Abstract
Numerous cases of glomerulonephritis manifesting shortly after SARS-CoV-2 vaccination have been reported, but causality remains unproven. Here, we studied the association between mRNA-based SARS-CoV-2 vaccination and new-onset glomerulonephritis using a nationwide retrospective cohort and a case-cohort design. Data from all Swiss pathology institutes processing native kidney biopsies served to calculate incidence of IgA nephropathy, pauci-immune necrotizing glomerulonephritis, minimal change disease, and membranous nephropathy in the adult Swiss population. The observed incidence during the vaccination campaign (January to August 2021) was not different from the expected incidence calculated using a Bayesian model based on the years 2015 to 2019 (incidence rate ratio 0.86, 95% credible interval 0.73–1.02) and did not cross the upper boundary of the 95% credible interval for any month. Among 111 patients 18 years and older with newly diagnosed glomerulonephritis between January and August 2021, 38.7% had received at least one vaccine dose before biopsy, compared to 39.5% of the general Swiss population matched for age and calendar-time. The estimated risk ratio for the development of new-onset biopsy-proven glomerulonephritis was not significant at 0.97 (95% confidence interval 0.66–1.42) in vaccinated vs. unvaccinated individuals. Patients with glomerulonephritis manifesting within four weeks after vaccination did not differ clinically from those manifesting temporally unrelated to vaccination. Thus, vaccination against SARS-CoV-2 was not associated with new-onset glomerulonephritis in these two complementary studies with most temporal associations between SARS-CoV-2 vaccination and glomerulonephritis likely coincidental.
Keywords: COVID vaccine, glomerulonephritis, IgA nephropathy, membranous nephropathy, minimal change disease, SARS-CoV-2 vaccination
Graphical abstract
Editor’s Note.
This excellent study discusses new-onset glomerular disease in the face of coronavirus disease 2019 (COVID-19) mRNA vaccination. The readership is reminded that there has been an abundance of reports suggesting activation or worsening of pre-existing glomerular diseases after receiving the vaccines, especially IgA nephropathy and minimal change disease. Although these reports do not provide mechanistic proof of causation, timing of disease exacerbation is consistent, and the association is plausible.
After emergency use authorization for the first vaccines developed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in many countries in late 2020, an unprecedented vaccination campaign was initiated worldwide in 2021. In Europe and North America, the majority of people received mRNA-based vaccines, mostly either BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna). Both vaccines had been tested in large randomized controlled trials with excellent safety profiles.1 , 2 However, these registration studies were not powered to detect rare vaccine side effects. During the worldwide vaccination campaign, numerous rare adverse events with a temporal association with SARS-CoV-2 vaccination were observed,3 including various autoimmune phenomena.4 Among such associations, many new-onset and recurrent cases of glomerulonephritis have been reported.5, 6, 7, 8, 9, 10, 11 Specific types of glomerulonephritis most often reported after SARS-CoV-2 vaccination include IgA nephropathy (IgAN), minimal change disease (MCD), membranous nephropathy (MN), and pauci-immune necrotizing glomerulonephritis (PINGN), the renal manifestation of antineutrophil cytoplasmic antibody–associated vasculitis.
Although mechanistically plausible,5 , 7 , 11 a causal link between SARS-CoV-2 vaccination and glomerulonephritis has not been formally established. Published case series lack control groups, and estimates of incidence of these glomerulonephritides before and during the vaccination campaign have not been published yet. Given the high number of vaccine doses administered, many cases of new-onset glomerulonephritis would be expected to occur in temporal proximity to vaccination by pure coincidence.
Here, we tested the hypothesis that SARS-CoV-2 mRNA vaccines increase the incidence of several types of glomerulonephritis against the null hypothesis that reported temporal associations can be attributed to a by-chance-effect. We compared the observed incidence of glomerulonephritis in Switzerland during the vaccination campaign in 2021 with the expected incidence based on a baseline period (2015–2019). We further compared the vaccination history of patients with glomerulonephritis newly diagnosed during the vaccination campaign to the general population matched for age and timepoint, and we characterized patients with new-onset glomerulonephritis in temporal association with vaccination.
Methods
Study design
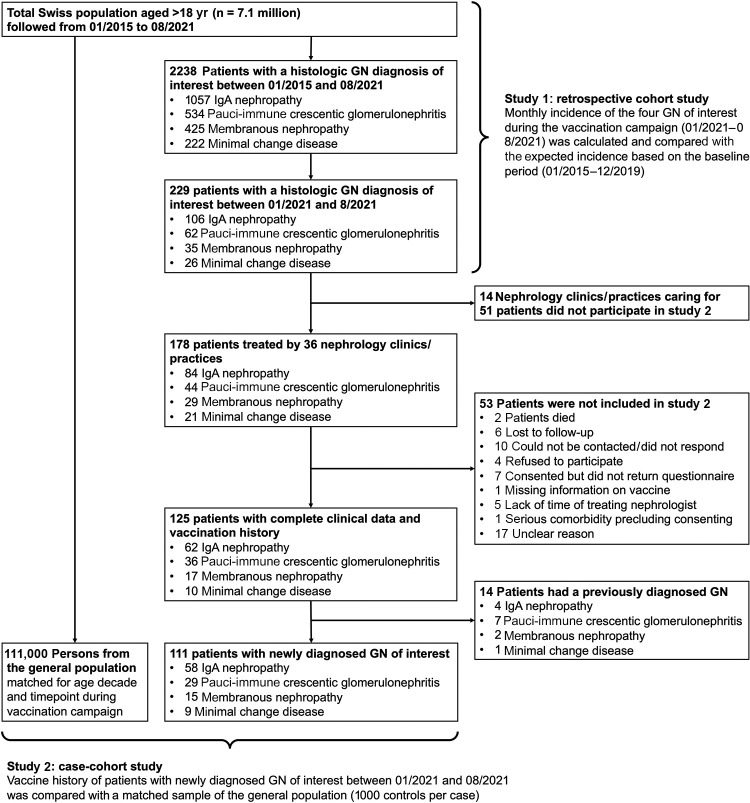
We performed 2 complementary, interconnected studies. For the first study with a retrospective cohort design (the cohort representing the entire adult Swiss population), all Swiss pathology institutes analyzing kidney biopsies provided biopsy date, age, sex, and histologic diagnosis for all patients aged >18 years with histologic diagnosis of IgAN, PINGN, MCD, or MN from January 1, 2015, through August 31, 2021. These data, together with census data for each year (2015–2021) on the Swiss population aged >18 years, were used to calculate the incidence of all 4 types of glomerulonephritis. Notably, only the histologic diagnosis was provided by pathologists, without information on extrarenal manifestations, such as IgA vasculitis or extrarenal symptoms of antineutrophil cytoplasmic antibody vasculitis. For the second study with a case-cohort design, all of the above-mentioned patients with biopsy date between January 1, 2021, and August 31, 2021, were eligible. Clinical information was gathered and vaccination history was compared with the general Swiss population, matched for age and timepoint during the vaccination campaign.
Both studies were approved by the Ethics Committee of Eastern Switzerland. The retrospective cohort study was exempt from patient consent because only minimal data were included and it would have been impossible to obtain consent from all patients, while inclusion of all cases was essential to calculate true incidence. For the case-cohort study, written informed consent was obtained from all cases, and the study was conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.12 We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies.13
Data collection
For the retrospective cohort study, local nephropathologists extracted relevant data from their local database (if existing) or performed a search of their electronic records. We limited our study to native kidney biopsies of persons aged >18 years with IgAN, PINGN, MCD, and MN. These are the most frequent glomerulonephritides that can be unambiguously diagnosed by histology and have been reported after SARS-CoV-2 vaccination. Lupus nephritis was not included because repeat biopsies are often performed for this glomerulonephritis, constituting a significant proportion of all lupus nephritis biopsies. We did not include focal segmental glomerulosclerosis (FSGS) because most FSGS cases are secondary, caused by distinct pathophysiological mechanisms and not reliably distinguishable from primary FSGS by histology.14 MN was included in the analysis because secondary MN constitutes a minority of cases,15 but lupus nephritis class V was excluded for reasons outlined above. As information on prior kidney biopsy was not available from the records of some pathology institutes and because repeat biopsies constitute only a small fraction of all biopsies, we included both new diagnoses and repeat biopsies in the first (retrospective cohort) study.
For the case-cohort study, nephropathologists provided the referring nephrologists’ names for all eligible patients (>18 years old with histologic diagnosis of IgAN, PINCG, MCD, or MN between January 1, 2021, and August 31, 2021) to the investigators. We contacted all nephrology divisions and practices that had sent biopsy specimens of eligible patients providing age, sex, histologic diagnosis, and date of biopsy to help them identify their patients and to contact them for study participation. Clinical and epidemiologic data were collected in a case report form completed by the treating nephrologist and in a questionnaire completed by the patient. All data were reviewed, and the timepoint of symptom onset or laboratory abnormalities attributable to the glomerular disease was adjudicated by the study investigators (see Supplementary Methods).
Pathologists provided complete data between September and December 2021, nephrologists were contacted between October and December 2021, and all questionnaires and case report form returned by February 22, 2022, were included in the analysis.
Data on the types and numbers of vaccine doses by calendar week and age decade for the general population (i.e., the entire cohort) were downloaded from the Swiss Federal Office of Public Health website.16
Statistical analysis
For the retrospective cohort study, a Bayesian Poisson regression model was used to predict the expected incidence of glomerulonephritis cases for each month in 2021 based on data for the years 2015–2019. The year 2020 was excluded from the baseline period because we expected underdiagnosis early in the pandemic due to lockdown measures. We calculated incidence rate ratios for the total of all 4 glomerulonephritides and for each diagnosis separately by dividing the observed cases in 2021 by the expected cases and expressed uncertainty using 95% credible intervals. In addition, we compared the average weekly incidence of glomerulonephritis during: (i) the entire vaccination campaign (January–August 2021) and (ii) the peak of the vaccination campaign (May–August 2021) with the corresponding months of the baseline period (2015–2019). Because the availability of serologic testing for anti-PLA2R autoantibodies may have influenced biopsy practice over time,17 we performed a sensitivity analysis excluding MN.
For the case-cohort study, we performed 2 analyses: we matched controls from the general population to patients with a new histologic diagnosis of glomerulonephritis for age and for the calendar week (=reference timepoint) of either kidney biopsy (primary endpoint, first analysis) or the onset of symptoms or laboratory abnormalities attributable to the disease (secondary endpoint, second analysis). For every patient, the percentage of the general population of the same age decade (the decade 20–29 was used for the few cases aged 18 or 19 years) who had received a first, second, or any vaccine dose in every 1-week interval before the reference timepoint was determined. Because the published general population vaccination data include cases (therefore, the study was designed as a case-cohort rather than a case-control study), we calculated and report risk ratios rather than odds ratios.18 Risk ratios were calculated by unconditional maximum likelihood estimation, using 1000 matched controls for every case, and confidence intervals (CIs) were computed by normal approximation using the R package epitools. In a sensitivity analysis, we repeated the calculations adjusting for age as the matching factor, using the Mantel-Haenszel approach.19 , 20 Discrete variables are expressed as counts (percentage) and continuous variables as median and interquartile range (IQR). Comparisons between the groups were made using the Kruskal-Wallis test and Pearson’s χ2 test, as appropriate.
Statistical analyses were performed using R software, version R 4.0.2 (R Core Team 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, https://www.R-project.org). A list of the packages used for this analysis is provided in the Supplementary Methods .
Results
Incidence of glomerulonephritis before and during the vaccination campaign
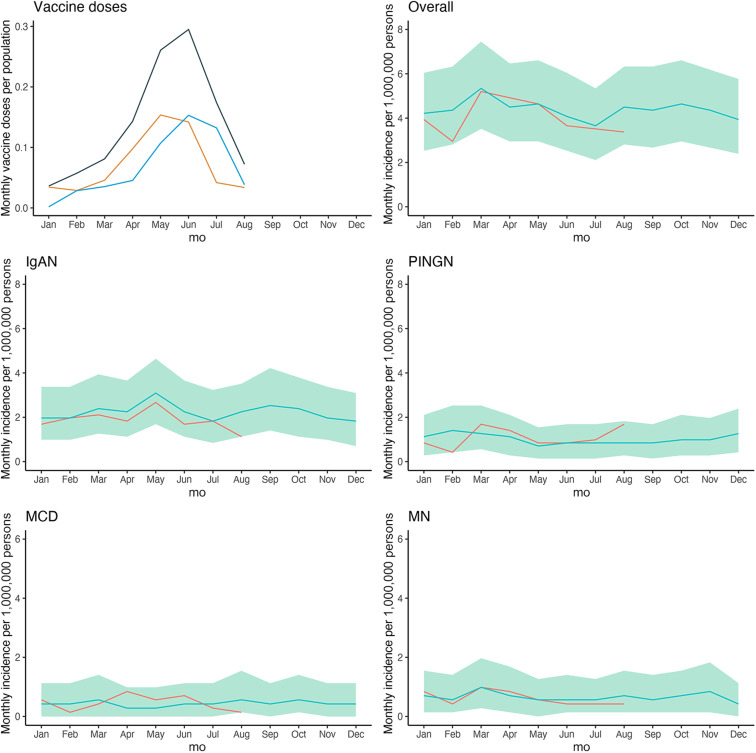
The study flowchart for both studies (retrospective cohort and case-cohort study) is shown in Figure 1 . During the baseline period (2015–2019), the incidence of IgAN, PINGN, MCD, and MN was 23.8, 11.9, 5.1, and 9.3 cases per million population per year, respectively, and remained stable over time (Supplementary Figure S1). In 2020, we found a reduced incidence during the first infection wave and the consecutive public lockdown measures, which was attributable to IgAN (Supplementary Figure S2). The first vaccines were administered in Switzerland in mid-December 2020; the number of vaccinations increased to reach a maximum in June 2021 and declined thereafter (Figure 2 , upper-left panel). The observed incidence of glomerulonephritis from January to August 2021 compared with the expected incidence is shown in Figure 2. The observed incidence was compatible with the expected incidence for all months during the vaccination campaign (Table 1 ) with an overall incidence rate ratio of 0.86 (95% credible interval: 0.73–1.02) for the entire study period (January–August 2021). The observed and expected incidences and incidence rate ratios for each individual glomerulonephritis are presented in Supplementary Table S1. In an additional analysis comparing the weekly incidence during the entire study period or the peak of the vaccination campaign (May–August 2021) with the corresponding calendar time of the baseline period, we also did not find any difference (Supplementary Table S2). Because the COVID pandemic may have influenced the accessibility of health care services, as the reduced incidence of glomerulonephritis suggested for the first infection wave in 2020, we also analyzed the total number of kidney biopsies per year by the pathology center. Although the total number of biopsies analyzed in 2020 tended to be lower, the total number of biopsies analyzed in 2021 was not different from the baseline period (Supplementary Table S3).
Figure 1.
Study flowchart of both studies (retrospective cohort and case-cohort). GN, glomerulonephritis.
Figure 2.
Expected and observed incidence of glomerulonephritis during the vaccination campaign. Shown is the number of first (orange), second (blue), and total (gray) doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines as a fraction of all patients aged ≥20 years (upper-left panel) and the observed incidence of glomerulonephritis in patients aged ≥18 years from January to August 2021 (red line) compared with the expected incidence based on the years 2015–2019 (blue line) with 95% credible intervals (green shading) for the sum of all 4 glomerulonephritides, and by diagnosis. IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy; PINGN, pauci-immune necrotizing glomerulonephritis.
Table 1.
Observed monthly incidence of GN during the vaccination campaign compared with the baseline period (2015–2019) and the expected incidence, relative risk ratio, and number of vaccine doses administered in the corresponding month
| Variable | January | February | March | April | May | June | July | August |
|---|---|---|---|---|---|---|---|---|
| Incidence 2021 | 3.94 | 2.95 | 5.2 | 4.92 | 4.64 | 3.65 | 3.51 | 3.37 |
| Observed incidence 2015–2019 | 4.05 | 4.18 | 5.11 | 4.29 | 4.37 | 3.92 | 3.51 | 4.26 |
| Expected incidence (95% credible intervals) | 4.22 (2.67–6.04) | 4.36 (2.67–6.32) | 5.34 (3.51–7.45) | 4.5 (2.81–6.47) | 4.5 (2.81–6.47) | 4.08 (2.53–5.90) | 3.65 (2.25–5.90) | 4.36 (2.81–6.47) |
| Relative incidence rate ratio (95% credible intervals) | 0.93 (0.64–1.47) | 0.68 (0.47–1.11) | 0.97 (0.69–1.48) | 1.09 (0.76–1.75) | 1.03 (0.70–1.65) | 0.90 (0.62–1.44) | 0.96 (0.66–1.56) | 0.77 (0.52–1.20) |
| Vaccine doses per population | 0.04 | 0.06 | 0.08 | 0.14 | 0.26 | 0.29 | 0.17 | 0.07 |
GN, glomerulonephritis.
Incidence is given as the number of biopsy-proven GN cases (IgA nephropathy, pauci-immune necrotizing glomerulonephritis, minimal change disease, and membranous nephropathy) per million population aged >18 years. Vaccine doses per population are given as doses per population. The expected incidence was calculated using a Bayesian model based on each month for the years 2015–2019. The relative incidence rate denotes the ratio between observed and expected cases.
Characteristics of patients with new-onset glomerulonephritis during the vaccination campaign
Overall, 229 patients aged >18 years had a biopsy-proven diagnosis of IgAN (n = 106), PINGN (n = 62), MCD (n = 26), or MN (n = 35) between January and August 2021 in Switzerland. A total of 125 (54.6%) of these patients could be included in the second study. Their characteristics were representative for the overall population of eligible patients (Supplementary Table S4); the reasons for noninclusion are shown in the study flowchart (Figure 1). Fourteen patients (11.2%) with repeat biopsies of a previously established diagnosis were not included in the analysis. Their clinical characteristics and reasons for repeat biopsy are shown in Supplementary Table S5. The clinical characteristics of all patients with a new diagnosis of glomerulonephritis (n = 111) are shown in Table 2 .
Table 2.
Clinical characteristics of patients with newly diagnosed GN during the study period
| Variable | All patients | IgAN | PINGN | MCD | MN |
|---|---|---|---|---|---|
| n | 111 | 58 | 29 | 9 | 15 |
| Median age (IQR), yr | 55 (43, 72) | 47 (37, 54) | 70 (57, 75) | 70 (61, 72) | 66 (60, 76) |
| Female sex | 42 (38) | 21 (36) | 12 (41) | 5 (56) | 4 (27) |
| Clinical syndrome | |||||
| Nephrotic syndrome | 29 (26)a | 5 (9)a | 0 (0) | 9 (100) | 15 (100)a |
| Nephritic syndrome | 60 (54)a | 30 (52)a | 27 (93) | 0 (0) | 3 (20)a |
| Acute GN | 21 (19) | 7 (12) | 13 (45) | 0 (0) | 1 (7) |
| Chronic GN | 24 (22) | 21 (36) | 1 (3) | 0 (0) | 2 (13) |
| RPGN | 15 (14) | 2 (3) | 13 (45) | 0 (0) | 0 (0) |
| Asymptomatic urinary abnormalities | 25 (23) | 23 (40) | 2 (7) | 0 (0) | 0 (0) |
| Asymptomatic microhematuria | 23 (21) | 21 (36) | 2 (7) | 0 (0) | 0 (0) |
| Asymptomatic proteinuria | 22 (20) | 20 (34) | 2 (7) | 0 (0) | 0 (0) |
| Isolated macrohematuria | 2 (2) | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| Laboratory parameters at the time of biopsy, median (IQR) | |||||
| Creatinine, μmol/l) | 128 (85, 190) | 127 (85, 165) | 162 (103, 276) | 130 (104, 263) | 86 (82, 173) |
| eGFR | 48 (29, 72) | 52 (37, 79) | 31 (15, 52) | 47 (15, 66) | 67 (38, 75) |
| Serum albumin, g/l | 34 (28, 40) | 39 (36, 43) | 34 (29, 39) | 21 (20, 24) | 25 (22, 28) |
| Proteinuria, g/d | 2 (1, 5) | 1 (1, 3) | 1 (0, 3) | 11 (5, 13) | 8 (6, 11) |
| History of COVID-19 | |||||
| COVID-19 before biopsy | 8 (7) | 4 (7) | 2 (7) | 1 (11) | 1 (7) |
| COVID-19 before onset of symptoms/signs | 4 (4) | 1 (2) | 2 (7) | 1 (11) | 0 (0) |
| Vaccination history | |||||
| Any vaccine before biopsy | 43 (39) | 20 (34) | 11 (38) | 5 (56) | 7 (47) |
| Any vaccine before onset of signs or symptoms | 22 (20) | 8 (14) | 5 (17) | 4 (44) | 5 (33) |
| Any vaccine within 4 wk of biopsy | 13 (12) | 9 (16) | 2 (7) | 2 (22) | 0 (0) |
| Any vaccine within 4 wk of symptom or sign onset | 12 (11) | 6 (10) | 3 (10) | 1 (11) | 2 (13) |
COVID, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; IgAN, IgA nephropathy; IQR, interquartile range; MCD, minimal change disease; MN, membranous nephropathy; PINGN, pauci-immune necrotizing glomerulonephritis; RPGN, rapidly progressive GN.
To convert the creatinine values from μmol/l to mg/dl, divide by 88.4.
Values are n (%) or median (IQR).
Five patients (2 IgAN and 3 MN) had nephrotic-nephritic syndrome and were counted in both categories.
Vaccination history of patients with new-onset glomerulonephritis and matched controls
Of the patients with a newly established histologic diagnosis of glomerulonephritis between January and August 2021, 43 (38.7%) had received at least 1 dose of an mRNA SARS-CoV-2 vaccine before kidney biopsy. In 21 of these patients, symptoms or laboratory abnormalities attributable to the glomerulonephritis or extrarenal manifestations of vasculitis had been present before receiving the first vaccine dose. Hence, 22 (19.8%) of the patients with a newly established histologic diagnosis of glomerulonephritis had received at least 1 vaccine dose before the onset of symptoms or laboratory abnormalities. The vaccination rates of the general population, matched for age and either date of biopsy or date of onset of symptoms or laboratory abnormalities, were similar (39.5% and 18.4%, respectively). The estimated risk ratios for the development of new-onset biopsy-proven glomerulonephritis and for the development of new symptoms or laboratory abnormalities attributable to glomerulonephritis were 0.97 (95% CI: 0.66–1.42, P = 0.95) and 1.10 (95% CI: 0.69–1.75, P = 0.79), respectively, in patients having received at least 1 dose of SARS-CoV-2 vaccine compared with unvaccinated persons matched for age and calendar date of biopsy or onset of symptoms or laboratory abnormalities, respectively. Risk ratios for the individual types of glomerulonephritis are shown in Table 3 . In a sensitivity analysis adjusting for age as the matching factor, the risk ratios for the development of new-onset biopsy-proven glomerulonephritis and symptoms or laboratory abnormalities of the disease were 1.02 (95% CI: 0.67–1.54, P = 0.93) and 1.11 (95% CI: 0.67–1.84, P = 0.77), respectively.
Table 3.
Estimated risk ratios for the development of each type of biopsy-proven GN and for the development of new symptoms or laboratory abnormalities attributable to the respective disease
| Diagnosis | Risk ratio for biopsy-proven GN | Risk ratio for symptoms or laboratory signs of GN |
|---|---|---|
| IgAN | 1.14 (95% CI: 0.67–1.97), P = 0.73 | 1.14 (95% CI: 0.54–2.41), P = 0.88 |
| PINGN | 0.54 (95% CI: 0.26–1.15), P = 0.15 | 0.61 (95% CI: 0.23–1.60), P = 0.42 |
| MCD | 1.72 (95% CI: 0.46–6.38), P = 0.63 | 2.20 (95% CI: 0.59–8.18), P = 0.41 |
| MN | 1.17 (95% CI: 0.43–3.23), P = 0.96 | 1.64 (95% CI: 0.56–4.79), P = 0.54 |
| Any GN | 0.97 (95% CI: 0.66–1.42), P = 0.95 | 1.10 (95% CI: 0.69–1.75), P = 0.79 |
CI, confidence interval; GN, glomerulonephritis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy; PINGN, pauci-immune necrotizing glomerulonephritis.
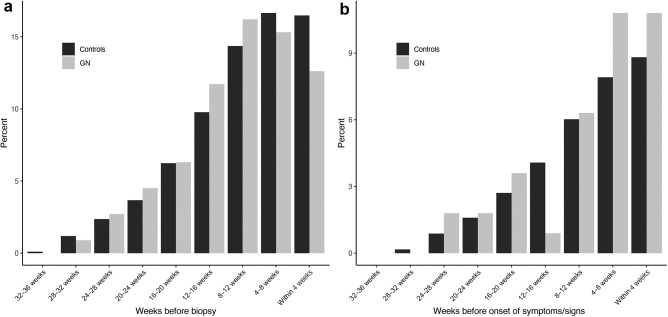
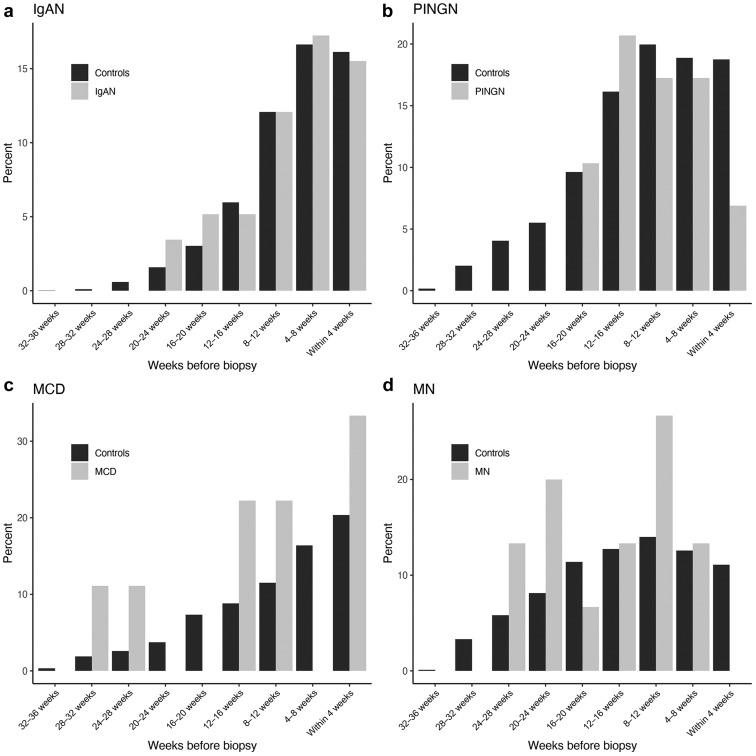
Figure 3 shows the percentage of individuals having received any vaccine dose in each 4-week time period before kidney biopsy or before the onset of symptoms or laboratory abnormalities as compared with matched-control groups. The analysis by the type of glomerulonephritis is shown in Figure 4 , and the analysis by the first or second vaccine dose in Supplementary Figure S3.
Figure 3.
Frequency and timing of vaccination in patients with glomerulonephritis (GN) compared with matched controls. Shown in gray is the percentage of patients with a new diagnosis of IgA nephropathy, pauci-immune necrotizing glomerulonephritis, minimal change disease, or membranous nephropathy during the study period, who have received any vaccine dose during each 4-week interval before (a) renal biopsy or (b) onset of symptoms or signs attributable to the renal disease or an extrarenal manifestation thereof. For comparison, the percentages of persons from the general population matched for age and timepoint during the vaccination campaign are shown in black.
Figure 4.
Frequency and timing of vaccination by histologic diagnosis in patients with glomerulonephritis compared with matched controls. Shown in gray is the percentage of patients with a new diagnosis of (a) IgA nephropathy (IgAN), (b) pauci-immune necrotizing glomerulonephritis (PINGN), (c) minimal change disease (MCD), or (d) membranous nephropathy (MN) during the study period, who have received any vaccine dose during each 4-week interval before renal biopsy. For comparison, the percentages of persons from a control population matched for age and timepoint during the vaccination campaign are shown in black.
Clinical characteristics of patients with a diagnosis of glomerulonephritis in temporal association with vaccination
Temporal association of glomerulonephritis with SARS-CoV-2 vaccination has not been uniformly defined in case reports and case series, with the largest case series defining it as the onset of symptoms within 1 month of any vaccine dose.6 In 15 patients of our cohort, glomerulonephritis definitely (n = 4) or possibly (n = 11) manifested within 28 days of a vaccine dose. The clinical characteristics of these patients compared with all other patients with a new diagnosis of glomerulonephritis during the study period are shown in Table 4 . Details of each individual patient are given in Supplementary Table S6. Patients with a new diagnosis of glomerulonephritis manifesting in temporal association with vaccination were older, but otherwise did not differ from patients with a new diagnosis of glomerulonephritis temporally unrelated to SARS-CoV-2 vaccination.
Table 4.
Characteristics of patients with newly diagnosed GN with versus without temporal association with SARS-CoV-2 vaccination
| Variable | Temporally unrelated to vaccination | Temporally related to vaccination | P value |
|---|---|---|---|
| n | 96 | 15 | |
| Median age (IQR), yr | 53 (42, 68) | 65 (58, 76) | 0.028 |
| Female sex | 39 (41) | 3 (20) | 0.213 |
| Histologic diagnosis | 0.920 | ||
| IgAN | 51 (53) | 7 (47) | |
| PINGN | 24 (25) | 5 (33) | |
| MCD | 8 (8) | 1 (7) | |
| MN | 13 (14) | 2 (13) | |
| Clinical syndrome | |||
| Nephrotic syndrome | 26 (27) | 3 (20) | 0.791 |
| Nephritic syndrome | 51 (53) | 9 (60) | 0.827 |
| Acute GN | 18 (19) | 3 (20) | 1.000 |
| Chronic GN | 22 (23) | 2 (13) | 0.616 |
| RPGN | 11 (11) | 4 (27) | 0.232 |
| Asymptomatic urinary abnormalities | 23 (24) | 2 (13) | 0.559 |
| Asymptomatic microhematuria | 21 (22) | 2 (13) | 0.677 |
| Asymptomatic proteinuria | 20 (21) | 2 (13) | 0.742 |
| Isolated macrohematuria | 1 (1) | 1 (7) | 0.632 |
| Presence of patient-reported symptoms of the disease (renal or extrarenal manifestation) | 61 (64) | 8 (53) | 0.637 |
| Extrarenal manifestations | 50 (52) | 12 (80) | 0.081 |
| IgA vasculitis | 3 (3) | 0 (0) | 1.000 |
| Laboratory parameters at the time of biopsy, median (IQR) | |||
| Creatinine, μmol/l | 127 (85, 178) | 136 (98, 439) | 0.133 |
| eGFR median (IQR) | 49 (30, 73) | 40 (13, 61) | 0.118 |
| Serum albumin, g/l | 35 (27, 41) | 34 (30, 37) | 0.985 |
| Proteinuria, g/d | 1.8 (0.8, 5.6) | 2.7 (1.8, 4.1) | 0.601 |
| History of COVID-19 | |||
| Before vaccination | 5 (5) | 1 (7) | 1.000 |
| Before biopsy | 6 (6) | 2 (13) | 0.662 |
COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; IgAN, IgA nephropathy; IQR, interquartile range; MCD, minimal change disease; MN, membranous nephropathy; PINGN, pauci-immune necrotizing glomerulonephritis; RPGN, rapidly progressive glomerulonephritis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Values are n (%) or median (IQR).
To convert the creatinine values from μmol/l to mg/dl, divide by 88.4.
Sensitivity analysis
The sensitivity analysis excluding all MN cases showed equivalent results (Supplementary Tables S2, S7, and S8; Supplementary Figures S4 and S5).
Discussion
Postmarketing surveillance is an important means to detect rare but relevant side effects that may have escaped detection in registration studies. However, while serving an important hypothesis-generating function, case reports and series are subject to post hoc fallacy and publication bias. In this study, which included incidence data of glomerulonephritis for 7.1 million individuals, 69% of whom had received at least 1 vaccine dose during the study period, we did not find evidence that mRNA-based vaccines against SARS-CoV-2 increase the risk for new-onset glomerulonephritis. On the epidemiologic level, incidence of 4 common types of glomerulonephritis did not exceed the expected rate during any month of the SARS-CoV-2 vaccination campaign in Switzerland. Patients with new-onset glomerulonephritis did not differ from age- and calendar-time–matched controls with respect to their vaccination history, and the characteristics of patients with glomerulonephritis temporally related to vaccination did not differ from those without temporal association with vaccination, except for higher age, probably due to preferential vaccination of older individuals.
Glomerulonephritis is a rare disease, and nationwide incidence rates have not been previously published for Switzerland. We found baseline incidence rates similar to those reported for other European countries.21, 22, 23 An interesting finding was the significantly reduced incidence of glomerulonephritis during public lockdown measures paralleling the first infection wave. Notably, this reduction was attributable to IgAN, the most frequent of the 4 glomerulonephritides, which often manifests with isolated, asymptomatic urinary abnormalities not necessitating urgent evaluation and more likely to be missed with reduced use of routine medical evaluations during the first pandemic phase. Importantly, this finding demonstrates the feasibility of our study to detect true changes of incidence. Likewise, the total number of kidney biopsies analyzed in Switzerland tended to be lower in 2020 compared with the baseline period. In contrast, the number of biopsies analyzed in 2021 was not different from the baseline period. Notably, SARS-CoV-2 infection rates were comparatively low during the peak of the vaccination campaign in Switzerland,24 and elective procedures were not restricted by the government. Thus, it is unlikely that an effect of vaccinations on the incidence of glomerulonephritis was counterbalanced by a reduced detection rate due to limited health care access during that phase of the pandemic.
Patients with a new diagnosis of histology-proven glomerulonephritis had similar vaccination histories to the matched population-based cohort, and the estimated risk for the development of glomerulonephritis was equal in vaccinated versus unvaccinated persons. In the time-based analysis, the proportion of patients having received a vaccine dose was virtually identical for every 4-week period before histologic diagnosis of a glomerulonephritis compared with the matched-control cohort. A trend toward less vaccines given to patients within 4 weeks before histologic diagnosis of glomerulonephritis is likely attributable to the fact that patients were less likely to schedule a vaccine appointment shortly before a scheduled kidney biopsy or when suffering symptoms of glomerulonephritis or vasculitis. In the two 4-week periods before symptom onset or first documented laboratory abnormalities, the percentages of patients having received a vaccine dose were nominally slightly higher compared with matched controls. However, this analysis has to be interpreted with caution, because the number of patients was relatively low and some patient-reported symptoms are unspecific and may have been incorrectly attributed to glomerulonephritis.
Our findings were generally consistent across all 4 types of glomerulonephritis, with the possible exception of MCD. Increased numbers of newly diagnosed MCD were recently reported for 2021 in a Dutch observational cohort.25 However, that cohort-based study could not calculate true population-based incidence, and only 5 of 11 cases had received any SARS-CoV-2 vaccine before presentation, which makes a true association with vaccination questionable. In our study, the incidence of MCD during the vaccination campaign did not cross the upper boundary of the 95% credible interval for the predicted incidence, and the risk ratio for the development of MCD in vaccinated versus unvaccinated persons was not significantly different from 1. However, both analyses showed a trend toward an increased risk, and 95% credible intervals/CIs were wide because of the overall low incidence of MCD. Thus, we cannot exclude an effect of SARS-CoV-2 mRNA vaccines on the development of MCD, but the absolute risk would be very small.
We identified 15 patients with new-onset glomerulonephritis in possible temporal association with SARS-CoV-2 vaccination. Although the limited number of cases precluded a detailed analysis, we did not find a distinct clinical manifestation in these patients. Macrohematuria has been frequently reported as clinical manifestation in patients with new-onset or relapsing glomerulonephritis temporally associated with SARS-CoV-2 vaccination.26 Episodes of gross hematuria, often in association with upper respiratory tract infection, are a known manifestation of IgAN27 and could theoretically be triggered by the systemic inflammatory response after mRNA-based SARS-CoV-2 vaccination. However, we did not find an increased rate of patient-reported gross hematuria in patients with newly diagnosed IgAN in temporal association with vaccination compared with the rest of the cohort. Furthermore, among a previously reported cohort of 88 patients with known IgAN who received at least 1 dose of mRNA-based SARS-CoV-2 vaccine, none had developed gross hematuria.28
Strengths of this study are the inclusion of virtually all biopsy-proven glomerulonephritis cases diagnosed in Switzerland, which allowed us to calculate true incidence rates, and the use of an unambiguous primary outcome (biopsy-proven glomerulonephritis) that limited the potential for bias. By linking the second (case-cohort) study to the epidemiologic (retrospective cohort) analysis, we were able to specifically approach potential participants and achieved a considerable inclusion rate (>50% of all Swiss patients with histologically confirmed glomerulonephritis during the study period). The availability of detailed information on vaccinations administered to the general Swiss population allowed precise matching to patients with regard to age and timepoint during the vaccination campaign. Because only mRNA-based vaccines were administered in Switzerland during the study period, our study specifically addresses this type of vaccine.
This study has several limitations. First, because of the limited population size of Switzerland and the low incidence of glomerulonephritis, we cannot exclude a small effect of vaccinations, in particular for MCD, as discussed above. Second, because of the decentralized health care system in Switzerland, patients were cared for by a variety of hospital-based and private practice nephrologists. Patient evaluation and care were thus not standardized, and the study relied on patient- and physician-reported data. Third, because of the retrospective design of the study, patient-reported symptoms were subject to potential recall bias and the onset of symptom dates was not precise in some patient questionnaires. Therefore, we chose biopsy-proven glomerulonephritis as the primary outcome. Fourth, we were not able to include all patients with biopsy-proven glomerulonephritis in the second study, which might theoretically introduce selection bias. However, the major reason for non-inclusion of patients was nonparticipation of their treating nephrology divisions or practices due to time constraints, which should not introduce bias. Also, patients included in the study were similar to those not included in terms of histologic diagnoses, age, gender, and timepoint of kidney biopsy. Fifth, although FSGS has been reported in temporal association with SARS-CoV-2 vaccination, we did not include patients with FSGS because the data available from pathology institutes did not allow a distinction between primary and secondary/adaptive FSGS. Sixth, we cannot exclude that some cases of IgA vasculitis and antineutrophil cytoplasmic antibody–associated vasculitis have been diagnosed on clinical grounds or by biopsy of the skin or other organs. Finally, our results are limited to new-onset glomerulonephritis and cannot answer the question, whether SARS-CoV-2 vaccination could trigger relapses in patients with previously diagnosed glomerulonephritis, because relapses are usually diagnosed clinically without repeat biopsy.
In conclusion, combining 2 complementary approaches, we did not find an association between mRNA-based vaccination against SARS-CoV-2 and an increased incidence of the 4 common glomerulonephritis types IgAN, PINGN, MCD, and MN. Most cases of new-onset glomerulonephritis manifesting shortly after vaccination against SARS-CoV-2 are likely attributable to temporal coincidence.
Disclosure
IF reports holding stocks from Pfizer. All the other authors declared no competing interests.
Data Statement
Data will be made available on reasonable request to the corresponding author after approval by the local ethics committee.
Acknowledgments
We thank Marcel Zwahlen and Julien Riou, both from the Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland, for epidemiologic and statistical support, in particular with the Bayesian regression model. Menno Prujim, Service of Nephrology and Hypertension, Department of Medicine, Lausanne University Hospital and University of Lausanne (CHUV), Switzerland, helped create the local biopsy database at the University of Lausanne and supervised GN. The following nephrologists from the indicated nephrology divisions and practices—in addition to those listed among the authors—have recruited patients for the second study and provided their clinical data: Bürgerspital Solothurn: S. Zschiedrich; CHUV: M. Stevanin; Dialyse Riviera (Vevey): T. Gauthier, R. Chazot; Ente Ospedaliero Cantonale (Lugano): G. Bedino; Gesundheitszentrum Fricktal: T. Öttl; Herz- und Nierenzentrum Aare (Solothurn): S. Farese; Hirslanden Klinik St. Anna (Luzern): A. Jehle; Hôpital du Jura (Delémont): P. Wilson; Hôpital Riviera Chablais (Rennaz): A. Rossier; Hôpitaux Universitaires de Genève: A. Faivre; Hôpital du Valais (Sion): G. Guzzo; Kantonsspital Baden: C. Gussone; Kantonsspital Baselland: F. Burkhalter, Y. Holzmann, C. Jäger, S. Kalbermatter; Kantonsspital Frauenfeld: D. Daiss, A. Keil, S. Flury; Kantonsspital Graubünden: A. Georgalis; Kantonsspital Olten: C. Lenherr; Kantonsspital Uri: D. Bruhin; Kantonsspital Winterthur: S. J. Rippin Wagner, L. Nigg Calanca; Luzerner Kantonsspital: A. Duss, A. Rali, S. Maloney, M. Neher; Nieren- und Dialysezentrum Männedorf: P. Rhyn; Nierenzentrum Rheintal (Altstätten): R. Eisel, C. Jäger; Quavitae Rive Gauche (Genève): V. Jotterand Drepper; Regionalspital Emmental (Burgdorf): I. Bergmann; Spital Lachen: R. Schorn; Spital Thun: B. Landtwing Lüscher; Spitalverbund Appenzell Auserrhoden: I. Koneth, T. Staub; Spitalzentrum Biel: N. Drivakos, A. Kruse; Spitalzentrum Oberwallis (Visp): D. Brodmann, C. Brun; Spital Zollikerberg (Zürich): B. Bergamin, M. Pechula Thut; Stadtspital Waid und Triemli (Zürich): A. Helmuth, A. Schleich, N. Weber, J. Meier; Universitätsspital Basel: M. Dickenmann, P. Hirt-Minkowski.
Author Contributions
ADK, EL, and MD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ADK developed the concept and designed the study and drafted the manuscript. MD and ADK did the statistical analysis. All authors contributed to the acquisition, analysis, and interpretation of data and the critical revision of the manuscript for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Supplementary Methods.
Table S1. Observed monthly incidence of each glomerulonephritis during the vaccination campaign and during the baseline period (2015–2019), expected incidence, and relative risk ratio in the given month.
Table S2. Weekly incidence of histologically diagnosed glomerulonephritis per 1,000,000 during the entire study period and during the peak of the vaccination campaign compared with the corresponding time of the year in the baseline period.
Table S3. Total yearly number of kidney biopsies analyzed between 2015 and 2021 by pathology centers.
Table S4. Characteristics of patients with consent and complete data for the second study compared with all patients with a histologic diagnosis of IgA nephropathy (IgAN), pauci-immune necrotizing glomerulonephritis (PINGN), minimal change disease (MCD), or membranous nephropathy (MN) between January and August 2021.
Table S5. Clinical details and indications for repeat biopsy of patients with previously known glomerulonephritis.
Table S6. Clinical details on glomerulonephritis cases temporally related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination.
Table S7. Observed monthly incidence of glomerulonephritis (excluding membranous nephropathy) during the vaccination campaign compared with the baseline period (2015–2019) and the expected incidence, relative risk ratio, and number of vaccine doses administered in the corresponding month.
Table S8. Estimated risk ratio for the development of biopsy-proven glomerulonephritis and for the development of new symptoms or laboratory abnormalities, excluding patients with membranous nephropathy.
Figure S1. Monthly incidence of IgA nephropathy (IgAN), pauci-immune necrotizing glomerulonephritis (PINGN), minimal change disease (MCD), and membranous nephropathy (MN) from January 2015 to August 2021.
Figure S2. Observed and expected incidence of glomerulonephritis during 2020, the first pandemic year.
Figure S3. Frequency and timing of vaccination by dose number in patients with glomerulonephritis compared with matched controls.
Figure S4. Observed and expected incidence of glomerulonephritis during the vaccination campaign, excluding membranous nephropathy.
Figure S5. Frequency and timing of vaccination in patients with glomerulonephritis (excluding membranous nephropathy) compared with matched controls.
Supplementary Material
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Xu Z., Wang P., et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 5.Bomback A.S., Kudose S., D'Agati V.D. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78:477–480. doi: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caza T.N., Cassol C.A., Messias N., et al. Glomerular disease in temporal association with SARS-CoV-2 vaccination: a series of 29 cases. Kidney360. 2021;2:1770–1780. doi: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan A.T.P., Tang S.C.W. De novo and relapsing glomerulonephritis after COVID-19 vaccination: how much do we know? Nephrology (Carlton) 2022;27:5–6. doi: 10.1111/nep.14013. [DOI] [PubMed] [Google Scholar]
- 8.Farooq H., Aemaz Ur Rehman M., Asmar A., et al. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: a systematic review. J Taibah Univ Med Sci. 2022;17:1–13. doi: 10.1016/j.jtumed.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klomjit N., Alexander M.P., Fervenza F.C., et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021;6:2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N.L., Coates P.T., Rovin B.H. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021;100:959–965. doi: 10.1016/j.kint.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izzedine H., Bonilla M., Jhaveri K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant. 2021;36:1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.De Vriese A.S., Sethi S., Nath K.A., et al. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29:759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco P., Beck L., Debiec H., et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7:69. doi: 10.1038/s41572-021-00303-z. [DOI] [PubMed] [Google Scholar]
- 16.Federal Office of Public Health FOPH Covid-19 Switzerland. https://www.covid19.admin.ch/de/vaccination/doses
- 17.Bobart S.A., Han H., Tehranian S., et al. Noninvasive diagnosis of PLA2R-associated membranous nephropathy: a validation study. Clin J Am Soc Nephrol. 2021;16:1833–1839. doi: 10.2215/CJN.05480421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrecque J.A., Hunink M.M.G., Ikram M.A., Ikram M.K. Do case-control studies always estimate odds ratios? Am J Epidemiol. 2021;190:318–321. doi: 10.1093/aje/kwaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 21.McGrogan A., Franssen C.F., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 22.Schena F.P., Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38:435–442. doi: 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Mohammad A.J. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford) 2020;59:iii42–iii50. doi: 10.1093/rheumatology/keaa089. [DOI] [PubMed] [Google Scholar]
- 24.Federal Office of Public Health FOPH Covid-19 Switzerland. https://www.covid19.admin.ch/en/overview
- 25.Timmermans S., Busch M.H., Abdul-Hamid M.A., et al. Primary podocytopathies after COVID-19 vaccination. Kidney Int Rep. 2022;7:892–894. doi: 10.1016/j.ekir.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter A., Helmchen B., Gaspert A., et al. Clinical spectrum of gross haematuria following SARS-CoV-2 vaccination with mRNA vaccines. Clin Kidney J. 2021;15:961–973. doi: 10.1093/ckj/sfab284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai K.N., Tang S.C., Schena F.P., et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 28.Lim C.C., Choo J., Tan C.S. COVID-19 vaccination in immunoglobulin A nephropathy. Am J Kidney Dis. 2021;78:617. doi: 10.1053/j.ajkd.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.