Abstract
Introduction Race, ethnicity, and socioeconomic status (SES) are complex, interconnected social determinants of health outcomes. This study uses multivariable analysis on a combination of large national datasets to examine the effects of these factors on 5-year disease-specific survival (DSS) and conditional DSS (CDSS) for nasopharyngeal carcinoma (NPC).
Methods A retrospective study of adults with NPC between 2000 and 2017 from the Surveillance, Epidemiology, End Results (SEER) registry was performed, using the National Cancer Institute Yost Index, a census tract–level composite score of SES to categorize patients. Kaplan–Meier analysis and Cox's regression for DSS and CDSS were stratified by SES. Logistic regression was conducted to identify risk factors for advanced cancer stage at time of diagnosis and receiving multimodal therapy.
Results Our analysis included 5,632 patients. DSS was significantly associated with race and SES ( p < 0.01). Asian/Pacific Islander patients exhibited increased survival when controlling for other variables (hazard ratio [HR] = 0.73, p < 0.01). Although Black patients were more likely to be diagnosed with advanced disease (Black odds ratio [OR] = 1.47, p < 0.01), Black patients were also less likely to receive multimodal therapy; however, this relationship lost statistical significance once SES was incorporated into the multivariable analysis. DSS was decreased among the lowest (first) and middle (second) tertiles of SES (first HR = 1.34, p < 0.01; second HR = 1.20, p < 0.01) compared with the highest (third).
Conclusion Our results indicate that race, ethnicity, and SES significantly affect survival, stage at diagnosis, and treatment of NPC. An interplay of tumor biology and inequalities in access to care likely drives these disparities.
Keywords: nasopharyngeal carcinoma, socioeconomic status, racial disparity, health outcomes
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignant neoplasm arising from the mucosal epithelium of the nasopharynx. The etiology of NPC involves a complex interplay of genetics, Epstein-Barr virus (EBV) infection, and environmental factors such as smoking and alcohol use. 1 2 Based on data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) national database, NPC accounts for only 2% of all head and neck cancers in the United States, with an incidence of less than 1 per 100,000 person-years. 2 Given its increased incidence in Asian countries, much of the epidemiologic focus on NPC in the United States has been centered around individuals of Asian descent; specifically, patients of Chinese descent account for 42.7% of NPC patients in the United States. 3
Patients diagnosed with early-stage NPC generally have a favorable prognosis with appropriate treatment. However, due to the innocuous nature of early symptoms, close to 80% of patients are diagnosed at later stages, with a 5-year survival of 70 to 80% after targeted therapy. 4 Despite standardized treatment guidelines, studies have demonstrated racial and socioeconomic disparities in outcomes for head and neck cancer patients. 5 6 7 These factors likely include barriers in access to specialty care, cancer stage at presentation, and other social determinants of health. While some studies have highlighted the effect of race alone on outcomes in NPC, none have examined the combined impact of race, ethnicity, and SES on the disease-specific survival (DSS) or conditional DSS (CDSS) for NPC. 5 6 8 9 At least one study has investigated the relationship between SES and NPC outcomes; a group from Taiwan reported a strongly significant association between NPC survival and SES. 10 In the United States, associations among race, ethnicity and SES are deeply interrelated and complex. Our composite analysis is designed to examine the way that these factors interact and modulate each other's effects on NPC outcomes.
Using data from the SEER registry and the National Cancer Institute Yost Index, this study explores the impact of sociodemographics on the 5-year DSS and CDSS for NPC. The Yost Index is a validated census tract–level composite score of SES integrating seven parameters: median household income, median home value, median rent, the percentage of individuals below 150% of the federal poverty line, unemployment percentage, working-class occupation percentage, and educational achievement index. 11 12 Because census tracts are smaller and more likely to contain individuals of similar SES, the Yost Index offers a more granular representation of SES than county-level measurements that have been commonly used in earlier analyses. Several studies have illustrated the validity of the Yost Index in cancer-related outcomes. 13 14 15 16
With this multivariable framework, we examine the following outcomes and data related to NPC: DSS, CDSS, cancer stage at diagnosis, and likelihood of receiving multimodal treatment. CDSS characterizes the probability of continued survival based on existing survival duration since the time of diagnosis which can offer more prospective insights than DSS alone. It has been established that race and SES affect overall and disease-specific mortality for cancer in general, so we hypothesized that race, ethnicity, and SES would similarly impact the DSS and CDSS of NPC . 13 14 15 16 Findings from this study could help to identify potentially modifiable variables that determine survival and prognosis for NPC.
Materials and Methods
Data Source and Study Population
We identified patients from the National Cancer Institute SEER Registry which compiles cancer-related survival data from 18 population-based registries in the country and represents approximately 30% of the U.S. population. SEER provides accurate and continuous data on patient demographics, cancer incidence, and survival rates. 9
NPC cases are identified as SEER site codes C110 to C119 and were extracted from the SEER database. Other information collected included the race, ethnicity, gender, age at diagnosis, staging, DSS, treatment regimens (surgery, radiation, and/or chemotherapy), and length of follow-up time. Staging of disease was based on AJCC guidelines at the time of diagnosis. A combined SEER stage was used for tumors diagnosed after 2016. Overall staging was used for analysis.
Inclusion criteria for this analysis included (1) patients diagnosed with NPC between 1973 and 2015; and (2) patients with complete data on age, sex, staging, race, ethnicity (Hispanic vs. non-Hispanic), treatment, and socioeconomic measures. After all criteria were applied, 5,632 patients remained, all of whom were diagnosed with NPC between 2000 and 2015.
The Yost Index
Within the study cohort, the Yost Index, available through the SEER Census-tract SES and Rurality Database, was used to characterize SES. 11 17 The Yost score is a composite index of SES that incorporates seven different factors based on geocoded, patient-level, location-based, and census-tract level data that is included in the 18 registries used in SEER. In the SEER database, this index is divided into tertiles whereby the first tertile represents the lowest SES, the second tertile represents the middle SES, and the third tertile represents the highest SES.
Primary and Secondary Outcomes
DSS is the primary survival outcome utilized. The SEER registry includes information on the cause of death; therefore, DSS was defined as the time from diagnosis to death from the primary tumor. Patients who died from unknown causes or causes other than NPC were censored in survival analysis. Five-year CDSS was calculated based on DSS estimates.
Secondary outcomes included (1) staging of the primary malignancy at the time of diagnosis; and (2) use of multimodal therapy (any combination of surgery, chemotherapy, and/or radiation).
Statistical Analysis
Kaplan–Meier and log-rank analyses were conducted to examine the effect of SES tertile on DSS. A Cox's proportional hazard regression model, controlling for age, sex, stage, the Yost Index, and treatment (surgery, radiation, and chemotherapy) was utilized to determine the independent effect of SES on DSS. Two models were used. Model 1 does not include the Yost Index and model 2 does. Using the two models helps illustrate the degree to which SES increases or mitigates the effects of factors such as race and ethnicity. Multivariable logistic regression models were then generated to determine the secondary outcomes of stage at time of diagnosis and the use of multimodal therapy.
A simplified Cox's proportional hazard (S(x)) regression model controlling for stage and the Yost Index was used to estimate CDSS. The 5-year CDSS is calculated using the following equation: CS (5 + t | t ) = S (5 + t ) / S( t ), where S is the function for DSS based on the Cox proportional hazard regression model. Conceptually, CDSS is the probability that a patient will live an additional 5 years, given that the patient has already survived t years since diagnosis. To calculate 5-year CDSS for a patient who has already survived four years since diagnosis, the survival rate at 4 + 5 years is divided by the survival rate at 4 years. The final calculation would therefore be S(9)/S(4).
Statistical analysis was conducted in R V4.03.1 (RStudio, PBC). Statistical significance was set at p < 0.05. This study was exempted by the Columbia University IRB committee due to the deidentified nature of the data.
Results
We analyzed data from 5,632 patients from the SEER registry. Demographics and outcomes of these patients are presented in Table 1 , stratified by the Yost Index. White was the most common race (2,707, 48%) and most patients were male (3,967, 70%). Patients were roughly balanced across the SES Yost tertile as follows: first tertile, 1,935 (34%); second tertile, 1,998 (35%); and third tertile, 1,699 (30%). A total of 1,567 (28%) patients died from their primary NPC during the follow-up period. DSS was lowest for the first Yost tertile, the lowest SES, at 68%.
Table 1. Patient demographics and mortality stratified by the SES tertiles (the Yost Index).
| Characteristic | First tertile ( n = 1,935) | Second tertile ( n = 1,998) | Third tertile ( n = 1,699_ | p -Value a |
|---|---|---|---|---|
| Age categories (y) | 0.021 | |||
| < 45 | 462 (24%) | 386 (19%) | 365 (21%) | |
| 45–59 | 748 (39%) | 776 (39%) | 673 (40%) | |
| 60–79 | 635 (33%) | 727 (36%) | 573 (34%) | |
| 80+ | 90 (4.7%) | 109 (5.5%) | 88 (5.2%) | |
| Race | <0.001 | |||
| White | 826 (43%) | 1,015 (51%) | 866 (51%) | |
| Black | 396 (20%) | 198 (9.9%) | 85 (5.0%) | |
| Asian/Pacific Islander | 691 (36%) | 763 (38%) | 726 (43%) | |
| Alaskan Native/Native American | 10 (0.5%) | 4 (0.2%) | 10 (0.6%) | |
| Other | 12 (0.6%) | 18 (0.9%) | 12 (0.7%) | |
| Hispanic ethnicity | <0.001 | |||
| Non-Hispanic | 1,721 (89%) | 1,844 (92%) | 1,626 (96%) | |
| Hispanic | 214 (11%) | 154 (7.7%) | 73 (4.3%) | |
| Sex | 0.563 | |||
| Female | 584 (30%) | 595 (30%) | 486 (29%) | |
| Male | 1,351 (70%) | 1,403 (70%) | 1,213 (71%) | |
| Mortality | <0.001 | |||
| Censored | 1,315 (68%) | 1,439 (72%) | 1,311 (77%) | |
| NPC | 620 (32%) | 559 (28%) | 388 (23%) |
Abbreviations: NPC, nasopharyngeal carcinoma; SES, socioeconomic status.
Statistical tests performed: Chi-square test of independence.
Note: Figures in bold indicate statistical significance p < 0.05.
Staging and therapy characteristics are detailed in Table 2 , stratified by the Yost Index. Patients in the Yost tertiles of higher SES tended to be diagnosed at earlier stages. Stage I/II made up 35% of cancers diagnosed in patients in the third tertile of the highest SES, but only 26% of cancers diagnosed in patients in the first tertile of lowest SES. Stage-IV malignancy was present on diagnosis in 44% of patients in the first tertile, 40% of patients in the second tertile, and 34% of patients in the third tertile.
Table 2. Tumor staging and treatment variables stratified by the SES tertiles (the Yost Index).
| Characteristic | First tertile ( n = 1,935) | Second tertile ( n = 1,998) | Third tertile ( n = 1,699) | p -Value a |
|---|---|---|---|---|
| Overall stage of disease | <0.001 | |||
| I | 136 (7.0%) | 208 (10%) | 186 (11%) | |
| II | 373 (19%) | 467 (23%) | 405 (24%) | |
| III | 567 (29%) | 536 (27%) | 522 (31%) | |
| IVa | 372 (19%) | 336 (17%) | 269 (16%) | |
| IVb | 227 (12%) | 211 (11%) | 143 (8.4%) | |
| IVc | 260 (13%) | 240 (12%) | 174 (10%) | |
| Chemotherapy (yes) | 1,514 (78%) | 1,574 (79%) | 1,372 (81%) | 0.151 |
| Surgery (yes) | 196 (10%) | 227 (11%) | 222 (13%) | 0.021 |
| Radiation (yes) | 1,607 (83%) | 1,699 (85%) | 1,488 (88%) | <0.001 |
Abbreviation: SES, socioeconomic status.
Statistical tests performed: Chi-square test of independence.
Note: Figures in bold indicate statistical significance p < 0.05.
Fig. 1 presents the Kaplan–Meier analysis of DSS stratified by the Yost Index which demonstrates a significant difference between the three tertiles ( p < 0.01) and an association between increased survival and higher SES. Fig. 2A presents the Kaplan–Meier analysis of DSS stratified by race which demonstrates a significant difference between races ( p < 0.0001), with Asian/Pacific Islander patients having the highest survival and Black patients having the lowest survival. Fig. 2B presents the Kaplan–Meier analysis of DSS stratified by ethnicity (Hispanic vs. non-Hispanic) which demonstrates lower DSS for Hispanic patients, diverging after 7 years from diagnosis, but this difference was not statistically significant overall.
Fig. 1.
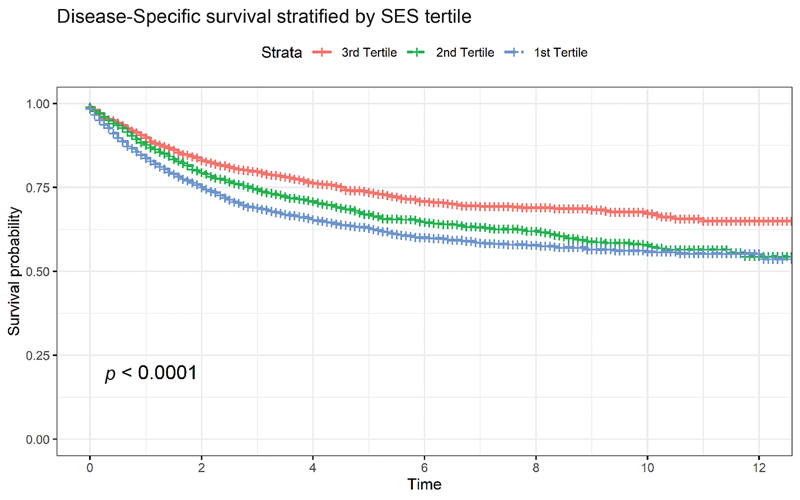
Kaplan–Meier curve stratified by SES tertiles, with log-rank statistic displayed. Median survival was not reached over follow-up period. SES, socioeconomic status.
Fig. 2.
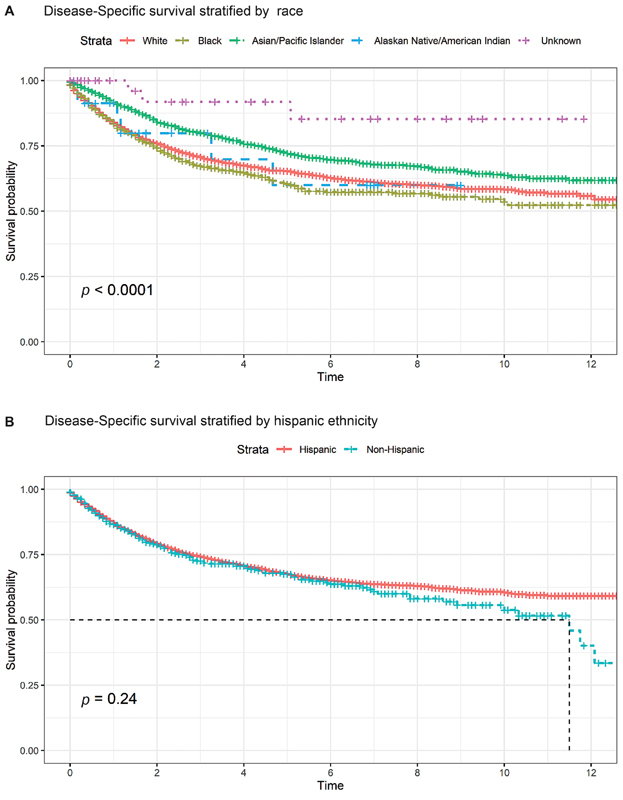
( A ) Kaplan–Meier curve stratified by race, with log-rank statistic displayed. Median survival was not reached over follow-up period. Time is measured by years. ( B ) Kaplan–Meier curve stratified by Hispanic ethnicity, with log-rank statistic displayed. Time is measured in years.
Tables 3 , 4 , 5 present step-wise addition of SES to the multivariable regression analyses for DSS, presenting with advanced NPC, defined as stage III or IV and receiving multimodal therapy, respectively. Comparing the two models within each table reveals the extent to which observed differences in outcomes across age, sex, race, and ethnicity can be statistically accounted for by underlying differences in SES between those groups. In other words, meaningful differences in the odds ratio (OR) or hazard ratio (HR) between the two models indicate that SES is a driver in the differences in outcomes for a certain age, sex, racial, or ethnic group.
Table 3. Cox's proportional hazards regression models for disease-specific survival. SES is incorporated in a step-wise analysis: model 1 without SES and model 2 with SES.
| Model 1 a | Model 2 a | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p -Value | HR | 95% CI | p -Value |
| Age categories (y) | ||||||
| – < 45 | – | – | – | – | ||
| 45–59 | 1.51 | 1.30, 1.74 | <0.001 | 1.51 | 1.30, 1.74 | <0.001 |
| 60–79 | 1.89 | 1.62, 2.20 | <0.001 | 1.88 | 1.62, 2.19 | <0.001 |
| 80+ | 3.32 | 2.64, 4.18 | <0.001 | 3.36 | 2.67, 4.23 | <0.001 |
| Race | ||||||
| White | – | – | – | – | ||
| Black | 1.1 | 0.94, 1.28 | 0.2 | 1.01 | 0.87, 1.19 | 0.9 |
| Asian/Pacific Islander | 0.74 | 0.66, 0.83 | <0.001 | 0.73 | 0.65, 0.82 | <0.001 |
| Alaskan Native/Native American | 0.93 | 0.42, 2.08 | 0.9 | 0.92 | 0.41, 2.07 | 0.8 |
| Other | 0.26 | 0.09, 0.82 | 0.022 | 0.26 | 0.08, 0.80 | 0.019 |
| Sex | ||||||
| Female | – | – | – | – | ||
| Male | 1.23 | 1.10, 1.38 | <0.001 | 1.23 | 1.10, 1.38 | <0.001 |
| Hispanic ethnicity | ||||||
| Non-Hispanic | – | – | – | – | ||
| Hispanic | 0.99 | 0.82, 1.20 | >0.9 | 0.94 | 0.78, 1.14 | 0.5 |
| The Yost Index | ||||||
| Third tertile | – | – | ||||
| Second tertile | 1.20 | 1.05, 1.37 | 0.007 | |||
| First tertile | 1.34 | 1.17, 1.52 | <0.001 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; SES, socioeconomic status.
Note: Figures in bold indicate statistical significance p < 0.05.
Both models control for stage and treatment (surgery, radiation, and chemotherapy).
Table 4. Logistic regression models predicting diagnosis at later stages of disease at presentation. SES is incorporated in a step-wise analysis: model 1 without SES and model 2 with SES.
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p -Value | OR | 95% CI | p -Value |
| Age categories (y) | ||||||
| – < 45 | – | — | – | – | ||
| 45–59 | 0.76 | 0.65, 0.89 | <0.001 | 0.76 | 0.65, 0.89 | <0.001 |
| 60–79 | 0.65 | 0.56, 0.77 | <0.001 | 0.66 | 0.56, 0.78 | <0.001 |
| 80+ | 0.59 | 0.45, 0.78 | <0.001 | 0.60 | 0.45, 0.79 | <0.001 |
| Sex | ||||||
| Female | – | – | – | – | ||
| Male | 1.18 | 1.04, 1.33 | 0.009 | 1.18 | 1.05, 1.34 | 0.008 |
| Race | ||||||
| White | – | – | – | – | ||
| Black | 1.62 | 1.33, 1.97 | <0.001 | 1.47 | 1.21, 1.80 | <0.001 |
| Asian/Pacific Islander | 1.10 | 0.97, 1.25 | 0.130 | 1.09 | 0.96, 1.24 | 0.180 |
| Alaskan Native/Native American | 1.26 | 0.54, 3.27 | 0.614 | 1.21 | 0.52, 3.15 | 0.676 |
| Other | 0.9 | 0.48, 1.77 | 0.746 | 0.9 | 0.48, 1.78 | 0.759 |
| Hispanic ethnicity | ||||||
| Non-Hispanic | – | – | – | – | ||
| Hispanic | 1.62 | 1.29, 2.06 | <0.001 | 1.53 | 1.21, 1.94 | <0.001 |
| The Yost-Index | ||||||
| Third tertile | – | – | ||||
| Second tertile | 1.03 | 0.90, 1.18 | 0.661 | |||
| First tertile | 1.38 | 1.19, 1.60 | <0.001 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; SES, socioeconomic status.
Note: Figures in bold indicate statistical significance p < 0.05.
Table 5. Logistic regression models predicting receiving multimodal therapy (any combination of surgery, chemotherapy, or radiation therapy).
| Model 1 a | Model 2 a | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p -Value | OR | 95% CI | p -Value |
| Age categories (y) | ||||||
| < 45 | – | – | – | – | ||
| 45–59 | 0.63 | 0.51, 0.77 | <0.001 | 0.62 | 0.50, 0.76 | <0.001 |
| 60–79 | 0.45 | 0.36, 0.55 | <0.001 | 0.44 | 0.36, 0.54 | <0.001 |
| 80+ | 0.11 | 0.08, 0.15 | <0.001 | 0.11 | 0.08, 0.15 | <0.001 |
| Sex | ||||||
| Female | – | – | – | – | ||
| Male | 0.99 | 0.85, 1.15 | 0.889 | 0.99 | 0.85, 1.15 | 0.865 |
| Race | ||||||
| White | – | – | – | – | ||
| Black | 0.79 | 0.64, 0.98 | 0.030 | 0.86 | 0.69, 1.08 | 0.195 |
| Asian/Pacific Islander | 1.14 | 0.97, 1.33 | 0.107 | 1.15 | 0.99, 1.35 | 0.074 |
| Alaskan Native/Native American | 0.68 | 0.27, 1.87 | 0.430 | 0.7 | 0.27, 1.94 | 0.469 |
| Other | 0.68 | 0.33, 1.43 | 0.289 | 0.67 | 0.33, 1.42 | 0.279 |
| Hispanic ethnicity | ||||||
| Non-Hispanic | – | – | – | – | ||
| Hispanic | 0.95 | 0.73, 1.24 | 0.679 | 1.01 | 0.77, 1.32 | 0.964 |
| The Yost Index | ||||||
| Third tertile | – | – | ||||
| Second tertile | 0.92 | 0.77, 1.09 | 0.335 | |||
| First tertile | 0.71 | 0.60, 0.85 | <0.001 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; SES, socioeconomic status.
Notes: Figures in bold indicate statistical significance p < 0.05.
SES is incorporated in a step-wise analysis: model 1 without SES and model 2 with SES.
Models also control for overall stage of disease.
In Table 3 , model 1 presents Cox's regression to evaluate the effects of stage, age, treatment, race, ethnicity, and sex on DSS. Asian/Pacific Islander patients exhibited increased survival when controlling for other factors (HR = 0.74, 95% confidence interval [CI]: 0.66–0.83, p < 0.01). In model 2, the Yost index was added. The first and second tertiles, representing lower SES, exhibited worse survival compared with patients in the third tertile (first HR = 1.34, 95% CI: 1.17–1.52, p < 0.01; second HR = 1.20, 95% CI: 1.05–1.37, p < 0.01). Survival by race was not dramatically different when adding in Yost tertiles, suggesting that SES does not further exacerbate the impact of race on DSS. Further investigating the interation between SES and other demographic factors, Table 6 shows the effect of race, ethnicity, and sex on DSS HR by SES cohort. There were no instances of gain or loss of statistical significance but there was one slight trend of HR for Black patients increasing with SES status, although none met the threshold for statistical significance (first tertile Black HR = 0.97, second = tertile Black HR = 1.11, and third tertile Black HR = 1.17).
Table 6. Cox's proportional hazards regression model for disease-specific survival, stratified by SES cohort.
| First-tertile (lowest SES) | 2nd-tertile (middle SES) | 3rd-tertile (highest SES) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p -Value | HR | 95% CI | p -value | HR | 95% CI | p -Value |
| Race | |||||||||
| –White | – | — | – | – | – | – | |||
| Black | 0.97 | 0.78, 1.20 | 0.8 | 1.11 | 0.85, 1.46 | 0.4 | 1.17 | 0.74, 1.84 | 0.5 |
| Asian/Pacific Islander | 0.78 | 0.64, 0.95 | 0.012 | 0.63 | 0.52, 0.77 | <0.001 | 0.79 | 0.64, 0.99 | 0.038 |
| American Indian/Alaskan Native | 0.59 | 0.15, 2.41 | 0.5 | 0.94 | 0.13, 6.76 | >0.9 | 1.35 | 0.43, 4.25 | 0.6 |
| Unknown | 0.38 | 0.09, 1.55 | 0.2 | 0.23 | 0.03, 1.67 | 0.15 | – | – | – |
| Sex | |||||||||
| Female | – | – | – | – | – | – | |||
| Male | 1.2 | 1.00, 1.45 | 0.053 | 1.24 | 1.02, 1.50 | 0.027 | 1.25 | 0.99, 1.58 | 0.063 |
| Ethnicity | |||||||||
| Non-Hispanic | – | – | – | – | – | – | |||
| Hispanic | 1.09 | 0.83, 1.42 | 0.5 | 0.81 | 0.58, 1.12 | 0.2 | 0.83 | 0.48, 1.42 | 0.5 |
Abbreviations: CI, Confidence Interval; HR, Hazard Ratio; SES, socioeconomic status.
Note: Figures in bold indicate statistical significance p < 0.05.
In Table 4 , model 1 presents logistic regression analysis to evaluate the effects of age, race, ethnicity, and sex on the likelihood of being diagnosed with advanced disease at the time of diagnosis. Model 1, which does not account for SES differences, indicates the following: (1) Black patients were more likely to be diagnosed with advanced disease (OR = 1.62, 95% CI: 1.33–1.97, p < 0.01), and (2) Hispanic patients were also more likely to be diagnosed with advanced disease (OR = 1.62, 95% CI: 1.29–2.06, p < 0.01). In model 2, with the Yost Index added to the model, patients in the first tertile (OR = 1.38, 95% CI: 1.19–1.60, p < 0.01) were more likely to present at later stages than the third tertile, suggesting that SES is a risk factor for presenting with advanced disease independent of race. Notably, the effect size on Black (OR = 1.47) and Hispanic (OR = 1.53) patients was reduced by approximately 10% when including SES. This finding indicates that some but not all of the racial/ethnic disparity in presenting with stage-III or -IV cancer can be accounted for by differences in SES between those groups.
In Table 5 , model 1 presents the logistic regression for predicting the likelihood of receiving multimodal therapy, as defined by any two of the three treatment modalities of surgery, radiation, or chemotherapy, without the Yost Index. Black patients were less likely to receive multimodal therapy than White patients (OR = 0.79, 95% CI: 0.64–0.98, p = 0.030). In model 2, with the Yost Index, patients in the first tertile with the lowest SES were significantly less likely to receive multimodal therapy (OR = 0.71, 95% CI: 0.60–0.85, p < 0.01). Notably, the effect of race on receiving multimodal therapy was not statistically significant when the Yost Index was added.
Finally, Fig. 3A–D illustrates CDSS curves based on Cox's regression models controlling for stage and the Yost Index. The difference in CDSS was most significant between the first and third tertiles. CDSS estimates converged for all stages except stage-IV disease in which the first and second tertiles exhibited worse CDSS persistently throughout survivorship. The difference in CDSS was largely the same for patients with stage–I to -III diseases.
Fig. 3.
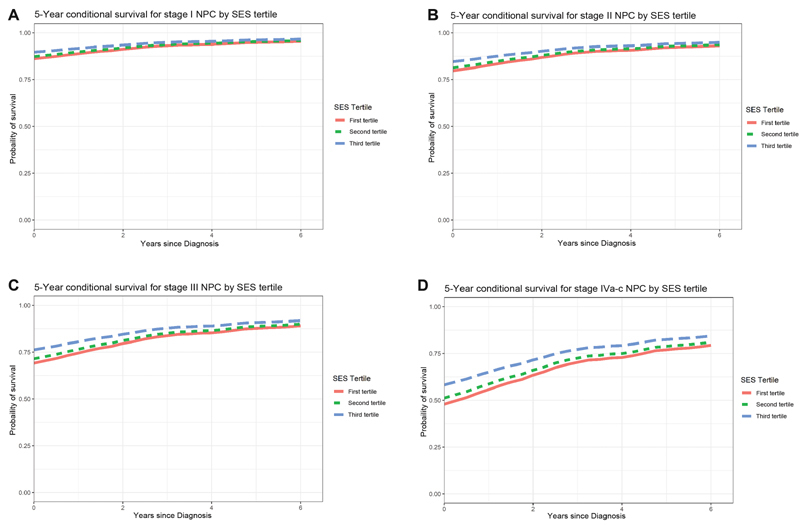
( A – D ) 5-year conditional survival graphs stratified by the Yost tertiles. NPC, nasopharyngeal carcinoma; SES, socioeconomic status.
Discussion
This study presents the effects of race, ethnicity, and SES on survival, stage at diagnosis, and treatment, in 5,632 patients with NPC. Our analysis presents both 5-year DSS as well as 5-year CDSS which represents the likelihood of continued survival based on the duration of patients' survival since time of diagnosis. The effect of race on incidence and survival of NPC is well described in the literature. 3 8 9 18 19 20 To our knowledge, this is the first report that incorporates socioeconomic status (SES) into the analysis of the effect of race on NPC prognosis, and the first to explore CDSS for NPC.
In the cohort we analyzed, we observed a decreased hazard of disease-specific death in Asian/Pacific Islander patients compared with White patients once controlling for other factors including stage, age, and SES. To understand the mechanism of this association, we analyzed the effect of race on the likelihood of presenting with advanced disease and receiving the standard of care. Race did impact the likelihood of having advanced disease, defined as stage III or IV at time of diagnosis, with Black patients significantly more likely to present with advanced NPC compared with White patients. Race was not significantly associated with receiving multimodal therapy when controlling for SES.
Racial disparities in access to care and disproportionate environmental exposure to carcinogens are known to drive disparities in outcomes for many cancers. Our results reflect the unique and complex epidemiology of NPC. Relatively high rates of advanced disease at time of diagnosis is likely an indicator of decreased access to health care. Although Black patients were more likely to have advanced NPC at time of diagnosis, survival among Black patients was less but not significantly lower compared with White patients who tended to present with earlier stage disease. Additionally, Black patients were significantly less likely to receive multimodal therapy; however, this effect was mitigated by SES, illustrated by the loss of significance of race when including the Yost Index ( Table 5 ). This complex relationship between race, stage at presenation, and survival has not been described before and may reflect the interrelated effects of both pathophysiological differences and social determinants of health.
The mixed picture of these results reflects not only the multifactorial impacts of social determinants of health but also the heterogenous etiology of NPC. NPC disproportionately affects Asian people, due in part to the endemic nature of EBV in some Asian nations, although other genetic and epigenetic mechanisms likely contribute as well. This burden persists among Asian Americans, with patients of Chinese descent accounting for nearly half of NPC cases in the United States. 3 Our results are consistent with previously published literature demonstrating that while the increased burden of EBV infection leads to higher rates of NPC among Asian/Pacific Islanders, the prognosis of EBV-associated NPC is better than NPC associated with smoking and alcohol. In other words, our findings suggest that the racial disparity in NPC survival is partly explained by racial differences in pathophysiology.
SES also has a powerful impact on the burden and prognosis for many cancers. In our study, we use the validated and census-based Yost Index to categorize patients into tertiles of SES. Our results indicate that SES has a significant impact on survival and other cancer outcome metrics and allows for some insights into the mechanism of that impact. Disease-specific death was dramatically different between SES tertiles, with mortality for patients in the lowest third of SES 39% higher than for patients in the highest third of SES. Patients in the lowest SES tertile were also 28% more likely to present with stage-III or -IV disease than patients in the highest SES tertile. Despite presenting with relatively advanced disease, patients in the lowest SES tertile were more likely to receive no therapy and less likely to receive combined surgical and radiation therapy.
Notably, SES does not substantially modulate the effect of race on DSS, but it does modulate the effect of race on therapy received and stage at diagnosis, both of which contribute to DSS. While these disparities partially characterize the relationship between race and DSS for patients with NPC, additional specific and controlled studies are necessary to substantiate these relationships. Decreased access to care and treatment appears to be an important driver of worse outcomes for patients of lower SES. However, our multivariable analysis reveals that even when controlling for stage and treatment, DSS was still lower for patients of lower SES. This disparity indicates a persistent burden of health inequity related to SES.
While improving access to care and treatment would likely decrease these disparities in survival and other metrics, our results suggest that there are perhaps additional mechanisms driving worse outcomes for patients of lower SES. In nearly all cases, SES appeared to correlate with survival and other cancer outcomes, with the metrics on the second tertile falling between the first and third tertiles. The level of clinical data in this study is limited to the basics of treatment and diagnosis and does not comprehensively characterize the nuances of management. Management of NPC is complex and depends on factors including care access, health literacy, and adherence, all of which likely correlate strongly with SES. Further, more detailed study of these factors are necessary to better elucidate the mechanisms of how SES effects NPC outcomes.
Our analysis on CDSS may shed some light on possible mechanisms for reduced survival by SES tertiles. Notably, SES tertiles largely converge for stage-I to -III diseases. On the other hand, SES tertiles do not converge to nearly the same degree for stage-IV disease, where factors, such as surveillance and adherence to complex treatment regiments, are necessary. Patients of lower SES have perpetually worse survival, even 6 years out from diagnosis. Further studies on disparities in long-term management of NPC in these patients is necessary to further elucidate this mechanism.
Limitations
Studies using large, national databases such as the SEER registry facilitate insights into the burden of rare diseases and may identify mechanisms for improving care for affected patients. While robust and generalizable, our study faces the limitations inherent to SEER registry research including lack of information on comorbidities, risk factors, detailed treatment, pathology, and recurrence. Although the Yost Index is rigorous and validated, stratifying patients roughly into thirds may not resolve more granular but important findings. Our study calls for further research into the social determinants of health for patients with NPC. We hope that these findings contribute to larger aims to reduce demographic disparities in the outcomes of sinonasal and skull base disease.
Conclusion
We have demonstrated that race, ethnicity, and SES significantly impact the presentation, treatment, and outcomes for NPC. Our analysis yielded important insights that corroborate and augment the results of other investigators. Notably, lower SES among Black and Hispanic patients can account only partially for the higher rates of advanced cancer at diagnosis among these groups, indicating that race and ethnicity may present barriers to accessing care. Additionally, patients of lower SES had persistently worse outcomes even when accounting for differences in stage on presentation and treatment, suggesting a complex mechanism of SES impact on NPC outcomes which warrants further investigation.
Conflict of Interest None declared.
Denotes equal authorship
References
- 1.Chan A TC, Teo P ML, Johnson P J. Nasopharyngeal carcinoma. Ann Oncol. 2002;13(07):1007–1015. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 2.Shah A B, Zulfiqar H, Nagalli N. Treasure Island, FL: StatPearls Publishing; 2021. Nasopharyngeal carcinoma. [Google Scholar]
- 3.Bhattacharyya N. The impact of race on survival in nasopharyngeal carcinoma: a matched analysis. Am J Otolaryngol. 2004;132(10):1035–104. doi: 10.1016/j.amjoto.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Osazuwa-Peters N, Christopher K M, Hussaini A S, Behera A, Walker R J, Varvares M A. Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head and Neck. 2016;38 01:E1826–E1832. doi: 10.1002/hed.24327. [DOI] [PubMed] [Google Scholar]
- 5.Richey L M, Olshan A F, George J. Incidence and survival rates for young blacks with nasopharyngeal carcinoma in the United States. Arch Otolaryngol Head Neck Surg. 2006;132(10):1035–1040. doi: 10.1001/archotol.132.10.1035. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Shen N, Li G, Ding J, Liu D, Huang X. The racial disparity of nasopharyngeal carcinoma based on the database analysis. Am J Otolaryngol. 2019;40(06):102288. doi: 10.1016/j.amjoto.2019.102288. [DOI] [PubMed] [Google Scholar]
- 7.Naghavi A O, Echevarria M I, Grass G D. Having Medicaid insurance negatively impacts outcomes in patients with head and neck malignancies. Cancer. 2016;122(22):3529–3537. doi: 10.1002/cncr.30212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang Y, Ma S. Racial differences in nasopharyngeal carcinoma in the United States. Cancer Epidemiol. 2013;37(06):793–802. doi: 10.1016/j.canep.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel V J, Chen N-W, Resto V A. Racial and ethnic disparities in nasopharyngeal cancer survival in the United States. Otolaryngol Head Neck Surg. 2017;156(01):122–131. doi: 10.1177/0194599816672625. [DOI] [PubMed] [Google Scholar]
- 10.Chang T S, Chang C M, Hsu T W. The combined effect of individual and neighborhood socioeconomic status on nasopharyngeal cancer survival. PLoS One. 2013;8(09):e73889. doi: 10.1371/journal.pone.0073889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Census tract-level SES and rurality database(2000–2015). Accessed November 25, 2021 at:https://seer.cancer.gov/seerstat/databases/census-tract/index.html
- 12.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States) Cancer Causes Control. 1998;9(04):369–380. doi: 10.1023/a:1008811432436. [DOI] [PubMed] [Google Scholar]
- 13.Shields C L, Kaliki S, Cohen M N, Shields P W, Furuta M, Shields J A. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 Doyne Lecture. Eye (Lond) 2015;29(08):1027–1035. doi: 10.1038/eye.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajeshuni N, Zubair T, Ludwig C A, Moshfeghi D M, Mruthyunjaya P. Evaluation of Racial, Ethnic, and Socioeconomic Associations With Treatment and Survival in Uveal Melanoma, 2004-2014. JAMA Ophthalmol. 2020;138(08):876–884. doi: 10.1001/jamaophthalmol.2020.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis L, Canchola A J, Spiegel D, Ladabaum U, Haile R, Gomez S L. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(01):25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodeib M, Chang J, Liu F. Socioeconomic status as a predictor of adherence to treatment guidelines for early-stage ovarian cancer. Gynecol Oncol. 2015;138(01):121–127. doi: 10.1016/j.ygyno.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(08):703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 18.Sun L M, Li C I, Huang E Y, Vaughan T L. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol. 2007;165(03):271–278. doi: 10.1093/aje/kwk008. [DOI] [PubMed] [Google Scholar]
- 19.Sultan I, Casanova M, Ferrari A, Rihani R, Rodriguez-Galindo C. Differential features of nasopharyngeal carcinoma in children and adults: a SEER study. Pediatr Blood Cancer. 2010;55(02):279–284. doi: 10.1002/pbc.22521. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Shen N, Li G, Ding J, Liu D, Huang X. The racial disparity of nasopharyngeal carcinoma based on the database analysis. Am J Otolaryngol. 2019;40(06):102288. doi: 10.1016/j.amjoto.2019.102288. [DOI] [PubMed] [Google Scholar]


