Abstract
Objectives
We sought to investigate the efficacy and safety of SpikoGen®, a subunit coronavirus disease 2019 (COVID-19) vaccine composed of a recombinant severe acute respiratory syndrome coronavirus 2 spike protein with Advax-CpG55.2™ adjuvant.
Methods
This randomized, placebo-controlled, double-blind, phase 3 trial was conducted on 16 876 participants randomized (3:1) to receive two intramuscular doses of SpikoGen® or a saline placebo 21 days apart. The primary outcome was to assess the efficacy of SpikoGen® in preventing symptomatic COVID-19. Secondary outcomes included safety assessments and evaluation of SpikoGen® vaccine's efficacy in preventing severe COVID-19. The study aimed for 147 COVID-19 symptomatic cases.
Results
Overall, 12 657 and 4219 participants were randomized to the SpikoGen® and placebo group and followed for a median of 55 days (interquartile range, 48–60 days) and 51 days (interquartile range, 46–58 days) after 14 days of the second dose, respectively. In the final per-protocol analysis, the number of COVID-19 cases was 247 of 9998 (2.4%) in the SpikoGen® group and 119 of 3069 (3.8%) in the placebo group. This equated to a vaccine efficacy of 43.99% (95% CI, 30.3–55.0%). The efficacy was calculated to be 44.22% (95% CI, 31.13–54.82%) among all participants who received both doses. From 2 weeks after the second dose, 5 of 9998 (0.05%) participants in the SpikoGen® group and 6 of 3069 (0.19%) participants in the placebo group developed severe COVID-19, equating to a vaccine efficacy against severe disease of 77.51% (95% CI, 26.3–93.1%). The SpikoGen® vaccine was well tolerated.
Discussion
A 2-dose regimen of SpikoGen® reduced the rate of COVID-19 and severe disease in the wave of the Delta variant.
Keywords: Delta, Phase 3, Spike protein, SpikoGen, Subunit protein vaccine
Introduction
As of August 2022, >580 million coronavirus disease 2019 (COVID-19) cases have been confirmed worldwide [1]. Vaccination can be an efficient strategy for controlling viral infections, and adequate global access to safe and effective COVID-19 vaccines remains a key priority.
Reduced vaccine effectiveness has been seen caused by waning vaccine immunity together with the development of vaccine-resistant variants, including Beta, Delta, and Omicron [2]. Given the high levels of uncertainty regarding future evolution of the virus, additional vaccine approaches may still offer benefits alongside the existing vaccines.
Recombinant subunit protein vaccines are a well-established platform known for their high efficacy, safety, and low reactogenicity [3]. SpikoGen® is a subunit spike protein vaccine formulated with Advax-CpG adjuvant.
Previous studies of the SpikoGen® have shown robust protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) challenge test along with the induction of neutralizing antibodies and T-cell responses [[4], [5], [6], [7], [8]].
Based on the positive phase 2 results, this phase 3 efficacy trial was undertaken to assess the efficacy and safety of the SpikoGen® vaccine. The prevalent variant of SARS-CoV-2 circulating in the community at the time of the trial was the Delta variant.
Materials and methods
Study design
This study was a phase 3, parallel, randomized, double-blind, placebo-controlled trial (3:1) with safety follow-up of 6 months after the second dose. The trial was conducted at the Espinas Palace Hotel, Tehran, Iran, in August and September 2021, which was converted into a large clinical trial site (please see supplementary material for a video link). Immunocompetent adults aged 18–50 years with stable medical conditions (not being hospitalized within 3 months before the screening visit) were enrolled in the study. The key exclusion criteria were active infection with clinical signs of SARS-CoV-2 during screening, history of previous vaccination with any type of SARS-CoV-2 vaccine, and receiving immunosuppressive medications. Please see supplementary material for detailed exclusion criteria and the rules for stopping the trial.
The study design was agreed with the Iranian Food and Drug Administration as the relevant regulator, conducted in compliance with Good Clinical Practice, approved by the Iran National Ethics Committee (IR.NREC.1400.005) and registered at ClinicalTrials.gov (NCT05005559) and the Iranian Registry of Clinical Trials (IRCT20150303021315N24).
Randomization and intervention
Eligible participants were randomized using R-CRAN 4.0.1, to either the vaccine candidate arm or the placebo comparator arm (allocation 3:1). The randomization was stratified by age (from 18 to <40 years or from 40 to <50 years). The appearance of the vaccine and placebo were identical, and the participants, investigators, and laboratory staff were blinded to the allocation. Please see supplementary material for details of the randomization process.
The SpikoGen® vaccine was administered as two 25-μg doses of recombinant spike protein with Advax-CpG55.2 adjuvant (15.5 mg Advax, 171 μg CpG). The two doses were administered 21 days apart in the deltoid muscle. Before-injection vital signs, including heart and respiratory rates, temperature, and oxygen saturation, were assessed. Moreover, serum samples were taken for later nucleocapsid antibody testing by ELISA (Pishtazteb, Iran).
Outcomes
The primary outcome was the occurrence of symptomatic COVID-19 starting from 14 days after the administration of the second dose based on the prespecified criteria (please see supplementary material).
Secondary outcomes included severe COVID-19 based on the specific criteria (please see supplementary material) and safety outcomes, including the incidence of local and systemic solicited adverse events for 7 days after each dose and the incidence of unsolicited adverse events up to 28 days after the second dose. Serious adverse events and suspected unexpected serious adverse events were evaluated up to 6 months after the second vaccination.
Follow-up and outcome assessment
The participants completed electronic diaries for local and systemic solicited adverse events daily for 7 days after each vaccination. Safety outcomes were reported based on the Medical Dictionary for Regulatory Activities classification. Each participant's severity score was assessed based on the Food and Drug Administration Toxicity Grading Scale along with the causality assessment of the adverse events [9].
Statistical analysis
A sample of 16 876 participants was calculated based on detecting 147 cases of COVID-19 to achieve 60% vaccine efficacy (VE) with 90% power and 0.025 significance level. An interim analysis was planned after reaching 50% of the target number of COVID-19 cases (i.e. 74 subjects). The sample size was calculated using R-CRAN.
The population sets for the analysis were defined as follows:
-
1.
Safety analysis dataset included participants who took at least one dose of the study intervention. Safety results were presented as incidence and percentages of solicited and unsolicited adverse events for each group.
-
2.
Sensitivity analysis dataset 1 (all participant population) included participants who took both doses of study intervention within the specified time window and were not discontinued/withdrawn from the study until 14 days after the second dose.
-
3.
Sensitivity analysis dataset 2 (per-protocol [PP] + nucleocapsid antibody–positive population) included a subset of the participants in the sensitivity analysis dataset 1 who had no major protocol deviations that may affect the study data; PP analysis dataset included a subset of the participants in the sensitivity analysis dataset 2 who were negative for anti-nucleocapsid IgG antibodies at baseline.
To facilitate the regulatory review of data related to VE as the earliest possible opportunity, an interim efficacy analysis was planned once the number of COVID-19 cases exceeded 74. The participants, investigators, and all other trial staff remained blinded throughout the interim analysis. VE was defined as (1 − relative risk) × 100, and the relative risk was estimated using the Poisson regression with robust error variance. We used R (version 3.6.0) and STATA 14 for all statistical analyses. The ‘Sandwich’ package in the R-CRAN version 4.0.1 was used for analysing robust standard error.
Results
Study participants
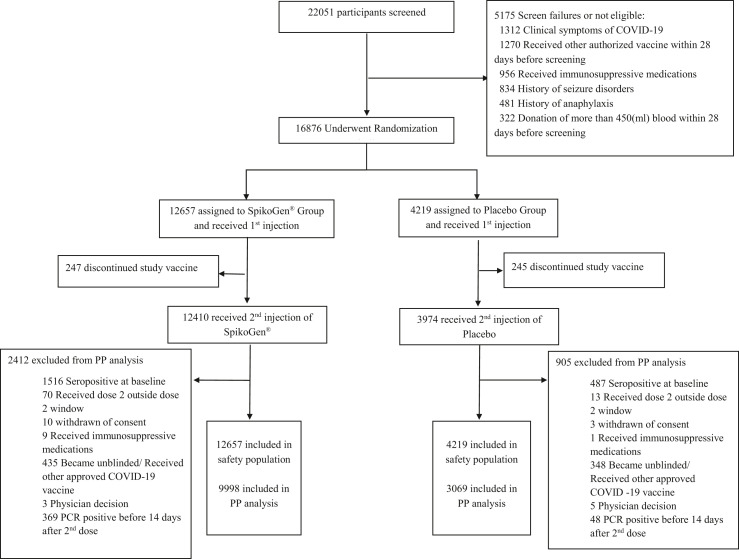
The trial was initiated on 7 August 2021. In total, 12 657 volunteers were randomized to the SpikoGen® group and 4219 volunteers to the placebo group. Fig. 1 depicts a Consolidated Standards of Reporting Trials diagram showing the flow of participants through the study. Demographic and baseline characteristics of the participants are provided in Table 1 . Detailed baseline medical histories are presented in Table S1.
Fig. 1.
Consolidated Standards of Reporting Trials diagram showing the participants' flow through trial screening, randomization, vaccination, and analysis steps.
Table 1.
Participant characteristics of the randomized population
| Characteristics | SpikoGen® (N = 12 657) | Placebo (N = 4219) |
|---|---|---|
| Sex (male), n (%) | 7120 (56.26) | 2431 (57.62) |
| Body mass index (kg/m2), mean ± SDa | 25.99 ± 4.56 | 25.99 ± 4.52 |
| Age (y), mean ± SD | 32.37 ± 6.79 | 35.21 ± 6.76 |
| Nucleocapsid antibody status (positive), n (%)b | 1516 (11.98) | 487 (11.54) |
| Medical history, n (%)c | ||
| Obesityd | 2343 (18.51) | 784 (18.59) |
| Anxiety/depression | 871 (6.88) | 253 (6) |
| Hepatic steatosis | 703 (5.55) | 227 (5.38) |
| Hypertension | 258 (2.04) | 89 (2.11) |
| Asthma | 203 (1.6) | 69 (1.64) |
| Malignancy | 164 (1.30) | 59 (1.40) |
| Diabetes mellitus | 95 (0.75) | 31 (0.73) |
| Cerebrovascular accident | 6 (0.05) | 1 (0.02) |
| Myocardial ischaemia | 5 (0.04) | 0 (0) |
| Dyslipidaemia | 5 (0.04) | 0 (0) |
| Deep vein thrombosis | 4 (0.03) | 2 (0.05) |
The body mass index is the weight in kilograms divided by the square of the height in metres.
Values of >1.1 were considered positive at baseline.
Malignancy includes ovarian cyst, breast cyst, uterine leiomyoma, fibroadenoma of breast, benign pituitary tumour, ovarian fibroma, benign bone neoplasm, uterine polyp, thyroid cancer, renal cyst, fibrocystic breast disease, breast cancer, and benign breast neoplasm.
Obesity is defined as body mass index of ≥30 kg/m2.
Efficacy outcomes
In the interim analysis 10 612 participants (8100 in the SpikoGen® group and 2512 in the placebo group) were analysed. Fourteen days after the second dose, 50 of 8100 (0.62%) participants in the SpikoGen® group and 37 of 2512 (1.47%) participants in the placebo group of the PP population had symptomatic COVID-19, indicating a VE of 59.69% (95% CI, 37.95–73.57%). In the PP + baseline nucleocapsid antibody–positive population analysis dataset, the VE was 64.36% (95% CI, 46.54–76.11%).
At the time of the final efficacy analysis, the median of follow-up after 14 days of the second dose in the SpikoGen® and placebo group were 55 days (interquartile range, 48–60 days) and 51 days (interquartile range, 46–58 days), respectively. The blinded part of the study was continued after the interim analysis until the emergency use authorization was granted, at which point, with agreement from the regulator, the trial was unblinded, and all participants in the placebo group were offered an active vaccine. By that time, 424 symptomatic infections had already occurred during the study as this was at the time of the massive wave of the Delta variant in Iran. Hence, all symptomatic infections that occurred up to the time of unblinding were considered in the final analysis.
In the PP analysis dataset, the VE was 43.99% (95% CI, 30.3–55.0%) (Table 2 ). The results of the VE in different populations are provided in Table 2.
Table 2.
Vaccine efficacy against symptomatic COVID-19
| Study population | No. of events/Total No. |
Rate ratio |
Vaccine efficacy (95% CI)a | |
|---|---|---|---|---|
| SpikoGen® | Placebo | (95% CI) | ||
| Per-protocol | 247/9998 | 119/3069 | 0.56 (0.45–0.70) | 43.99 (30.30–55.00) |
| Per-protocol + nucleocapsid antibody–positive | 264/11 417 | 128/3502 | 0.56 (0.45–0.69) | 43.81 (30.61–54.51) |
| All participants | 266/11 760 | 128/3603 | 0.56 (0.45–0.69) | 44.22 (31.13–54.82) |
COVID-19, coronavirus disease 2019.
Vaccine efficacy and 95% CIs of the SpikoGen® in preventing COVID-19 in different populations with the use of Poisson regression with robust error variance.
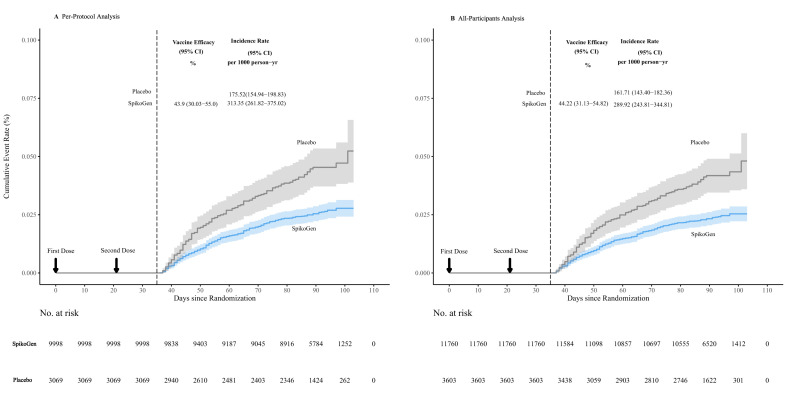
Of those with PCR-confirmed COVID-19, 50 were successfully sequenced, and all isolates were confirmed to be the B.1.617.2 (Delta) variant. Fig. 2 shows the Kaplan-Meier curve of VE in preventing any symptomatic COVID-19. The incidence rate in the SpikoGen® group was 175.52 versus 313.35 per 1000 person/y in the placebo group.
Fig. 2.
Kaplan-Meier curve of efficacy of the SpikoGen® vaccine in preventing coronavirus disease 2019.
Eleven participants developed severe COVID-19, from 2 weeks after the second dose: 5 of 9998 (0.05%) in the SpikoGen® group and 6 of 3069 (0.19%) in the placebo group. The efficacy of SpikoGen® in preventing severe COVID-19 was 77.51% (95% CI, 26.32–93.14%). No COVID-19–related death was reported from 2 weeks after the second dose in participants belonging to either group.
Safety outcomes
The vaccine was well tolerated. Most adverse events were graded as mild and lasting for no longer than 1–2 days. Table 3 shows the incidence of local and systemic main solicited adverse events after the first and second injection.
Table 3.
Percentage of participants experiencing main solicited local and systemic adverse events by symptoms, vaccination dose, vaccine group, and maximum toxicity grading scale
| Symptom | Grade | Vaccination 1 |
Vaccination 2 |
||
|---|---|---|---|---|---|
| SpikoGen® | Placebo | SpikoGen® | Placebo | ||
| Any solicited local adverse event | 1 | 9833 (77.69) | 1053 (24.96) | 8096 (63.96) | 791 (18.75) |
| 2 | 853 (6.74) | 35 (0.83) | 1203 (9.50) | 48 (1.14) | |
| 3 | 1 (0.01) | 0 (0) | 7 (0.05) | 0 (0) | |
| Injection site pain | 1 | 9417 (74.40) | 992 (23.51) | 7522 (61.48) | 755 (19.22) |
| 2 | 678 (5.36) | 30 (.71) | 944 (7.72) | 45 (1.15) | |
| 3 | 0 (0) | 0 (0) | 1 (.008) | 0 (0) | |
| Injection site swelling/induration | 1 | 2092 (16.53) | 116 (2.75) | 1999 (16.34) | 59 (1.50) |
| 2 | 195 (1.54) | 5 (0.12) | 304 (2.49) | 3 (0.08) | |
| 3 | 1 (0.01) | 0 (0) | 4 (0.03) | 0 (0) | |
| Injection site erythema | 1 | 473 (3.74) | 54 (1.28) | 577 (4.72) | 23 (0.58) |
| 2 | 46 (0.36) | 1 (0.02) | 156 (1.27) | 1 (0.03) | |
| 3 | 0 (0) | 0 (0) | 5 (0.04) | 0 (0) | |
| Any solicited systemic adverse event | 1 | 6782 (55.58) | 2031 (48.14) | 5193 (41.03) | 1226 (29.06) |
| 2 | 3173 (25.07) | 970 (22.10) | 2896 (22.89) | 657 (15.57) | |
| 3 | 328 (2.60) | 82 (1.95) | 367 (2.90) | 79 (1.88) | |
| Fatigue | 1 | 3974 (31.40) | 1125 (26.67) | 2986 (24.41) | 666 (16.95) |
| 2 | 1478 (11.68) | 430 (10.19) | 1373 (11.22) | 300 (7.64) | |
| 3 | 192 (1.52) | 53 (1.26) | 245 (2.00) | 48 (1.22) | |
| Headache | 1 | 2717 (21.47) | 929 (22.02) | 1921 (15.70) | 490 (12.47) |
| 2 | 1654 (13.07) | 551 (13.06) | 1538 (12.57) | 388 (9.88) | |
| 3 | 11 (0.09) | 1 (0.02) | 17 (0.14) | 4 (0.10) | |
| Myalgia | 1 | 2184 (17.25) | 567 (13.44) | 1415 (11.57) | 296 (7.53) |
| 2 | 822 (6.49) | 178 (4.22) | 751 (6.14) | 141 (3.59) | |
| 3 | 74 (0.58) | 10 (0.24) | 122 (1) | 19 (0.49) | |
| Arthralgia | 1 | 1222 (9.65) | 335 (7.94) | 821 (6.71) | 185 (4.71) |
| 2 | 385 (3.04) | 93 (2.20) | 379 (3.10) | 60 (1.53) | |
| 3 | 36 (0.28) | 9 (0.21) | 62 (0.51) | 12 (0.31) | |
| Chills | 1 | 463 (3.66) | 118 (2.80) | 446 (3.65) | 88 (2.24) |
| 2 | 144 (1.14) | 39 (0.92) | 241 (1.97) | 37 (0.94) | |
| Pyrexia | 1 | 346 (2.73) | 81 (1.92) | 220 (1.80) | 39 (0.99) |
| 2 | 68 (0.54) | 17 (0.40) | 34 (0.28) | 6 (0.15) | |
| 3 | 71 (0.56) | 19 (0.45) | 43 (0.35) | 10 (0.26) | |
Please see supplementary material for detailed data on the incidence of the solicited and unsolicited adverse events, serious adverse events, grading, and the causality of the adverse events (Tables S2, S3, S4, and S5).
Discussion
This phase 3 trial confirmed that SpikoGen® vaccine when used as a primary 2-dose course is safe and able to significantly reduce the rate of COVID-19 in baseline seronegative participants. The VE was calculated as 44% (95% CI, 30.30–55.0%) against any symptomatic COVID-19 and as 77.51% (95% CI, 26.32–93.14%) against severe COVID-19. Similar levels of VE were observed when baseline nucleocapsid antibody–positive volunteers were included, suggesting that the SpikoGen® vaccine can boost protection even in those with natural immunity acquired through previous infection.
It is not possible to directly compare our efficacy results with that of other vaccine trials, given the differences in populations, endpoints, and timing. Furthermore, our VE was measured in response to a major wave of Delta variant in Iran, which contrasts with phase 3 trials for earlier approved vaccines where the interim analyses leading to approval were conducted before the Delta variant surge. Overall, the COVID-19 vaccine effectiveness against the Delta variant has been found to be reduced compared with the more ancestral strains with, for example, a test-negative case-control study showing two-dose effectiveness of the AstraZeneca ChAdOx1 to be only 59.8% (95% CI, 28.9–77.3%) against the B.1.617.2 (Delta) variant versus 66.1% (95% CI, 54.0–75.0%) for the earlier B.1.1.7 (Alpha) variant [10]. The same study showed that the effectiveness of two doses of BNT162b2 mRNA vaccine dropped from 93.7% against the Alpha variant to 88.0% against the Delta variant. Similarly, a U.S. Department of Veterans Affairs study showed the effectiveness in September 2021 of two doses of vaccine against the Delta variant was just 13.1% for the Janssen adenoviral vector vaccine, 43.3% for Pfizer mRNA vaccine, and 58% for Moderna mRNA vaccine, having been earlier measured in March 2021 against the earlier strains (predominantly Alpha) as 86.4%, 86.9%, and 89.2%, respectively [11].
Algorithms have been developed based on the extrapolations from antibody levels and virus neutralization assays to predict the overall VE against ancestral strains [12,13]. Therefore, we evaluated our previously published phase 2 trial data [7] to determine what might have been the likely efficacy of SpikoGen® against the ancestral strains. Earle et al. [14], developed an algorithm based on the ratio of the mean vaccine-induced neutralizing antibodies to mean convalescent levels to predict VE. Based on the phase 2 data, baseline seropositive participants had a geometric mean concentration (GMC) of 9.92 RU/mL for S1 IgG. We then used this measure as our convalescent level to compare the levels after the administration of two doses of SpikoGen®, where the GMC was 29.12 RU/mL. Hence, the ratio of mean spike IgG levels after vaccination to mean convalescent levels was 2.93 (29.12:9.92). Applying this ratio to the Fig. 2B in the study by Earle et al. [14] would predict the efficacy of SpikoGen® at approximately 85% (Fig. S1a).
Next, we performed a similar evaluation using surrogate virus neutralizing antibody levels from the phase 2 trial. The baseline seropositive participants had a GMC of 1.61 μg/mL. By contrast, participants who received two doses of SpikoGen® had a GMC of 19.71 μg/mL, or 12.24 times the convalescent level. Plotting this 12.24 ratio on Fig. 2A in the study by Earle et al. [14] would predict the efficacy of SpikoGen at >95% (Fig. S1b). Similarly, plotting this ratio on Fig. 1 in the study by Khoury et al. [12] would similarly predict the efficacy of SpikoGen at >95% (Fig. S1c).
Lastly, 191 of 302 (63.25%) of the SpikoGen®-immunized participants in the phase 2 trial achieved S1-binding IgG levels of >60 binding antibody units/mL. Based on supplementary Fig. 3 presented in the study by Goldblatt et al. [15], this would predict the efficacy of SpikoGen® at 63.25% (Fig. S1d).
These algorithms predict SpikoGen® vaccine's efficacy against the ancestral strain to be in the range of 60% to 95% depending on the actual algorithm and antibody levels used.
SpikoGen® vaccine significantly reduced the risk of severe COVID-19 by 77.5%. Given the small number of such events, there were wide CIs around this estimated effect that ranged from 93.1% at the high side to 26.3% on the low side. Although phase 3 trials for other vaccines reported higher point estimates of efficacy against severe disease of up to 95%, these estimates were similarly based on extremely small numbers with consequent wide CIs that overlap with ours and their data that was largely collected pre-Delta [[16], [17], [18]].
An important consideration for COVID-19 vaccines is that in addition to efficacy, they should have high levels of safety and tolerability. Notably, the SpikoGen® vaccine exhibited normal vaccine-associated reactions, including pain at injection site, headache, myalgia, and fatigue, that were predominantly mild and short lasting. Reassuringly, no episodes of central venous thrombosis, myocarditis/pericarditis, or autoimmune phenomena, such as Guillain-Barre syndrome, were seen in our study. Recombinant protein vaccines have a long history of safety and tolerability, established over many decades of use [19]. This history of safe long-term use could be an advantage for regular COVID-19 boosters, if required.
This study had some limitations, including the enrolment of adults aged 18–50 years only; a restriction imposed by the ethics for conducting a placebo-controlled phase 3 trial against COVID-19 at a time when other vaccines were starting to become available.
Based on the interim analysis results, which confirmed the VE on a PP basis, SpikoGen® vaccine received an emergency-use approval from the Iranian Food and Drug Administration. After this approval, the number of participants requesting to be unblinded to determine whether they had received a placebo and should thereby seek active vaccination became too much. Moreover, with the agreement of the regulator, the whole trial was unblinded and the placebo group received active vaccination. Before unblinding the participants, an efficacy analysis on the whole symptomatic COVID-19 cases was performed, which showed a VE of 44.0% (95% CI, 30.30–55.0%) against the symptomatic disease, which was lower than the results obtained at the interim analysis. Several potential factors might have contributed to this lower second result. The first is that because other approved vaccines were available to them, an increasing number of trial participants were requesting unblinding. This could have created imbalances among the originally randomized groups, and the reduction in active participant numbers diluted the power of the study at the later timepoints. The use of a saline placebo may lead to a degree of unblinding because of the complete lack of reactogenicity of the saline compared with the known local reactogenicity of an active vaccine. This problem would also be an issue for most of the other phase 3 COVID-19 vaccine trials that also used a saline placebo [16,17]. An exception was the Oxford trials of the AstraZeneca vaccine that used an active meningococcal vaccine comparator [18]. At the time of the study, Iran was in the middle of a devastating Delta-driven outbreak, and we hypothesized that the measured VE could be reduced during periods of extremely high infection rates. Unlike the normal situation where a small number of trial participants may get exposed to the virus just once or twice, with extremely high community infection rates, trial participants may be repeatedly exposed to high levels of virus given the number of infected people around them. The higher the viral challenge dose, the greater the likelihood of infection. High community infection rates may act to reduce the measured VE, with breakthrough infections more likely in such an environment. Notably, the observed disease rate in our trial was many times higher than the predicted rate used in the initial trial power calculations. A study of immune correlates of protection indicated a higher baseline exposure risk of SARS-CoV-2 infections and predicted higher probability of all infection outcomes, except for asymptomatic infections, which would fit with our hypothesis [20].
Overall, this phase 3 trial highlights the challenges of undertaking a pivotal VE study in the middle of a pandemic, with rapidly changing circumstances necessitating adaptations to trial design and analysis. Despite these many challenges, this pivotal phase 3 trial confirmed that two doses of SpikoGen® given 3 weeks apart provide significant efficacy against COVID-19 and a significant reduction in the risk of severe disease caused by the Delta variant. The SpikoGen® vaccine had a positive safety profile, and solicited adverse events were predominantly mild and short lived. With the rate of global infections not abating and with the ongoing waves of the disease caused by novel variants, the SpikoGen® vaccine provides an additional protein-based vaccine to assist in the global battle against COVID-19.
Author contributions
PT performed the research and conceptualized the study. NA was involved in data collection and coordination of the study. RSH performed statistical analysis. MM, AS, BY, HK, NF, AE, AT, and NP were involved in organization, conduct, and technical support of the study. SB was involved in the trial design, conceptualization, and drafted the manuscript. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and take the final responsibility for the decision to submit for publication.
Transparency declaration
This study was funded by CinnaGen Co. NA, RSH, AS, BY, HK, NF, AE, AT, and SB are members of the Orchid Pharmed Medical department, which is in collaboration with CinnaGen company with respect to conducting clinical trials. NP is associated with Vaxine Pty Ltd. The other authors declare that they have no conflicts of interest.
Acknowledgements
The authors are thankful to study participants, site research staff, and members of the steering and data and safety monitoring board committee.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.09.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rezaei S., Fatemi B., Karimi Majd Z., Minaei H., Peikanpour M., Anjidani N., et al. Efficacy and safety of Tocilizumab in severe and critical COVID-19: a systematic review and meta-analysis. Expert Rev Clin Immunol. 2021;17:499–511. doi: 10.1080/1744666X.2021.1908128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Z., Zhu H., Wang X., Jing B., Li Z., Xia X., et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L., Honda-Okubo Y., Huang Y., Jang H., Carlock M.A., Baldwin J., et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39:5940–5953. doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabarsi P., Anjidani N., Shahpari R., Mardani M., Sabzvari A., Yazdani B., et al. Safety and immunogenicity of SpikoGen®, an advax-cpg55.2-adjuvanted sars-cov-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022;28:1263–1271. doi: 10.1016/j.cmi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., Honda-Okubo Y., Baldwin J., Bowen R., Bielefeldt-Ohmann H., Petrovsky N. Covax-19/Spikogen® vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine. 2022;40:3182–3192. doi: 10.1016/j.vaccine.2022.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popmihajlov Z., Pang L., Brown E., Joshi A., Su S.C., Kaplan S.S., et al. A post hoc analysis utilizing the FDA toxicity grading scale to assess injection site adverse events following immunization with the live attenuated Zoster Vaccine (ZVL) Hum Vaccin Immunother. 2018;14:2916–2920. doi: 10.1080/21645515.2018.1502517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn B.A., Cirillo P.M., Murphy C.C., Krigbaum N.Y., Wallace A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331–336. doi: 10.1126/science.abm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin J., Piplani S., Sakala I.G., Honda-Okubo Y., Li L., Petrovsky N. Rapid development of analytical methods for evaluating pandemic vaccines: a COVID-19 perspective. Bioanalysis. 2021;13:1805–1826. doi: 10.4155/bio-2021-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldblatt D., Fiore-Gartland A., Johnson M., Hunt A., Bengt C., Zavadska D., et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40:306–315. doi: 10.1016/j.vaccine.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jilg W., Schmidt M., Deinhardt F. Four-year experience with a recombinant hepatitis B vaccine. Infection. 1989;17:70–76. doi: 10.1007/BF01646879. [DOI] [PubMed] [Google Scholar]
- 20.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.