Abstract
Background
Research on the effectiveness of COVID-19 booster-based vaccine schedule is ongoing and real-world data on vaccine effectiveness (VE) in comorbid patients are limited. We aimed to estimate booster dose VE against SARS-CoV-2 infection and COVID-19 severity in the general population and in comorbid patients.
Method
A retrospective test-negative control study was undertaken in Galicia-Spain (December 2020–November 2021). VE and 95% confidence interval (CI) were estimated using multivariate logistic regression models.
Results
1,512,415 (94.13%) negative and 94,334 (5.87%) positive SARS-CoV-2 test results were included. A booster dose of COVID-19 vaccine is associated with substantially higher protection against SARS-CoV-2 infection than vaccination without a booster [VEboosted = 87% (95%CI: 83%; 89%); VEnon-boosted = 66% (95%CI: 65%; 67%)]. The high VE was observed in all ages, but was more pronounced in subjects older than 65 years. VE against COVID-19 severity was analyzed in a mixed population of boosted and non-boosted individuals and considerable protection was obtained [VE: hospitalization = 72% (95%CI: 68%; 75%); intensive care unit administration = 83% (95%CI: 78%; 88%), in-hospital mortality = 66% (95%CI: 53%; 75%)]. Boosted comorbid patients are more protected against SARS-CoV-2 infection than those who were non-boosted. This was observed in a wide range of major diseases including cancer (81% versus 54%), chronic obstructive pulmonary disease (84% versus 61%), diabetes (84% versus 65%), hypertension (82% versus 65%) and obesity (91% versus 67%), among others.
Conclusions
A booster dose of COVID-19 vaccine increases the protection against SARS-CoV-2 infection and COVID-19 severity in the general population and in comorbid patients.
Keywords: COVID-19 booster vaccine effectiveness, Comorbidities, Population-based study, SARS-CoV-2, Spain
1. Introduction
Vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) remains the fundamental preventive measure against spreading the virus and developing severe Coronavirus Disease 2019 (COVID-19) disease, but whether a booster dose is necessary remains a point of debate (Shekhar et al., 2021). Studies on COVID-19 vaccine effectiveness (VE) suggested declining protection over time, yet no global consensus has been reached on the speed of this decrease (Feikin et al., 2022). A study in Israel reported that antibody titer reached the climax after one month of the second dose of BNT162b2, and then declined rapidly (Khoury et al., 2021). In their study, Naaber and colleagues documented that the antibody levels declined six months after the second dose of BNT162b2, revealing a decrease in the immune response over time (Naaber et al., 2021). A study in Northern California showed that mRNA-1273 VE against SARS-CoV-2 infection moderately decreased from 88% to 76% at 6–8 months after vaccination (Florea et al., 2022).
The impact of booster dose administration on COVID-19 is ongoing research. The World Health Organization (WHO), continues to review the emerging evidence on the need for and timing of a booster dose for the currently available COVID-19 vaccines (World Health Organization, 2021), but studies are still limited. Besides, the external validity of findings on VE is influenced by setting-specific parameters such as adherence to vaccine doses and time intervals for dose administration, access to healthcare, number of undertaken SARS-CoV-2 tests, the threshold for COVID-19 hospitalization and intensive care admission, as well as COVID-19 management strategy and the applied non-pharmaceutical interventions at different time points of the pandemic (e.g., lockdowns, facemasks, social distancing, etc.). Studies in different settings and populations would therefore aid in assessing the impact of booster administration.
Moreover, although it has been widely reported that comorbid patients are very likely to develop serious COVID-19 outcomes (CDC: Center for Disease Control and Prevention, 2019; He et al., 2022; Chiner-Vives et al., 2022; Gonzalez-Barcala et al., 2022; Adab et al., 2022; Ejaz et al., 2020), several vulnerable groups were not sufficiently included in clinical trials on COVID-19 vaccines. Therefore, the degree of protection offered by recall COVID-19 vaccine doses in individuals with medical conditions requires further investigation.
Spain was among the countries most affected by SARS-CoV-2 pandemic with 5,111,842 cases and 87,904 deaths as of November 24, 2021 (Ministerio de Sanidad Consumo y Bienestar Social [Ministry of Health Consumption and Social Welfare], 2021). It prioritized vaccination of inmates, social and healthcare workers and residents of long-term care facilities, front-line healthcare staff and elderly individuals to reduce the risk of COVID-19 related morbidity and mortality in these populations, and thereafter decrease the burden on public health facilities (Gobierno de España [Spanish Government], 2022). Vaccination of the other groups of the population was introduced successively following a priority order according to age, medical status, and occupation (Gobierno de España [Spanish Government], 2022).
Monge and colleagues reported a moderate VE of booster mRNA vaccine dose against SARS-CoV-2 infections; however, the study was limited to individuals older than 40 years and excluded individuals at risk of infection (Monge et al., 2022). Using real-world data of around 3,000,000 Polymerase Chain Reaction (PCR)-based SARS-CoV-2 tests, we extend the study of Monge by investigating the impact of a booster dose administration against SARS-CoV-2 infection as well as COVID-19 severe illness resulting in hospitalization, admission to intensive care unit (ICU), and death. We undertook a population-based study in Galicia, Northwest Spain that involved individuals older than 11 years and explored the impact of a booster dose on a large variety of comorbidities.
2. Methods
2.1. Settings
This study was initiated within the framework of a project on COVID-19 VE in Galicia, a region located in Northwest Spain (Pardo-Seco et al., 2022). Galicia is an autonomous community with a total inhabitants of 2,694,245 (Instituto Galego de Estatística, 2021a), for 29,576 km2 (Instituto Galego de Estatística, 2019) i.e,., its population density is similar to that of the European population. The gender distribution in Galicia is 48% (N = 1,296,602) males and 52% (N = 1,397,643) females. Almost 9% of the Galician population are aged 80 and above (N = 236,788), 17% are in the age range of 65–79 (N = 457,245), 22% are aged between 50 and 64 (N = 596,034), while the rest are younger adults and children (Instituto Galego de Estatística, 2021b).
The Galician Healthcare Service (SERGAS) is a public health system with universal access to healthcare at low or no cost. Galicia has 6,571 public hospital beds distributed in 35 hospitals (data of 2020) (Instituto Galego de Estatística, 2020). COVID-19 vaccine has been made freely available for all the population, and it was administered by priority order according to occupation, age, and medical status (Gobierno de España [Spanish Government], 2022). During the study period (December 26, 2020–November 23, 2021), the following four vaccines were administered in Spain: Pfizer-BioNTech vaccine (BNT162b2), Vaxzevria (ChAdOx1 nCoV-19), Spikevax (mRNA-1273), and Janssen (Ad26. COV2–S) (European Medicines Agency, 2022). A full vaccine course of BNT162b2, mRNA-1273, or ChAdOx1 nCoV-19 consisted of two injections with a predefined time separation between the first and the second injection, while Ad26. COV2–S was given as a single injection. Until the start of the study, the vaccination campaign encompassed individuals 11 years or above, hence, those younger than that age were excluded from the study.
2.2. Ethics
The study did not involve an intervention or the use of human biological samples. SERGAS provided the authors with anonymized data, waiving the need for written informed consent. SERGAS did not take part in data analysis. The study protocol was approved by the clinical research ethics committee of Galicia (CEIC, protocol number: 2022–175).
2.3. Study design
A retrospective test-negative case-control study was conducted to determine the effectiveness of COVID-19 vaccines administered in Galicia-Spain, against SARS-CoV-2 infection as well as against severe COVID-19 causing hospitalization, ICU admission, or death.
To estimate VE in fully susceptible people, we excluded from the analysis those individuals who had a previous positive SARS-CoV-2 PCR, antigen or antibody test result at any time before the enrolment date in the study. Antibody response to the vaccine in infection-naive individuals takes more time than in those who had been infected with the virus in the past (Tut et al., 2021).
A positive SARS-CoV-2 PCR test result represented a case, while a negative test result was deemed a control. An individual could contribute to the study by one or more negative tests; however, only one positive SARS-CoV-2 PCR test was considered per patient. During the study period, SARS-CoV-2 PCR test results were performed at no cost by SERGAS health centers for clinical motives such as presenting COVID-19-related symptoms or being in close contact with a SARS-CoV-2 infected individual in the last two weeks.
2.4. Exposure definition
In our settings, the exposure was defined as receiving any injection of COVID-19 vaccine. Individuals were categorized into unvaccinated, partially vaccinated, non-boosted and boosted according to their exposure status, in order to focus on SARS-CoV-2 infection acquired since vaccination after a sufficient interval for biological protection. Unvaccinated individuals are those who did not receive any COVID-19 vaccine injection during the study period. Partially vaccinated individuals are those who received a single dose of BNT162b2, mRNA-1273 or ChAdOx1 nCoV-19, as well as those who were given the two injections of these vaccines or the single injection of Ad26. COV2–S, but with less than seven days after the last injection. Non-boosted individuals are those who received the complete course of the vaccine counting since the 7th day after the last injection. Boosted individuals are those who received an additional dose of the vaccine counting at least seven days after the injection.
2.5. Outcome definition
The primary outcome of the study consisted of SARS-CoV-2 infection confirmed by a PCR-based test. The date of SARS-CoV-2 infection was considered the date of the first positive PCR test.
Secondary outcomes included hospitalization, ICU admission, or mortality attributed to severe COVID-19. Only in-hospital death events were considered for the mortality analysis.
SARS-CoV-2 was deemed a cause of hospitalization if a patient had a positive PCR test result in the 30 days preceding the hospital admission or within three days after hospitalization (Yeo et al., 2021; Mehta et al., 2021). To account for nosocomial infections, patients with a positive SARS-CoV-2 PCR test after three days of hospitalization were not included in the analysis. ICU admissions and in-hospital mortality were ascertained among the population of COVID-19 hospitalized patients.
2.6. Statistical analysis
Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using multivariate logistic regression models. Unvaccinated individuals were used as a reference. The models were directly adjusted for age and sex due to their biological plausibility, and other variables were added to control confounding. To account for socioeconomic differences the following three variables were used as a proxy of this indicator: 1) sanitary area which represents the area of residence; 2) pharmaceutical cost contribution defined as the percentage of medicine cost paid by the participants according to their income tax; and 3) receiving social support. Data on comorbidities associated with each individual were also collected. The comorbidities included: atrial fibrillation, cancer, chronic obstructive pulmonary disease (COPD), dementia, depression, diabetes, epilepsy, heart failure, human immunodeficiency virus (HIV), hypertension, ischemic cardio-pathology, kidney failure, obesity, stroke, and Parkinson disease. Information on receiving any of pneumococcal conjugate vaccine, pneumococcal polysaccharide vaccine, or flu vaccine was also retrieved. The evolution of the epidemiological situation of the pandemic was considered by computing the time period between the PCR test date and the COVID-19 vaccination date. We examined the effect of each potentially confounding variable using the change-in-estimate method. For this purpose, a univariate analysis was performed and the covariables with a p-value <0.2 were consecutively added to the model (Greenland, 1989). A covariable was kept in the model if it changed the originally estimated OR by at least 10%. The effectiveness of COVID-19 vaccination status was then calculated as follows: VE = (1 - ORadjusted) × 100. When the number of observations to estimate the effectiveness of the booster against a certain outcome was not sufficient, a mixed population of boosted and non-boosted individuals was used.
The analysis was stratified by age group and comorbidities.
All analyses were undertaken using STATA v.12 (Stata Statistical Software: Release 12; StataCorp LP; College Station, TX, USA).
3. Results
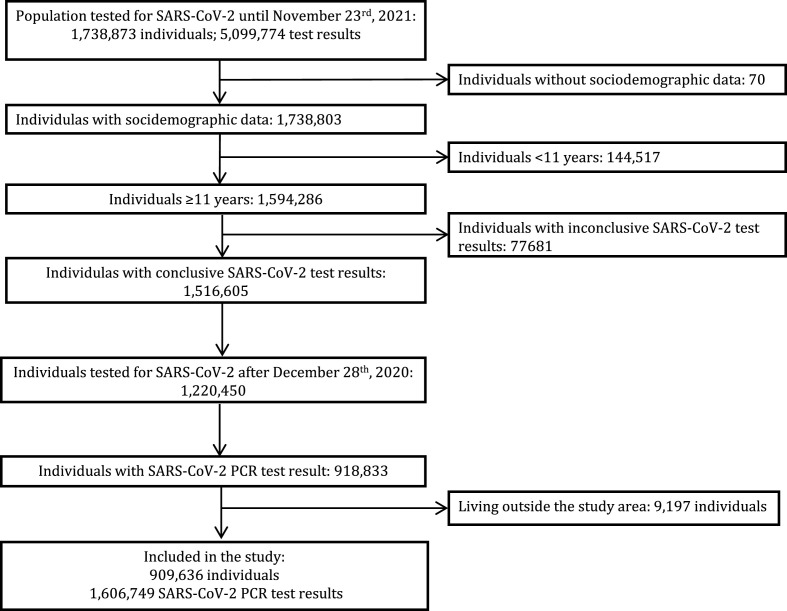
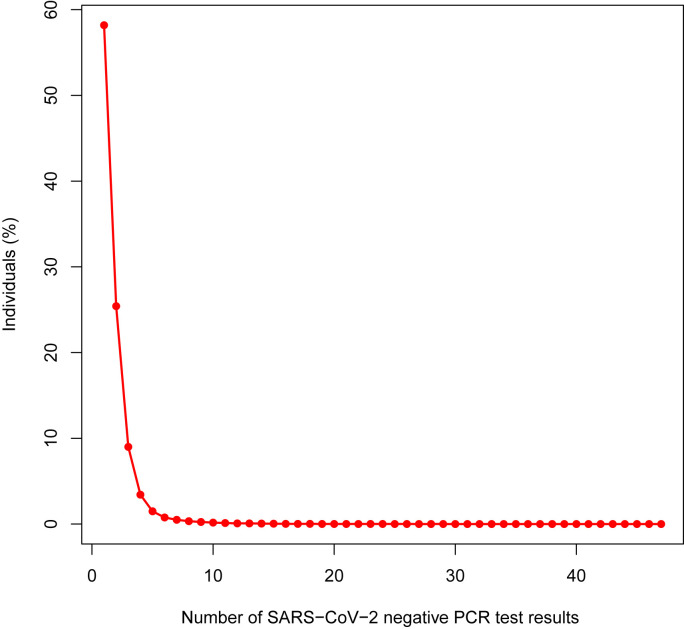
Fig. 1 summarizes how the sample size of the analysis was reached. A total of 909,636 individuals fulfilled the inclusion criteria and contributed 1,606,749 SARS-CoV-2 test results to the analysis. Among the included individuals, 94,334 were infected with SARS-CoV-2, whereas the remaining 815,302 individuals were negative for SARS-CoV-2. The number of SARS-CoV-2 infected individuals was the same as that of the positive SARS-CoV-2 test results (94,334; 5.87% of total PCR tests) as only one SARS-CoV-2 PCR positive test was considered per individual. The 815,302 SARS-CoV-2 negative people, provided 1,512,415 (94.13% of total PCR tests) test results to the analysis since each individual could contribute one or more negative SARS-CoV-2 PCR test results to the study. The analysis unit in our study is the test, not the person. Fig. 2 represents the distribution of the negative tests across the study population. Of the negatively tested individuals, 58.2% contributed only one negative test result, 25.4% provided two negative test results, 16.4% shared more than two negative test results, and 2.5% gave more than 5 negative test results (Fig. 2).
Fig. 1.
Flow diagram of participants and SARS-COV-2 PCR test results entry the study.
Fig. 2.
Distribution of SARS-CoV-2 PCR negative test results per individual.
In 1,155 infected individuals, COVID-19 was not deemed the cause of hospitalization as SARS-CoV-2 infection was either nosocomial or the hospitalization took place more than 30 days after infection. Accordingly, 93,179 infected individuals were included in the analysis of VE against COVID-19 hospitalization, ICU admission and in-hospital mortality. Of the 93,179 infected individuals, 5,871 (6.30%) were hospitalized for COVID-19, and 925 (0.99%) were further admitted to ICU. One thousand and eight (1.08%) in-hospital deaths for COVID-19 were also registered.
The general and clinical characteristics of the study population are presented in Table 1 .
Table 1.
Sociodemographic and clinical characteristics of the study population per each of the outcomes: infection with SARS-CoV-2; hospitalization for COVID-19; intensive care unit (ICU) admission for COVID-19; and in-hospital death for COVID-19.
| Characteristic | Infection N (%) |
Hospitalization N (%) |
ICU Admission N (%) |
In-hospital Death N (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | No | Yes | Total | No | Yes | Total | |
| N (%) | 1,512,415 (94.13) | 94,334 (5.87) | 1,606,749 (100) | 87,308 (93.70) | 5871 (6.30) | 93,179 (100) | 92,254 (99.01) | 925 (0.99) | 93,179 (100) | 92,340 (98.92) | 1008 (1.08) | 93,348 (100) |
| Age (years) | ||||||||||||
| Mean (SD) | 49.40 (21.68) | 42.63 (21.10) | 45.0 (21.70) | 40.82 (20.20) | 64.28 (19.24) | 42.30 (20.93) | 42.12 (20.91) | 60.05 (14.0) | 42.30 (20.93) | 41.93 (20.65) | 81.75 (10.61) | 42.36 (20.98) |
| Range | 11–111 | 11–107 | 11–111 | 11–107 | 11–102 | 11–107 | 11–107 | 13–94 | 11–107 | 11–107 | 29–104 | 11–107 |
| Age quartiles† | ||||||||||||
| 1st quartile: 11 - 32 | 375,231 (24.81) | 35,850 (38.00) | 411,081 (25.58) | 35,354 (40.49) | 438 (7.46) | 35,792 (38.41) | 35,750 (38.75) | 42 (4.54) | 35,792 (38.41) | 35,791 (38.76) | 1 (0.10) | 35,792 (38.34) |
| 2nd quartile: 33 - 48 | 392,178 (25.93) | 23,334 (24.74) | 415,512 (25.86) | 22,397 (25.65) | 844 (14.38) | 23,241 (24.94) | 23,093 (25.03) | 148 (16.00) | 23,241 (24.94) | 23,234 (25.16) | 7 (0.69) | 23,241 (24.90) |
| 3rd quartile: 49 - 65 | 373,152 (24.67) | 19,874 (21.07) | 393,026 (24.46) | 18,096 (20.73) | 1524 (25.96) | 19,620 (21.06) | 19,266 (20.88) | 354 (38.27) | 19,620 (21.06) | 19,556 (21.18) | 84 (8.33) | 19,640 (21.04) |
| 4th quartile: 66 - 111 | 371,854 (24.59) | 15,276 (16.19) | 387,130 (24.09) | 11,461 (13.13) | 3065 (52.21) | 14,526 (15.59) | 14,145 (15.33) | 381 (41.19) | 14,526 (15.59) | 13,759 (14.90) | 916 (90.87) | 14,675 (15.72) |
| Gender∗† | ||||||||||||
| Male | 672,291 (44.45) | 45,229 (47.95) | 717,520 (55.34) | 41,398 (47.42) | 3194 (54.40) | 44,592 (47.86) | 43,969 (47.66) | 623 (67.35) | 44,592 (47.86) | 44,139 (47.80) | 554 (54.96) | 44,693 (47.88) |
| Female | 840,124 (55.55) | 49,105 (52.05) | 889,229 (55.34) | 45,910 (52.58) | 2677 (45.60) | 48,587 (52.14) | 48,285 (52.34) | 302 (32.65) | 48,587 (52.14) | 48,201 (52.20) | 454 (45.04) | 48,655 (52.12) |
| COVID-19 vaccine∗‡ | ||||||||||||
| Unvaccinated | 917,498 (60.66) | 69,816 (74.01) | 987,314 (61.45) | 64,251 (73.59) | 4609 (78.50) | 68,860 (73.90) | 68,067 (73.78) | 793 (85.73) | 68,860 (73.90) | 68,279 (73.94) | 730 (72.42) | 69,009 (73.93) |
| Partially vaccinated | 158,103 (10.45) | 8937 (9.47) | 167,040 (10.40) | 8459 (9.69) | 431 (7.34) | 8890 (9.54) | 8843 (9.98) | 47 (5.08) | 8890 (9.54) | 8770 (9.50) | 124 (12.30) | 8894 (9.53) |
| Fully vaccinated | 428,756 (28.35) | 15,498 (16.43) | 444,254 (27.65) | 14,522 (16.63) | 827 (14.09) | 15,349 (16.47) | 15,264 (16.55) | 85 (9.19) | 15,349 (16.47) | 15,211 (16.47) | 154 (15.28) | 15,365 (16.46) |
| Fully vaccinated and booster | 8058 (0.53) | 83 (0.09) | 8141 (0.51) | 76 (0.09) | 4 (0.07) | 80 (0.09) | 80 (0.09) | 0 (0.00) | 80 (0.09) | 80 (0.09) | 0 (0.00) | 80 (0.09) |
| Flu vaccine∗† | ||||||||||||
| Vaccinated | 661,912 (43.77) | 29,005 (30.75) | 690,917 (43.00) | 24,830 (28.44) | 3396 (57.84) | 28,226 (30.29) | 27,740 (30.07) | 486 (52.54) | 28,226 (30.29) | 27,251 (29.80) | 836 (82.94) | 28,357 (30.38) |
| Unvaccinated | 850,503 (56.23) | 65,329 (69.25) | 915,832 (57.00) | 62,478 (71.56) | 2475 (42.16) | 64,953 (69.71) | 64,514 (69.93) | 439 (47.46) | 64,953 (69.71) | 64,819 (70.20) | 172 (17.06) | 64,991 (69.62) |
| Pneumococcal conjugate vaccine**,† | ||||||||||||
| Vaccinated | 165,174 (10.92) | 9207 (9.76) | 174,381 (10.85) | 8416 (9.64) | 631 (10.75) | 9047 (9.71) | 8933 (9.68) | 114 (12.32) | 9047 (9.71) | 8868 (9.60) | 200 (19.84) | 9068 (9.71) |
| Unvaccinated | 1,347,241 (89.08) | 85,127 (90.24) | 1,432,368 (89.15) | 78,892 (90.36) | 5240 (89.25) | 84,132 (90.29) | 83,321 (90.32) | 811 (87.68) | 84,132 (90.29) | 83,472 (90.40) | 808 (80.16) | 84,280 (90.29) |
| Pneumococcal polysaccharide vaccine∗† | ||||||||||||
| Vaccinated | 178,026 (11.77) | 7008 (7.43) | 185,034 (11.52) | 5404 (6.19) | 1286 (21.90) | 6690 (7.18) | 6484 (7.03) | 206 (22.27) | 6690 (7.18) | 6419 (6.95) | 321 (31.85) | 6740 (7.22) |
| Unvaccinated | 1,334,389 (88.23) | 87,326 (92.57) | 1,421,715 (88.48) | 81,904 (93.81) | 4585 (78.10) | 86,489 (92.82) | 85,770 (92.97) | 719 (77.73) | 86,489 (92.82) | 85,921 (93.05) | 687 (68.15) | 86,608 (92.78) |
| Presence of comorbidities (yes) | ||||||||||||
| Obesity∗† | 236,286 (15.62) | 12,737 (13.50) | 249,023 (15.50) | 10,515 (12.04) | 1829 (31.15) | 12,344 (13.25) | 11,960 (12.96) | 384 (41.51) | 12,344 (13.25) | 12,108 (13.11) | 287 (28.47) | 12,395 (13.28) |
| Depression∗† | 204,577 (13.53) | 8270 (8.77) | 212,847 (13.25) | 6839 (7.83) | 1156 (19.69) | 7995 (8.58) | 7849 (8.51) | 146 (15.78) | 7995 (8.58) | 7826 (8.48) | 204 (20.24) | 8030 (8.60) |
| Diabetes∗† | 168,868 (11.17) | 7115 (7.54) | 175,983 (10.95) | 5274 (6.04) | 1488 (25.34) | 6762 (7.26) | 6510 (7.06) | 252 (27.24) | 6762 (7.26) | 6490 (7.03) | 344 (34.13) | 6834 (7.32) |
| COPD∗† | 75,539 (4.99) | 2366 (2.51) | 77,905 (4.85) | 1562 (1.79) | 637 (10.85) | 2199 (2.36) | 2125 (2.30) | 74 (8.00) | 2199 (2.36) | 2064 (2.24) | 161 (15.97) | 2225 (2.38) |
| Atrial fibrillation∗† | 89,838 (5.94) | 2906 (3.08) | 92,744 (5.77) | 1825 (2.09) | 827 (14.09) | 2652 (2.85) | 2559 (2.77) | 93 (10.05) | 2652 (2.85) | 2397 (2.60) | 317 (31.45) | 2714 (2.91) |
| Hypertension∗† | 390,812 (25.84) | 16,672 (17.67) | 407,484 (25.36) | 12,959 (14.84) | 2980 (50.76) | 15,939 (17.11) | 15,479 (16.78) | 460 (49.73) | 15,939 (17.11) | 15,342 (16.61) | 734 (72.82) | 16,076 (17.22) |
| Heart failure∗† | 81,349 (5.38) | 2191 (2.32) | 83,540 (5.20) | 1173 (1.34) | 756 (12.88) | 1929 (2.07) | 1878 (2.04) | 51 (5.51) | 1929 (2.07) | 1659 (1.80) | 332 (32.94) | 1991 (2.13) |
| Kidney failure∗† | 79,955 (5.29) | 2185 (2.32) | 82,140 (5.11) | 1247 (1.43) | 728 (12.40) | 1975 (2.12) | 1896 (2.06) | 79 (8.54) | 1975 (2.12) | 1739 (1.88) | 293 (29.07) | 2032 (2.18) |
| Cancer∗† | 159,940 (10.58) | 5289 (5.61) | 165,229 (10.28) | 4120 (4.72) | 895 (15.24) | 5015 (5.38) | 4120 (4.72) | 895 (15.24) | 5015 (5.38) | 4799 (5.20) | 270 (26.79) | 5069 (5.43) |
| Ischemic cardio-pathology∗† | 73,100 (4.83) | 2585 (2.74) | 75,685 (4.71) | 1785 (2.04) | 617 (10.51) | 2402 (2.58) | 2310 (2.50) | 92 (9.95) | 2402 (2.58) | 2244 (2.43) | 203 (20.14) | 2447 (2.62) |
| Stroke∗† | 65,833 (4.35) | 2075 (2.20) | 67,908 (4.23) | 1431 (1.64) | 493 (8.40) | 1924 (2.06) | 1866 (2.02) | 58 (6.27) | 1924 (2.06) | 1775 (1.92) | 198 (19.64) | 1973 (2.11) |
| Epilepsy**† | 23,544 (1.56) | 963 (1.02) | 24,507 (1.53) | 778 (0.89) | 141 (2.40) | 919 (0.99) | 901 (0.98) | 18 (1.95) | 919 (0.99) | 889 (0.96) | 39 (3.87) | 928 (0.99) |
| Dementia**† | 55,383 (3.66) | 1859 (1.97) | 57,242 (3.56) | 1250 (1.43) | 490 (8.35) | 1740 (1.87) | 1736 (1.88) | 4 (0.43) | 1740 (1.87) | 1517 (1.64) | 250 (24.80) | 1767 (1.89) |
| Parkinson disease† | 15,017 (0.99) | 463 (0.49) | 15,480 (0.96) | 289 (0.33) | 132 (2.25) | 421 (0.45) | 413 (0.45) | 8 (0.86) | 421 (0.45) | 366 (0.40) | 64 (6.35) | 430 (0.46) |
| HIV | 4339 (0.29) | 143 (0.15) | 4482 (0.28) | 127 (0.15) | 14 (0.24) | 141 (0.15) | 140 (0.15) | 1 (0.11) | 141 (0.15) | 141 (0.15) | 0 (0.00) | 141 (0.15) |
| Multi-medicated (yes)∗† | 57,037 (3.77) | 1741 (1.85) | 58,788 (3.66) | 1161 (1.33) | 458 (7.80) | 1619 (1.74) | 1569 (1.70) | 50 (5.41) | 1619 (1.74) | 1495 (1.62) | 150 (14.88) | 1645 (1.76) |
| Proxy of socioeconomic status | ||||||||||||
| Receiving social protection∗† | ||||||||||||
| No | 1,465,683 (96.91) | 92,883 (98.46) | 1,558,566 (97.00) | 86,303 (98.85) | 5521 (94.04) | 91,824 (98.55) | 90,933 (98.57) | 891 (96.32) | 91,824 (98.55) | 91,058 (98.61) | 921 (91.37) | 91,979 (98.53) |
| Yes | 46,732 (3.09) | 1451 (1.54) | 48,183 (3.00) | 1005 (1.15) | 350 (5.96) | 1355 (1.45) | 1321 (1.43) | 34 (3.68) | 1355 (1.45) | 1282 (1.39) | 87 (8.63) | 1396 (1.47) |
| Contribution to paying medicine costs according to income∗‡ | ||||||||||||
| 50%–60% of medicine cost | 275,993 (19.21) | 20,257 (22.25) | 296,250 (19.39) | 19,649 (23.02) | 550 (11.36) | 20,199 (22.39) | 20,082 (22.45) | 117 (15.92) | 20,199 (22.39) | 20,195 (22.39) | 4 (33.33) | 20,199 (22.39) |
| 30%–40% of medicine cost | 634,009 (44.13) | 45,661 (50.15) | 679,670 (44.49) | 44,081 (51.64) | 1410 (29.12) | 45,491 (50.43) | 25,274 (50.60) | 217 (29.52) | 45,491 (50.43) | 45,490 (50.44) | 1 (8.33) | 45,491 (50.43) |
| 10% of medicine cost | 247,385 (17.22) | 10,997 (12.08) | 258,382 (16.91) | 9138 (10.71) | 1507 (31.12) | 10,645 (11.80) | 10,414 (11.64) | 231 (31.43) | 10,645 (11.80) | 10,640 (11.80) | 5 (41.67) | 10,645 (11.80) |
| 0% of medicine cost | 279,206 (19.44) | 14,140 (15.53) | 293,346 (19.20) | 12,493 (14.64) | 1375 (28.40) | 13,868 (15.37) | 13,698 (15.31) | 170 (23.13) | 13,868 (15.37) | 13,866 (15.37) | 2 (16.67) | 13,868 (15.37) |
| Unknown or missing | 75,822 (5.00) | 3279 (3.50) | 79,101 (4.90) | 1947 (2.20) | 1029 (17.5) | 2976 (3.20) | 2786 (3.00) | 190 (20.50) | 2976 (3.20) | 2149 (2.30) | 12 (1.20) | 3145 (3.40) |
N: the number of PCR tests; ICU: intensive care unit; Hospitalization, ICU, and in-hospital death were determined in the subpopulation of SARS-CoV-2 positive PCR tests.
All variables listed in Table 1 showed a statistically significant association with SARS-CoV-2 infection (Χ2 p-value <0.001). They also showed a statistically significant association with hospitalization for COVID-19 (Χ2 p-value of Pneumococcal conjugate vaccine = 0.005; Χ2 p-value of the other variables <0.001), except HIV (p-value = 0.076).
Χ2 p-value of the association with ICU admission for COVID-19 > 0.05.
Χ2 p-value of the association with ICU admission for COVID-19 > 0.05.
:Χ2p-value of the association with ICU admission for COVID-19 < 0.001.
:Χ2p-value of the association with ICU admission for COVID-19 < 0.005. Variables without any asterisk showed.
Χ2p-value of the association with in-hospital death for COVID-19 < 0.001.
Χ2p-value of the association with in-hospital death for COVID-19 < 0.005. Variables without any dagger showed.
Impact of COVID-19 booster-based vaccination on SARS-CoV-2 infection and COVID-19 severity.
Receiving a booster dose of COVID-19 vaccine increased the protection against infection by SARS-CoV-2 from 66% in non-boosted individuals to 87% [VEnon-boosted = 66% (95%CI: 65%; 67%); VEboosted = 87% (95%CI: 83%; 89%)] (Table 2 ).
Table 2.
Vaccine effectiveness (VE) against SARS-CoV-2 infection.
| Covid-19 vaccination | Infection |
||
|---|---|---|---|
| No N (%) | Yes N (%) | VE∗ (95%CI); p-value | |
| ≥ 11 years | |||
| Unvaccinated | 917,498 (60.66) | 69,816 (74.01) | Reference |
| Partially vaccinated | 158,103 (10.45) | 8937 (9.47) | 36% (35%; 38%); p-value <0.0001 |
| Non-boosted | 428,756 (28.35) | 15,498 (16.43) | 66% (65%; 67%); p-value <0.0001 |
| Boosted | 8058 (0.53) | 83 (0.09) | 87% (83%; 89%); p-value <0.0001 |
| 11–17 years | |||
| Unvaccinated | 99,133 (91.70) | 9997 (92.68) | Reference |
| Partially vaccinated | 4516 (4.18) | 705 (6.54) | 1% (−9%, 9%); p-value = 0.849 |
| Non-boosted/boosted | 4454 (4.12) | 85 (0.79) | 76% (69%; 82%); p-value <0.0001 |
| 18–30 years | |||
| Unvaccinated | 180,126 (78.11) | 18,785 (83.98) | Reference |
| Partially vaccinated | 21,694 (9.41) | 2503 (11.19) | 23% (19%; 26%); p-value <0.0001 |
| Non-boosted/boosted | 28,781 (12.48) | 1080 (4.83) | 69% (67%; 71%); p-value <0.0001 |
| 31–45 years | |||
| Unvaccinated | 243,684 (69.21) | 15,082 (70.59) | Reference |
| Partially vaccinated | 35,834 (10.18) | 2924 (13.69) | 8% (3%; 12%); p-value <0.0001 |
| Non-boosted/boosted | 72,552 (20.61) | 3359 (15.72) | 52% (50%; 54%); p-value <0.0001 |
| 46–55 years | |||
| Unvaccinated | 145,184 (59.23) | 9432 (66.46) | Reference |
| Partially vaccinated | 25,117 (10.25) | 718 (5.06) | 64% (61%; 67%); p-value <0.0001 |
| Non-boosted/boosted | 74,801 (30.52) | 4043 (28.49) | 51% (48%; 55%); p-value <0.0001 |
| 56–65 years | |||
| Unvaccinated | 111,703 (54.57) | 6805 (65.78) | Reference |
| Partially vaccinated | 30,547 (14.92) | 867 (8.38) | 53% (49%; 57%); p-value <0.0001 |
| Non-boosted/boosted | 62,435 (30.50) | 2673 (25.84) | 51% (47%; 53%); p-value <0.0001 |
| 66–80 years | |||
| Unvaccinated | 94,054 (43.75) | 6474 (65.82) | Reference |
| Partially vaccinated | 21,520 (10.01) | 606 (6.16) | 41% (35%; 46%); p-value <0.0001 |
| Non-boosted | 97,155 (45.20) | 2735 (27.81) | 67% (64%; 70%); p-value <0.0001 |
| Boosted | 2228 (1.04) | 21 (0.21) | 85% (77%; 91%); p-value <0.0001 |
| ≥ 81 years | |||
| Unvaccinated | 43,614 (27.80) | 3241 (59.58) | Reference |
| Partially vaccinated | 18,875 (12.03) | 614 (11.29) | 48% (43%; 53%); p-value <0.0001 |
| Non-boosted | 90,037 (57.39) | 1535 (28.22) | 79% (77%; 81%); p-value <0.0001 |
| Boosted | 4371 (2.79) | 50 (0.92) | 82% (75%; 87%); p-value <0.0001 |
VE was adjusted for age, sex, and time between outcome occurrence and pandemic initiation.
Only four (0.07%) boosted individuals who had been tested positive for SARS-CoV-2 were hospitalized for COVID-19. VE against COVID-19 hospitalization was then estimated in the mixed subpopulation of non-boosted and boosted individuals and a protection exceeding 70% was observed [VE = 72% (95%CI: 68%; 75%)] (Table 3 ).
Table 3.
Vaccine effectiveness (VE) against COVID-19-related hospitalization.
| Covid-19 vaccination | Hospitalization |
||
|---|---|---|---|
| No N (%) | Yes N (%) | VE∗ (95%CI); p-value | |
| ≥ 11 years | |||
| Unvaccinated | 64,251 (73.59) | 4609 (78.50) | Reference |
| Partially vaccinated | 8459 (9.69) | 431 (7.34) | 42% (35%; 48%); p-value <0.0001 |
| Non-boosted/boosted | 14,598 (16.72) | 831 (14.15) | 72% (68%; 75%); p-value <0.0001 |
| 11–17 years | |||
| Unvaccinated | 9945 (92.65) | 45 (97.83) | |
| Partially vaccinated | 704 (6.56) | 1 (2.17) | NA |
| Non-boosted/boosted | 85 (0.79) | 0 (0.00) | NA |
| 18–30 years | |||
| Unvaccinated | 18,462 (83.91) | 283 (88.44) | Reference |
| Partially vaccinated | 2468 (11.22) | 30 (9.38) | 29% (−5%; 51%); p-value = 0.086 |
| Non-boosted/boosted | 1072 (4.87) | 7 (2.19) | 69% (29%; 87%); p-value = 0.006 |
| 31–45 years | |||
| Unvaccinated | 14,424 (70.08) | 601 (84.53) | Reference |
| Partially vaccinated | 2848 (13.84) | 69 (9.70) | 58% (45%; 68%); p-value <0.0001 |
| Non-boosted/boosted | 3311 (16.09) | 41 (5.77) | 76% (66%; 83%); p-value <0.0001 |
| 46–55 years | |||
| Unvaccinated | 8666 (65.13) | 670 (85.68) | Reference |
| Partially vaccinated | 681 (5.12) | 35 (4.48) | 46% (22%; 63%); p-value = 0.001 |
| Non-boosted/boosted | 3959 (29.75) | 77 (9.85) | 84% (78%; 88%); p-value <0.0001 |
| 56–65 years | |||
| Unvaccinated | 5888 (63.85) | 769 (81.20) | Reference |
| Partially vaccinated | 788 (8.54) | 69 (7.29) | 45% (27%; 59%); p-value <0.0001 |
| Non-boosted/boosted | 2546 (27.61) | 109 (11.51) | 76% (68%; 83%); p-value <0.0001 |
| 66–80 years | |||
| Unvaccinated | 4828 (62.26) | 1298 (78.24) | Reference |
| Partially vaccinated | 492 (6.34) | 105 (6.33) | 29% (11%; 44%); p-value = 0.004 |
| Non-boosted/boosted | 2435 (31.40) | 256 (15.43) | 71% (62%; 78%); p-value <0.0001 |
| ≥ 81 years | |||
| Unvaccinated | 2038 (54.99) | 943 (67.07) | Reference |
| Partially vaccinated | 478 (12.90) | 122 (8.68) | 48% (35%; 59%); p-value <0.0001 |
| Non-boosted/boosted | 1190 (32.11) | 341 (24.25) | 73% (64%; 80%); p-value <0.0001 |
: VE was adjusted for age, sex, and time between outcome occurrence and pandemic initiation. NA: VE estimation is not applicable due to a lack of observations.
None of those individuals who tested positive for SARS-CoV-2 and had received a booster dose of the vaccine was admitted to ICU or died for COVID-19, thus VE against these outcomes was estimated in the mixed subpopulation of non-boosted and boosted individuals. VE against ICU admission was 83% (95%CI: 78%; 88%), and that against in-hospital mortality was 66% (95%CI: 53%; 75%).
In our study population, only mRNA type vaccines were administered as a booster. Restricted analysis of individuals who had received mRNA-type vaccines exclusively, showed 88% VE against SARS-CoV-2 infections [VE = 88% (95%CI: 85%; 90%)]. Among individuals who had received the mRNA booster dose, only four were hospitalized and none was admitted to ICU or died of COVID-19. In a mixed subpopulation of non-boosted and boosted individuals, VE against hospitalization for COVID-19 was 74% (95%CI: 70%; 77%); ICU admission 86% (95%CI: 80%; 90%) and in-hospital mortality 71% (95%CI: 59%; 79%) (Table S2).
Impact of COVID-19 booster-based vaccination on SARS-CoV-2 infection and COVID-19 severity stratified by age.
When stratifying the study population by age, sufficient observations of boosted individuals were only obtained for those older than 65 years. Receiving a booster dose of the COVID-19 vaccine offers more than 80% protection against SARS-CoV-2 infection in the elderly population [66–80 years: VE = 85% (95%CI: 77%; 91%); ≥81 years: VE = 82% (95%CI: 75%; 87%)] (Table 2). For the younger age categories, VE was estimated in the mixed subpopulation of non-boosted and boosted individuals due to insufficient number of observations, and ranged between 51% and 76% (Table 2).
Likewise, the effectiveness of receiving a booster dose of COVID-19 vaccine against hospitalization was more than 70% in people aged over 65 years [66–80 years: VE = 71% (95%CI: 62%; 78%); ≥81 years: VE = 73% (95%CI: 64%; 80%)] (Table 3). VE against hospitalization in the younger age groups (≥18 years) was estimated in the mixed subpopulation of non-boosted and boosted individuals and ranged between 69% and 84% (Table 2).
Comparing the mixed subpopulation of non-boosted and boosted individuals to unvaccinated individuals showed a substantial VE against ICU admission for COVID-19 in individuals aged between 46 and 65 years [VE = 86% (95%CI: 77%; 91%)] and in those older than 65 years [VE = 83% (95%CI: 73%; 89%)]. The data also revealed considerable protection against death for COVID-19 in individuals older than 65 years [VE = 65% (95%CI: 50%; 75%)].
Impact of COVID-19 booster-based vaccination on SARS-CoV-2 infection and COVID-19 severity stratified by comorbidity type.
Individuals with any of the following comorbidities and who received a booster dose of COVID-19 vaccine are more protected against SARS-CoV-2 infection than those who were non-boosted: cancer [VEnon-boosted = 54% (95%CI: 49%; 59%); VEboosted = 81% (95%CI: 68%; 88%)], COPD [VEnon-boosted = 61% (95%CI: 55%; 67%); VEboosted = 84% (95%CI: 66%; 93%)], depression [VEnon-boosted = 62% (95%CI: 59%; 65%); VEboosted = 83% (95%CI: 74%; 89%)], diabetes [VEnon-boosted = 65% (95%CI: 62%; 68%); VEboosted = 84% (95%CI: 75%; 90%)], hypertension [VEnon-boosted = 65% (95%CI: 63%; 68%); VEboosted = 82% (95%CI: 76%; 86%)], ischemic heart disease [VEnon-boosted = 56% (95%CI: 49%; 63%); VEboosted = 79% (95%CI: 60%; 90%)], kidney failure [VEnon-boosted = 67% (95%CI: 61%; 72%); VEboosted = 85% (95%CI: 70%; 92%)], obesity [VEnon-boosted = 67% (95%CI: 65%; 69%); VEboosted = 91% (95%CI: 83%; 95%)], and stroke [VEnon-boosted = 66% (95%CI: 60%; 71%); VEboosted = 85% (95%CI: 68%; 93%)] (Table 4 ).
Table 4.
Vaccine effectiveness (VE) against SARS-CoV-2 infection and COVID-19 hospitalization stratified by comorbidity.
| Covid-19 vaccination | Infection |
||
|---|---|---|---|
| No N (%) | Yes N (%) | VE∗ (95%CI); p-value | |
| Obesity | |||
| Unvaccinated | 127,335 (53.89) | 9223 (72.41) | Reference |
| Partially vaccinated | 25,207 (10.67) | 1077 (8.46) | 41% (37%; 45%); p-value <0.0001 |
| Non-boosted | 81,711 (34.58) | 2426 (19.05) | 67% (65%; 69%); p-value <0.0001 |
| Boosted | 2033 (0.86) | 11 (0.09) | 91% (83%; 95%); p-value <0.0001 |
| Depression | |||
| Unvaccinated | 99,231 (48.55) | 5383 (65.09) | Reference |
| Partially vaccinated | 22,995 (11.24) | 811 (9.81) | 36% (30%; 41%); p-value <0.0001 |
| Non-boosted | 79,917 (39.06) | 2056 (24.86) | 62% (59%; 65%); p-value <0.0001 |
| Boosted | 2344 (1.15) | 20 (0.24) | 83% (74%; 89%); p-value <0.0001 |
| Diabetes | |||
| Unvaccinated | 73,600 (43.58) | 4681 (65.79) | Reference |
| Partially vaccinated | 19,514 (11.56) | 597 (8.39) | 44% (38%; 49%); p-value <0.0001 |
| Non-boosted | 73,354 (43.44) | 1819 (25.57) | 65% (62%; 68%); p-value <0.0001 |
| Boosted | 2400 (1.42) | 18 (0.25) | 84% (75%; 90%); p-value <0.0001 |
| COPD | |||
| Unvaccinated | 31,329 (41.47) | 1532 (64.75) | Reference |
| Partially vaccinated | 8278 (10.96) | 161 (6.80) | 48% (38%; 56%); p-value <0.0001 |
| Non-boosted | 34,691 (45.92) | 666 (28.15) | 61% (55%; 67%); p-value <0.0001 |
| Boosted | 1241 (1.64) | 7 (0.30) | 84% (66%; 93%); p-value <0.0001 |
| Atrial fibrillation | |||
| Unvaccinated | 33,498 (37.29) | 1882 (64.76) | Reference |
| Partially vaccinated | 10,004 (11.14) | 228 (7.85) | 43% (35%; 51%); p-value <0.0001 |
| Non-boosted | 44,643 (49.69) | 781 (26.88) | 64% (58%; 69%); p-value <0.0001 |
| Boosted | 1693 (1.88) | 15 (0.52) | 73% (54%; 85%); p-value <0.0001 |
| Hypertension | |||
| Unvaccinated | 169,915 (43.48) | 10,930 (65.56) | Reference |
| Partially vaccinated | 44,592 (11.41) | 1341 (8.04) | 45% (41%; 48%); p-value <0.0001 |
| Non-boosted | 170,803 (43.70) | 4348 (26.08) | 65% (63%; 68%); p-value <0.0001 |
| Boosted | 5502 (1.41) | 53 (0.32) | 82% (76%; 86%); p-value <0.0001 |
| Cardiac failure | |||
| Unvaccinated | 30,688 (37.72) | 1441 (65.77) | Reference |
| Partially vaccinated | 9201 (11.31) | 210 (9.58) | 33% (22%; 43%); p-value <0.0001 |
| Non-boosted | 39,902 (49.05) | 530 (24.19) | 70% (64%; 75%); p-value <0.0001 |
| Boosted | 1558 (1.92) | 10 (0.46) | 80% (61%; 90%); p-value <0.0001 |
| Kidney failure | |||
| Unvaccinated | 30,755 (38.47) | 1411 (64.58) | Reference |
| Partially vaccinated | 9021 (11.28) | 183 (8.38) | 39% (28%; 48%); p-value <0.0001 |
| Non-boosted | 38,152 (47.72) | 581 (26.59) | 67% (61%; 72%); p-value <0.0001 |
| Boosted | 2027 (2.54) | 10 (0.46) | 85% (70%; 92%); p-value <0.0001 |
| Cancer | |||
| Unvaccinated | 73,865 (46.18) | 3445 (65.14) | Reference |
| Partially vaccinated | 18,558 (11.60) | 426 (8.05) | 35% (28%; 48%); p-value <0.0001 |
| Non-boosted | 65,200 (40.77) | 1403 (26.53) | 54% (49%; 59%); p-value <0.0001 |
| Boosted | 2317 (1.45) | 15 (0.28) | 81% (68%; 88%); p-value <0.0001 |
| Ischemic heart disease | |||
| Unvaccinated | 30,813 (42.15) | 1686 (65.22) | Reference |
| Partially vaccinated | 8202 (11.22) | 193 (7.47) | 38% (27%; 47%); p-value <0.0001 |
| Non-boosted | 32,960 (45.09) | 697 (26.96) | 56% (49%; 63%); p-value <0.0001 |
| Boosted | 1125 (1.54) | 9 (0.35) | 79% (60%; 90%); p-value <0.0001 |
| Stroke | |||
| Unvaccinated | 25,764 (39.14) | 1328 (64.00) | Reference |
| Partially vaccinated | 7782 (11.82) | 243 (11.71) | 29% (18%; 38%); p-value <0.0001 |
| Non-boosted | 31,093 (47.23) | 496 (23.90) | 66% (60%; 71%); p-value <0.0001 |
| Boosted | 1194 (1.81) | 8 (0.39) | 85% (68%; 93%); p-value <0.0001 |
| Epilepsy | |||
| Unvaccinated | 11,238 (47.73) | 653 (67.81) | Reference |
| Partially vaccinated | 2794 (11.87) | 123 (12.77) | 26% (9%; 40%); p-value = 0.004 |
| Non-boosted/boosted | 9512 (40.40) | 187 (19.42) | 66% (58%; 73%); p-value <0.0001 |
| Dementia | |||
| Unvaccinated | 15,244 (27.52) | 1047 (56.32) | Reference |
| Partially vaccinated | 7621 (13.76) | 320 (17.21) | 38% (29%; 45%); p-value <0.0001 |
| Non-boosted | 31,054 (56.07) | 477 (25.66) | 81% (77%; 84%); p-value <0.0001 |
| Boosted | 1464 (2.64) | 15 (0.81) | 88% (78%; 90%); p-value <0.0001 |
| Parkinson | |||
| Unvaccinated | 4790 (31.90) | 275 (59.40) | Reference |
| Partially vaccinated | 2011 (13.39) | 70 (15.12) | 35% (15%; 50%); p-value = 0.002 |
| Non-boosted/boosted | 8216 (54.71) | 118 (25.49) | 74% (64%; 81%); p-value <0.0001 |
| HIV | |||
| Unvaccinated | 2350 (54.16) | 90 (62.94) | Reference |
| Partially vaccinated | 531 (12.24) | 10 (6.99) | 35% (−30%; 67%); p-value = 0.229 |
| Non-boosted/boosted | 1458 (33.60) | 43 (30.07) | 30% (−16%; 57%); p-value = 0.166 |
COPD: chronic obstructive pulmonary disease. HIV: human immunodeficiency virus.
: VE was adjusted for sex, age, and time between outcome occurrence and pandemic initiation.
Few individuals with epilepsy or Parkinson who had received the booster dose of COVID-19 vaccine tested positive for SARS-CoV-2, therefore VE against infection in these subgroups was estimated in the mixed population of non-boosted and boosted individuals. Substantial protection against SARS-CoV-2 infection was observed for epilepsy [VE = 66% (95%CI: 58%; 73%)] and Parkinson patients [VE = 74% (95%CI: 64%; 81%)] (Table 4).
Few patients with comorbidity who had received the booster dose of the COVID-19 vaccine were hospitalized for COVID-19, hence VE against hospitalization was estimated by comparing the odds of hospitalization in the mixed population to that of unvaccinated individuals. Vaccinated comorbid patients showed lower odds of COVID-19 related hospitalization than unvaccinated ones. VE ranged between 49% and 79% (Table 5 ).
Table 5.
Vaccine effectiveness (VE) against COVID-19 hospitalization stratified by comorbidity.
| Covid-19 vaccination | Hospitalization |
||
|---|---|---|---|
| No N (%) | Yes N (%) | VE∗ (95%CI); p-value | |
| Obesity | |||
| Unvaccinated | 7436 (70.72) | 1459 (79.77) | Reference |
| Partially vaccinated | 929 (8.83) | 135 (7.38) | 43% (30%; 54%); p-value <0.0001 |
| Non-boosted/boosted | 2150 (20.45) | 235 (12.85) | 78% (73%; 83%); p-value <0.0001 |
| Depression | |||
| Unvaccinated | 4303 (62.92) | 856 (74.05) | Reference |
| Partially vaccinated | 713 (10.43) | 88 (7.61) | 52% (38%; 63%); p-value <0.0001 |
| Non-boosted/boosted | 1823 (26.66) | 212 (18.34) | 69% (59%; 77%); p-value <0.0001 |
| Diabetes | |||
| Unvaccinated | 3267 (61.95) | 1124 (75.54) | Reference |
| Partially vaccinated | 476 (9.03) | 108 (7.26) | 48% (34%; 59%); p-value <0.0001 |
| Non-boosted/boosted | 1531 (29.03) | 256 (17.20) | 74% (65%; 80%); p-value <0.0001 |
| COPD | |||
| Unvaccinated | 931 (59.60) | 461 (72.37) | Reference |
| Partially vaccinated | 122 (7.81) | 35 (5.49) | 43% (14%; 63%); p-value = 0.008 |
| Non-boosted/boosted | 509 (32.59) | 141 (22.14) | 71% (51%; 82%); p-value <0.0001 |
| Atrial fibrillation | |||
| Unvaccinated | 1076 (58.96) | 592 (71.58) | Reference |
| Partially vaccinated | 161 (8.82) | 57 (6.89) | 42% (19%; 58%); p-value = 0.001 |
| Non-boosted/boosted | 588 (32.22) | 178 (21.52) | 68% (51%; 79%); p-value <0.0001 |
| Hypertension | |||
| Unvaccinated | 8074 (62.30) | 2247 (75.40) | Reference |
| Partially vaccinated | 1110 (8.57) | 209 (7.01) | 47% (37%; 55%); p-value <0.0001 |
| Non-boosted/boosted | 3775 (29.13) | 524 (17.58) | 73% (67%; 78%); p-value <0.0001 |
| Cardiac failure | |||
| Unvaccinated | 682 (58.14) | 543 (71.83) | Reference |
| Partially vaccinated | 140 (11.94) | 58 (7.67) | 52% (33%; 66%); p-value <0.0001 |
| Non-boosted/boosted | 351 (29.92) | 155 (20.50) | 72% (54%; 53%); p-value <0.0001 |
| Kidney failure | |||
| Unvaccinated | 716 (57.42) | 519 (71.29) | Reference |
| Partially vaccinated | 137 (10.99) | 39 (5.36) | 66% (50%; 77%); p-value <0.0001 |
| Non-boosted/boosted | 394 (31.60) | 170 (23.35) | 78% (60%; 88%); p-value <0.0001 |
| Cancer | |||
| Unvaccinated | 2545 (61.77) | 666 (74.41) | Reference |
| Partially vaccinated | 364 (8.83) | 56 (6.26) | 45% (25%; 60%); p-value <0.0001 |
| Non-boosted/boosted | 1211 (29.39) | 173 (19.33) | 66% (50%; 56%); p-value <0.0001 |
| Ischemic heart disease | |||
| Unvaccinated | 1094 (61.29) | 443 (71.80) | Reference |
| Partially vaccinated | 145 (8.12) | 40 (6.48) | 39% (10%; 58%); p-value = 0.014 |
| Non-boosted/boosted | 546 (30.59) | 134 (21.72) | 49% (19%; 67%); p-value = 0.004 |
| Stroke | |||
| Unvaccinated | 848 (59.26) | 357 (72.41) | Reference |
| Partially vaccinated | 191 (13.35) | 42 (8.52) | 55% (35%; 69%); p-value <0.0001 |
| Non-boosted/boosted | 392 (27.39) | 94 (19.07) | 70% (49%; 83%); p-value <0.0001 |
| Epilepsy | |||
| Unvaccinated | 518 (66.58) | 103 (73.05) | Reference |
| Partially vaccinated | 100 (12.85) | 20 (14.18) | 8% (−62%; 47%); p-value = 0.785 |
| Non-boosted/boosted | 160 (20.57) | 18 (12.77) | 32% (−55%; 71%); p-value = 0.355 |
| Dementia | |||
| Unvaccinated | 634 (50.72) | 322 (65.71) | Reference |
| Partially vaccinated | 251 (20.08) | 64 (13.06) | 48% (29%; 62%); p-value <0.0001 |
| Non-boosted/boosted | 365 (29.20) | 104 (21.22) | 79% (62%; 89%); p-value <0.0001 |
| Parkinson | |||
| Unvaccinated | 149 (51.56) | 96 (72.73) | Reference |
| Partially vaccinated | 61 (21.11) | 8 (6.06) | 80% (60%; 91%); p-value <0.0001 |
| Non-boosted/boosted | 79 (27.34) | 28 (21.21) | 73% (−4%; 93%); p-value = 0.058 |
| HIV | |||
| Unvaccinated | 79 (62.20) | 9 (64.29) | Reference |
| Partially vaccinated | 9 (7.09) | 1 (7.14) | NA |
| Non-boosted/boosted | 39 (30.71) | 4 (28.57) | NA |
COPD: chronic obstructive pulmonary disease. HIV: human immunodeficiency virus. NA: VE estimation is not applicable due to a lack of observations.
: VE was adjusted for sex, age, and time between outcome occurrence and pandemic initiation.
VE against ICU admission for COVID-19 was estimated in the mixed population of non-boosted and boosted individuals relative to unvaccinated people. Significant protection against COVID-19-related ICU admission was observed in patients with cancer [VE = 87% (95%CI: 71%; 94%)], depression [VE = 87% (95%CI: 73%; 94%)], diabetes [VE = 83% (95%CI: 70%; 91%)], hypertension [VE = 83% (95%CI: 72%; 89%)], and obesity [VE = 84% (95%CI: 73%; 91%)]. VE against COVID-19-related ICU admission could not be estimated for other comorbid groups due to the limited number of observations.
4. Discussion
The present population-based study showed high effectiveness of a third-dose booster schedule in preventing SARS-CoV-2 infection in Galicia-Spain. We reported that people who received a booster dose of COVID-19 vaccine are substantially more protected against SARS-CoV-2 infection than non-boosted individuals who had received any of the authorized vaccines but not the booster dose (VE = 87% versus VE = 66%). Our data support the utility of a booster dose of COVID-19 vaccine in developed country settings such as that of Spain in both age and co-morbidity-based indications.
During our study period, most individuals who had received the booster dose were older than 65 years as this age range represents a priority group for vaccination in Spain. Upon stratification by age, elevated VE (>80%) from a booster dose against SARS-CoV-2 infection was maintained in people aged 66–80 years, as well as those 81 years and above. As for the younger age categories, VE was estimated in a mixed subpopulation of non-boosted and boosted individuals due to insufficient number of observations among individuals with a booster dose, and a protection against infection ranging between 51% and 76% was observed. We also showed that administering a booster dose is also associated with an important decrease in the likelihood of hospitalization for COVID-19 among people over 65 years (VE >70%). VE against ICU admission and in-hospital mortality was evaluated in the mixed vaccinated population of non-boosted and boosted individuals due to lack of observations among the boosted group. Vaccination against COVID-19 contributed to lowering the odds of ICU admission for COVID-19 by more than 80% in people aged between 46 and 65 years as well as in those older than 65 years. It also revealed 65% protection against death for COVID-19 in individuals older than 65 years.
Studies on the impact of administering a booster dose are emerging and our findings are comparable to reports available so far. A study in Chile showed that a three-dose schedule prevents infection with SARS-CoV-2 between 79% and 97%, depending on the vaccine type (Jara et al., 2022). The study also reported considerable VE that ranged between 86% and 99% against hospitalization, ICU admission and death in individuals who received a booster dose (Jara et al., 2022). A preliminary study in Israel, reported 86% reduction in the odds of testing positive for SARS-CoV-2 in individuals who received a booster dose of BNT162b2 relative to those who received only two doses of the vaccine (Patalon et al., 2022). A second study in Israel also demonstrated that the rates of SARS-CoV-2 infection and COVID-19 related hospitalization was lower in the boosted group than those in the non-boosted group by factors of 11.3 and 19.5, respectively (Bar-On et al., 2021). Arbel and colleagues showed that the COVID- 19-related mortality rate is 90% lower in Israeli people with a booster dose of BNT162b2 (Arbel et al., 2021). Barda et al. evaluated the effectiveness of a booster dose of BNT162b2 in Israel and estimated VE of 93% against COVID-19-related hospitalization and 81% against COVID-19-related death (Barda et al., 2021). A study in England reported that the effectiveness of a booster dose against symptomatic COVID-19 ranged from 94% to 97% and was similar in all age groups, and that against hospitalization or death oscillated between 97% and 99% in all age groups (Andrews et al., 2022). In the United States, VE against COVID-19–associated hospitalization was at least 90% among persons who had received a third dose of mRNA vaccine ≥14 days earlier (Thompson et al., 2022). In Spain, to the best of our knowledge, this is the first report on the effectiveness of the boosted dose of COVID-19 vaccine.
As several particularly vulnerable groups were not included in sufficient numbers in clinical trials on COVID-19 vaccines, quantifying real-world VE, including both biological and behavioral effects is essential. In the present study, we found that administering a booster dose in comorbid patients importantly increments the protection against SARS-CoV-2 infection. The increase in protection was the most noticeable in boosted patients with major health problems like cancer (from 54% to 81%) and obesity (from 67% to 91%) as compared to non-boosted patients. VE against severe COVID-19 (hospitalization, ICU admission or death) was assessed in the mixed population of non-boosted and boosted people. The odds of COVID-19 related hospitalization decreased between 49% and 79% after vaccinating comorbid patients. Important VE against COVID-19-related ICU admission (>80%) was seen in patients with cancer, depression, diabetes, hypertension, or obesity. Considering the deficit of studies that evaluated COVID-19 VE in subpopulations of comorbid patients, the findings of the present study could prove useful for future systematic reviews and meta-analyses.
The main strength of our study lies in its population-based nature where all individuals vaccinated in Galicia-Spain during the study period were assessed for their inclusion in the study; thus, selection bias is only remotely probable. Exposure misclassification is also improbable to have occurred as in Galicia-Spain, all population had access to free of charge COVID-19 vaccine. Vaccination was managed by the regional health care organism and corresponding data were electronically registered. The estimated VE was adjusted for a wide range of possible confounding variables including socio-demographic, socioeconomic, and clinical variables. The extent of exposure to SARS-CoV-2 varies across settings which if present would bias VE estimates, and residual confounding from unmeasured factors such as health seeking behavior, application of different non-pharmaceutical measures during the study period and adherence to the use of facemasks might have still been present. The outcomes, SARS-CoV-2 infection and disease severity, in our study were ascertained through clinical and medical based records, yet the possibility of outcome misclassification from a false positive or a false negative test result or from inaccurate diagnosis codes cannot be ruled out. If it occurred, this non-differential misclassification could have underestimated the VE. The imperfect sensitivity of PCR testing could cause misclassification, which could attenuate VE estimates (Fonseca et al., 2021). We excluded from the analysis individuals who were infected with SARS-CoV-2 before the study initiation in order to account for acquired immunity from a past infection. Nonetheless, asymptomatic patients are likely not to be tested for SARS-CoV-2 which if present could overestimate our findings on VE. The time passed since booster dose administration until outcome development could influence VE estimates. We did not stratify for this variable due to insufficient observations, however, we controlled the analysis for the time between COVID-19 pandemic initiation and outcome occurrence. Other studies are encouraged to control for time since booster dose administration. Our data lacks information on SARS-CoV-2 variant in positively tested individuals, accordingly VE stratified by SARS-CoV-2 variant is lacking. SARS-CoV-2 variants might affect COVID-19 VE, consequently, a continuous assessment of vaccine performance is needed. Future studies are required to estimate the effectiveness of booster-based schedule on COVID-19 severity in young adults.
5. Conclusions
The need for booster dose(s) of COVID-19 vaccines remains an issue of debate. Vaccine performance varies across settings and populations, hence determining the extent and duration of VE using real-world data is crucial to inform related authorities and design specific prevention programs that take into account the vaccination calendar, the need for booster dose/s and the vulnerable populations. Our findings suggest that, in settings like Spain, booster-based vaccine schedule increments the protection against to SARS-CoV-2 infection and COVID-19 severity. Importantly, COVID-19 vaccine booster administration considerably protects patients with major comorbidities.
Funding
This work was supported by Framework Partnership Agreement between the Consellería de Sanidad de la XUNTA de Galicia and GENVIP-IDIS-2021–2024 (SERGAS-IDIS March 2021; Spain); and consorcio Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CB21/06/00103; F.M-T), DIAVIR (Instituto de Salud Carlos III (ISCIII)/DTS19/00049/Cofinanciado FEDER; Proyecto de Desarrollo Tecnológico en Salud), Resvi-Omics (Instituto de Salud Carlos III (ISCIII)/PI19/01039/Cofinanciado FEDER), BI-BACVIR (PRIS-3; Agencia de Conocimiento en Salud (ACIS)—Servicio Gallego de Salud (SERGAS)—Xunta de Galicia; Spain), Programa Traslacional COVID-19 (ACIS—Servicio Gallego de Salud (SERGAS)—XUNTA de Galicia; Spain) and Axencia Galega de Innovación (GAIN; IN607B 2020/08—XUNTA de Galicia; Spain) [A.S]; and ReSVinext (Instituto de Salud Carlos III (ISCIII)/PI16/01569/Cofinanciado FEDER), Enterogen (Instituto de Salud Carlos III (ISCIII)/PI19/01090/Cofinanciado FEDER), and Axencia Galega de Innovación (GAIN; IN845D 2020/23—Xunta de Galicia; Spain) [F.M-T].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.114252.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adab P., Haroon S., O'Hara M.E., Jordan R.E. Comorbidities and covid-19. BMJ. 2022;377:o1431. doi: 10.1136/bmj.o1431. [DOI] [PubMed] [Google Scholar]
- Andrews N., Stowe J., Kirsebom F., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022;28(4):831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel R., Hammerman A., Sergienko R., et al. BNT162b2 vaccine booster and mortality due to covid-19. N. Engl. J. Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N. Engl. J. Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC: Center for Disease Control and Prevention COVID-19, people with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available at: Accessed.
- Chiner-Vives E., Cordovilla-Perez R., de la Rosa-Carrillo D., et al. Short and long-term impact of COVID-19 infection on previous respiratory diseases. Arch. Bronconeumol. 2022;58(Suppl. 1):39–50. doi: 10.1016/j.arbres.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz H., Alsrhani A., Zafar A., et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency COVID-19 vaccines: authorised. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised Available at: Accessed June 01, 2022.
- Feikin D.R., Higdon M.M., Abu-Raddad L.J., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea A., Sy L.S., Luo Y., et al. Durability of mRNA-1273 against COVID-19 in the time of Delta: interim results from an observational cohort study. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0267824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca E., Ferreira L.C., Loureiro B.M.C., et al. Chest computed tomography in the diagnosis of COVID-19 in patients with false negative RT-PCR. Einstein (Sao Paulo) 2021;19 doi: 10.31744/einstein_journal/2021AO6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobierno de España [Spanish Government] Estrategia de vacunación COVID-19 [COVID-19 vaccination strategy] https://www.vacunacovid.gob.es Available at: Accessed June 012022.
- Gonzalez-Barcala F.J., Nieto-Fontarigo J.J., Mendez-Brea P., Salgado F.J. The polyhedric reality of the interaction between COVID-19, asthma and inhaled corticosteroids. ERJ Open Res. 2022;8(2) doi: 10.1183/23120541.00179-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am. J. Publ. Health. 1989;79(3):340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.F., Zhong N.S., Guan W.J. Impact of chronic respiratory diseases on the outcomes of COVID-19. Arch. Bronconeumol. 2022;58(1):5–7. doi: 10.1016/j.arbres.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2019. Instituto Galego de Estatística [Galician Statistical Office]https://www.ige.eu/igebdt/esqv.jsp?ruta=verTabla.jsp?OP=1&B=1&M=&COD=77&R=9915 [Google Scholar]
- Instituto Galego de Estatística [Galician Statistical Office] 2020. Hospitais [hospitals]https://www.ige.eu/igebdt/esqv.jsp?ruta=verTabla.jsp?OP=1&B=1&M=&COD=2922&R=9915 [12];1 &C=0[all]&F=&S=&SCF= Accessed. Accessed. [Google Scholar]
- Instituto Galego de Estatística [Galician Statistical Office] 2021. Población [population]http://www.ige.eu/web/index.jsp?idioma=gl Available at. [Google Scholar]
- Instituto Galego de Estatística [Galician Statistical Office] 2021. Poboación por sexo e grupos quinquenais de idade. Ano 2021 [Population by sex and five-year age groups. Year 2021.https://www.ige.eu/igebdt/esq.jsp?paxina=002001&c=0201001002&ruta=verPpalesResultados.jsp?OP=1&B=1&M=&COD=1373&R=2%5Ball%5D&C=1%5Ball%5D&F=T Available at: [Google Scholar]
- Jara A., Undurraga E.A., Zubizarreta J.R., et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Global Health. 2022;10(6):E798–E806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury J., Najjar-Debbiny R., Hanna A., et al. COVID-19 vaccine - long term immune decline and breakthrough infections. Vaccine. 2021;39(48):6984–6989. doi: 10.1016/j.vaccine.2021.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H.B., Li S., Goodwin J.S. Risk factors associated with SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw. Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Sanidad Consumo y Bienestar Social [Ministry of Health Consumption and Social Welfare] Informe nº 106. Situación de COVID-19 en España 24 de noviembre de 2021 [Report nº 106. COVID-19 situation in Spain. November 24. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/-COVID-19.-Informes-previos.aspx Available at: Accessed June 01 2021 2022.
- Monge S., Rojas-Benedicto A., Olmedo C., et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect. Dis. 2022;22(9):1313–1320. doi: 10.1016/S1473-3099(22)00292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P., Tserel L., Kangro K., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Seco J., Mallah N., Lopez-Perez L.R., et al. Evaluation of BNT162b2 vaccine effectiveness in Galicia, northwest Spain. Int. J. Environ. Res. Publ. Health. 2022;19(7) doi: 10.3390/ijerph19074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patalon T., Gazit S., Pitzer V.E., Prunas O., Warren J.L., Weinberger D.M. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern. Med. 2022;182(2):179–184. doi: 10.1001/jamainternmed.2021.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar R., Garg I., Pal S., Kottewar S., Sheikh A.B. COVID-19 vaccine booster: to boost or not to boost. Infect. Dis. Rep. 2021;13(4):924–929. doi: 10.3390/idr13040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.G., Natarajan K., Irving S.A., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 States, august 2021-january 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71(4):139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tut G., Lancaster T., Krutikov M., et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. Lancet Healthy Longev. 2021;2(9):e544–e553. doi: 10.1016/S2666-7568(21)00168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Interim statement on booster doses for COVID-19 vaccination. https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination---update-22-december-2021 Available at: Accessed.
- Yeo I., Baek S., Kim J., et al. Assessment of thirty-day readmission rate, timing, causes and predictors after hospitalization with COVID-19. J. Intern. Med. 2021;290(1):157–165. doi: 10.1111/joim.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.