Abstract
Background
The BNT162b2 (Pfizer-BioNTech) two-dose vaccine regiment for children and the BNT162b2 third dose for adolescents were approved shortly before the SARS-CoV-2 omicron (B.1.1.529) outbreak in Israel. We aimed to estimate the effects of these vaccines on the rates of confirmed infection against the omicron variant in children and adolescents.
Methods
In this observational cohort study, we extracted data for the omicron-dominated (sublineage BA.1) period. We compared rates of confirmed SARS-CoV-2 infection between children aged 5–10 years 14–35 days after receiving the second vaccine dose with an internal control group of children 3–7 days after receiving the first dose (when the vaccine is not yet effective). Similarly, we compared confirmed infection rates in adolescents aged 12–15 years 14–60 days after receiving a booster dose with an internal control group of adolescents 3–7 days after receiving the booster dose. We used Poisson regression, adjusting for age, sex, socioeconomic status, calendar week, and exposure.
Findings
Between Dec 26, 2021, and Jan 8, 2022, we included 1 158 289 participants. In children aged 5–10 years, the adjusted rate of confirmed infection was 2·3 times (95% CI 2·0–2·5) lower in children who received a second dose than in the internal control group. The adjusted infection rate in children who received a second dose was 102 infections per 100 000 risk-days (94–110) compared with 231 infections per 100 000 risk-days (215–248) in the corresponding internal control cohort. In adolescents aged 12–15 years, the booster dose decreased confirmed infection rates by 3·3 times (2·8–4·0) compared with in the internal control group. The adjusted infection rate of the booster cohort was 70 per 100 000 risk-days (60–81) compared with 232 per 100 000 risk-days (212–254) in the internal control cohort.
Interpretation
A recent two-dose vaccination regimen with BNT162b2 and a recent booster dose in adolescents substantially reduced the rate of confirmed infection compared with the internal control groups. Future studies are needed to assess the duration of this protection and protection against other outcomes such as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 and long-COVID.
Funding
None.
Introduction
The effectiveness of the BNT162b2 (Pfizer–BioNTech) vaccine has been shown to be poorer against the omicron (B.1.1.529) variant than against the delta (B.1.617.2) variant and other variants in terms of infection and hospitalisation.1 However, little evidence supports the real-world effectiveness of the BNT162b2 vaccine against the omicron variant in children and adolescents.
In Israel, the two-dose BNT162b2 vaccine regimen was approved for adolescents aged 12–15 years on June 2, 2021, and a booster dose was approved starting Aug 29, 2021, for individuals who received the second dose at least 5 months previously. For children aged 5–11 years, the two-dose vaccination (using a third of the dosage given for individuals aged ≥12 years) was administered starting Nov 23, 2021.
For children, studies from the USA estimated vaccine effectiveness against confirmed SARS-CoV-2 infection of 65% in the first 2 weeks after vaccination, followed by rapid waning.3, 4 The vaccine effectiveness was lower for children aged 5–11 years than for those aged 12–15 years. A report from the US Centers for Disease Control and Prevention (CDC)5 that was based on a cohort of about 1000 children aged 5–11 years estimated vaccine effectiveness against SARS-CoV-2 infection to be 31% up to 82 days from the date of vaccination. In adolescents aged 16–18 years, a booster dose was shown to lower the confirmed infection rate by 3·7 times compared with two doses during the delta wave.6 For adolescents aged 12–15 years, a booster dose was shown to improve protection against omicron infection by 2·9 times compared with two doses.4 In this study, we estimated (1) the adjusted rates of confirmed infection following two doses of the BNT162b2 vaccine in children aged 5–10 years up to 35 days from the second dose, and (2) the adjusted rates of confirmed infections following a third dose of the BNT162b2 vaccine in adolescents aged 12–15 years up to 60 days from this dose.
Research in context.
Evidence before this study
We searched PubMed, Google Scholar, medRxiv and relevant journals for studies of COVID-19 vaccine effectiveness in children and adolescents, using search terms such as “COVID-19 vaccine effectiveness” and search terms that included the younger age groups, including “COVID-19 vaccine effectiveness children”, and “COVID-19 vaccine effectiveness adolescents”, without any language restrictions. The search was done on June 20, 2022. For each relevant paper, we further looked at its references and papers that cited it. Because vaccinations for children were only approved on Oct 29, 2021, we only searched for studies from Dec 1, 2021, or later. We found three highly relevant papers—one analysing New York (USA) data, and two reports by the US Centers for Disease Control and Prevention (CDC). These reports showed that a two-dose BNT162b2 vaccine regimen in children aged 5–11 years reduced confirmed infection rates of the omicron variant (B.1.1.529) by around 2 times in the first weeks after vaccination, and that a booster dose in adolescents aged 12–15 years reduced confirmed infection rates by around 3 times compared with a second dose.
Added value of this study
Our findings add to the existing evidence on the vaccine effectiveness of BNT162b2 against confirmed SARS-CoV-2 infection in young age groups, which is scarce. Our results show that the BNT162b2 vaccine provided an initial increased protection of around 2 times against infection in children aged 5–10 years. The estimated protection is in line with the vaccine effectiveness results estimated in the USA for a similar study period and time from receipt of the vaccine. Vaccine effectiveness in our study was somewhat higher than that reported by the CDC, possibly because of waning immunity, because more time had passed since vaccination in the CDC study. Our analysis further showed that a recent booster dose in adolescents decreased infections by around 3–4 times compared with in the internal control, which is similar to estimates from the reported by the CDC.
Implications of all the available evidence
The findings of the current study join existing evidence of the BNT162b2 vaccine in children and adolescents against confirmed SARS-CoV-2 infection while omicron was dominant and can support policy making decisions regarding vaccination regimes for young age groups. Future studies are needed to assess the duration of this protection and protection against other outcomes such as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 and long-COVID.
Methods
Study design and participants
In this observational study, we analysed observational data on SARS-CoV-2 infections collected during Israel's fifth wave of infection, during which omicron (BA.1 sublineage) was dominant.2 We used the Israeli Ministry of Health database (appendix p 2) that includes information on all vaccinations and tests that had been done in Israel.
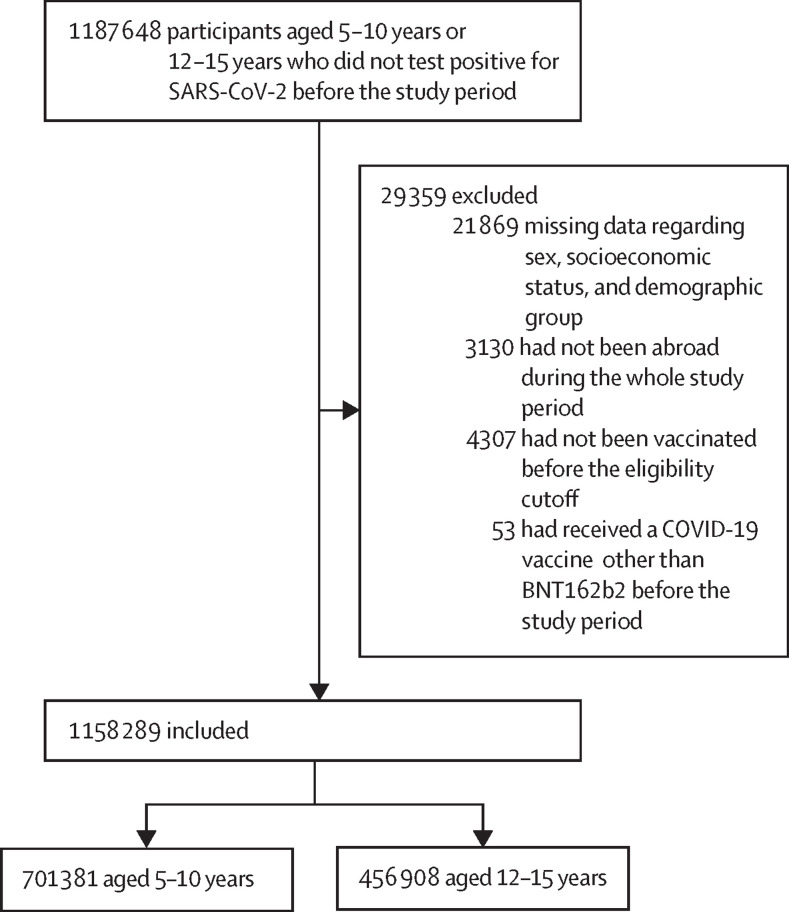
The study population included children aged 5–10 years and adolescents aged 12–15 years who were either vaccinated or took at least one SARS-CoV-2 test (PCR or state-regulated antigen) before Dec 1, 2021. We excluded individuals aged 11 years because our data included age in years only (according to each individual's age on June 1, 2021), and vaccination eligibility dates differed between 11-year-olds and 12-year-olds. Thus, some individuals in the 11-year-old group turned 12 years old before or during the study period and became eligible to receive the vaccine. We excluded individuals who had documented positive PCR or state-regulated antigen results before the study period; had stayed abroad during the entire study period; had missing data regarding sex, demographic group, or socioeconomic status; or had received a vaccine different from BNT162b2 before the end of the study period (figure 1 ).
Figure 1.
Study population
The study population included individuals aged 5–10 years or 12–15 years, who had no documented positive PCR for SARS-CoV-2 infection or a positive state-regulated antigen result before the study period, had not stayed abroad during the whole study period, had not been vaccinated before their age-group eligibility time, and had not been vaccinated with a vaccine other than BNT162b2 before the beginning of the study period.
Procedures
We compared the confirmed SARS-CoV-2 infection rates in different cohorts during a 2-week study period (Dec 26, 2021, to Jan 8, 2022) in which national surveillance testing was functioning well and before home antigen testing became common. Because of policy changes in testing and isolation of contacts and quarantine in schools, reliable estimates of effectiveness are difficult to obtain for the period after Jan 8, 2022.
On each day of the follow-up, children aged 5–10 years were divided into three cohorts: those unvaccinated, those who received the second dose of vaccine at least 14 days previously (with respect to each day of the study period), and an internal control cohort of those who received their first dose 3–7 days previously. Adolescents aged 12–15 years were divided into six cohorts: those unvaccinated, those who received two doses of the vaccine only (divided according to time since the second dose [14–59, 60–119, and ≥120 days]), those who received a booster (third) dose at least 14 days previously, and an internal control cohort of those who received the booster dose 3–7 days previously. Cohort membership was dynamic—ie, during the study period an individual could leave one cohort and join another.
Differences in behavioural and exposure characteristics,7, 8 and possibly in the proportion of undocumented previous infections,9 might bias comparisons between the two-dose and the unvaccinated cohorts in children and between the booster-dose cohorts and other cohorts in adolescents. To mitigate such biases, our main analysis focused on comparisons with the internal control cohorts that had received the vaccine, but before the vaccine was expected to affect their risk of confirmed infection.10 We designed the internal control groups to be similar to the reference group to which the protection conferred by the vaccine is being compared—ie, to unvaccinated individuals in the case of children aged 5–10 years (who have received two doses) and to second dose (≥5 months) in the case of adolescents aged 12–15 years (who would be receiving a third booster dose). Therefore, for children aged 5–10 years, the internal control cohort included children on days 3–7 since receiving the first dose, when their protection was similar to that of unvaccinated individuals. For adolescents aged 12–15 years, the internal control cohort included adolescents on days 3–7 since receiving the booster dose because they were expected to have similar protection to that of individuals who received their second dose several months earlier (those with waned protection).
Because the two age groups became eligible to receive either the two-dose vaccination or the third dose not long before the study period, we estimated only the short-term protection conferred by these vaccinations (appendix p 3). The main analysis of adolescents focused on assessing the relative protection conferred by the third dose compared with that of the second dose because a small proportion of individuals in this age group received the second dose during the relevant time period. The main analysis included only the general Jewish population, as vaccination rates were very low in the Arab and Jewish ultra-orthodox populations (appendix p 2).
The study was approved by the Institutional Review Board of the Sheba Medical Center, Helsinki approval number SMC-8228-21. The requirement for consent was waived. The investigators did not have access to de-anonymised information.
Our primary measure was the adjusted rate of confirmed infection in each of the cohorts, adjusted for age (by year), sex, socioeconomic group, calendar week, and an exposure risk measure.
Statistical analysis
We analysed the data using methodology similar to that used in our previous studies.11 We counted the number of confirmed infections and the number of days at risk during the study period for each cohort. For each age group, we used a separate Poisson regression model to estimate the adjusted rate of confirmed infections per 100 000 risk days, adjusting for age (1-year categories), sex, socioeconomic status (low, medium, or high), calendar week, and an exposure risk measure. We calculated the exposure risk measure for each person on each follow-up day according to the proportion of new confirmed infections during the past 7 days in their area of residence; the measure was then divided into five categories according to quintiles (see Bar-On et al11 for details). A national mean risk was imputed to individuals with missing data on residency (only 25 individuals were missing this data). The population (general Jewish, ultra-orthodox Jewish, and Arab) and socioeconomic status (SES) were determined by the Israel Central Bureau of Statistics on the basis of the statistical area of residence (similar to a census block). Specifically, the Central Bureau of Statistic classified municipalities into ten clusters of SES on the basis of information such as demographics, education, and employment. Our analysis considers clusters 1–3 as low, clusters 4–6 as medium. and clusters 7–10 as high SES.
We did a secondary statistical analysis using a matching approach, similar to that used by Dagan and colleagues.13 In this approach, individuals who received the vaccine were matched with individuals who did not. Specifically, on each day of the study period, each individual aged 5–10 years who became vaccinated (14 days after the second dose) was matched with another individual from that age group who had not yet received any vaccine dose. Similarly, each individual aged 12–15 years who received the booster dose 14 days previously was matched with an individual who was eligible to receive the third dose but had not yet received it. Matching was done on the basis of the following characteristics: age (by year), sex, city, and SES. Follow-up for matched individuals ended at the time of infection. Both individuals in a pair were censored at the end of the study or at the time the control matched individual received a vaccine dose (first dose for individuals aged 5–10 years or third dose for those aged 12–15 years). For each treatment group, we calculated the probability of being free of infection using the Kaplan-Meier estimator. We used the ratio between the probabilities of the treatment group and the control group as an estimate for the risk ratio for our population over the study period. We generated 95% CIs around this estimate using the percentile bootstrap method with 400 repetitions.
We did several sensitivity analyses. To examine the effect of including all population sectors (general Jewish, Jewish ultra-orthodox, and Arab), we repeated the analysis on the whole population adding the sector as a covariate in the analysis. While our main analysis included only 2 weeks until Jan 8, after which state-regulated tests were supplemented by undocumented home tests for vaccinated individuals, a second analysis extended the study period by an additional week (to Jan 15, 2022). We also analysed the rate of confirmed infection over a shorter study period, including only the week of Jan 2, to Jan 8, 2022. The shorter time period has the benefit of maintaining a similar rate of exposure throughout the study period because the number of cases in the second week of the main study period was much higher than that in the first week. All analyses were done using R (version 4.1).
Role of the funding source
There was no funding source for this study.
Results
In the main analysis, we included 190 058 individuals (128 522 aged 5–10 years and 61 536 aged 12–15 years.(table 1 ; details on other cohorts are given in the appendix pp 5–7). The proportion of days at risk for female participants was similar in all cohorts and varied between 48·3% and 48·9% (table 1), and the proportion of days at risk for male participants varied between 51·1% and 51·7%. We found 347 726 (94·9%) of 366 364 person-days at risk in children aged 5–10 years who received their second dose and 171 281 (95·8%) of 178 780 person-days at risk in adolescents aged 12–15 years who received their booster were from the general Jewish sector (table 1). 259 866 (70·9%) of 366 364 person-days at risk in children aged 5–10 years were from the high SES group, and 25 641 (7·0%) of 366 364 person-days at risk were from the low SES group. This difference was more pronounced in adolescents aged 12–15 years who received their booster, among whom 136 121 (76·1%) of 178 780 person-days at risk were in the high SES group, whereas only 9473 (5·3%) of 178 780 person-days at risk were in the low SES group (table 1).
Table 1.
Characteristics of the study groups included in the main analysis
|
Ages 5–10 years (second-dose effect) |
Ages 12–15 years (third-dose effect) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Internal control (3–7 days from first dose); 367 168 person-days at risk; 71 703 individuals |
Second dose (14–35 days from second dose) 366 364 person-days at risk; 56 819 individuals |
Internal control (3–7 days from third dose); 190 139 person-days at risk; 43 375 individuals |
Third dose (14–60 days from third dose); 178 780 person-days at risk; 24 393 individuals |
|||||
| Person-days at risk (%) | Infections, n | Person-days at risk (%) | Infections, n | Person-days at risk (%) | Infections, n | Person-days at risk (%) | Infections, n | |
| Sex | ||||||||
| Female | 178 174 (48·5%) | 411 | 177 130 (48·3%) | 287 | 92 113 (48·4%) | 265 | 87 392 (48·9%) | 99 |
| Male | 188 994 (51·5%) | 411 | 189 234 (51·7%) | 315 | 98 026 (51·6%) | 253 | 91 388 (51·1%) | 80 |
| Population | ||||||||
| General Jewish | 318 513 (86·7%) | 743 | 347 726 (94·9%) | 576 | 180 100 (94·7%) | 494 | 171 281 (95·8%) | 166 |
| Ultra-orthodox | 24 140 (6·6%) | 69 | 11 871 (3·2%) | 21 | 5979 (3·1%) | 20 | 4875 (2·7%) | 13 |
| Arab | 24 515 (6·7%) | 10 | 6767 (1·8%) | 5 | 4060 (2·1%) | 4 | 2624 (1·5%) | 0 |
| Socioeconomic status | ||||||||
| Low | 54 804 (14·9%) | 83 | 25 641 (7·0%) | 31 | 12 513 (6·6%) | 36 | 9473 (5·3%) | 13 |
| Medium | 84 710 (23·1%) | 220 | 80 857 (22·1%) | 119 | 38 293 (20·1%) | 107 | 33 186 (18·6%) | 28 |
| High | 227 654 (62·0%) | 519 | 259 866 (70·9%) | 452 | 139 333 (73·3%) | 375 | 136 121 (76·1%) | 138 |
Table shows the proportion of person-days at risk instead of the proportion of individuals. Values are presented for the study period, Dec 26, 2021, to Jan 8, 2022.
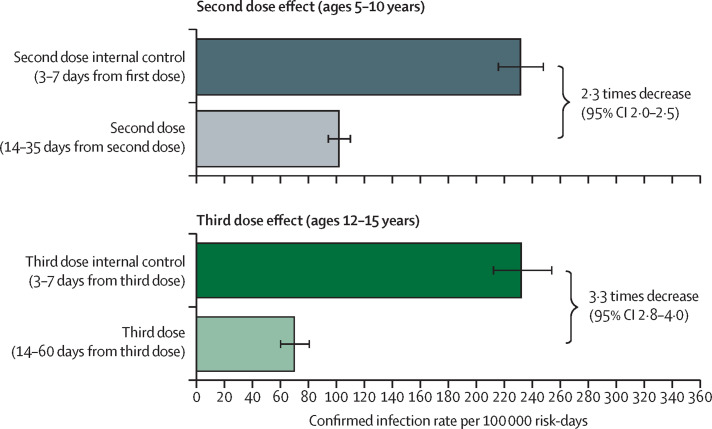
We found that, for children aged 5–10 years, two doses provided a decrease of 2·3 times (95% CI 2·0–2·5) in the rate of confirmed SARS-CoV-2 infection compared with the internal control cohort (figure 2 ; table 2 ). The estimated infection rate in children who received a second dose was 102 infections per 100 000 risk-days (94–110), compared with 231 infections per 100 000 risk-days (215–248) in the corresponding internal control cohort (table 2; appendix p 6). In adolescents aged 12–15 years, the third dose decreased the confirmed SARS-CoV-2 infection rates by 3·3 times (2·8–4·0; figure 2). The estimated infection rate in the booster cohort was 70 per 100 000 risk-days (60–81) compared with 232 per 100 000 risk-days (212–254) in the internal control cohort (table 3 ; figure 2).
Figure 2.
Adjusted rate of confirmed infections for the main study cohorts
Adjusted rate of confirmed infections per 100 000 risk-days obtained from a Poisson regression analysis for the study period Dec 26, 2021, to Jan 8, 2022, adjusted for age category, sex, socioeconomic status (low, medium, or high), calendar week, and exposure. Wings represent 95% CIs, which were not adjusted for multiplicity.
Table 2.
Adjusted rate ratios of study cohort versus the vaccinated cohorts of interest in children aged 5–10 years
| Confirmed infections (at-risk days), n | Adjusted rate per 100 000 at-risk days (95% CI) | Adjusted rate ratio vs second dose | |
|---|---|---|---|
| Unvaccinated | 10 048 (4 420 027) | 239 (235–244) | 2·4 (2·2–2·6) |
| Internal control | 743 (318 513) | 232 (215–248) | 2·3 (2·0–2·5) |
| Second dose (14–35 days) | 576 (347 726) | 102 (94–110) | Ref |
Adjusted for age category, sex, socioeconomic status (low, medium, or high), calendar week, and an exposure risk measure, for the study period, Dec 26, 2021, to Jan 8, 2022. Results are shown for the main study population and thus include only the general Jewish population.
Table 3.
Adjusted rate ratios of study cohort versus the vaccinated cohorts of interest in adolescents aged 12–15 years
| Confirmed infections (at-risk days), n | Adjusted rate per 100 000 at-risk days (95% CI) | Adjusted rate ratio vs third dose (95% CI) | |
|---|---|---|---|
| Unvaccinated | 2684 (834 149) | 349 (336–363) | 5·0 (4·3–5·9) |
| Second dose (14–60 days) | 153 (115 371) | 155 (132–182) | 2·2 (1·8–2·8) |
| Second dose (60–120 days) | 1999 (815 036) | 267 (257–280) | 3·8 (3·3–4·5) |
| Second dose (>120 days) | 5983 (2 003 011) | 291 (284–299) | 4·2 (3·6–4·9) |
| Internal control | 494 (180 100) | 232 (212–254) | 3·3 (2·8–4·0) |
| Third dose (14–60 days) | 166 (171 281) | 70 (60–81) | Ref |
Adjusted for age category, sex, socioeconomic status (low, medium, or high), calendar week, and an exposure risk measure, for the study period, Dec 26, 2021, to Jan 8, 2022. Results are shown for the main study population and thus include only the general Jewish population.
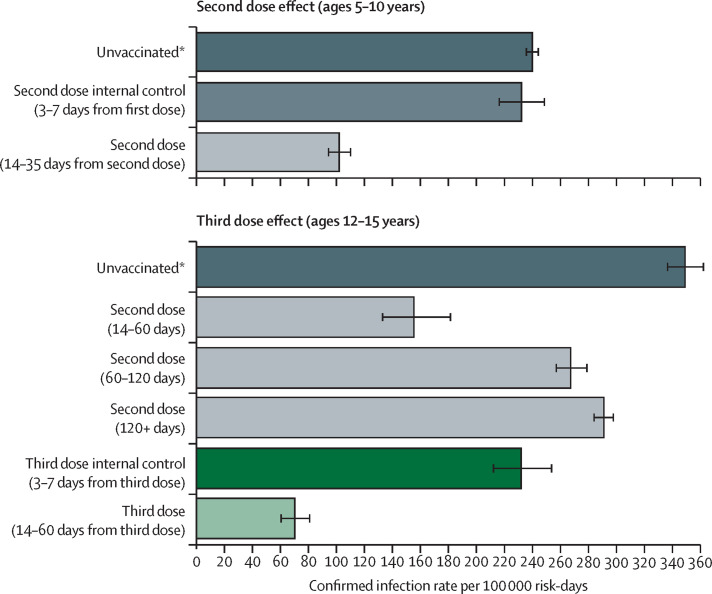
The estimated infection rate in unvaccinated children was 239 infections per 100 000 risk-days (235–244; figure 3 ). The protection rate provided by the second dose compared with the unvaccinated cohort was 2·4 (2·2–2·6; figure 3). These results are similar to that of the main analysis. In adolescents aged 12–15 years, the protection from infection of the two-dose vaccine regimen waned quickly over time, with infection rates rising from 155 per 100 000 risk-days (95% CI 132–182) among those who received their second dose 14–60 days previously to 291 per 100 000 risk-days (284 to 299) among those receiving the second dose more than 120 days previously (table 3). Protection was restored and substantially increased with receipt of the third dose, with an adjusted rate of confirmed infection of 70 per 100 000 person-days at risk (60–81; table 2; figure 3). Comparing the effects of the third dose with individuals who received the second dose more than 120 days previously yielded a protection rate of 4·2 (3·6–4·9). This estimate is slightly higher than the protection rate compared with the internal control group, which was 3·3 (2·8–4·0; table 3)
Figure 3.
Adjusted rate of confirmed infections including the unvaccinated cohort
Adjusted rate of confirmed infections per 100 000 risk-days obtained from a Poisson regression analysis for the study period Dec 26, 2021, to Jan 8, 2022, stratified by age groups and adjusted for age category, sex, socioeconomic status (low, medium, or high), calendar week, and an exposure risk measure. Wings represent 95% CIs, which were not adjusted for multiplicity. *All groups, and in particular the unvaccinated group, probably had proportions of individuals with undocumented previous infections. Therefore, the adjusted confirmed infection rates are likely to be underestimated.
The secondary analysis yielded similar results (table 4 ). Confirmed SARS-CoV-2 infection rates decreased by 2·2 times (95% CI 2·0–2·4) in children aged 5–10 years and by 4·0 times (3·5–4·5) in adolescents aged 12–15 years (table 4). The sensitivity analyses that considered different study periods and population groups yielded similar results (table 4). To ensure that the lower confirmed infection rates in the vaccinated cohorts compared with the unvaccinated cohorts were not explained by different testing behaviour, we further compared testing rates in the different cohorts (appendix p 4). In children aged 5–10 years, the testing rate in the unvaccinated cohort (approximately 19 000 individuals who tested at least once per 100 000 people) was lower than that in both the internal control group (approximately 30 000) and the vaccinated cohort (approximately 23 000). In adolescents, the testing rates behaved similarly in the corresponding cohorts with rates of around 16 000 for the unvaccinated, 26 000 in the internal control group and 30 000 in the booster cohort.
Table 4.
Results from sensitivity analyses
|
Ages 5–10 years (second-dose effect) |
Ages 12–15 years (third-dose effect) |
|||||
|---|---|---|---|---|---|---|
| Second dose confirmed infections (at-risk days), n | Internal control confirmed infections (at-risk days), n | Adjusted rate ratio internal control vs second dose (95% CI) | Third dose confirmed infections (at-risk days), n | Internal control confirmed infections (at-risk days), n | Adjusted rate ratio internal control vs third dose (95% CI) | |
| Main analysis | 576 (347 726) | 743 (318 513) | 2·3 (2·0–2·5) | 166 (171 281) | 494 (180 100) | 3·3 (2·8–4·0) |
| All population groups | 602 (366 364) | 822 (367 168) | 2·3 (2·1–2·6) | 179 (178 780) | 518 (190 139) | 3·2 (2·7–3·8) |
| Longer study period | 3142 (840 479) | 1699 (430 094) | 1·9 (1·8–2·0) | 911 (410 853) | 1379 (285 267) | 2·9 (2·6–3·1) |
| Shorter study period | 530 (286 144) | 524 (148 127) | 2·2 (2·0–2·5) | 148 (120 433) | 395 (103 922) | 3·2 (2·7–3·9) |
| Matching | .. | .. | 2·2 (2·0–2·4) | .. | .. | 4·0 (3·5–4·5) |
Results from sensitivity analyses including all population groups (general Jewish, ultra-orthodox Jewish, and Arab), longer (Dec 26, 2021, to Jan 15, 2022), and shorter (Jan 2, 2022, to Jan 8, 2022) study periods.
Discussion
Our results show that the BNT162b2 vaccine provided an initial increased protection of around two times against SARS-CoV-2 infection in children aged 5–10 years old. The estimated protection is in line with the results of vaccine effectiveness against infection estimated in the USA for a similar study period and time from receipt of the vaccine.3, 4 Vaccine effectiveness was somewhat higher than that reported by the CDC,5 possibly because of waning immunity, because more time had passed since vaccination in the CDC study. Our analysis further showed that a recent booster dose in adolescents decreased the proportion of infections by 3–4 times compared with in the internal control, which is similar to estimates from the USA.4
We note that the lower confirmed SARS-CoV-2 infection rates in the vaccinated cohorts compared with in the unvaccinated cohorts are not explained by different testing behaviour. Specifically, the proportion of individuals who were tested at least once was smaller in the unvaccinated cohorts in both age groups than in the vaccinated cohorts, suggesting that the estimated protection compared with that of unvaccinated individuals might be underestimated. Although the unvaccinated cohorts had lower testing rates than the vaccinated cohorts did, in children aged 5–10 years, the internal control group had a somewhat higher testing rate than those who received a second dose did, which might lead to an overestimation of the protection conferred by the vaccine. In vaccinated adolescents aged 12–15 years, the internal control group had a lower testing rate than the booster cohort did, which might suggest a higher level of protection conferred by the booster than that estimated in our analysis. We also note that in adolescents aged 12–15 years, the internal control cohort (who received the second dose >150 days previously) had a lower infection rate than did the two cohorts who received the vaccine more than 60 days previously. This finding might be related to the so-called healthy vaccinee bias,14 when people who feel ill tend not to get vaccinated in the following days, or to other behavioural biases (eg, adhering to social distancing or mask wearing.).
The study had several limitations. Our results only provide an estimate of the short-term protection against confirmed SARS-CoV-2 infection conferred by the BNT162b2 vaccine. Extending the study period and obtaining reliable estimates for a longer follow-up time was not possible for several reasons. First, from Jan 6, 2022, changes were made to the testing and isolation policies in Israel. These changes, which included the extended use of home testing, make estimating the rate of confirmed infection in children and adolescents difficult. Second, using the internal control cohorts in later time periods might lead to biases because rates of vaccination declined. Moreover, individuals who enter these internal control cohorts after the study period might have higher rates of undocumented previous infections compared with those who were vaccinated earlier because the high exposure during the omicron wave. Additionally, the vaccination rates for the two-dose vaccine in children aged 5–10 years and for the third dose in adolescents aged 12–15 years were fairly low, making the vaccinated individuals a fairly selected group. This selection bias is addressed through the comparisons with the internal control groups; the absolute number of individuals in each cohort was sufficient for obtaining robust estimates.
Although we estimated protection against confirmed SARS-CoV-2 infection, the effect of the vaccine on other outcomes in these age groups remains unclear. In particular, estimation of the protection against paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-215 and long-COVID, as well as vaccine side-effects can provide additional important information for policy making regarding vaccination in these age groups.
Data sharing
The individual-level data used in this study are sensitive and cannot be publicly shared.
Declaration of interests
We declare no competing interests.
Acknowledgments
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Contributors
The final manuscript has been read and approved by all authors, who have taken care to ensure the integrity of this work. OA, LF, YG, AH, MM, and RM were responsible for the study design and writing of the manuscript. OA, OB, YB, YG, and MM analysed the data. NA, SA, OB, YB, and YG were responsible for collecting the data and for data management. NA, OA, SA-P, OB, LF, AH, and RM did the literature survey. OA, LF, YG, and MM developed the statistical analysis. OA and YG verified all the data. All the authors contributed to conceiving the study, critically reviewed the results, approved the final version, and made the decision to submit the manuscript for publication. All authors had access to the study data.
Supplementary Material
References
- 1.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Our World in Data SARS-CoV-2 variants in analyzed sequences. https://ourworldindata.org/grapher/covid-variants-area
- 3.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5–11 and 12–17 years in New York after the emergence of the omicron variant. medRxiv. 2022 doi: 10.1101/2022.02.25.22271454. published online Feb 28. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327:2210–2219. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (delta) variant predominance—eight US locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amir O, Goldberg Y, Mandel M, et al. Protection following BNT162b2 booster in adolescents substantially exceeds that of a fresh 2-dose vaccine. Nat Commun. 2022;13 doi: 10.1038/s41467-022-29578-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. COVID-19 dynamics after a national immunization program in Israel. Nat Med. 2021;27:1055–1061. doi: 10.1038/s41591-021-01337-2. [DOI] [PubMed] [Google Scholar]
- 8.Muhsen K, Na'aminh W, Lapidot Y, et al. A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020-February 2021. Lancet Reg Health Eur. 2021;7 doi: 10.1016/j.lanepe.2021.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn R, Schrag SJ, Verani JR, Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in post-marketing evaluations of COVID-19 Vaccines. Am J Epidemiol. 2022;191:800–811. doi: 10.1093/aje/kwac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On YM, Goldberg Y, Mandel M, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a Nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remschmidt C, Wichmann O, Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis. 2015;15:429. doi: 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royal College of Paediatrics and Child Health Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS)—guidance for clinicians. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual-level data used in this study are sensitive and cannot be publicly shared.