Abstract
Objectives
Refractory hypoxemia can occur in patients with acute respiratory distress syndrome from COVID-19 despite support with venovenous (VV) extracorporeal membrane oxygenation (ECMO). Parallel ECMO circuits can be used to increase physiologic support. We report our clinical experience using ECMO circuits in parallel for select patients with persistent severe hypoxemia despite the use of a single ECMO circuit.
Methods
We performed a retrospective cohort study of all patients with COVID–19-related acute respiratory distress syndrome who received VV-ECMO with an additional circuit in parallel at Vanderbilt University Medical Center between March 1, 2020, and March 1, 2022. We report demographic characteristics and clinical characteristics including ECMO settings, mechanical ventilator settings, use of adjunctive therapies, and arterial blood gas results after initial cannulation, before and after receipt of a second ECMO circuit in parallel, and before removal of the circuit in parallel, and outcomes.
Results
Of 84 patients with COVID-19 who received VV-ECMO during the study period, 22 patients (26.2%) received a circuit in parallel. The median duration of ECMO was 40.0 days (interquartile range, 31.6-53.1 days), of which 19.0 days (interquartile range, 13.0-33.0 days) were spent with a circuit in parallel. Of the 22 patients who received a circuit in parallel, 16 (72.7%) survived to hospital discharge and 6 (27.3%) died before discharge.
Conclusions
In select patients, the additional use of an ECMO circuit in parallel can increase ECMO blood flow and improve oxygenation while allowing for lung-protective mechanical ventilation and excellent outcomes.
Key Words: extracorporeal membrane oxygenation, acute respiratory distress syndrome, coronavirus disease 2019, hypoxemic respiratory failure, parallel circuits
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; Fr, French; IJ, internal jugular; IQR, interquartile range; IVC, inferior vena cava; VV, venovenous
Graphical abstract

VV-ECMO with the additional use of an ECMO circuit in parallel.
Central Message.
In select patients with refractory hypoxemia and high cardiac output, the additional use of an ECMO circuit in parallel can increase ECMO blood flow and improve oxygenation.
Perspective.
The management of patients with COVID–19-related ARDS is challenging. Refractory hypoxemia can develop during VV-ECMO in the setting of a hyperinflammatory state, hyperdynamic circulation, and severely impaired gas exchange through the native lungs. The additional use of a parallel ECMO circuit can result in increased ECMO blood flow and improved oxygenation while allowing for lung-protective mechanical ventilation.
See Commentary on page XXX.
Extracorporeal membrane oxygenation (ECMO) has been widely used for patients with COVID–19-related acute respiratory distress syndrome (ARDS) refractory to conventional management. Yet, outcome data are inconsistent. Initial studies showed exceedingly high mortality rates among cohorts with COVID-19 who were receiving ECMO.1 , 2 Recent large multicenter cohort studies have shown outcomes for patients with COVID–19-related ARDS comparable with outcomes in patients with non-COVID–19-related ARDS who were receiving ECMO.3 , 4 Some data suggest that ECMO confers a survival benefit in this population compared with patients who are treated without ECMO.5 , 6 Other data show that survival might be declining over time,7 and the most recent mortality rate reported by the Extracorporeal Life Support Organization is nearly 50%.8 These conflicting data might reflect, in part, challenges of ECMO management that are unique to, or especially prevalent in, patients with COVID-19.
One such challenge is the management of patients with persistent hypoxemic respiratory failure despite the maximum respiratory support provided by venovenous (VV)-ECMO in addition to the use of adjunctive therapies such as deep sedation, neuromuscular blockade, red blood cell transfusion, inhaled pulmonary vasodilators, prone positioning, and/or mechanical ventilator settings that exceed criteria considered to be lung-protective.9 In these patients, a single ECMO circuit fails to provide the oxygen delivery necessary to meet the physiologic demand.10
For patients with refractory hypoxemia, the use of a second ECMO circuit in parallel to improve oxygen delivery has been previously reported, but data are limited to small case series.11 , 12 In this retrospective cohort study, we show the clinical characteristics and outcomes of 22 patients with COVID-19 ARDS for whom circuits in parallel were used to provide additional respiratory support.
Methods
Study Design, Setting, and Participants
We performed a retrospective cohort study of consecutive patients with COVID–19-related ARDS who received a second ECMO circuit in parallel at Vanderbilt University Medical Center between March 1, 2020, and March 1, 2022. We aimed to describe the clinical characteristics in patients for whom a circuit was used in parallel, assess the feasibility of the intervention, and measure patient outcomes. This retrospective study was approved by the institutional review board at Vanderbilt University with waiver of informed consent (approved: May 23, 2022; 220849).
Data were obtained from the electronic medical record. We report baseline characteristics, clinical characteristics including ECMO settings, mechanical ventilator settings, arterial blood gas results, and receipt of adjunctive therapies at discrete time points: 4 hours after initial ECMO cannulation, 4 hours before receipt of the circuit in parallel, 24 hours after receipt of the circuit in parallel, and 24 hours before the removal of parallel circuit, and clinical outcomes such as in-hospital survival, ECMO duration, and length of hospital stay. Complications including bleeding and thromboembolic events as previously defined13 are also reported. For a convenience sample of 10 patients, cardiac output data were estimated using the LiDCOrapid monitor14 (LiDCO Ltd). Continuous variables are reported as median with interquartile range (IQR). Categorical variables are reported as frequency with percentage. Analyses were performed using STATA 16.1 (StataCorp).
Patient Selection
The diagnosis of COVID-19 was confirmed using a nasopharyngeal swab and a multiplexed nucleic acid amplification test. Patients were evaluated, cannulated, and managed by a multidisciplinary team of perfusionists, nurses, advanced practice providers, respiratory and physical therapists, intensivists, and surgeons.
For patients with COVID-19, patient selection for ECMO followed a standardized framework with stringent criteria and decisions were made by a multidisciplinary team.13 Indications reflected the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial inclusion criteria including a ratio of partial pressure of oxygen to fraction of inspired oxygen of <80 mm Hg for ≤6 hours or <50 mm Hg for <3 hours or a pH of <7.25 with a partial pressure of arterial carbon dioxide of >60 mm Hg. If inclusion criteria were met the patient was assessed for absolute contraindications including age older than 60 years, active solid or liquid malignancy, moribund condition, irreversible neurologic injury, body mass index >55, >7 days of mechanical ventilation before ECMO, multiorgan failure, and high-grade shock. Age and body mass index thresholds were adjusted on the basis of referral volume and need for triage. Relative contraindications included the presence of comorbidities, prolonged use of noninvasive positive pressure ventilation and high-flow nasal cannula, acute kidney injury, and hypotension requiring vasopressors. Details of the selection process have been reported previously.15 In the absence of absolute contraindications or >4 relative contraindications, the patient was considered medically eligible to receive ECMO. Subsequently, a separate systematic assessment of hospital resources was conducted and if resources were available to provide ECMO care to the patient, the patient received ECMO.5 , 15
The decision to treat patients with a circuit in parallel was determined on the basis of evidence of physiologic need. A circuit in parallel was reserved for patients who maintained or developed severe hypoxemia despite maximal ECMO support with a single circuit requiring a combination of mechanical ventilator settings exceeding those considered lung-protective and adjunctive therapies including deep sedation, neuromuscular blocking agents, and inhaled pulmonary vasodilators. As clinically appropriate, other noninvasive therapies such as cooling, antipyretics, and red blood cell transfusion were trialed before using a circuit in parallel. Further, we consistently monitored for the presence of clinically meaningful recirculation and oxygenator dysfunction and addressed these mechanical problems before evaluating the need for a circuit in parallel. If patients developed an absolute contraindication or multiple relative contraindications from the time of initial cannulation to the time the patient was being considered for a circuit in parallel, the circuit in parallel was not provided.
ECMO Circuit and Configuration
Several ECMO platforms were deployed on the basis of diagnosis, anticipated duration of ECMO, and resources during periods of high demand. For patients with COVID-19, we used either the Cardiohelp system (Maquet Cardiopulmonary) with the HLS Advanced 7.0 disposable set, CentriMag device (Abbott Laboratories) with the Nautilus Smart ECMO Oxygenator (Medtronic), or the Spectrum Quantum ECLS system with the Quantum CP22 centrifugal pump and Nautilus Smart Oxygenator.
A standard percutaneous cannulation technique was used for all patients and performed at the bedside. For patients referred from outside hospitals considered too unstable to transport without ECMO, cannulation occurred at the outside hospital by our team and the patient was transported with ECMO to our center. Patients in the study underwent dual-site cannulation via a femoral vein for drainage and the internal jugular (IJ) vein for reinfusion. On the basis of our evolving experience, we anticipated high blood flow requirements and selected larger cannulas at the time of initial cannulation. We routinely used the largest cannulas available at our institution: a 25- to 27-French (Fr) multistage femoral drainage cannula and a 22- to 25-Fr reinfusion cannula. The femoral drainage cannula terminated in the intrahepatic inferior vena cava (IVC) close to the right atrial-IVC junction. The reinfusion cannula was inserted with the tip in the superior vena cava close to the right atrial-superior vena cava junction. Chest radiography was used immediately after cannulation to ensure proper cannula positioning (Figure E1).
Figure E1.
Representative chest roentgenogram in 3 patients showing cannula positioning and progression during extracorporeal membrane oxygenation (ECMO) support: (A) after initial cannulation, (B) during use of parallel circuits, and (C) immediately after decannulation. ∗ Identifies the tips of drainage and reinfusion cannulas.
For the additional use of a circuit in parallel, a 23- to 25-Fr drainage cannula was placed in the contralateral femoral vein and positioned in the intrahepatic IVC. The second drainage cannula served as the inflow for the additional circuit and a 20- to 22-Fr reinfusion cannula was placed in the contralateral IJ vein (Figure 1 ). As our experience grew, and to avoid cannulation of both IJ veins, we used a single 23- to 25-Fr IJ cannula for reinfusion from both circuits using a Y connector (Figure 2 ). Blood flow, revolutions per minute of the pump, and sweep gas flow were balanced evenly between the 2 circuits and adjusted as needed to target physiologic goals. We maintained our usual anticoagulation strategy for patients receiving VV-ECMO of intravenous heparin titrated to a goal partial thromboplastin time of 40 to 60 seconds. In the presence of bleeding, anticoagulation was deferred; in the presence of thrombosis, anticoagulation was increased per standard clinical protocols.
Figure 1.
Parallel circuit cannulation for venovenous extracorporeal membrane oxygenation using bilateral femoral veins and bilateral internal jugular veins.
Figure 2.
Parallel circuit cannulation for venovenous extracorporeal membrane oxygenation using bilateral femoral veins. Return from both circuits is through a single, large cannula placed in the internal jugular vein.
ECMO Weaning and Decannulation
For patients who received circuits in parallel, when signs of lung recovery such as improvements in pulmonary compliance and gas exchange were identified, weaning was initiated by reducing ECMO blood flow and sweep gas flow. When patients were able to tolerate a blood flow rate of <5.0 L/min and <10.0 L/min of sweep gas flow while receiving lung protective ventilation with a fraction of inspired oxygen of ≤0.6, the parallel circuit was reconfigured to a single circuit while preserving both femoral drainage cannulas. After conversion to a single circuit, usual weaning processes16 of a single circuit commenced and both drainage cannulas and the return cannula were removed at the time of decannulation.
Results
Of the 84 patients with a confirmed diagnosis of COVID-19 admitted who received VV-ECMO at the institution during the study period, 22 patients (26.2%) received a circuit in parallel. The median time from initial cannulation to parallel circuit placement was 9 days (5-12 days). Among patients who received a circuit in parallel, the median age was 40.5 years (IQR, 34.0-47.0 years) and 18 (81.8%) were male. The median height was 177.8 cm (IQR, 167.6-182.9 cm), weight was 120.3 kg (93.4-133.0 kg), and body mass index was 37.6 (IQR, 30.9-45.5). The median simplified acute physiology score was 23.5 (IQR, 17.0-24.0). Preexisting comorbidities in the study cohort included hypertension (n = 4), diabetes mellitus (n = 2), asthma (n = 4), and obstructive sleep apnea (n = 3) as shown in Table 1 .
Table 1.
Baseline characteristics
| Characteristic | Whole cohort (N = 22) | Survivors (n = 16) | Nonsurvivors (n = 6) |
|---|---|---|---|
| Age, y | 40.5 (34.0-47.0) | 36.5 (30.0-48.0) | 42.0 (40.0-44.0) |
| Male sex | 18 (81.8) | 14 (87.5) | 4 (66.7) |
| Height, cm | 177.8 (167.6-182.9) | 181.5 (171.5-184.2) | 170.3 (167.6-175.3) |
| Weight, kg | 120.3 (93.4-133.0) | 112.3 (92.5-132.8) | 127.6 (108.9-142.0) |
| Body mass index | 37.6 (30.9-45.5) | 35.3 (29.7-41.4) | 45.6 (36.4-46.7) |
| Body surface area, m2 | 2.3 (2.2-2.5) | 2.3 (2.1-2.5) | 2.3 (2.2-2.4) |
| Simplified acute physiology score II | 23.5 (17.0-24.0) | 18.5 (17.0-24.0) | 24.0 (23.0-27.0) |
| Comorbidities | |||
| Hypertension | 4 (18.2) | 3 (18.75) | 1 (16.7) |
| Diabetes mellitus | 2 (9.1) | 2 (12.5) | 0 (0.0) |
| Asthma | 4 (18.2) | 2 (12.5) | 2 (33.3) |
| Obstructive sleep apnea | 3 (13.6) | 2 (12.5) | 1 (16.7) |
| Chronic lung disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Congestive heart failure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Postpartum | 1 (4.6) | 1 (4.5) | 0 (0.0) |
| Before receipt of ECMO | |||
| Days of mechanical ventilation | 2.5 (1.4-3.0) | 2.2 (1.3-2.9) | 3.1 (2.0-4.0) |
| Respiratory rate, breaths per minute | 30.0 (24.0-32.0) | 28.0 (24.0-34.0) | 31.0 (21.0-32.0) |
| Tidal volume, mL/kg | 5.8 (5.1-6.8) | 5.2 (4.6-6.6) | 6.1 (5.4-7.1) |
| Positive end expiratory pressure, cm H2O | 15.0 (14.0-16.0) | 15.0 (14.0-18.0) | 14.5 (12.0-15.0) |
| Fraction of inspired oxygen | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) |
| pH | 7.29 (7.24-7.35) | 7.30 (7.21-7.35) | 7.28 (7.27-7.29) |
| Partial pressure of arterial carbon dioxide, mm Hg | 65.0 (54.0-77.0) | 65.5 (61.0-77.0) | 54.0 (52.0-75.0) |
| Partial pressure of arterial oxygen, mm Hg | 63.0 (55.0-69.0) | 63.0 (55.0-70.0) | 58.0 (52.0-69.0) |
| Oxygen saturation, % | 82.0 (80.0-87.0) | 82.5 (80.0-87.0) | 81.0 (81.0-87.0) |
| Vasopressor use | 6 (27.3) | 6 (37.5) | 0 (0.0) |
| Continuous renal replacement therapy | 1 (4.6) | 1 (6.25) | 1 (16.7) |
| Neuromuscular blocking agent | 22 (100.0) | 16 (100.0) | 6 (100.0) |
| Inhaled pulmonary vasodilators | 9 (40.9) | 6 (37.5) | 3 (50.0) |
| Prone positioning | 11 (50.0) | (56.3) | 2 (33.3) |
Data are expressed as n (%) or median (interquartile range). Patients remaining in hospital, n = 1. ECMO, Extracorporeal membrane oxygenation.
Characteristics obtained within the 6 hours before ECMO cannulation are shown in Table 1. The median pH was 7.29 (IQR, 7.24-7.35), partial pressure of arterial carbon dioxide was 65.0 mm Hg (IQR, 54.0-77.0 mm Hg), and ratio of the partial pressure of arterial oxygen to fraction of inspired oxygen was 63.0 mm Hg (IQR, 55.0-69.0 mm Hg). Median time from endotracheal intubation to ECMO cannulation was 2.1 days (IQR, 1.4-3.0 days). Mechanical ventilator settings before receipt of ECMO included a median respiratory rate of 30 breaths per minute (IQR, 24-32 breaths per minute), median tidal volume of 5.8 mL/kg of predicted body weight (IQR, 5.1-6.8 mL/kg), and positive end-expiratory pressure of 15 cm H2O (IQR, 14-16 cm H2O). All 22 patients received neuromuscular blocking agents before ECMO, 9 (40.9%) received inhaled pulmonary vasodilators, and 11 (50.0%) underwent prone positioning.
ECMO settings, mechanical ventilator settings, arterial blood gas results, and the presence of adjunctive therapies after initial ECMO cannulation, before receipt of parallel circuit, after receipt of parallel circuit, and before the removal of the parallel circuit are shown in Table 2 . ECMO settings tended to increase before parallel circuit placement, further increase after parallel circuit placement, and decrease by 24 hours before removal of the parallel circuit. The blood flow rate continued to increase after parallel circuit placement while the mechanical ventilator settings were reduced. The blood flow rate after initial cannulation was 4.6 L/min (IQR, 4.1-5.1 L/min) and 6.0 L/min (IQR, 5.5-6.2 L/min) before parallel circuit placement. After parallel circuit placement, the blood flow rate increased to a median of 7.0 L/min (IQR, 6.4-7.4 L/min) and decreased to a median of 4.9 L/min (IQR, 4.2-5.1 L/min) before parallel circuit removal. Mechanical ventilator settings tended to increase before parallel circuit placement and decrease after parallel circuit placement and before parallel circuit removal. For example, the fraction of inspired oxygen increased from a median of 0.6 (IQR, 0.6-0.7) after initial cannulation to 1.0 (IQR, 1.0-1.0) and returned to a median of 0.6 (IQR, 0.6-0.6) after parallel circuit placement. Similarly, driving pressure was increased from a median of 12 cm H20 (IQR, 12-14 cm H2O) after initial cannulation to a median of 14 cm H2O (IQR, 14-16 cm H2O) before parallel circuit placement.
Table 2.
ECMO settings, mechanical ventilator settings, and arterial blood gas results during ECMO among 22 patients
| Characteristic | 4 hours after initial ECMO cannulation | 4 hours before receipt of parallel circuit | 24 hours after receipt of parallel circuit | 4 hours before removal of parallel circuit |
|---|---|---|---|---|
| ECMO settings | ||||
| Blood flow rate, L/min | 4.6 (4.1-5.1) | 6.0 (5.5-6.2) | 7.0 (6.4-7.4) | 4.9 (4.2-5.1) |
| Sweep gas flow rate, L/min | 4.0 (3.0-5.0) | 7.0 (5.0-10.0) | 6.0 (5.0-7.0) | 6.0 (4.0-8.0) |
| Fraction of delivered oxygen | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) |
| Mechanical ventilator settings | ||||
| Respiratory rate, breaths per minute | 16 (14-20) | 18 (16-20) | 18 (16-22) | 22.0 (18.0-28.0) |
| Tidal volume, mL/kg | 3.5 (2.4-4.8) | 1.7 (1.5-2.3) | 2.0 (1.2-2.9) | 4.4 (3.6-5.1) |
| Driving pressure, cm H2O | 12.0 (12.0-14.0) | 14.0 (14.0-16.0) | 14.0 (12.0-14.0) | 14.0 (12.0-16.0) |
| Positive end expiratory pressure, cm H2O | 12.0 (12.0-12.0) | 12.0 (12.0-14.0) | 12 (10.0-12.0) | 10.0 (10.0-12.0) |
| Fraction of inspired oxygen | 0.6 (0.6-0.70) | 1.0 (1.0-1.0) | 0.6 (0.6-0.6) | 0.6 (0.5-0.6) |
| Arterial blood gas results | ||||
| pH | 7.38 (7.36-7.42) | 7.37 (7.32-7.40) | 7.39 (7.35-7.45) | 7.37 (7.36-7.41) |
| Partial pressure of arterial carbon dioxide, mm Hg | 44.5 (40.0-51.0) | 62.0 (55.0-73.0) | 50.0 (47.0-63.0) | 57.0 (49.0-71.0) |
| Partial pressure of arterial oxygen, mm Hg | 97.0 (86.0-121.0) | 56.6 (53.0-65.0) | 90.5 (77.0-104.0) | 83.0 (72.0-106.0) |
| Oxygen saturation, % | 97.5 (95.0-98.0) | 85.5 (84.0-88.0) | 96.0 (95.0-99.0) | 97.0 (95.0-99.0) |
| Receipt of adjunctive therapies | ||||
| Neuromuscular blocking agent | 1 (4.6) | 14 (63.6) | 0 (0.0) | 0 (0.0) |
| Inhaled pulmonary vasodilators | 11 (50.0) | 21 (95.5) | 17 (77.3) | 2 (10.5) |
| Prone positioning | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Data are expressed as n (%) or median (interquartile range). ECMO, Extracorporeal membrane oxygenation.
The presence of adjunctive therapies tended to decrease after initial cannulation, increase before parallel circuit placement, and decrease after parallel circuit placement (Table 2). For example, 4 hours before parallel circuit placement, neuromuscular blocking agents were present in 14 patients (63.6%) and 24 hours after parallel circuit placement, neuromuscular blocking agents were not used in any patients (Table 2). Median ECMO and mechanical ventilator settings and arterial blood gas thresholds at which patients were removed from the parallel circuit are shown in Table 2. At the time of parallel circuit removal, there were no patients receiving neuromuscular blocking agents or inhaled pulmonary vasodilators.
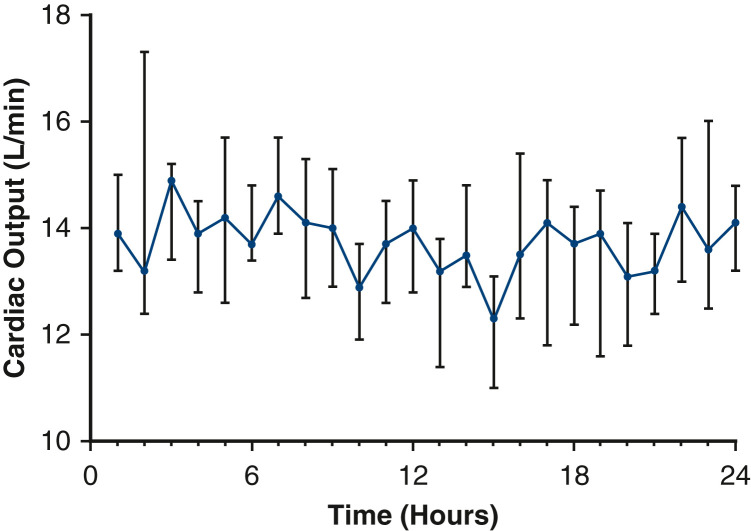
Among the 10 patients in whom cardiac output was measured, the estimated cardiac output during the 24-hour period immediately preceding the additional use of a circuit in parallel was a median of 13.8 L/min (IQR, 13.4-14.1 L/min) as shown in Figure 3 . Characteristics while receiving ECMO are shown in Table 3 . Nearly all patients (n = 21) underwent a tracheostomy while receiving ECMO. One patient received an arterial cannula in the setting of a sudden cardiac arrest of unclear etiology. Ten patients (45.5%) were able to participate in regular physical therapy including recumbent bicycle and bed-level exercises while receiving the circuit in parallel. The median number of circuit exchanges was 2 (IQR, 2-4). Bleeding events during ECMO occurred in 8 (40.0%) patients and thromboembolic events occurred in 10 (45.5%) patients. Among decannulated patients for whom data were available (n = 16) there were 5 (31.3%) cannula-associated deep vein thromboses (DVTs). Inadvertent decannulation occurred in 1 patient; the patient was recannulated emergently and survived to discharge.
Figure 3.
Median cardiac output of 10 patients over a 24-hour period before additional use of a second extracorporeal membrane oxygenation circuit in parallel. Vertical bars represent the interquartile range.
Table 3.
Characteristics∗ during receipt of ECMO and outcomes
| Characteristic | Value |
|---|---|
| Number of circuit exchanges during ECMO run | 2.0 (1.5-3.5) |
| Bleeding event during ECMO† | 8 (38.1) |
| Thrombotic event during ECMO‡ | 10 (45.5) |
| Inadvertent decannulation | 1 (4.8) |
| Cannula-associated DVT§ | 5 (31.3) |
| Receipt of ECMO, d | 40.0 (31.6-53.1) |
| Receipt of parallel circuit, d | 19.0 (13.0-33.0) |
| Receipt of mechanical ventilation, d | 55.0 (39.2-70.0) |
| Tracheostomy during ECMO | 21 (95.5) |
| Receipt of lung transplant | 2 (9.1) |
| Intensive care unit length of stay, d | 58.1 (43.5-73.0) |
| Hospital length of stay, d | 61.0 (43.5-75.1) |
| Survival to decannulation | 17 (77.3) |
| Survival to hospital discharge | 16 (72.7) |
Data are expressed as n (%) or median (interquartile range). ECMO, Extracorporeal membrane oxygenation; DVT, deep vein thrombosis.
N = 22 patients.
Defined as overt bleeding associated with either a decrease in hemoglobin concentration of 2 g/dL or a transfusion of at least 2 units of packed red blood cells in 24 hours, bleeding at any critical site (eg, intracranial bleeding), or bleeding requiring a procedural intervention.
Defined as cerebral stroke, intracardiac thrombus, acute pump head thrombosis, acute oxygenator failure, pulmonary emboli, or deep vein thrombosis.
Among 16 decannulated patients.
The median duration of ECMO was 40.0 days (IQR, 31.6-53.1 days) and the median duration of circuits in parallel was 19.0 days (IQR, 13.0-33.0 days). Of the 22 patients who received a circuit in parallel, 16 (72.7%) survived to hospital discharge and 6 (27.3%) died. Of the 6 deaths before discharge, 3 occurred because of refractory septic shock and multiple organ failure, 2 were secondary to hemorrhagic shock due to a hemothorax and intra-abdominal bleed, and 1 was in the setting of a devastating intracranial hemorrhage. Among the patients who survived to discharge, 1 was discharged home, 13 were discharged to a long-term acute care facility, 1 was transferred after evaluation and acceptance for lung transplant, and 1 was transferred back to the referring hospital after ECMO decannulation, ventilator weaning, and conditioning. Two patients underwent a lung transplant and were discharged alive. The median length of intensive care unit stay and hospital stay was 58.1 days (IQR, 43.5-73.0 days) and 61.0 days (IQR, 43.5-75.1 days), respectively. Among patients discharged from the hospital, 8 (53.3%) were still undergoing weaning from the ventilator and did not yet tolerate 24 hours of delivery of oxygen via tracheostomy collar. Five patients were weaned to <5 L of oxygen delivery through a nasal cannula including 3 patients who were discharged with no supplemental oxygen.
Discussion
In this retrospective cohort study we found that, among patients with refractory hypoxemia despite VV-ECMO support for COVID-19 ARDS, the additional use of a circuit in parallel was feasible, improved oxygenation, decreased ventilator settings and rescue therapies, and was associated with an overall good rate of survival to hospital discharge (Figure 4 ). In select patients with severe respiratory failure, the additional use of an ECMO circuit in parallel might allow for greater total ECMO flow, facilitate lung-protective mechanical ventilation, and reduce receipt of potentially injurious adjunctive therapies.
Figure 4.
Overview of study and results. COVID-19, Coronavirus disease 2019; VV, venovenous; ECMO, extracorporeal membrane oxygenation.
More than a quarter of total patients with COVID–19-related ARDS who received VV-ECMO at our center were treated with a circuit in parallel. Yet, the use of additional mechanical support for refractory hypoxemia during VV-ECMO for ARDS has been rarely described. The severity of hypoxemia in COVID–19-related ARDS might be partly explained by a hyperkinetic circulatory profile and pulmonary vascular dysfunction unique to COVID-19 ARDS.17 , 18 The mechanism of refractory hypoxemia might be due to hyperactivation of the immune system leading to a hyperinflammatory cell state.19 Because of the extent of lung injury in some patients, gas exchange through the native lungs was severely compromised, and patients were completely dependent on ECMO for respiratory support. In the setting of a hyperdynamic circulation and an elevated cardiac output, hypoxemia can result if the ratio of ECMO blood flow to cardiac output is <60%.20 The exceedingly high estimated cardiac outputs in the 24-hour period before placement of a circuit in parallel among the convenience sample led us to speculate that an appreciable mismatch between ECMO blood flow and cardiac output might have been present in our cohort despite high ECMO blood flows through a single circuit. With a circuit in parallel, greater ECMO flows were achieved and the total undrained systemic venous return was reduced thereby increasing the ECMO flow:patient cardiac output ratio.
The limits of oxygen delivery of a VV-ECMO platform and cannulation configuration using a single circuit are determined by several factors including maximum achievable blood flow, oxygen transfer at the blood-membrane interface, increasing shunt fraction within the oxygenator at greater flow, and the recirculation fraction of a circuit.21 The additional use of an ECMO circuit in parallel addresses these limitations in several ways. First, the additional use of a drainage cannula increases the maximum achievable blood flow, while limiting negative drainage pressure and reducing recirculation fraction.22 Incorporating a second centrifugal pump and oxygenator in parallel enables greater total flow. Second, the blood flow through each individual oxygenator is decreased relative to the blood flow through a single oxygenator. Thus, the shunt fraction within each oxygenator is decreased, optimizing oxygen transfer at the blood-membrane interface. This might explain why, in our study, only modest increases in total flow through circuits in parallel appeared to have had a greater effect on oxygenation than might be expected through a single circuit. Third, parallel circuits reduce the revolutions per minute required by a single centrifugal pump to achieve the ECMO flow needed for oxygen delivery, which would be expected to limit mechanical trauma and shear stress on blood components.23 , 24 Minimizing blood trauma might be especially important considering the toxic effects of cell-free hemoglobin and its potential to exacerbate lung injury and multiorgan dysfunction.25 In these ways, ECMO circuits in parallel improved oxygen delivery while minimizing negative effects of high-flow VV-ECMO.
Other reported strategies to address refractory hypoxemia during VV-ECMO26 include higher red blood cell transfusion targets to increase oxygen delivery,27 cannula repositioning to reduce recirculation and optimize blood flow,28 therapeutic hypothermia,29 use of β-blockers to reduce metabolic demand,30 and prone positioning during ECMO31 to improve intrapulmonary shunt. However, none of these have shown efficacy in this population, many remain controversial,32 , 33 and some might be inappropriate in certain clinical circumstances and result in additional complications.34 The additional use of a second drainage cannula has been used to improve total flow, limit negative drainage pressure, and minimize recirculation,22 but the additional use of a circuit in parallel might provide an even greater increase in total flow allowing for improved oxygen delivery. We did not convert VV-ECMO to venoarterial or VV arterial ECMO for any patient in the study because no patient exhibited signs of cardiac failure. Therefore, the additional use of peripheral arterial support would have likely worsened hypoxemia and introduced risk of additional complications unnecessarily.
Among decannulated patients, 5 (31.3%) patients had cannula-associated DVTs; the incidence of DVTs was lower in our cohort of patients than what has been recently reported in the literature.35 Despite prolonged ECMO runs and using large cannulas, bleeding at cannulation sites was minimized by careful dilation of the vessel and making the smallest possible skin incision to facilitate insertion of the cannula. Two patients had major thoracic bleeding and 1 patient had intraperitoneal bleeding because of non-ECMO procedural complications. It should be noted that 2 circuits might further exacerbate ECMO-induced coagulopathy and increase the propensity for bleeding.23 There was 1 major cannula-associated bleeding complication from dislodgement of an IJ return cannula because of long-term ECMO support and compromised skin integrity. After this, we modified our cannulation strategy to avoid cannulation of both IJ veins by using a Y connector to join the reinfusion lines of both circuits into a single cannula placed in the right IJ. We began placing larger 23-to 25-Fr IJ outflow cannulas at the time of initial cannulation for all COVID-19 patients to obviate the requirement for a second reinfusion cannula should the patient need parallel ECMO support later in their course.
This study has limitations. The single-center, retrospective nature of the study and small sample size limits the interpretation and generalizability of the results. Patient characteristics that increase the likelihood they will require increased mechanical support or for whom a circuit in parallel might benefit could not be identified from this study. Further, although the survival rate in this cohort of severely ill patients is promising, no definitive conclusion can be made about the safety or efficacy of this cannulation strategy. Further, the length of intensive care unit and hospital stay was prolonged in this cohort. ECMO is a limited and resource-demanding therapy. Triaging scarce resources during a pandemic has practical and ethical implications. Other considerations include the increased burden of care on bedside staff and cost of disposables.
Conclusions
The management of patients with COVID–19-related ARDS is challenging and the optimal use of ECMO in this population has not been determined. Refractory hypoxemia can develop in the setting of a hyperinflammatory state, hyperdynamic circulation, and severely impaired gas exchange through the native lungs. In select patients, additional use of a parallel ECMO circuit can result in increased ECMO blood flow and improved oxygenation while allowing for lung-protective mechanical ventilation.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
References
- 1.Cheng W., Ma X.D., Su L.X., Long Y., Liu D.W., Du B., et al. Retrospective study of critically ill COVID-19 patients with and without extracorporeal membrane oxygenation support in Wuhan, China. Front Med (Lausanne) 2021;8:659793. doi: 10.3389/fmed.2021.659793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization Registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramanathan K., Shekar K., Ling R.R., Barbaro R.P., Wong S.N., Tan C.S., et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25:211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gannon W.D., Stokes J.W., Francois S.A., Patel Y.J., Pugh M.E., Benson C., et al. Association between availability of ECMO and mortality in COVID-19 patients eligible for ECMO: a natural experiment. Am J Respir Crit Care Med. 2022;205:1354–1357. doi: 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urner M., Barnett A.G., Bassi G.L., Brodie D., Dalton H.J., Ferguson N.D., et al. Venovenous extracorporeal membrane oxygenation in patients with acute COVID-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377:e068723. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaro R.P., MacLaren G., Boonstra P.S., Combes A., Agerstrand C., Annich G., et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ELSO Registry dashboard of ECMO-supported COVID patient data. https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx
- 9.Abrams D., Schmidt M., Pham T., Beitler J.R., Fan E., Goligher E.C., et al. Mechanical ventilation for acute respiratory distress syndrome during extracorporeal life support. Research and practice. Am J Respir Crit Care Med. 2020;201:514–525. doi: 10.1164/rccm.201907-1283CI. [DOI] [PubMed] [Google Scholar]
- 10.Caravita S., Baratto C., Di Marco F., Calabrese A., Balestrieri G., Russo F., et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur J Heart Fail. 2020;22:2228–2237. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik A., Shears L.L., Zubkus D., Kaczorowski D.J. Parallel circuits for refractory hypoxemia on venovenous extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2017;153:e49–e51. doi: 10.1016/j.jtcvs.2016.10.067. [DOI] [PubMed] [Google Scholar]
- 12.Patel Y.J., Stokes J.W., Gannon W.D., Francois S.A., Wu W.K., Rice T.W., et al. Extracorporeal membrane oxygenation circuits in parallel for refractory hypoxemia in COVID-19: a case series. ASAIO J. 2022;68:1002–1009. doi: 10.1097/MAT.0000000000001706. [DOI] [PubMed] [Google Scholar]
- 13.Aubron C., McQuilten Z., Bailey M., Board J., Buhr H., Cartwright B., et al. Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: a pilot randomized trial. Crit Care Med. 2019;47:e563–e571. doi: 10.1097/CCM.0000000000003780. [DOI] [PubMed] [Google Scholar]
- 14.Pearse R.M., Ikram K., Barry J. Equipment review: an appraisal of the LiDCO plus method of measuring cardiac output. Crit Care. 2004;8:190–195. doi: 10.1186/cc2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gannon W.D., Trindade A.J., Stokes J.W., Casey J.D., Benson C., Patel Y.J., et al. Extracorporeal membrane oxygenation selection by multidisciplinary consensus: the ECMO Council. ASAIO J. Published online May 7, 2022 doi: 10.1097/MAT.0000000000001757. [DOI] [PubMed] [Google Scholar]
- 16.Tonna J.E., Abrams D., Brodie D., Greenwood J.C., Mateo-Sidron J.A.R., Usman A., et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the Extracorporeal Life Support Organization (ELSO) ASAIO J. 2021;67:601–610. doi: 10.1097/MAT.0000000000001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahjoub Y., Rodenstein D.O., Jounieaux V. The hyperdynamic circulatory profile of patients with COVID-19-related acute vascular distress syndrome. Letter regarding the article ‘Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization'. Eur J Heart Fail. 2021;23:493. doi: 10.1002/ejhf.2089. [DOI] [PubMed] [Google Scholar]
- 18.Mahjoub Y., Rodenstein D.O., Jounieaux V. Severe Covid-19 disease: rather AVDS than ARDS? Crit Care. 2020;24:327. doi: 10.1186/s13054-020-02972-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rendeiro A.F., Ravichandran H., Bram Y., Chandar V., Kim J., Meydan C., et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593:564–569. doi: 10.1038/s41586-021-03475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt M., Tachon G., Devilliers C., Muller G., Hekimian G., Bréchot N., et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013;39:838–846. doi: 10.1007/s00134-012-2785-8. [DOI] [PubMed] [Google Scholar]
- 21.Zanella A., Salerno D., Scaravilli V., Giani M., Castanga L., Magni F., et al. A mathematical model of oxygenation during venovenous extracorporeal membrane oxygenation support. J Crit Care. 2016;36:178–186. doi: 10.1016/j.jcrc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Zakhary B., Vercaemst L., Mason P., Lorusso R., Brodie D. How I manage drainage insufficiency on extracorporeal membrane oxygenation. Crit Care. 2020;24:151. doi: 10.1186/s13054-020-02870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W., Wang S., Chen Z., Zhang J., Li T., Aria K., et al. Impact of high mechanical shear stress and oxygenator membrane surface on blood damage relevant to thrombosis and bleeding in a pediatric ECMO circuit. Artif Organs. 2020;44:717–726. doi: 10.1111/aor.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toomasian J.M., Bartlett R.H. Hemolysis and ECMO pumps in the 21st century. Perfusion. 2011;26:5–6. doi: 10.1177/0267659110396015. [DOI] [PubMed] [Google Scholar]
- 25.Meegan J.E., Bastarache J.A., Ware L.B. Toxic effects of cell-free hemoglobin on the microvascular endothelium: implications for pulmonary and nonpulmonary organ dysfunction. Am J Physiol Lung Cell Mol Physiol. 2021;321:L429–L439. doi: 10.1152/ajplung.00018.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montisci A., Maj G., Zangrillo A., Winterton D., Pappalardo F. Management of refractory hypoxemia during venovenous extracorporeal membrane oxygenation for ARDS. ASAIO J. 2015;61:227–236. doi: 10.1097/MAT.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 27.Abbasciano R.G., Yusuff H., Vlaar A.P.J., Lai F., Murphy G.J. Blood transfusion threshold in patients receiving extracorporeal membrane oxygenation support for cardiac and respiratory failure-a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021;35:1192–1202. doi: 10.1053/j.jvca.2020.08.068. [DOI] [PubMed] [Google Scholar]
- 28.Abrams D., Bacchetta M., Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–121. doi: 10.1097/MAT.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 29.Luc J.G.Y., Meyer S.R., Murtha W.J., Singh G. Therapeutic hypothermia as an adjunct to extracorporeal membrane oxygenation for acute respiratory distress and refractory hypoxemia. Perfusion. 2019;34:422–424. doi: 10.1177/0267659118822941. [DOI] [PubMed] [Google Scholar]
- 30.Guarracino F., Zangrillo A., Ruggeri L., Pieri M., Calabrò M.G., Landoni G., et al. β-Blockers to optimize peripheral oxygenation during extracorporeal membrane oxygenation: a case series. J Cardiothorac Vasc Anesth. 2012;26:58–63. doi: 10.1053/j.jvca.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Garcia B., Cousin N., Bourel C., Jourdain M., Poissy J., Duburcq T., et al. Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 2020;24:428. doi: 10.1186/s13054-020-03162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netzer G., Shah C.V., Iwashyna T.J., Lanken P.N., Finkel B., Fuchs B., et al. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132:1116–1123. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 33.Agerstrand C.L., Burkart K.M., Abrams D.C., Bacchetta M.D., Brodie D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg. 2015;99:590–595. doi: 10.1016/j.athoracsur.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Polderman K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 35.Menaker J., Tabatabai A., Rector R., Dolly K., Kufera J., Lee E., et al. Incidence of cannula-associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J. 2017;63:588–591. doi: 10.1097/MAT.0000000000000539. [DOI] [PubMed] [Google Scholar]