing fragment defines which fragment contains the insertion, and the sizes of the new fragments indicate two possible locations of the insertion. By comparing the insertion sites on the two enzyme maps, it is generally possible to determine unequivocally the location of the transposon insertion.
For more efficient transposition, TnV.AS was placed onto bacteriophage P4 for delivery into M. xanthus. Studies (B. Julien and D. Kaiser, personal communication) have shown that the efficiency of transfection of the satellite bacteriophage P4 into M. xanthus is over 100-fold higher than the efficiency of P1 transduction. Since the inverted repeat regions of TnV are identical to those of Tn5, TnV.AS was cloned into P4 by replacing the 3.9-kb NheI fragment of P4::Tn5 with the 4.1-kb NheI fragment from pTF1.AS. The resulting plasmid was designated P4::TnV.AS. The P4::Tn5 DNA and the P4::TnV.AS phage were prepared according to the protocol supplied by B. Julien. All of the manipulations of plasmid DNA were performed using standard protocols (42).
Transposon mutagenesis.
To obtain the ssp mutant strains, P4::TnV.AS was introduced into DK6621 by transfection according to the protocol supplied by B. Julien. The mutants with transposon insertions in their chromosomes were selected by growth on kanamycin-containing CTT nutrient agar. Briefly, 0.4 ml (∼2 × 108 cells per ml) of exponentially growing DK6621 cells was mixed with 100 μl (∼3 × 1011 PFU per ml) of P4::TnV.AS and 10 μl of 0.5 M CaCl2, and the mixture was incubated at room temperature without agitation for 30 min. Then, 3 ml of CTT soft agar (0.7% agar), was added, and the mixture was poured onto kanamycin-containing CTT agar plates and incubated at 32°C for 5 days. Then, each plate was overlaid with 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) in 2.5 ml of CTT soft agar.
Mapping of the TnV.AS transposon insertions.
The TnV.AS insertions were mapped by Southern analysis according to standard protocols (42). Generalized transduction mediated by phage Mx4, performed according to the procedures described by Bowden and Kaplan (8), was also used for mapping. To locate the TnV.AS insertions on the physical map of the M. xanthus chromosome, pulsed-field gel electrophoresis was used. Chromosomal DNA was prepared and digested with restriction enzymes AseI and SpeI as described by Chen et al. (11) and He et al. (24). The chromosomal fragments were separated using Beckman GeneLine System II according to the methods recommended by the manufacturer. The electrophoresis conditions were as follows: 350 mA, 5 h 30 s; 370 mA, 8 h 45 s; 370 mA, 8 h 60 s; and 390 mA, 5 h 90 s. The gels were stained with ethidium bromide and photographed while UV illuminated. Southern blot analysis was performed on the transverse alternating field electrophoresis (TAFE) gels to confirm the location of the transposons.
One-step cloning of the DNA flanking the TnV.AS insertions.
The DNA flanking each TnV.AS insertion was cloned from the M. xanthus chromosome using the one-step cloning procedure (18). First, a DNA restriction map of each mutant chromosome was prepared by Southern blot analysis using the kanamycin resistance gene nptII as a probe. Depending on the Southern hybridization results, the chromosomal DNA was digested with restriction enzyme ApaI or MluI, and the appropriately sized DNA fragments were purified from agarose gels, self-ligated, and used to transform E. coli DH5α by electroporation. The transformants were selected on LB agar plates containing kanamycin. The recombinant plasmids purified from the E. coli cells contained TnV.AS and the flanking M. xanthus DNA.
DNA sequence determination and computer analysis.
To sequence the M. xanthus chromosomal DNA flanking the transposon insertions, (i) the majority of the transposon was removed from the initial recombinant plasmids, (ii) the flanking DNA fragments were separated into different pBluescript KS plasmids, and (iii) the subclones were sequenced using a primer designed from the sequences at the left end of IS50 and the T7 or T3 extended primers. To remove the majority of the transposon, the initial recombinant plasmids were digested with restriction enzyme PstI or XhoI, which cuts at the ends of TnV.AS, and then ligated with pBluescript KS digested with the appropriate enzyme. Each resulting plasmid was digested with ApaI or MluI and PstI or XhoI. A plasmid containing one flanking DNA fragment was generated in each case by self-ligation of this digested DNA. A plasmid containing the other flanking DNA fragment was generated by cloning the fragment without an origin into another pBluescript KS vector digested with the appropriate enzymes. The DNA sequence of each plasmid was determined at the DNA Core Facility of the Department of Microbiology and Molecular Genetics, University of Texas—Houston Medical School, with a 373A or 373 Prism DNA sequencer (Perkin-Elmer, Applied Biosystems Division) and Taq polymerase in a thermal cycling reaction.
The DNA sequences were aligned and edited with the SeqEd 675 DNA sequence editor program (Perkin-Elmer, Applied Biosystems Division). The Genetics Computer Group sequence software package, version 9.1 (14), was used for the sequence analysis. The Codonpreference program predicted the open reading frames (ORFs) based on the G+C codon bias of the third position (5) in this high (67.5 mol%)-G+C-content organism (35). Searches were performed at the National Center for Biotechnology Information with the BLAST network service (1).
RESULTS
Isolation of ssp mutants which express 4521 at low levels.
The expression of 4521 under normal circumstances is restricted to early development. However, the loss of the LPS O antigen allows 4521 expression on nutrient agar plates, such that colonies of LPS O-antigen mutants containing Tn5 lac Ω4521 (Tcr) are Lac+ (21, 28). These Lac+ mutants provided a useful parent strain from which to isolate mutants that express 4521 at low levels. Mutations that inactivate positive regulators of 4521 expression would be expected to abolish or reduce 4521 expression.
The LPS O-antigen biosynthesis mutant DK6621 [sasA1 asgB480 Tn5 lac Ω4521 (Tcr)] was used as the parent strain for transposon mutagenesis with a modified Tn5 transposon designated TnV.AS (see Materials and Methods). Colonies of this strain are blue when overlaid with the chromogenic substrate for β-galactosidase, X-Gal (28). To obtain mutations in genes encoding possible positive regulators of 4521 expression, we screened for tan Lac− TnV.AS-containing transductants among the blue Lac+ parent-like colonies. From 18,000 kanamycin-resistant colonies screened, 40 tan colonies were found. Southern analysis of these 40 colonies excluded transposon insertions in the 4521 promoter and the lacZ gene, leaving 11 mutants for further study.
Eight of the TnV.AS insertions cluster in the sasB locus, and one identifies a known positive regulator of 4521 expression, SasS.
An effective screen for positive regulators of 4521 expression was expected to identify the one known positive regulator of 4521 expression, SasS. We screened for the presence of the TnV.AS transposon in the sasS gene by Southern hybridization analysis of the chromosomal DNA of the 11 ssp mutants. One insertion mutant, designated ssp1, was determined to be located in the sasS gene, because a 7.1-kb 32P-labeled SalI fragment of the sasB locus containing the sasS gene hybridized to a band of 6.7 kb rather than the expected 1.6 kb when the ssp1 chromosome was digested with ApaI and SalI (Fig. 1). This result indicated that the screen worked as expected.
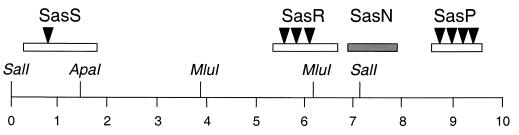
FIG. 1.
Physical map of the sasB locus. The line represents the M. xanthus chromosome. The boxes indicate the Sas ORFs. The open boxes represent the positive regulators, and the shaded box represents the negative regulator. The arrowheads indicate the general locations of the TnV.AS insertions. The ssp1 insertion is in sasS; the ssp2, ssp3, and ssp4 insertions are in sasR; and the ssp5, ssp6, ssp7, and ssp8 insertions are in sasP. All of the genes are transcribed from left to right, and genetic studies have indicated that they are each independent transcription units. The relevant restriction enzyme sites are shown. The numbers indicate kilobases.
The ssp2, ssp3, and ssp4 insertions were determined to be inserted in an ORF approximately 3.5 kb downstream of sasS (Fig. 1). This conclusion was based on Southern analysis of chromosomal DNA of the ssp2, ssp3, and ssp4 mutants digested with restriction enzyme MluI and hybridized with the 7.1-kb 32P-labeled fragment described above. Subsequent analysis determined that these transposon insertions were located in a gene encoding an NtrC-like response regulator. This gene became the subject of a separate investigation (C. Yang, D. Guo, J. Rivera, and H. B. Kaplan, submitted for publication) and will not be discussed further here.
The ssp5, ssp6, ssp7, and ssp8 transposon insertions were found to cluster together about 8 kb downstream of sasS and about 2 kb downstream of sasN (Fig. 1). This conclusion was based on the application of Wu's formula to the results of generalized transduction experiments (45). The ssp5, ssp6, ssp7, and ssp8 TnV.AS insertions were cotransduced with the sasS mutant allele in about 60% of the transductants assayed and were cotransduced with the sasN mutant allele in about 90% of the transductants assayed. These data suggest that these four mutations mapped to a single genetic locus. We observed that all of the phenotypes of these four mutants were identical, and the DNA sequence data (see below) confirmed that these four mutants contained TnV.AS inserted into one ORF. As a result, only the data for the ssp5 mutant will be included here.
Expression of 4521 in ssp mutants.
The effect of each insertion mutation on 4521 gene expression was studied by examining the β-galactosidase activity patterns during growth and development. First, to ensure that the Lac− phenotype was a result of the insertions, ssp mutations (ssp5, ssp9, ssp10, and ssp11) were reintroduced into parent strain DK6621 by Mx4-mediated transduction. All of the newly isolated ssp mutant colonies (more than 100 were tested for each transduction) were Lac−, indicating that the insertions were 100% linked to the mutant phenotype and thus were responsible for the phenotype.
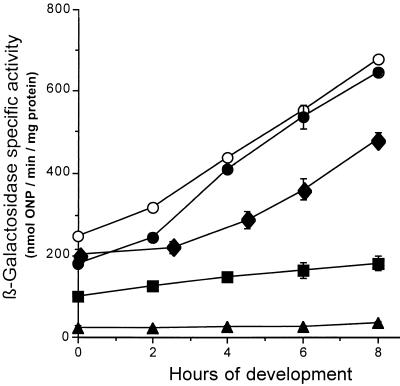
The β-galactosidase specific activities of the new transductants were analyzed from broth-grown cells and from cells developing on starvation agar. As shown in Fig. 2, mutants containing ssp5, ssp9, and ssp11 showed abolished β-galactosidase activity during growth (time zero of development) and development; in contrast, the parent strain showed increased β-galactosidase activity throughout development. The extent of 4521 expression in ssp10 mutant HK1110 was reduced during development compared to that in the parent strain (Fig. 2). The β-galactosidase level in HK1110 reached about 50 U by 8 h of development, a value which is about 10% the level in the parent. The 4521 expression pattern of the ssp10 mutant is similar to that of the parent strain of DK6621, DK6600, which expresses 4521 at a low level because of a defect in A-signal generation due to the asgB480 mutation (28). Thus, by genetic criteria, the ssp10 mutation is considered a suppressor, because its presence returns the strain to its parental phenotype.
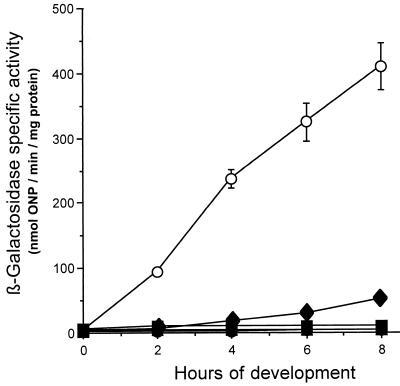
FIG. 2.
Effects of the ssp mutations on 4521 expression in the absence of the LPS O antigen during early development. β-Galactosidase specific activity levels of parent strain DK6621 (asgB480 sasA1) (○) are compared to those of the following strains: HK1105 (asgB480 sasA1 ssp5) (●), HK1109 (asgB480 sasA1 ssp9) (▴), HK1110 (asgB480 sasA1 ssp10) (⧫), and HK1111 (asgB480 sasA1 ssp11) (■). β-Galactosidase specific activity was measured as described in Materials and Methods. The data represent the average of at least three independent experiments; error bars indicate standard deviations. ONP, o-nitrophenol.
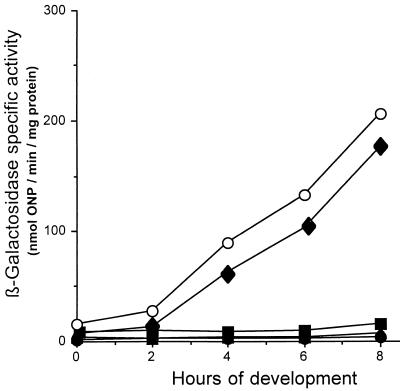
Mutants containing mutations in genes encoding positive regulators of 4521 expression would be expected to reduce or abolish 4521 expression in an otherwise wild-type background. To generate such strains, the ssp5, ssp9, ssp10, and ssp11 mutations were introduced by Mx4 transduction into strain DK6620, an essentially wild-type strain containing Tn5 lac Ω4521 (Tcr). As in the original DK6621 background, the presence of the ssp5, ssp9, and ssp11 mutations in the wild-type background abolished 4521 expression during growth and development (Fig. 3). These data suggest that the genes identified by the ssp5, ssp9, and ssp11 mutations are good candidates for positive regulators of 4521 expression. In contrast to these results, the ssp10 mutation had no significiant effect on 4521 expression in the wild-type background (Fig. 3). The expression of 4521 during growth and development in the ssp10 mutant was similar to its expression in the wild-type background. The β-galactosidase activity level increased after about 2 h of development and reached nearly 200 U by 8 h. These data confirm that the ssp10 mutation is a specific suppressor of the LPS O-antigen biosynthesis mutation, sasA1, because it reduced 4521 expression only in the presence of the sasA1 mutation.
FIG. 3.
Effects of the ssp mutations on 4521 expression in an otherwise wild-type background. β-Galactosidase specific activity levels of wild-type strain DK6620 (○) are compared to those of the following strains: HK1112 (ssp5::TnV.AS) (●), HK1116 (ssp9::TnV.AS) (▴), HK1117 (ssp10::TnV.AS) (⧫), and HK1118 (ssp11::TnV.AS) (■). β-Galactosidase specific activity was measured as described in Materials and Methods. The data represent the average of at least three independent experiments. ONP, o-nitrophenol.
Some of the ssp mutations cause growth defects.
Analysis of the ssp mutant colony morphologies and growth rates revealed a variety of phenotypes. The colonies of strains HK1130, HK1131, and HK1132, containing ssp9, ssp10, and ssp11 mutations, respectively, in an otherwise wild-type DK1622 background, were glossy compared with the rough-surfaced colonies of the wild type (data not shown). The ssp9 and ssp10 colonies were small (about half the diameter of the wild type) after incubation for 72 h at 32°C on CTT agar containing kanamycin. Interestingly, the ssp11 colonies were even smaller than those of ssp9 and ssp10. In CTT liquid cultures, the doubling times of the ssp9, ssp10, and ssp11 colonies were 8.5, 6, and 17 to 18 h, respectively, compared to the 5-h doubling time of the wild type. The colony morphology and growth rate in liquid cultures of the ssp5 mutant, HK1126, were similar to those of the wild type.
Some of the ssp mutations cause developmental defects.
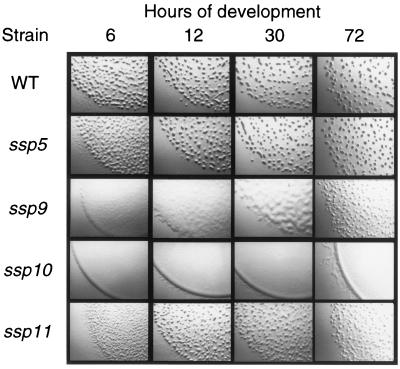
Examination of fruiting body formation (Fig. 4) and sporulation (Table 2) of strains containing the ssp mutations in a wild-type DK1622 background revealed developmental defects for the ssp9-, ssp10-, and ssp11-containing strains. Strain HK1130, containing the ssp9 mutation, was severely delayed (more than 24 h) in developmental aggregation. Aggregation was initiated by 30 h, but the fruiting bodies generated were more numerous and smaller than those in the wild type. This mutant was also severely defective in sporulation. The sporulation level was less than 0.001% the wild-type level. The fruiting body formation of strain HK1131, containing the ssp10 mutation, was completely abolished. No developmental aggregation was detected, and the sporulation efficiency was reduced to 0.006% the wild-type level. Strain HK1132, containing the ssp11 mutation, was slightly delayed in early developmental aggregation. The late-forming HK1132 aggregates failed to condense or darken, and the sporulation efficiency was reduced to 0.02% the wild-type level. Strain HK1126, containing the ssp5 mutation, formed wild-type fruiting bodies and sporulated at a wild-type level.
FIG. 4.
Developmental morphologies of the ssp mutants. The developmental morphologies of the wild type and the mutants, containing the indicated mutations in an otherwise wild-type background, on starvation agar at various times during development are shown. The wild-type strain is DK1622, and the mutants are HK1126 (ssp5::TnV.AS), HK1130 (ssp9::TnV.AS), HK1131 (ssp10::TnV.AS), and HK1132 (ssp11::TnV.AS). The magnification is approximately 2.7-fold.
TABLE 2.
Sporulation of ssp mutants
| Strain | Relevant genotype | CFU ml−1a | % Sporesb |
|---|---|---|---|
| DK1622 | Wild type | 7.62 × 106 | 100 |
| HK1126 | ssp5 | 7.03 × 106 | 92.3 |
| HK1130 | ssp9 | <102 | <0.001 |
| HK1131 | ssp10 | 4.73 × 102 | 0.006 |
| HK1132 | ssp11 | 1.52 × 103 | 0.02 |
The number of heat- and sonication-resistant viable spores was calculated from the number of CFU per milliliter assayed after 7 days of development. The values presented are from one representative experiment. Each experiment was repeated at least three times, and the standard error from these independent experiments was between 10 and 50%.
Reported as a percentage of wild-type parent strain spore levels.
Expression of 4521 in ssp sasS* double mutants.
To determine the relative dependency of 4521 expression on the SasS protein kinase sensor and the putative positive regulators identified by use of the ssp mutations, epistasis experiments were performed. A series of double mutants were constructed by pairing the Lac+ sasS gain-of-function mutation, sasB7 (sasS*), with each of the Lac− ssp5, ssp9, ssp10, and ssp11 mutations. The SasS gain-of-function point mutation is proposed to cause unrestrained phosphate transfer to a downstream cognate response regulator (47). It is unclear how any of the ssp mutations functions. However, if Lac+ double-mutant colonies arise with expression patterns similar to that of the sasS* parent, then the ssp gene product in that strain likely functions upstream of SasS; if Lac− double-mutant colonies arise, then the ssp gene product in that strain likely functions downstream of SasS (37). These interpretations are based on the assumption that the proteins function in a linear and dependent pathway. It is not possible to suggest an order of function for mutants with intermediate phenotypes.
To carefully assess the phenotypes of the double mutants, in vitro β-galactosidase activities were determined during growth and development (Fig. 5). The expression of 4521 in the ssp5 sasS* double mutant was essentially the same as that in the sasS* parent. These data indicate that 4521 expression is primarily dependent on SasS and suggest that the gene product(s) affected by ssp5 normally functions upstream of SasS. The expression of 4521 in the ssp9 sasS* double mutant was abolished, just as in the ssp9 background. These data indicate that 4521 expression is primarily dependent on the gene product(s) affected by the ssp9 mutation and suggest that ssp9 encodes a positive regulator that functions downstream of SasS. The expression of 4521 in the ssp10 sasS* and ssp11 sasS* double mutants was intermediate relative to that in the parent strains.
FIG. 5.
Effects of the ssp mutations on 4521 expression in a strain carrying a gain-of-function SasS sensor mutation. β-Galactosidase specific activity levels of the gain-of-function sasS suppressor strain DK6625 (asgB480 sasB7) (○) are compared to those of the following strains: HK1119 (asgB480 sasB7 ssp5) (●), HK1123 (asgB480 sasB7 ssp9) (▴), HK1124 (asgB480 sasB7 ssp10) (⧫), and HK1125 (asgB480 sasB7 ssp11) (■). β-Galactosidase specific activity was measured as described in Materials and Methods. The data represent the average of at least three independent experiments; error bars indicate standard deviations. ONP, o-nitrophenol.
Location of the ssp insertion mutations on the physical map of the M. xanthus chromosome.
Locating the ssp insertion mutations on the M. xanthus physical map was rapid due to the addition of the SpeI and AseI restriction sites to the TnV transposon. AseI and SpeI restriction enzyme digests of chromosomal DNA of strains containing each of the ssp5, ssp9, ssp10, and ssp11 mutations were electrophoresed on a TAFE gel apparatus, and the resulting restriction patterns were compared to the restriction pattern of wild-type DK1622 DNA. Definitive locations were possible for all of the ssp mutations except ssp9 (Table 3). The ssp9 insertion was at either kb 5475 or kb 5675. However, since the ssp9 mutation mapped to the same AseI and SpeI fragments as the 4521 gene, we used Mx4-mediated generalized transduction to map the distance between the ssp9 insertion and the 4521 gene. Tetracycline resistance conferred by Tn5 lac Ω4521 (Tcr) and kanamycin resistance conferred by TnV.AS inserted in ssp9 were cotransduced at a frequency of 36%. Applying Wu's formula (45) to these data predicted that these two loci are separated by about 14 kb. None of the other insertions mapped close to any known loci.
TABLE 3.
Location of ssp TnV.AS insertions on the M. xanthus chromosome
| Mutation | Locationa (kb) | Restriction fragment into which the transposon was inserted (sizes of new fragments generated, in kb)
|
|
|---|---|---|---|
| AseI | SpeI | ||
| ssp5 (sasB locus) | 1637 | M (100 + 850) | U (500 + 1,800) |
| ssp9 | 5475 or 5675 | I (100 + 300) | L (55 + 250) |
| ssp10 | 4376 | P (750 + 1,000) | S (520 + 520) |
| ssp11 | 4076 | P (450 + 1,300) | S (220 + 820) |
Estimated location of ssp TnV.AS insertions on the physical map of the M. xanthus wild-type strain DK1622 genome.
Cloning and sequencing of the chromosomal DNA flanking the TnV.AS transposon insertions.
For each TnV.AS insertion, the DNA flanking the insertion was cloned and the sequence of 350 to 400 bp of the DNA flanking each side of TnV.AS was determined. All 11 TnV.AS insertions were predicted to be located in ORFs, and four of these ORFs contained regions of similarity with sequences in databases. As detailed previously, ssp1 was inserted in sasS (47), and ssp2, ssp3, and ssp4 were inserted in sasR (Yang et al., submitted). The ssp10 TnV.AS insertion was in a putative ORF whose product has regions similar to Arabidopsis thaliana dTDP-glucose 4,6-dehydratase (55% identity over 236 amino acids) (31). The Salmonella enterica serovar Typhimurium equivalent of this enzyme is required for rhamnose biosynthesis (25). The ssp11 TnV.AS insertion was in an ORF whose product is similar to a number of periplasmic thioredoxin-like proteins. The highest identity was to the Rhodobacter capsulatus HelX protein (40% identity over 156 amino acids) (3). The DNA sequences flanking the transposon insertions in ssp5, ssp6, ssp7, and ssp8 were overlapping and appeared to be inserted in the same putative ORF, which is about 1 kb downstream of sasN. The predicted amino acid sequences of the ORFs identified by the ssp5 and ssp9 mutations do not show significant homology to any known protein sequences in databases.
DISCUSSION
A genetic screen has identified at least three new putative positive regulators of 4521, a gene normally expressed during early M. xanthus development, and the only previously known positive regulator of gene 4521 developmental expression, sasS. Eleven transposon insertion mutations that abolished or reduced 4521 expression were isolated using a newly modified Tn5-based transposon. Physical mapping of the transposon insertions on the M. xanthus chromosome and sequencing of the DNA flanking each insertion revealed that at least five new genes have been identified in four unlinked loci. All of the genes identified appear to affect 4521 expression by functioning either downstream or independently of A-signal generation because wild-type levels of the A signal were detected in all of the mutant cell supernatants (data not shown). In addition, the ssp suppressor mutations do not appear to interfere with the specific suppression resulting from the sasA1 allele because none of them restored LPS O-antigen biosynthesis (data not shown).
The ssp1 TnV.AS insertion mutation mapped to the already identified positive regulator of 4521, sasS, encoding a histidine protein kinase sensor (47). SasS is a major regulator of 4521 expression and has been proposed to sense the M. xanthus cell density signal, designated the A signal (39, 47). As a typical two-component system sensor, it would be expected to serve as the phosphate donor for a cognate response regulator protein when the A signal surpasses its threshold concentration. An NtrC-like response regulator (36), designated SasR, that appears to function with SasS was identified by the ssp2, ssp3, and ssp4 insertions. These TnV.AS insertions place the sasR gene about 3.5 kb downstream of sasS in the sasB locus. This gene is the focus of another report (Yang et al., submitted).
The ssp5, ssp6, ssp7, and ssp8 TnV.AS insertions all mapped to one ORF, designated here as sasP. The sasP gene is directly downstream of the negative regulator of 4521 expression, sasN. Mutations in sasP abolished 4521 expression in the wild-type and LPS O-antigen mutant backgrounds. However, the sasP mutations did not block 4521 expression in the presence of the sasS* mutation. These data suggest that SasP is a positive regulator of 4521 expression that functions upstream of SasS. It is unclear why the sasP mutations conferred no obvious vegetative or developmental defects.
The ssp9 mutation abolished 4521 expression in all of the backgrounds tested. These results suggest that ssp9 encodes a positive regulator of 4521 expression that functions downstream of SasS. If SasS and SasR form a two-component signal transduction system, Ssp9 may function downstream of SasR. Alternatively, Ssp9 may function in an independent pathway that regulates 4521 expression.
The ssp11 mutation abolished 4521 expression in the wild-type and LPS O-antigen mutant backgrounds. DNA sequence analysis indicated that the ssp11 insertion identified a gene encoding a new member of the family of periplasmic thioredoxin-like proteins. Other members of the family include E. coli CcmG (also called DsbE) (17) and R. capsulatus HelX (3). These proteins are proposed to function as thiol:disulfide oxidoreductases in a cyctochrome c-specific dithiol pathway that reduces apocytochrome c for heme attachment. The ssp11 mutant grew extremely slowly and was defective in fruiting body development. It is likely that the ssp11 gene product is only indirectly involved in M. xanthus development.
The ssp10 mutation is the most unique among this group of 11 mutations. This mutation reduced 4521 expression in the absence of LPS O antigen to a level close to that in the sasA+ parent strain and had no significant effect on 4521 expression in a wild-type background. Thus, the ssp10 mutation appears to specifically suppress the LPS O-antigen biosynthesis mutation sasA1. DNA sequence analysis suggested that the ssp10 insertion identified an enzyme required for rhamnose biosynthesis. Rhamnose appears to be one of the monosaccharide components of the M. xanthus LPS core (2) and of the extracellular polysaccharide, protein-associated material termed fibrils (4). It is likely that the ssp10 mutation affects the M. xanthus cell surface. Immunoslot blot assay results indicate that the ssp10 mutation, like all of the other ssp mutations, does not restore LPS O-antigen biosynthesis in the sasA1 background. It is unclear how the ssp10 mutation specifically restores 4521 expression to a low level in the presence of a mutation that normally increases 4521 expression due to the absence of LPS O antigen. The effects of the ssp10 mutation on developmental gene expression are likely to be distinct from its effects on fruiting body formation and sporulation. The latter effects, similar to those of the LPS O-antigen mutations (8), may result from possible motility defects due to alterations of the cell surface.
Strain DK6621, which is defective in LPS O-antigen biosynthesis, was an excellent parent for the mutagenesis experiments, since it is unusual in that its colonies are Lac+ on nutrient agar. As a result, it was relatively simple to screen for mutations that reduced or abolished 4521 expression. Presumptive candidates were transfectants that grew on nutrient agar plates containing kanamycin and that were tan when overlaid with X-Gal. It is interesting to note that although DK6621 colonies are Lac+, cells grown in nutrient broth express 4521 at a low level and are essentially Lac−. This apparent discrepancy may reflect the fact that in vegetative colonies, many cells are already starving. The mechanism by which the absence of the LPS O antigen bypasses the cell density requirement for 4521 expression is unknown. It appears to stimulate the SasS sensor by a novel mechanism that is intrinsic to the cell (6). This kind of stimulation of the sensor transduction system is ideal for such a screen because it should not bias the type of positive regulators to be identified; positive regulators functioning upstream or downstream of the SasS kinase should be identifiable. If a strain containing the gain-of-function mutation sasS* were used as the parent strain, then no elements that function upstream of SasS would be expected to be identified.
This mutagenesis study has been successful in identifying a number of positive regulators of 4521 expression. The facts that a known positive regulator was identified and that other genes were identified by multiple insertions suggest that mutagenesis may have been saturating. The ability to map the genes on the M. xanthus physical map was very useful. It indicated that three of the six genes map to one locus, sasB, clearly showing that this is an important region for the control of developmental gene expression. Another putative positive regulator was found to be linked to the 4521 reporter itself. These data indicate that only ssp10 may be responsive to the cell surface-mediated increase in 4521 expression. All of these genes are candidates for further studies to understand how M. xanthus cells integrate information on their nutrient status, cell density, and cell surface to determine if conditions are appropriate to initiate the complex behavioral response of fruiting body formation.
ACKNOWLEDGMENTS
We thank B. Julien for P4 phage and plasmids and technical advice, S. Kaplan for use of the TAFE apparatus, and J. Eraso and J. Gibson for comments on the manuscript. C. Yang, D. Xu, and J. Rivera supplied information concerning the sasB locus.
This investigation was supported by Public Health Service grant GM47444 to H.B.K. from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ashton A. Structural studies of the lipopolysaccharide from Myxococcus xanthus and lipopolysaccharide mutants. Ph.D. thesis. Minneapolis: University of Minnesota; 1993. [Google Scholar]
- 3.Beckman D L, Kranz R G. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc Natl Acad Sci USA. 1993;90:2179–2183. doi: 10.1073/pnas.90.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behmlander R M, Dworkin M. Biochemical and structural analysis of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6295–6303. doi: 10.1128/jb.176.20.6295-6303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 6.Bowden M G. Cell surface regulation of Myxococcus xanthus social motility and multicellular development. Ph.D. thesis. Houston: University of Texas—Houston Health Sciences Center; 1999. [Google Scholar]
- 7.Bowden M G, Kaplan H B. The Myxococcus xanthus developmentally expressed asgB-dependent genes can be targets of the A signal-generating or A signal-responding pathway. J Bacteriol. 1996;178:6628–6631. doi: 10.1128/jb.178.22.6628-6631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowden M G, Kaplan H B. The Myxococcus xanthus LPS O-antigen is required for social motility and multicellular development. Mol Microbiol. 1998;30:275–284. doi: 10.1046/j.1365-2958.1998.01060.x. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage Mx4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen H W, Kuspa A, Keseler I M, Shimkets L J. Physical map of the Myxococcus xanthus chromosome. J Bacteriol. 1991;173:2109–2115. doi: 10.1128/jb.173.6.2109-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho K, Zusman D. AsgD, a new two-component regulator required for A-signalling and nutrient sensing during early development of Myxococcus xanthus. Mol Microbiol. 1999;34:268–281. doi: 10.1046/j.1365-2958.1999.01594.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis J M, Mayor J, Plamann L. A missense mutation in rpoD results in an A signaling defect in Myxococcus xanthus. Mol Microbiol. 1995;18:943–952. doi: 10.1111/j.1365-2958.1995.18050943.x. [DOI] [PubMed] [Google Scholar]
- 14.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dworkin M, Kaiser D. Myxobacteria II. Washington, D.C.: American Society for Microbiology; 1993. [Google Scholar]
- 17.Fabianek R A, Hennecke H, Thony-Meyer L. The active-site cysteines of the periplasmic thioredoxin-like protein CcmG of Escherichia coli are important but not essential for cytochrome c maturation in vivo. J Bacteriol. 1998;180:1947–1950. doi: 10.1128/jb.180.7.1947-1950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuichi T, Inouye M, Inouye S. Novel one-step cloning vector with a transposable element: application to the Myxococcus xanthus genome. J Bacteriol. 1985;164:270–275. doi: 10.1128/jb.164.1.270-275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisselsoder J, Campos J M, Zusman D R. Physical characterization of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:179–189. doi: 10.1016/0022-2836(78)90432-1. [DOI] [PubMed] [Google Scholar]
- 20.Gulati P, Xu D, Kaplan H B. Identification of the minimum regulatory region of a Myxococcus xanthus A-signal-dependent developmental gene. J Bacteriol. 1995;177:4645–4651. doi: 10.1128/jb.177.16.4645-4651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo D, Bowden M G, Pershad R, Kaplan H B. The Myxococcus xanthus rfbABC operon encodes an ABC transporter homolog required for O-antigen biosynthesis and multicellular development. J Bacteriol. 1996;178:1631–1639. doi: 10.1128/jb.178.6.1631-1639.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Harris B Z, Kaiser D, Singer M. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 1998;12:1022–1035. doi: 10.1101/gad.12.7.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, Chen H, Kuspa A, Chen Y, Kaiser D, Shimkets L J. A physical map of the Myxococcus xanthus chromosome. Proc Natl Acad Sci USA. 1994;91:9584–9587. doi: 10.1073/pnas.91.20.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X M, Neal B, Santiago F, Lee S J, Romana L K, Reeves P R. Structure and sequence of the rfb (O-antigen) gene cluster of Salmonella serovar typhimurium (strain LT2) Mol Microbiol. 1991;5:695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 26.Kahn M L, Ziermann R, Deho G, Ow D W, Sunshine M G, Calender R. Bacteriophage P2 and P4. Methods Enzymol. 1991;204:264–280. doi: 10.1016/0076-6879(91)04013-e. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan H B, Kuspa A, Kaiser D. Suppressors that permit A-signal-independent developmental gene expression in Myxococcus xanthus. J Bacteriol. 1991;173:1460–1470. doi: 10.1128/jb.173.4.1460-1470.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 30.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushnir S, Babiychuk E, Kampfenkel K, Belles-Boix E, Van Montagu M, Inze D. Characterization of Arabidopsis thaliana cDNAs that render yeasts tolerant toward the thiol-oxidizing drug diamide. Proc Natl Acad Sci USA. 1995;92:10580–10584. doi: 10.1073/pnas.92.23.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuspa A, Kroos L, Kaiser D. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev Biol. 1986;117:267–276. doi: 10.1016/0012-1606(86)90369-6. [DOI] [PubMed] [Google Scholar]
- 33.Kuspa A, Plamann L, Kaiser D. Identification of heat-stable A-factor from Myxococcus xanthus. J Bacteriol. 1992;174:3319–3326. doi: 10.1128/jb.174.10.3319-3326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuspa A, Plamann L, Kaiser D. A-signalling and the cell density requirement for Myxococcus xanthus development. J Bacteriol. 1992;174:7360–7369. doi: 10.1128/jb.174.22.7360-7369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesbah M, Premachandran U, Whitman W B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 36.North A, Klose K, Stedman K, Kustu S. Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol. 1993;175:4267–4273. doi: 10.1128/jb.175.14.4267-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkinson J S. Genetic approaches for signaling pathways and proteins. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 9–23. [Google Scholar]
- 38.Plamann L, Davis J M, Cantwell B, Mayor J. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J Bacteriol. 1994;176:2013–2020. doi: 10.1128/jb.176.7.2013-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plamann L, Kaplan H B. Cell-density sensing during early development in Myxococcus xanthus. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 67–82. [Google Scholar]
- 40.Plamann L, Kuspa A, Kaiser D. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J Bacteriol. 1992;174:3311–3318. doi: 10.1128/jb.174.10.3311-3318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plamann L, Li Y, Cantwell B, Mayor J. The Myxococcus xanthus asgA gene encodes a novel signal transduction protein required for multicellular development. J Bacteriol. 1995;177:2014–2020. doi: 10.1128/jb.177.8.2014-2020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Singer M, Kaiser D. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 1995;9:1633–1644. doi: 10.1101/gad.9.13.1633. [DOI] [PubMed] [Google Scholar]
- 44.Wall D, Kolenbrander P E, Kaiser D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J Bacteriol. 1999;181:24–33. doi: 10.1128/jb.181.1.24-33.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu T T. A model for three-point analysis of random generalized transduction. Genetics. 1966;54:405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu D, Yang C, Kaplan H B. Myxococcus xanthus sasN encodes a regulator that prevents developmental gene expression during growth. J Bacteriol. 1998;180:6215–6223. doi: 10.1128/jb.180.23.6215-6223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, Kaplan H B. Myxococcus xanthus sasS encodes a sensor histidine kinase required for early developmental gene expression. J Bacteriol. 1997;179:7759–7767. doi: 10.1128/jb.179.24.7759-7767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]