Summary
Background
Solid organ transplant recipients have attenuated immune responses to SARS-CoV-2 vaccines. In this study, we report on immune responses to 3rd- (V3) and 4th- (V4) doses of heterologous and homologous vaccines in a kidney transplant population.
Methods
We undertook a single centre cohort study of 724 kidney transplant recipients prospectively screened for serological responses following 3 primary doses of a SARS-CoV2 vaccine. 322 patients were sampled post-V4 for anti-spike (anti-S), with 69 undergoing assessment of SARS-CoV-2 T-cell responses. All vaccine doses were received post-transplant, only mRNA vaccines were used for V3 and V4 dosing. All participants had serological testing performed post-V2 and at least once prior to their first dose of vaccine.
Findings
586/724 (80.9%) patients were infection-naïve post-V3; 141/2586 (24.1%) remained seronegative at 31 (21-51) days post-V3. Timing of vaccination in relation to transplantation, OR: 0.28 (0.15-0.54), p=0.0001; immunosuppression burden, OR: 0.22 (0.13-0.37), p<0.0001, and a diagnosis of diabetes, OR: 0.49 (0.32-0.75), p=0.001, remained independent risk factors for non-seroconversion. Seropositive patients post-V3 had greater anti-S if primed with BNT162b2 compared with ChAdOx1, p=0.001.
Post-V4, 45/239 (18.8%) infection-naïve patients remained seronegative. De novo seroconversion post-V4 occurred in 15/60 (25.0%) patients. There was no difference in anti-S post-V4 by vaccine combination, p=0.50. T-cell responses were poor, with only 11/54 (20.4%) infection-naive patients having detectable T-cell responses post-V4, with no difference seen by vaccine type.
Interpretation
A significant proportion of transplant recipients remain seronegative following 3- and 4- doses of SARS-CoV-2 vaccines, with poor T-cell responses, and are likely to have inadequate protection against infection. As such alternative strategies are required to provide protection to this vulnerable group.
Funding
MW/PK received study support from Oxford Immunotec.
Keywords: COVID-19, Kidney transplant, Immunosuppression, Vaccination
Research in context.
Evidence before this study
We searched PubMed from inception to 12th May 2022 for studies in English reporting responses to 3rd and 4th dose SARS-CoV-2 vaccine in kidney transplant recipients (KTR) using the search terms “vaccine”, “kidney transplant”, “immune response”, “SARS-CoV-2”, “Covid-19”. There are currently a small number of published studies investigating immune responses to fourth dose SARS-CoV-2 vaccination in KTR with all but one study limited to patients receiving mRNA vaccines. Results from other studies show that a proportion (10-50%) of patients with no detectable serological response after 3 doses of vaccination can seroconvert after a 4th but they are unlikely to have significant anti-spike antibody concentrations or possess neutralising capabilities. There are no data available comparing heterologous and homologous vaccine dosing schedules.
Added value of this study
This study is the first to report fourth dose SARS-CoV-2 vaccine immune responses in transplant recipients receiving heterologous dosing schedules. We show that 24% and 19% of kidney transplant recipients without prior natural infection, do not have any detectable spike protein antibody in response to 3rd and 4th doses of vaccine respectively. T cell responses are poor following fourth dose vaccination regardless of prior infection status. In contrast to three-dose vaccination, there was no benefit of heterologous dosing schedule on either the proportion or magnitude of serological responses.
Implications of all the available evidence
Repeated vaccinations will not result in immune responses or protection from infection in all kidney transplant recipients. However, there is a now a broad spectrum of responses related to the diverse combination of prior infection and vaccine schedules used, baseline immunosuppression and patient comorbidities. We recommend a more personalised approach to the management of transplant recipients, initially using serological screening to identify vaccine non-responders who are most likely to be at risk of adverse outcomes following infection.
Alt-text: Unlabelled box
Introduction
There will now be significant heterogeneity in the immune repertoire against COVID-19 in the population, reflecting a combination of evolving vaccination policies over the last 2 years and infection due to an array of different variants.1 In the general population, additional booster vaccinations have served to ensure adequate protection against severe infection with the emergence of the Omicron variant. The immune signature against COVID-19 in immunocompromised people could be considered even more diverse than that of the general population, with vaccine responses being additionally dependent upon the underlying condition and treatment.2,3 Recognised as having attenuated immune responses to COVID-19 vaccines, immunocompromised people in the UK are now being offered their 5th vaccine dose, coupled with eligibility for community therapeutic interventions, including monoclonal antibody treatment, should they become infected.2,4
Some immunocompromised individuals will fail to mount an immune response to vaccination, but there remains no policy for the clinical testing of vaccine responses in this or wider population, which is largely due to the lack of a definition of an adequate response, or correlate of protection. Herein we report on the immune responses to 3rd- and 4th- doses of heterologous and homologous vaccines in a kidney transplant population, to inform the immune landscape in this severely immunosuppressed population prior to their 5th vaccine dose.
Methods
Study population
The study included 724 kidney transplant recipients, under the care of the Imperial College Renal and Transplant Centre, London. Patients were sampled at their first routine clinic appointment after their 3rd- and 4th vaccine. Patients had previously provided consent for prospective follow up following 1st and 2nd vaccine doses, as previously described.2 As such, all participants had serological testing performed following 2- (V2), and 3- (V3) vaccines, and at least once prior to their 1st dose of vaccine. An additional 322 patients were investigated for immune responses following their 4th dose (V4). All vaccines were received post-transplant, and sampling occurred between September 2021 and April 2022. The study ‘The effect of COVID-19 on Renal and Immunosuppressed patients’, sponsored by Imperial College London, was approved by the Health Research Authority, Research Ethics Committee (Reference: 20/WA/0123).
Serological testing
Serum was tested for antibodies to nucleocapsid protein (anti-NP) using the Abbott Architect SARS-CoV-2 IgG 2 step chemiluminescent immunoassay (CMIA) according to manufacturer's instructions. This is a non-quantitative assay and samples were interpreted as positive or negative with a threshold index value of 1.4. Spike protein antibodies (anti-S IgG) were detected using the Abbott Architect SARS-CoV-2 IgG Quant II CMIA. Anti-S antibody titres are quantitative with a threshold value for positivity of 7.1 BAU/ml, to a maximum value of 5680 BAU/ml.
Infection diagnosis
Infection was defined serologically or via confirmation with RT-PCR or lateral flow testing. The detection of anti-NP on current or historic samples, or the presence of anti-S at baseline (pre-vaccine) or historic samples, was required for the definition of prior infection by serological methods. Prior to December 2021, prior infection was determined by the presence of anti-NP or receptor binding domain (RBD) antibodies, using an in-house double binding antigen ELISA (Imperial Hybrid DABA; Imperial College London, London, UK), which detects total RBD antibodies.
T cell ELISpot
SARS-CoV-2 specific T-cell responses were detected using the T-SPOT® Discovery SARS-CoV-2 (Oxford Immunotec) according to the manufacturer's instructions, and as previously described.2 In brief, peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples with the addition of T-Cell Select™ (Oxford Immunotec) where indicated. 250,000 PBMCs were plated into individual wells of a T-SPOT® Discovery SARS-CoV-2 plate. The assay measures immune responses to SARS-CoV-2 structural peptide pools; S1 protein, S2 protein, and positive PHA (phytohemagglutinin) and negative controls. Cells were incubated and interferon-γ secreting T cells were detected. Spot forming units (SFU) were detected using an automated plate reader (Autoimmun Diagnostika). Infection-naïve, unvaccinated participants were used to identify a threshold for a positive response using mean +3 standard deviation SFU/106 PBMC, as previously described.2 This resulted in a cut-off for positivity of 40 SFU/106 PBMC.
Statistical analysis
Statistical analysis was conducted using Prism 9.3.1 (GraphPad Software Inc., San Diego, California). Unless otherwise stated, all data are reported as median with interquartile range (IQR). Where appropriate, Mann-Whitney U and Kruskal-Wallis tests were used to assess the difference between 2 or >2 groups, with Dunn's post-hoc test to compare individual groups. For serological responses post-V3, multivariable analysis was carried out using multiple logistic regression using variables which were found to be significant on univariable analysis, p<0.05 . The univariable factors considered are shown in Table 1. For serological and cellular responses post-V4, given the smaller sample size, we considered all of the variables shown in Table 2, and included all variables with a p value of <0.15 in the multivariable model, as shown in Supplemental Information, Table S2 and S5.
Table 1.
Clinical characteristics in 586 infection-naïve transplant recipients by serostatus following 3rd primary vaccine dose.
| Characteristics | No seroconversion | Seroconversion | p value | |
|---|---|---|---|---|
| N= 141 (%) | N= 445 (%) | |||
| Gender | Male | 93 (66.0) | 291 (65.4) | 0.90 |
| Female | 48 (34.0) | 154 (34.6) | ||
| Age at 1st vaccine | Years (Median) | 61 (51-68) | 60 (49-67) | 0.39 |
| Ethnicity | Caucasiana | 73 (51.8) | 221 (49.7) | 0.66 |
| Black | 13 (9.2) | 27 (6.1) | ||
| Indoasian | 38 (27.0) | 137 (30.8) | ||
| Other | 17 (12.1) | 60 (13.5) | ||
| Cause of ESKD | Polycystic kidney disease | 17 (12.1) | 52 (11.7) | 0.41 |
| Glomerulonephritisa | 41 (29.1) | 146 (32.8) | ||
| Diabetic nephropathy | 27 (19.1) | 67 (15.1) | ||
| Urological | 8 (5.7) | 43 (9.7) | ||
| Unknown | 30 (21.3) | 93 (20.9) | ||
| Other | 18 (12.8) | 44 (9.9) | ||
| Number of transplants received | 1 | 117 (83.0) | 395 (88.8) | 0.072 |
| ≥2 | 24 (17.0) | 50 (11.2) | ||
| 1st vaccine <1 year post- | No | 116 (82.3) | 418 (93.9) | <0.0001 |
| Transplant | Yes | 25 (17.7) | 27 (6.1) | |
| Type of transplant | Deceased Donor | 83 (58.9) | 240 (53.9) | 0.07 |
| Living Donora | 49 (34.8) | 193 (43.4) | ||
| Simultaneous Pancreas-Kidney | 9 (6.4) | 12 (2.7) | ||
| Induction agent | Alemtuzumaba | 76 (53.9) | 325 (73.0) | <0.0001 |
| IL2 receptor antagonist | 32 (22.7) | 41 (9.2) | ||
| None | 5 (3.5) | 16 (3.6) | ||
| Unknown | 28 (19.9) | 63 (14.2) | ||
| Immunosuppression type | CNI Monotherapya | 29 (20.6) | 244 (54.8) | <0.0001 |
| CNI/MMF (orAza) | 57 (40.4) | 99 (22.2) | ||
| CNI/MMF/Prednisolone | 42 (29.8) | 57 (12.8) | ||
| CNI/Prednisolone | 9 (6.4) | 42 (9.4) | ||
| MMF (or Aza)/Prednisolone | 1 (0.7) | 1 (0.2) | ||
| Other | 3 (2.1) | 2 (0.4) | ||
| Diabetes | No | 79 (56.0) | 307 (69.0) | 0.005 |
| Yes | 62 (44.0) | 138 (31.0) | ||
| Priming vaccine type | BNT162b22 | 61 (43.3) | 249 (56.0) | 0.0086 |
| ChAdOx12 | 80 (56.7) | 196 (44.0) | ||
| Time between 1st 2 vaccinations | Days (median) | 74 (63-78) | 75 (67-78) | 0.51 |
| Time between 2nd -3rd vaccinations | Days (median) | 167 (145-189) | 174 (156-189) | 0.033 |
| Time of serological test post-V3 | Days (median) | 24 (21-43) | 33 (21-53) | 0.007 |
Comparator. CNI (Calcineurin inhibitor); MMF (mycophenolate); Aza (Azathioprine).
Table 2.
Clinical characteristics in 239 infection-naïve transplant recipients by serostatus following 4th vaccine dose.
| Characteristics | No seroconversion | Seroconversion | p value | |
|---|---|---|---|---|
| N= 45 (%) | N= 194 (%) | |||
| Gender | Male | 27 (60.0) | 122 (62.9) | 0.72 |
| Female | 18 (40.0) | 72 (37.1) | ||
| Age at 1st vaccine | Years (Median) | 58 (50-66) | 61 (53-68) | 0.22 |
| Ethnicity | Caucasian | 28 (62.2) | 121 (62.4) | 0.48 |
| Black | 5 (11.1) | 10 (5.2) | ||
| Indoasian | 8 (17.8) | 42 (21.6) | ||
| Other | 4 (8.9) | 21 (10.8) | ||
| Cause of ESKD | Polycystic kidney disease | 5 (11.1) | 28 (14.4) | 0.78 |
| Glomerulonephritis | 17 (37.8) | 66 (34.0) | ||
| Diabetic nephropathy | 4 (8.9) | 26 (13.4) | ||
| Urological | 4 (8.9) | 20 (10.3) | ||
| Unknown | 8 (17.8) | 36 (18.6) | ||
| Other | 7 (15.6) | 18 (9.3) | ||
| Number of transplants received | 1 | 32 (71.1) | 168 (86.6) | 0.014 |
| ≥2 | 13 (28.9) | 26 (13.4) | ||
| 1st vaccine <1 year post-transplant | No | 39 (86.7) | 183 (94.3) | 0.07 |
| Yes | 6 (13.3) | 11 (5.7) | ||
| Type of transplant | Deceased Donor | 25 (55.6) | 92 (47.4) | 0.45 |
| Living Donor | 17 (37.8) | 93 (47.9) | ||
| Simultaneous Pancreas-Kidney | 3 (6.7) | 9 (4.6) | ||
| Induction agent | Alemtuzumaba | 25 (55.6) | 123 (63.4) | 0.049 |
| IL2 receptor antagonist | 12 (26.7) | 25 (12.9) | ||
| None | 0 | 13 (6.7) | ||
| Unknown | 8 (17.8) | 33 (17.0) | ||
| Immunosuppression type | CNI Monotherapya | 9 (20.0) | 88 (45.4) | <0.0001 |
| CNI/MMF (orAza) | 11 (24.4) | 64 (33.0) | ||
| CNI/MMF/Prednisolone | 21 (46.7) | 20 (10.3) | ||
| CNI/Prednisolone | 3 (6.7) | 19 (9.8) | ||
| MMF (or Aza)/Prednisolone | - | 1 (0.5) | ||
| Other | 1 (2.2) | 2 (1.0) | ||
| Diabetes | No | 30 (66.7) | 136 (70.1) | 0.65 |
| Yes | 15 (33.3) | 58 (29.9) | ||
| Vaccine type | BNT162b2 | 20 (44.4) | 111 (57.2) | 0.12 |
| ChAdOx1 | 25 (55.6) | 83 (42.8) | ||
| Vaccine combination | BNT162b2/ mRNA-1273 | 1 (2.2) | - | 0.001 |
| BNT162b2/ mRNA-1273/ | 3 (6.7) | 12 (6.4) | ||
| BNT162b2 | 16 (35.6) | 99 (52.9) | ||
| BNT162b2 | 5 (11.1) | 2 (1.1) | ||
| ChAdOx1/mRNA-1273 | 4 (8.9) | 8 (4.3) | ||
| ChAdOx1/mRNA-1273/ BNT162b2 | 16 (35.6) | 73 (37.6) | ||
| ChAdOx1/ BNT162b2 | ||||
| Time between 1st and 2nd vaccinations | Days (median) | 71 (63-77) | 75 (65-78) | 0.21 |
| Time between 2nd and 3rd vaccinations | Days (median) | 160 (142-189) | 167 (153-184) | 0.41 |
| Time between 3rd and 4th vaccinations | Days (median) | 116 (95-130) | 98 (92-112) | 0.005 |
| Time of serological test post-V4 | Days (median) | 38 (28-53) | 42 (23-66) | 0.66 |
Comparator. CNI (Calcineurin inhibitor); MMF (mycophenolate); Aza (Azathioprine).
Role of funding source
The funders had no role in the study design, collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. All authors had full access to all the data in the study and responsibility for the decision to submit for publication.
Results
We assessed 724 kidney transplant recipients following 3rd vaccine doses (V3); 586 (80.9%) were infection-naïve, with 138 (19.1%) having evidence of prior infection (Figure S1). A further 322 patients were sampled following a 4th vaccine dose; 239 (74.2%) were infection-naïve, and 83 (25.8%) had evidence of prior infection.
Serological responses in infection-naïve patients post-V3 and V4
Following V3, 141 (24.1%) infection-naïve patients remained seronegative at a median time of 31 (21-51) days post-vaccination. De novo seroconversion post-V3 occurred in 138/279 (49.5%) patients who were seronegative post-V2, (Figure S2). Patients who were seropositive post-V3 were more likely to have received their 1st dose of vaccine more than one-year post-transplant (p<0.001), be maintained on tacrolimus monotherapy (p<0.001), primed (V1 and V2) with BNT162b2 (p=0.0086) and not have a diagnosis of diabetes (p=0.005) (Table 1). Sampling of seropositive, infection-naïve patients post-V3 occurred significantly later than seronegative patients, at a median time of median 33 (21-53) and 24 (21-43) days respectively, p=0.007.
On multivariable analysis, timing of vaccination after transplantation, OR: 0.28 (0.15-0.54), p=0.0001; immunosuppression burden, OR: 0.22 (0.13-0.37), p<0.0001, and a diagnosis of diabetes, OR: 0.49 (0.32-0.75), p=0.001, remained independent risk factors for non-seroconversion, (Table S1).
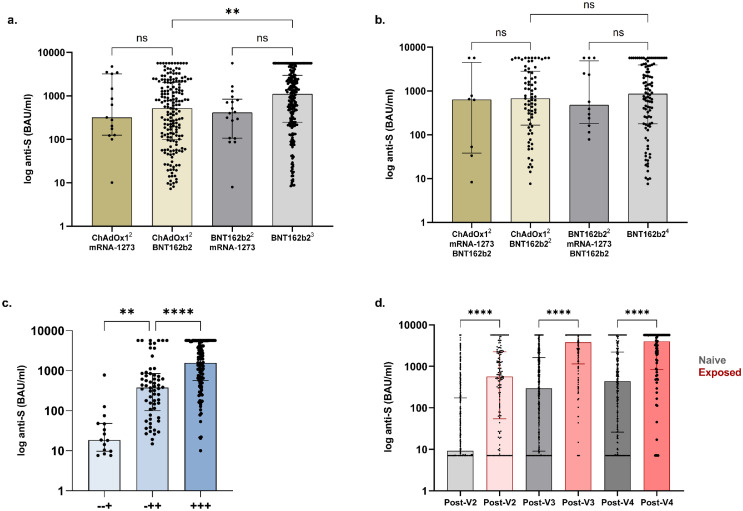
Following receipt of the vaccine combinations ChAdOx1(V1/2)-mRNA1273(V3), ChAdOx1(V1/2)-BNT162b2(V3), BNT162b2(V1/2)-mRNA1273(V3) or all BNT162b2(V1/2/3), the proportion of patients seropositive post-V3 was 15/31 (48.4%), 181/245 (73.9%), 18/25 (72.0%) and 231/285 (81.1%) respectively. Of the patients who received BNT162b2 as V3, a significantly higher proportion of those who received BNT162b2 compared with ChAdOx1 for priming were seropositive, p=0.048. Of the 445 seropositive patients post-V3, anti-S concentrations in patients receiving ChAdOx1(V1/2)-mRNA1273(V3), ChAdOx1(V1/2)-BNT162b2(V3), BNT162b2(V1/2)-mRNA1273(V3) and BNT162b2(V1/2/3), were 319 (125-3213), 518 (98-2049), 412 (106-841) and 1110 (246-2969) BAU/ml respectively, with significantly higher levels in those primed with BNT162b2 compared with ChAdOx1, p=0.0011, (Figure 1A).
Figure 1.
Serological responses to 3rd and 4th dose vaccination.
(A) Anti-S concentrations post-V3 in infection-naïve patients receiving ChAdOx12-mRNA1273, ChAdOx12-BNT162b2, BNT162b22-mRNA1273 and BNT162b23, were 319 (125-3213), 518 (98-2049), 412 (106-841) and 1110 (246-2969) BAU/ml respectively. Significantly higher concentrations were seen with BNT162b2 as V3 following priming with BNT162b2 compared with ChAdOx1, p=0.0011 (B) Anti-S concentrations post-V4 in infection-naïve patients receiving ChAdOx12-mRNA1273-BNT162b2, ChAdOx12-BNT162b22, BNT162b22-mRNA1273-BNT162b2 and BNT162b24, were no different between groups. (C) Anti-S concentrations post-V4 by dose of vaccine seroconverted. +++, -++, and -+ denote seroconversion post V2, V3 and V4 respectively. The median anti-S in infection-naïve patients post-V4 in those seroconverting post V2-, V3- or V4- were 1561 (567-5211), (101-851) and 19 (9.7-48) BAU/ml respectively. (D) Anti-S concentrations post 2nd-, 3rd- and 4th vaccinations by infection exposure Anti-S concentrations were greater in patients with prior infection (568 (54-2237) post-V2, 3791 (1142-5680) post-V3 and 3993 (835-5680) BAU/ml post-V4) compared with infection-naïve patients (9.2 (7.1-173) post-V2, 295 (9.1-1611) post-V3 or 437 (26-2211) BAU/ml post-V4). There was no difference between post-V2 concentrations in patients with prior infection compared with infection naïve individuals post-V3, p=0.06 or post-V4, p=0.99.
Following V4, 45/239 (18.8%) infection-naïve patients remained seronegative after a median period of 41 (25-64) days (Figure S2). De novo seroconversion post-V4 occurred in 15/60 (25.0%) patients who were seronegative post-V3. Clinical characteristics associated with lack of seroconversion post-V4 included number of transplants received, immunosuppression type, vaccine combination, and time between 3rd and 4th doses (Table 2). On multivariable analysis receiving ≥2 different classes of immunosuppression medications, OR: 0.41(0.17-0.90), p=0.033 was associated with non-seroconversion; whilst seropositivity was more likely with shorter time intervals between doses 3 and 4, OR: 0.99 (0.97-0.99), p=0.039, (Table S2).
There was no difference in the proportion of patients who were seropositive post-V4 following BNT162b2(V1-4) compared with ChAdOx1(V1/2)-BNT162b2(V3/4), at 99/115 (86.1%) versus 73/89 (82.0%) respectively, p=0.43 (Figure 1B). Serostatus post-V4 in the other vaccine combinations maybe found in the Supplemental Information (Table S3). Of the 194 seropositive patients post-V4, anti-S concentrations in patients receiving ChAdOx1-BNT162b2, 678 (167-284) BAU/ml, were no different compared with those patients who received BNT162b2, 865 (179-3936) BAU/ml, p=0.50. Anti-S concentrations for the other vaccine combinations are shown in the Supplemental Information (Table S3).
There was a significant difference in anti-S concentration in seropositive patients post-V4 in relation to which vaccine dose led to seroconversion. The median anti-S post-V4 in infection-naïve patients who seroconverted post-V2 was 1561 (567-5211) BAU/ml, which was significantly higher than the median concentration of 379 (101-851) BAU/ml in patients who had seroconverted post-V3, p<0.0001, (Figure 1C). This was in turn significantly greater compared with those patients who only seroconverted post-V4, with a median anti-S of 19 (9.7-48) BAU/ml, p=0.0013 (Figure 1C).
In those patients who responded post-V2, anti-S was significantly higher post-V3, with concentrations of 148 (30-617) and 1401 (472-3213) BAU/ml respectively, p<0.0001. However, no differences were seen post-V4, 1561 (567-5211) BAU/ml, compared with post-V3, p=0.17 (Figure S3).
Comparison of anti-S concentrations by vaccination and infection status
Of 138 patients with prior infection, 6/138 (4.3%) remained seronegative post-V3 at a median time of 34 (21-48) days. Five of 6 patients had infection confirmed via RT-PCR testing, with the remaining patient having positive serology pre-vaccination. At a median time of 36 (21-59) days post-V4, 4/83 (4.8%) patients with prior infection remained seronegative, all 4 patients had infection diagnosed via RT-PCR testing.
Comparing all (seronegative and seropositive) anti-S concentrations following each vaccine, patients with a history of SARS-CoV-2 infection had significantly higher anti-S compared with infection-naïve patients (Figure 1D). Post-V2 anti-S was 568 (54-2237) and 9.2 (7.1-173) BAU/ml in those with a history of SARS-CoV-2 infection compared with infection-naïve patients respectively, p<0.0001. Post-V3 concentrations in infection exposed were 3791 (1142-5680) BAU/ml compared with 295 (9.1-1611) BAU/ml in infection-naïve patients, p<0.0001; whilst post-V4 concentrations were 3993 (835-5680) and 437 (26-2211) BAU/ml respectively, p<0.0001 (Figure 1D). There was no difference between post-V2 concentrations in patients with prior exposure compared with infection-naïve individuals post-V3, p=0.06 or post-V4, p=0.99.
Cellular responses post-V4
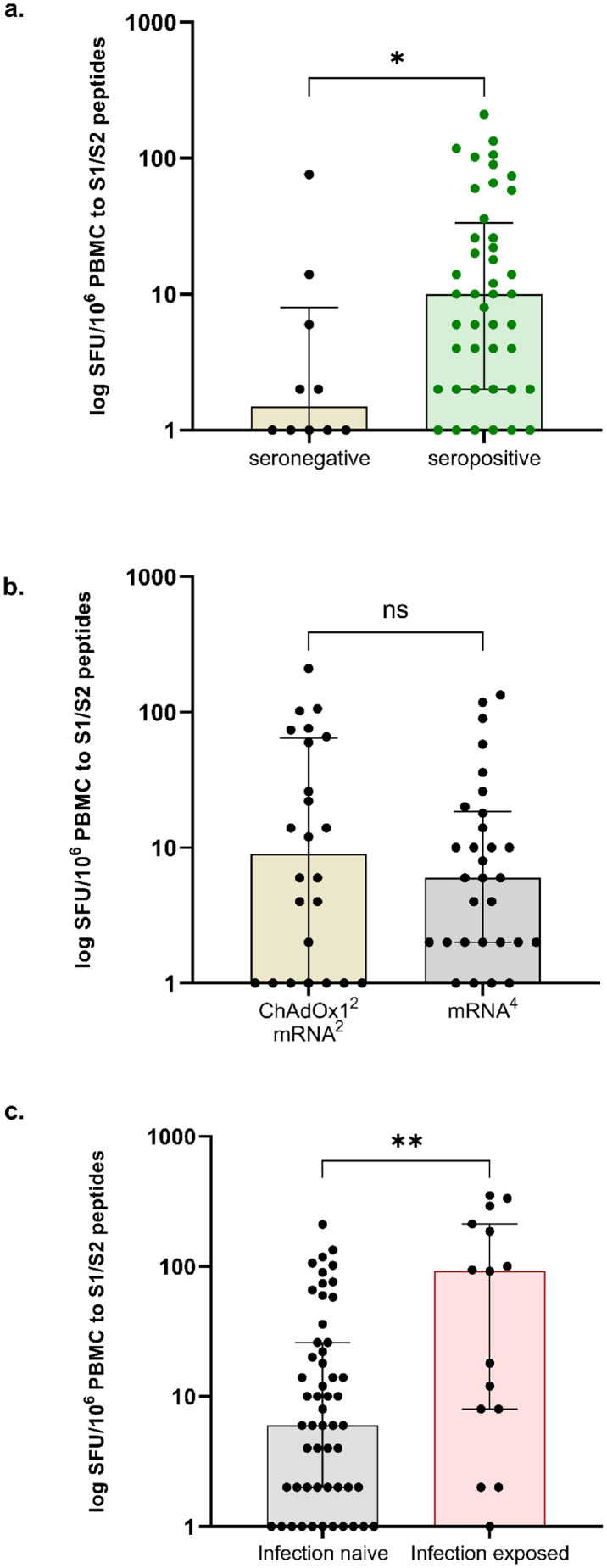
Fifty-four infection-naïve patients were assessed for T-cell responses post-V4. T-cell responses were detectable in only 11/54 (20.4%) of patients sampled at 38 (27-55) days post-V4. Clinical characteristics associated with T-cell response included younger age and being of non-Caucasian background (Table S4). On multivariable analysis, increasing age, OR: 0.88 (0.77-0.97), p=0.026 and Caucasian ethnicity, OR: 0.03 (0.00-0.33), p=0.08, remained independent factors associated with no detectable T-cell responses (Table S5). Patients who failed to seroconvert had significantly lower T cell responses than those with a detectable serological response post-V4 (Figure 2A). There was no difference in T cell responses between infection naïve patients receiving difference vaccine combinations (Figure 2B).
Figure 2.
Cellular responses to 4th dose vaccination.
(A) T-cell responses were greater in infection-naïve individuals who were seropositive post-V4, 10 (2-34) SFU/106 PBMC, compared with those who were seronegative, 1 (0-8) SFU/106 PBMC. (B) There was no difference in the magnitude of cellular responses between those patients who were primed with ChAdOx12 compared with BNT162b22, with a median 9 (1-65) and 6 (2-19) SFU/106 PBMC respectively, p=0.72. (C) T-cell responses were greater in infection exposed compared with infection-naïve individuals, with a median SFU/106 PBMC of 92 (8-212) and 6 (2-26) respectively, p=0.0098.
*For purposes of data representation, values of 0 were replaced by 1.
Fifteen of 83 (18.1%) infection-exposed individuals underwent T-cell assessment post-V4; 8/15 (53.3%) patients had detectable T-cell responses, which was proportionately higher than infection-naïve individuals post-V4, p=0.012. Overall T-cell responses were greater in infection exposed compared with infection-naïve individuals, with a median SFU/106 PBMC of 92 (8-212) and 6 (2-26) respectively, p=0.0098 (Figure 2C).
Discussion
This study shows that 24% and 19% of kidney transplant recipients do not have any detectable spike protein antibody in response to 3rd and 4th doses of vaccine respectively. For those patients who seroconvert after 3 or 4 doses, antibody concentrations remain lower than those patients who responded after 2 doses. Following 3rd dose vaccination, timing of first vaccination after transplantation, immunosuppression burden, and a diagnosis of diabetes, were independent risk factors for non-seroconversion. Following 4th dose vaccination, receiving ≥2 different classes of immunosuppression medications was associated with non-seroconversion; whilst seropositivity was more likely with shorter time intervals between doses 3 and 4. Furthermore, T-cell responses are poor post-V4, which is compatible with the universal use of calcineurin inhibitors in this group of KTRs and the majority of solid organ transplant recipients across the globe. The use of such agents together with other potent immunosuppressants in transplant recipients, probably also explains the weak T-cell responses to natural infection. Therefore, for solid organ transplant recipients, routine clinical testing for anti-S response will help identify those with no response, who maybe at highest risk of an adverse outcome if infected.
Previous studies have reported on immune responses to 4 doses of mRNA-based vaccines in transplant recipients with similar findings that patients with no detectable anti-S response post-V3 can seroconvert post-V4 in 10-50% of cases, but they are unlikely to have significant anti-spike concentrations or possess neutralising capabilities.5, 6, 7, 8, 9 This is in contrast to the general population in whom there is evidence for booster doses of vaccination leading to enhanced immunological responses and protection from infection.10 Our data also analyse comparative V4 data in transplant recipients receiving heterologous vaccines. Given evidence suggesting that heterologous vaccination dosing may result in at least equivocal, if not enhanced, serological and cellular responses in both the general population and transplant recipients post-V3, comparing vaccination schedules is an important consideration.11,12 After V4, we found no immune advantage of heterologous versus homologous vaccinations. However, we recognise that the ELISpot assay we utilise uses IFN-γ as the sole read out for T-cell reactivity, underestimating T-cell responses overall, and we do not report on antibody neutralising capabilities.13,14
Limitations of our study include the cross-sectional, non-randomised design including patients from a single centre which may not be representative of the wider population of KTR. Of specific relevance is our use of a steroid sparing immunosuppression protocol, with steroids only being introduced to treat rejection, should it occur. Therefore, patients receiving triple immunosuppression are a surrogate for those who have experienced rejection in our population. As tacrolimus monotherapy use was associated with seroconversion post vaccination, it maybe that our data underestimate serological responses in other solid organ transplant populations. Furthermore, data on other clinical factors such as renal function, body mass index, immunosuppression levels, are not available for this study, and may further influence immunological responses to vaccines in this population. In addition, T cell responses following V4 were only measured in a subset of patients. We are also unable to make any conclusions regarding vaccine efficacy against infection or disease, however anti-S IgG is acknowledged to be a surrogate for clinically relevant outcomes.
Consistent with this immunogenicity data, real world vaccine efficacy has been shown to be inferior in immunocompromised people, who have been at highest risk of breakthrough infections and severe disease, in the pre-Omicron era.15, 16, 17 So, what does this mean for the strategic forward planning to protect transplant recipients? The data shown in this study suggest that a proportion of transplant recipients who have not responded to the first 4 vaccines, are unlikely to develop meaningful protection with a fifth. Whilst for other immunocompromised people, mostly those on B-cell directed therapies, robust SARS-CoV-2 T-cell responses have been demonstrated in the absence of antibodies, for solid organ transplant patients who are commonly maintained on both B-cell and T-cell inhibiting agents, this will not necessarily be the case.3,18 With treatment options also limited in this group, related to relative contraindications and drug interactions, alternative strategies are required to provide protection to this vulnerable group. Modulating immunosuppression regimens at time of vaccination has been shown to be effective in patients being treated for inflammatory conditions, however this strategy may be of greater risk and consequences in transplant recipients at risk of rejection.19,20 Novel vaccine platforms such as those designed to induce mucosal immunity, or altered antigen sequences, targeting current variants or incorporating conserved epitopes beyond the spike region may also be of benefit. However, pre-exposure prophylaxis with passive immunity from neutralising monoclonal antibodies whilst they remain effective against the current dominant variant, is likely to currently be the best option in those with impaired responses.21
In summary, we have shown that repeated vaccinations will not adequately protect all transplant recipients. However, there is a spectrum of immune responses in patients in relation to vaccination and infection. It will disadvantage many immunocompromised people if they are managed as a uniform cohort irrespective of underlying disease, treatment or infection status. We recommend developing a more personalised approach to their management, starting with antibody screening which is widely available clinically to identify the vaccine non-responders who are likely to be the most immune suppressed and at risk of an adverse outcome with infection.
Contributors
MP, CLC, SPM, LL, PK, and MW contributed to the conception and design of this study. TT, MP, SG, PM, KS, RCA, BS, CS, JG, CLC, SL. GP, DT, SPM, LL, AC, PK, and MW contributed to the acquisition of the clinical and laboratory data. TT, MP, PK, and MW contributed to the interpretation of the data. MP and MW contributed to statistical analysis. TT, MP, and MW had full access to and verify all of the data in the study and take responsibility for the integrity of the data. TT, MP, and MW prepared the manuscript. All authors have full access to all the data in the study and accept responsibility to submit for publication. All authors critically reviewed and approved the final version.
Data sharing statement
All data will be made available from the corresponding author upon reasonable request following completion of the study.
Declaration of interests
DT has received consulting fees and honoraria from AZ and Novartis, SPM has received consulting fees and honoraria from GSK, Vifor, and Celltrion, LL has received consulting fees and honoraria from GSK, BMS, Aurinia, Pfizer, Roche, and Alexion; PK and MW received study support from Oxford Immunotec. Other authors have nothing to disclose.
Acknowledgments
Funding
MW/PK received study support from Oxford Immunotec.
Acknowledgements
This research is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The authors would like to thank the West London Kidney Patient Association, all the patients and staff at ICHNT (The Imperial COVID vaccine group and dialysis staff, and staff within the North West London Pathology laboratories). The authors are also grateful for support from The Nan Diamond Fund, Sidharth and Indira Burman, and the Auchi Charitable Foundation. MP is supported by an NIHR clinical lectureship. Work in DT's lab is supported by a Wellcome Trust Clinical Career Development Fellowship.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101642.
Appendix. Supplementary materials
References
- 1.Reynolds CJ, Gibbons JM, Pade C, et al. Heterologous infection and vaccination shapes immunity against SARS-CoV-2 variants. Science. 2022;375(6577):183–192. doi: 10.1126/science.abm0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prendecki M, Thomson T, Clarke CL, et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398(10310):1482–1484. doi: 10.1016/S0140-6736(21)02096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80(10):1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willicombe M, Scanlon M, Loud F, Lightstone L. Should we be clinically assessing antibody responses to Covid vaccines in immunocompromised people? BMJ. 2022;377:o966. doi: 10.1136/bmj.o966. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, Abravanel F, Marion O, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA–based vaccine in recipients of a solid organ transplant. JAMA Netw open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benotmane I, Bruel T, Planas D, Fafi-Kremer S, Schwartz O, Caillard S. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. 2022;101(5):1073–1076. doi: 10.1016/j.kint.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alejo JL, Mitchell J, Chiang TP, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105(12) doi: 10.1097/TP.0000000000003934. e280-e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osmanodja B, Ronicke S, Budde K, et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med. 2022;11(9):2565. doi: 10.3390/jcm11092565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midtvedt K, Vaage JT, Heldal K, Munthe LA, Lund-Johansen F, Asberg A. Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant. 2022 doi: 10.1111/ajt.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386(18):1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21(12):3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 14.Swanson PA, 2nd, Padilla M, Hoyland W, et al. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific T(H)1 response with a diverse TCR repertoire. Sci Transl Med. 2021;13(620):eabj7211. doi: 10.1126/scitranslmed.abj7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon JH, Tenforde MW, Gaglani M, et al. mRNA vaccine effectiveness against COVID-19 hospitalization among solid organ transplant recipients. J Infect Dis. 2022 doi: 10.1093/infdis/jiac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106(3):436–446. doi: 10.1097/TP.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SV, Whitaker HJ, Mumford L, et al. Effectiveness of COVID-19 vaccines against hospitalization and death with the SARS-CoV-2 delta variant in solid organ and islet transplant recipients. Transplantation. 2022;106(6):e310–e311. doi: 10.1097/TP.0000000000004104. Epub 2022 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SH, Stuart B, Joseph-Pietras D, et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer. 2022;3(5):552–564. doi: 10.1038/s43018-022-00364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med. 2022 doi: 10.1016/S2213-2600(22)00186-2. S2213-2600(22)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrezenmeier E, Rincon-Arevalo H, Jens A, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination–specific humoral and cellular immunity in kidney transplant recipients. JCI Insight. 2022;7(9):e157836. doi: 10.1172/jci.insight.157836. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.