Abstract
This work was designed to evaluate the efficacy of a postbiotic compound produced by stabilized non-viable Lactobacilli on the health, growth performance, immunity, and gut status against Escherichia coli (E. coli) challenge of broiler chickens. A total of 400, day-old broiler chicks were allocated into 4 equal groups (1–4) consisting of 100; each assigned into 2 equal replicates (50 each). Chickens in the 1st group were received the dry form of the compound at doses of 1 kg and 0.5 kg/ton feed for starter and grower, and the finisher diets, respectively. Chickens in the 2nd group were given the aqueous form of the compound in a dose of 4 mL/L of the drinking water during the first 3 days of life and at a day before and after each vaccination. Feed and water treatment regimens were administered to chickens in the 3rd group. Group 4 was kept without treatment. Each bird in the 1st, 2nd, 3rd, and 4th group was challenged with E. coli (O78) at 1-week-old. All groups were kept under observation till 5-week-old. Statistical analysis included one-way ANOVA and other methods as described with significant differences at P ≤ 0.05. The results indicated that feed and water treatments with the postbiotic compound induced more significant (P ≤ 0.05) amelioration of a disease picture, enhancement of growth performance, boosting of immune response, improvement of bursa of Fabricius/body weight ratio, and reduction of intestinal coliform count in challenged chickens when compared with challenged non-treated chickens. In conclusion, the postbiotic compound either in a dry and/or an aqueous form is recommended for improving the health, performance, and immunity of colisepticaemic broiler chickens.
Keywords: Chickens, E. coli, Growth performance, Immunity, Stabilized non-viable Lactobacillus
Introduction
The poultry industry is considered as one of the most important sources of income all over the world. Avian colibacillosis is an infectious disease that is caused by Escherichia coli (E. coli) (Lutful Kabir 2010). The disease is associated with heavy economic losses including mortality, decrease in productivity, and food-borne illnesses (Koutsianos et al. 2021). The most common avian pathogenic serogroups of E. coli are O78, O1, O2, and to some extent O15 and O55 (Ali et al. 2019). Antibiotic treatment is commonly used for the control of such infection. However, concern has been expressed that excessive use of antimicrobials, including those used at subclinical levels as growth promoters, is associated with the production of antibiotic-resistant strains of E. coli (Roth et al. 2019; Breijyeh et al. 2020; Radwan et al. 2021). These resistant strains can proliferate in the environment (Manyi-Loh et al. 2018) and infect humans either directly or via consumption of contaminated carcass (Valiakos and Kapna 2021). Mitigation strategies have been discussed (Ma et al. 2019), but successful strategies have been lacking. Thus, Food and Drug Administration Veterinary Feed Directive in 2017 prohibited the usage of antibiotics in farm animals as growth promotors (Veterinary Feed Directive 2019). Now, the urgent challenge of the poultry industry all over the world is to promote optimal production in parallel with providing safe product to the consumer. Some antibiotic alternatives have recently been used to improve poultry health while encouraging the production of food-borne disease-free products (Abd El-Ghany 2020).
Competitive exclusion compounds such as probiotics, prebiotics, and synbiotics are extensively used to prevent or reduce the intestinal colonization with E. coli especially in young broilers (Mohamed and Younis 2018). Although probiotics have been used to promote a healthy gut environment as well as growth performance and immune response of poultry, there is risk that they may acquire and transfer antibiotic resistance genes among microorganisms (Shazali et al. 2014). For instance, some probiotics of Lactobacilli species carry antibiotic-resistant genes for tetracycline and chloramphenicol that transfer among bacteria (Sharma et al. 2014). Besides, probiotics may have a negative influence on the host by increasing the severity of tissue inflammation (Tsilingiri et al. 2012). Additionally, many of these inoculants must be temperature controlled to maintain patency of live bacterial colonization. Subsequently, probiotics as living bacteria might not be used in the future. Recently, soluble non-viable probiotic metabolites or postbiotics have been used as a promising and potential substitute for both antibiotics and probiotics in poultry industry (Loh et al. 2010). Postbiotics are defined as secondary metabolites from probiotics without viable or living cells (Thanh et al. 2009). Postbiotics may be part of cell wall or cytoplasmic extracts of Lactobacilli species, stabilized bacteria, cellular products, or metabolic byproducts of fermentation (Johnson et al. 2019). They are not affected by acids, pH, or environmental conditions, so they can be kept safely, even under extreme temperature conditions, with longevity. Moreover, postbiotics of Lactobacilli origin have valuable components such as organic acids and bacteriocin which improve lactic acid bacteria growth (Loh et al. 2014). Components of cell wall and cytoplasmic extracts of numerous Lactobacillus (L.) species such as L. acidophilus, L. plantarum, L. fermentum, L. casei, L. rhamnosus, L. paracasei, L. rhamnosus, L. delbrueckil subsp. Bulgaricus, L. gasseri, L. helveticus, L. reuteri, and L. johnsonni were found to be highly effective postbiotics (Cicenia et al. 2016; Tiptiri-Kourpeti et al. 2016). Bacteria used for postbiotic production should be nonpathogenic, technologically suitable for industrial processes, acid and bile resistant, and good producers for antimicrobial substances that modulate immune responses and influence the metabolic activities of the gut (Dunne et al. 1999).
Therefore, the present study was planned to assess the efficacy of a postbiotic compound produced by Lactobacilli and including stabilized non-viable Lactobacilli on the health, growth performance, immunity, and gut status of challenged broiler chickens with E. coli.
Materials and methods
The experiment was carried out in strict compliance with the recommendations of the National Regulations on Animal Welfare and following the guidelines approved by the Institutional Animal Care and Use Committee and all efforts were made to minimize suffering.
Postbiotic compound
Stabilized non-viable Lactobacilli fermentation product (Culbac®, TransAgra’s International Inc., Storm Lake, Iowa, USA) is produced either in a dry or an aqueous form. The recommended manufacture dose of the dry form (Culbac® Animal Dry) is 1 kg/ton of the starter and grower diets and 0.5 kg/ton of the finisher diet. However, the dose of the aqueous form (Culbac® Animal Healthy Start) is 4 mL/L of the drinking water during the first 3 days of age and at 1 day before and after routine vaccination program.
Chicks and experimental design
A total of 410, day-old broiler chicks (Hubbard breed) of mixed sex were obtained from a local hatchery in Giza governorate, Egypt. On arrival, ten birds were sacrificed and subjected for bacteriological culture examination on the selective media which proved absence of E. coli growth and the birds were free from E. coli infection. The remaining 400 chicks were allocated into 4 equal groups (1–4) consisting of 100; each assigned into 2 equal replicates (50 each). All the birds were kept in deep litter system under restricted hygienic measures for 5 weeks. Vaccination was done against Newcastle disease virus (NDv) using live HB1 and La Sota strains at 6- and 18-day-old, respectively, inactivated highly pathogenic avian influenza virus (HPAIv) H5N1 strain at 7-day-old, and infectious bursal disease virus (IBDv) using intermediate live strain at 13-day-old. All the vaccines were given via eye drop method, except influenza vaccine was given subcutaneously (S/C) at the back of the neck. Chickens were fed on commercial balanced diets containing local feed ingredients and formulated to meet the National Research Council requirements (NRC 1994) (Table 1). Birds were fed on starter, grower, and finisher diets at ages 1–15, 16–28, and 29–35 days, respectively. Feed and drinking water were provided ad libitum. The dry form of Culbac® was applied as feed treatment in groups 1 and 3, while the aqueous form was given in the drinking water for groups 2 and 3. Each chick in groups 1, 2, 3, and 4 was challenged S/C with 0.3 mL nutrient broth including 1 × 108/mL of E. coli serotype (O78). Identified field strain of E. coli serotype (O78) was isolated from chickens with systemic colisepticaemia. The bacterial culture concentration was adjusted to 108 colony forming unit (CFU) of E. coli/mL with previously defined nutrient broth (Fernandez et al. 2002).
Table 1.
Composition of diet ingredients given for Hubbard broiler chickens during 5 weeks observation period
| Composition | Starter | Grower | Finisher |
|---|---|---|---|
| Metabolized energy (kcal/kg) | 3000 | 3150 | 3200 |
| Crude protein % | 23.0 | 22.0 | 19.0 |
| Soybean meal (45%) | 330.5 | 302.7 | 250.9 |
| Yellow maize (9%) | 57.94 | 57.94 | 57.94 |
| Maize gluten meal (60%) | 70.2 | 70.1 | 65.7 |
| Fat | 32 | 45 | 41 |
| Lysine | 2.9 | 2.7 | 3.5 |
| Methionine | 2.2 | 2.0 | 2.2 |
| Dicalcium phosphate | 18 | 18 | 18 |
| Sodium chloride | 4 | 4 | 4 |
Each gram of the mineral mixture of the diet contained: IU: vit. A 9000, vit. D3 2500, vit. E 17; mg, vit. K3 2.5, vit. B1 1.7, vit. B2 6.6, vit. B6 2.4, vit. B12 0.015; mg choline chloride 400, Mn 80, Fe 40, Zn 70, Cu 8, Se 0.3
Measured parameters
Clinical observations
All groups were kept under complete observation for 5 weeks recording clinical signs, mortalities, and post-mortem lesions after E. coli challenge.
Growth performance
On arrival, random chicks were collected and the initial body weight was measured. The body weights, feed intake, and feed conversion ratio (FCR) were weekly assessed in all groups through the 5-week experimental period (Sainsbury 1984).
Immune response
Ten blood samples were collected from each group at 1-day-old and weekly until 5 weeks of age. The blood was kept in refrigerator for several hours and then centrifuged for serum separation. The antibody titers against NDv and HPAIv (H5N1) vaccines were estimated using haemagglutination inhibition (HI) test with 4 haemagglutinating units (HAU) (OIE 2002). Briefly, in each well of HI plates, 4 HAU of virus/antigen were added, kept for 30 min, and then 0.050 mL of 0.5% chicken red blood cells was added for 30 min. Serum samples including positive and negative controls were distributed in wells. Inhibition at a dilution of 1/16 (4 log2 when expressed as the reciprocal) or more against 4 HAU was considered as positive HI. Moreover, the antibody titers against IBDv were measured at the 2nd, 3rd, 4th, and 5th week of age using enzyme-linked immunosorbent assay (ELISA) test (Snyder et al. 1984).
Bursa/body weight ratio
On weekly basis, 5 randomly selected chickens from each group were sacrificed, weighed, and the bursae of Fabricius were incised and weighed to determine bursa/body weight ratio.
Total coliform count
Five birds from each group were sacrificed at 5 weeks old. One hundred grams of the intestinal contents from different parts of the intestine (duodenum, ileum, and caecum) were homogenized with 0.5 mL of sterile buffered peptone, and tenfold serial dilutions for each homogenate from the initial dilution (10−1) were made. Approximately 0.1 mL of each dilution was incubated on a MacConkey agar plate at 37 °C for 24 h. The results of the count were expressed as the number of the organism log10 CFU/g of the intestinal content (Huang et al. 2019).
Histopathological examination
At 5-week-old, 5 birds from each group were sacrificed. Specimens from liver and intestine were collected, fixed in 10% neutral buffered formalin, dehydrated in different grades of ethyl alcohol, and embedded in paraffin wax. Microtetomy of the tissue to 5 µm thickness was done and stained with hematoxylin and eosin stain for histopathological examination by the light microscope (Bancroft and Gamble 2007).
Statistical analysis
The results were statistically analyzed according to Snedecor and Corchran (1980). One-way ANOVA was adopted using SAS® software general liner models procedure. Significant differences between treatment means were considered significant at level P ≤ 0.05.
Results
Clinical observations
Signs of depression, off food, ruffling, congestion of mucous membrane of the buccal cavity, conjunctiva, and comb and wattle, increasing the consumption of water, and greenish diarrhea were observed 2 days post E. coli challenge. The signs picture was absent in the treated groups as compared to the non-treated group. The effects of different postbiotic treatments on the mortality and protection rates of E. coli-challenged chickens are presented in Table 2. Chicken’s mortality began 3 days post-challenge and continued for 4 days in non-treated birds, while it subsided in the treated chickens 2 days post-challenge. Combined feed and water treatments with the postbiotic compound showed the highest protection rate of 93%, whereas the non-treated challenged group revealed protection rate of 73%. The protection rates of postbiotic treatment in feed and water were 90% and 88%, respectively. The post-mortem lesions of E. coli-challenged chickens were septicemia, hemorrhages on the internal organs, liver congestion and necrosis, and enteritis. However, this lesions picture was not seen in postbiotic-treated chickens.
Table 2.
Effect of postbiotic treatments on clinical status in different groups
| Group | Treatment | E. coli | Mortality % | Protection % |
|---|---|---|---|---|
| 1 | Feed | + | 10 | 90 |
| 2 | Water | + | 12 | 88 |
| 3 | Feed and water | + | 7 | 93 |
| 4 | - | + | 27 | 73 |
Number of birds/group (n = 100)
Mortality % = number of dead birds/total number of birds (n = 100)
Protection % = number of survived birds/total number of birds (n = 100)
Growth performance
The results of the growth performance parameters of different groups are shown in Table 3. Application of postbiotic compound in feed and water significantly (P ≤ 0.05) improved the performance parameters of broilers. The highest significant average body weights were recorded in chickens treated with combined postbiotic treatments in feed and water (1360.7 g), followed by feed (1245.9 g) and water (1196.5 g) treatments when compared with non-treated chickens (987.8 g). The FCR (1.6) was recorded in all the postbiotic-treated groups which was better than (2.2) of the non-treated group.
Table 3.
Effect of postbiotic treatments on performance parameters in different groups
| Group | Treatment | E. coli | Average body weight/g (mean ± SD)/age/week | FCR | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||||
| 1 | Feed | + | 140.1 ± 7.6a | 290.3 ± 6.9b | 578.9 ± 22.6b | 890.6 ± 65.3a | 1245.9 ± 65.0a | 1.61b |
| 2 | Water | + | 138.9 ± 3.2a | 270.9 ± 8.1ab | 556.1 ± 25.4ab | 863.9 ± 54.7b | 1196.5 ± 46.8b | 1.63b |
| 3 | Feed and water | + | 142.5 ± 5.4a | 320.6 ± 5.7a | 607.5 ± 27.1a | 910.9 ± 49.6a | 1360.7 ± 40.2a | 1.61b |
| 4 | - | + | 135.1 ± 3.1a | 299.9 ± 7.9b | 530.6 ± 19.8ab | 815.7 ± 54.8b | 987.8 ± 70.1c | 2.2 a |
Means with different letters (a, b, c) within the same column are significantly different at P ≤ 0.05
FCR, feed conversion ratio
Immune response
The results of immune response against NDv vaccine are showed in Table 4. The HI test results revealed that the geometric mean maternal antibody titer of day-old-chicks was 6.8. The HI titers against NDv vaccine were significantly (P ≤ 0.05) the highest in chickens treated with mixed feed and water postbiotic treatment along 5 weeks experimental period when compared single water or feed postbiotic treatment as well as the non-treated group. Moreover, the lowest significant (P ≤ 0.05) means of HI titers were recorded in the non-treated group in comparison with the treated groups.
Table 4.
Effect of postbiotic treatments on haemagglutination inhibition titers to Newcastle disease virus vaccine in different groups
| Group | Treatment | E. coli | HI titers (mean ± SD) (log2)/age/week | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |||
| 1 | Feed | + | 7.71 ± 0.2a | 7.56 ± 0.2a | 7.30 ± 0.1ab | 7.06 ± 0.1ab | 6.75 ± 0.1ab |
| 2 | Water | + | 7.37 ± 0.1b | 7.20 ± 0.1ab | 7.07 ± 0.2ab | 6.79 ± 0.3b | 6.28 ± 0.1b |
| 3 | Feed and water | + | 7.97 ± 0.2a | 7.80 ± 0.1a | 7.76 ± 0.1a | 7.21 ± 0.1a | 6.96 ± 0.2a |
| 4 | - | + | 6.88 ± 0.1b | 6.98 ± 0.3b | 6.79 ± 0.1b | 6.22 ± 0.2b | 5.89 ± 0.1b |
Means with different letters (a, b, c) within the same column are significantly different at P ≤ 0.05
Haemagglutination inhibition: (HI)
Regarding the humoral immunity against HPAIv (H5N1) vaccine, the results are presented in Table 5. The humoral immunity against HPAIv (H5N1) vaccine showed significant (P ≤ 0.05) difference between postbiotic-treated and non-treated chickens. However, combined feed and water treatment of postbiotic gave the best immune response along the whole experiment.
Table 5.
Effect of postbiotic treatments on haemagglutination inhibition titers to HPAI (H5N1) in different groups
| Group | Treatment | E. coli | HI titers (mean ± SD) (log2)/age/week | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |||
| 1 | Feed | + | 8.21 ± 0.57 ab | 8.65 ± 0.20ab | 9.66 ± 0.33b | 8.78 ± 0.16ab | 8.95 ± 0.05ab |
| 2 | Water | + | 8.53 ± 0.41ab | 8.94 ± 0.25ab | 8.33 ± 0.71ab | 9.65 ± 0.30b | 9.90 ± 0.52a |
| 3 | Feed and water | + | 8.61 ± 0.24ab | 8.97 ± 0.39ab | 9.79 ± 0.74b | 10.35 ± 0.16a | 10.12 ± 0.34a |
| 4 | - | + | 8.03 ± 0.23bc | 7.81 ± 0.54bc | 7.18 ± 0.30c | 6.89 ± 0.11c | 7.06 ± 0.41c |
Means with different letters (a, b, c) within the same column are significantly different at P ≤ 0.05
Haemagglutination inhibition: (HI)
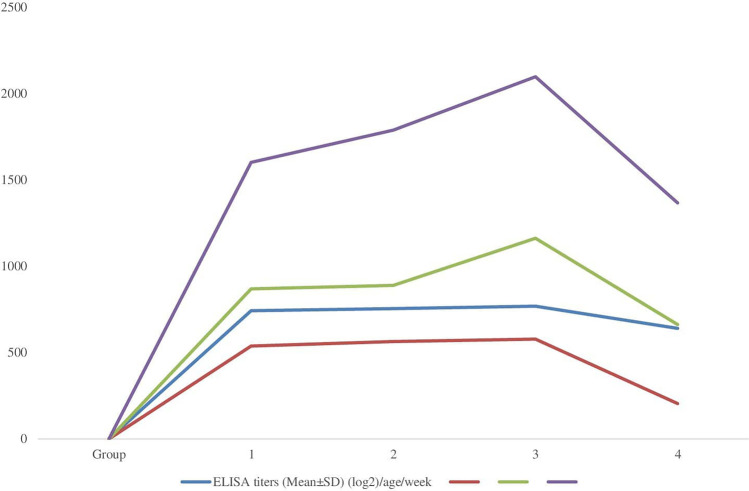
The humoral immune response to IBDv was measured from the 2nd till the 5th week of life and the mean ELISA titers showed significant (P ≤ 0.05) increase in all the postbiotic-treated groups when compared with the challenged non-treated group (Fig. 1). However, chickens given a postbiotic in feed and water showed the best means from the 2nd till the 5th week of age.
Fig. 1.
Effect of postbiotic treatments on the humoral immune response to IBDv using ELISA test in different groups
Bursa/body weight ratio
The measured mean bursae of Fabricius/body weight ratio in Table 6 indicated that the highest significant (P ≤ 0.05) ratios were in the postbiotic-treated groups when compared with the non-treated group. At the end of the study, the mean bursae/body weight ratios were 0.291, 0.288, and 0.272 in combined feed and water, feed, and water in the postbiotic-treated groups, respectively; however, this ratio was 0.203 in the non-treated group.
Table 6.
Effect of postbiotic treatments on bursa of Fabricius/body weight ratio in different groups
| Group | Treatment | E. coli | Bursa/body weight ratio (g) (mean ± SD)/age/week | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |||
| 1 | Feed | + | 0.086 ± 0.02ab | 0.192 ± 0.05a | 0.199 ± 0.01ab | 0.226 ± 0.07ab | 0.288 ± 0.03a |
| 2 | Water | + | 0.080 ± 0.06ab | 0.156 ± 0.01ab | 0.194 ± 0.07ab | 0.220 ± 0.08ab | 0.272 ± 0.07ab |
| 3 | Feed and water | + | 0.099 ± 0.05a | 0.194 ± 0.08a | 0.228 ± 0.02a | 0.253 ± 0.06a | 0.291 ± 0.09a |
| 4 | - | + | 0.069 ± 0.09b | 0.135 ± 0.07b | 0.151 ± 0.03b | 0.193 ± 0.01b | 0.203 ± 0.05b |
Means with different letters (a, b, c) within the same column are significantly different at P ≤ 0.05
Bursa/body weight ratio = [Bursa weight (g) / body weight (g)] × 100
Total bacterial count
In comparison with non-treated challenged chickens, postbiotic-treated chickens showed significant (P ≤ 0.05) reduction in the total intestinal coliform count at the end of observation period (Table 7). The intestinal coliform counts were 3.98 × 105, 4.72 × 105, and 4.10 × 105 in combined feed and water treatment, water treatment, and feed treatment, respectively. Meanwhile, the total coliform count in non-treated chickens was 8.06 × 106.
Table 7.
Effect of postbiotic treatments on intestinal coliform count in different groups
| Group | Treatment | E. coli | Intestinal coliform count log10 (CFU/g) |
|---|---|---|---|
| 1 | Feed | + | 4.10 × 105 ± 0.01a |
| 2 | Water | + | 4.72 × 105 ± 0.03a |
| 3 | Feed and water | + | 3.98 × 105 ± 0.02a |
| 4 | - | + | 8.06 × 106 ± 0.03b |
Means with different letters (a, b, c) within the same column are significantly different at P ≤ 0.05
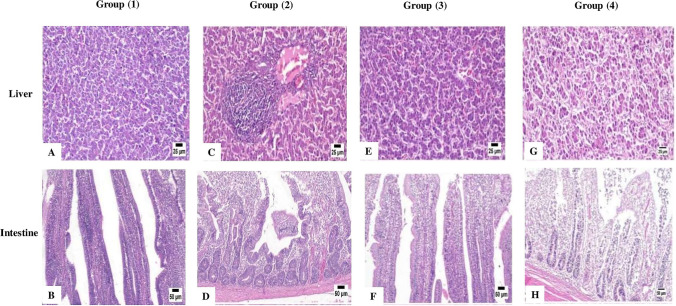
Histopathological examination
Figure 2 shows the results of the histopathological examination of liver and intestine in different groups. In group treated with the postbiotic in feed, the liver showed apparently normal hepatic parenchyma (A), while the intestine showed mild inflammation (B). Postbiotic treatment in water revealed focal portal hepatitis (C) and moderate enteritis (D). However, in a group that received combined feed and water treatments, apparently normal hepatic parenchyma (E) and mild enteritis (F) were observed. The E. coli-challenged group with E. coli that received no treatment exhibited disorganization of the hepatic plates admixed with numerous locations of scattered sporadic cell necrosis (G) and severe necrosis of the intestinal villi with accumulation of inflammatory cells and tissue debris in the submucosa (H).
Fig. 2.
The results of the histopathological examination of liver and intestine in different groups
Discussion
The devastating effects of avian colibacillosis are particularly prevalent in the poultry field, and poor biosecurity and husbandry practices, especially in developing countries, create a difficult paradigm for improvement without some easily applied intervention strategies (Ebrahimi-Nik et al. 2018). The disease induces significant economic annual losses in the global poultry industry (Roth et al. 2019). The resulting emergence and rapid dissemination of antibiotic-resistant E. coli, believed to be a result of low levels of antibiotics in feed, have resulted in a reduced efficacy of specific antibiotic classes and thus might pose a substantial risk in humans’ health (Belanger et al. 2011). Accordingly, it was necessary to find viable alternative resources to protect production levels and still maintain the health of the birds (Seal et al. 2013). One of these substitutes is “biotic feed additives” such as probiotics, prebiotics, and postbiotics (Klemashevich et al. 2014). The compound used in this study (Culbac®) is produced by fermentation process of proprietary strain of Lactobacilli and then a unique, proprietary method for protecting surface proteins and stabilization method for rendering the bacteria non-viable or dead. The non-viable bacterial fermentation product (postbiotic) is exposed to extensive processing to modify the cell contents and allow exposure of the cell wall to the digestive bacteria.
There was a notable decrease in the severity of the E. coli clinical observations after feed and water postbiotic treatments. Similarly, previous studies indicated postbiotic metabolites of specific strains of Pediococcus acidilactici, Enterococcus faecium, L. reuteri, and L. acidophilus reduced mortality and lesion scores of C. perfringens-challenged broiler chickens as compared to challenged non-treated chickens (Johnson et al. 2019). Moreover, the postbiotic from specific L. plantarum strains showed a trend of lower mortality in broiler chickens as compared with other treatments such as antibiotic growth promotor and ascorbic acid (Humam et al. 2019). These studies, in conjunction with the current study, indicate options for productive antibiotic-free production of poultry globally.
In this study, postbiotic treatment of broiler chickens improved the weight gain and the FCR while minimizing effects from exposure to pathogenic E. coli (078). It has been documented that postbiotics can be used as feed additives to promote the health and growth performance in broilers (Kareem et al. 2017) and layers (Choe et al. 2012; Loh et al. 2014). Thanh et al. (2009) reported that chickens fed combinations of metabolites produced by L. plantarum had higher final body weight and weight gain compared with those fed the negative control diet. Heat-stressed broiler chickens fed on a diet containing postbiotics of L. plantarum showed elevated weight gain and superior feed conversion efficiency as a result of increasing hepatic insulin-like growth factor 1 mRNA expression level (Humam et al. 2019). In addition, a combined mixture of postbiotic and prebiotic in the diet of broiler chickens enhanced the total body weight and the feed efficiency in conjunction with increasing liver insulin like growth factor 1 and growth hormone receptor mRNA expressions (Kareem et al. 2016). Improvement in weight gain was observed after treatment of C. perfringens-challenged broiler chickens with a postbiotic when compared with non-treated control (Johnson et al. 2019). However, Rosyidah et al. (2011) found no significant difference in body weight or weight gain of chickens fed on metabolites and combination of metabolite and acidifier and those fed positive and negative control diets. It is likely to mention that Hubbard broilers that used in this study have an excellent FCR and robustness which may support the action of the tested postbiotic.
These findings may be a result of the ability of postbiotics to reduce the number of pathogenic intestinal microorganisms, leading to better gut health and growth performance. Postbiotics may be also similar to probiotics in regard to the enhancement of nutrient transporter gene expression (Na + -dependent glucose, galactose transporter, and long-chain acyl CoA dehydrogenase genes) which promote broiler growth performance (Jahromi et al. 2016). Additionally, Lactobacilli species may increase the utilization of nutrients due to upregulation of nutrient gene expression resulting in improvement of the bodyweight of broilers (Kalavathy et al. 2003). Organic acids and bacteriocins are antimicrobial metabolites of postbiotics that may decrease the pH and prevent the proliferation of pathogens in the gut of animals (Aguilar-Toalá et al. 2018). Moreover, supplementation with postbiotics could enhance growth performance and health via improvement of physiological parameters including immune status, gut health, intestinal villus, increasing lactic acid bacteria, and decreasing Enterobacteriaceae and intestinal pH (Loh et al. 2010; Kareem et al. 2016).
The immune response against NDv, HPAIv, and IBDv vaccines was modulated after postbiotic treatments in the present study. Moreover, improved bursa of Fabricius/body weight ratios were observed in postbiotic-treated chickens as compared with non-treated chickens. It is known that E. coli infection is an immunosuppressive pathogen of poultry (McGruder and Moore 1998) as this pathogen can damage the immune system of chickens in terms of lymphocyte depletion in both bursa and thymus tissues (Nakamura et al. 1990). Hegazy et al. (2010) found that infection of chickens with E. coli prior to vaccination with IBDv vaccine resulted in more decrease in ELISA antibody titers in comparison with vaccinated non-infected chickens. The current results agree with those of Johnson et al. (2019) who found that postbiotic metabolites mixture of Pediococcus acidilactici, L. reuteri, Enterococcus faecium, and L. acidophilus was able to stimulate the immune response in C. perfringens infected broiler chickens. Moreover, the levels of immunoglobulin M (IgM) and IgG were significantly higher in broilers that received postbiotics in feed than those that received ascorbic acid. Stimulation of immune response in birds after postbiotic treatment might be related to the presence of peptidoglycan (β-glucan) in 90% of the dry weight of Lactobacilli with lipopolysaccharides and teichoic and lipoteichoic acids in the bacterial cell wall (Adams 2010). In addition, after Lactobacilli lysis or degradation by host gastric acid, the bacterial DNA (CpG motifs) released and recognized by the host as a foreign antigen has been shown to stimulate both the cell-mediated and humoral immune responses (Kant et al. 2014).
The current results showed that the total intestinal coliform count was reduced in the postbiotic-treated groups while it was high in the non-treated group. The bacteriostatic and bactericidal activities of postbiotics have been reported. During fermentation of Lactobacilli bacteria, metabolites are produced, released, and stimulated the growth of beneficial microorganisms (Caldwell 2016). Some of beneficial bacteria are fastidious and require a lot of nutrients such as amino acids, energy (sugars), and metabolites (vitamin B complex) provided by the host. Accordingly, postbiotics can provide these bacteria by nutrients such as inulin or cellulose that present in their cell walls. In addition, postbiotics can reduce the multiplication of harmful bacterial in the gut. Parallel results were found in pigs which showed a reduction in E. coli count after treatment with cell free extracts of Lactobacilli fermentation process (Pollman et al. 1982; Blomberg et al. 1993; Thu et al. 2011). A postbiotic metabolite of L. plantarum either alone or in combination with a prebiotic showed inhibitory effects of some pathogens such as E. coli, Salmonella typhimurium, vancomycin-resistant enterococci, and Listeria monocytogens (Thanh et al. 2009; Van Thu et al. 2011; Choe et al. 2013; Kareem et al. 2014). A significant increase in lactic acid bacteria and decrease in Enterobacteriaceae count were observed in broiler chickens fed on L. plantarum (Rosyidah et al. 2011) or a mixture of postbiotic and inulin (Kareem et al. 2016). Furthermore, the postbiotic produced from a mixture of L. plantarum strains significantly increased the total bacteria and Lactobacilli counts but decreased Salmonella, E. coli, and Enterobacteriaceae counts in broilers when compared to the control groups (Humam et al. 2019). Studies in humans showed that lipoteichoic acids of L. acidophilus and L. johnsonni supported human gut homeostasis and treated diseases caused by Gram-negative bacteria (Vidal et al. 2002). Also, L. paracasei cells supernatant protected tissues from invasive Salmonella (Tsilingiri et al. 2012).
The histopathologic examinations in this study revealed that the E. coli-challenged group exhibited disorganization and necrosis of the hepatic cells plus severe necrosis of the intestinal villi with inflammatory cells. The current observation is in tandem with that of Abalaka et al. (2017). In contrast, chickens treated with combined postbiotic treatment in feed and water or in feed alone showed apparently normal hepatic parenchyma and mild intestinal inflammation. Previous studies have shown that improvement of intestinal villi height was observed in broiler chickens treated with metabolite combinations in feed (Thanh et al. 2009; Loh et al. 2010). Moreover, addition of postbiotics and inulin combinations to the diet of broiler chickens beneficially altered the intestinal mucosal architecture in terms of longer villi (Kareem et al. 2016). Recently, Humam et al. (2019) demonstrated that a mixture of L. plantarum metabolites significantly increased the intestinal villus height to crypt depth ratio. Positive modification of liver architecture after postbiotic treatment may be attributed to the ability of Lactobacillus species to alleviate the severe damage of the liver by increasing the antioxidant enzyme activities in the blood (Chen et al. 2022). These findings provide new hope for growing healthy, productive animals while minimizing use of traditional antibiotics in production operations.
The reviewed postbiotic compound utilized in this study has not been previously tested in such a virulent challenge model, but neither have probiotics, phytobiotics, nor synbiotics been compared in a challenge model with E. coli that is demonstrated to produce colisepticaemia in broilers. Defining new opportunities for growth support in poultry while not inducing additional potential pressures for antibiotic-driven antibacterial-resistance mechanism proliferation, nor probiotic proliferation of resistance mechanisms, would be a great asset for poultry production globally. This study demonstrates an early inroad to the use of postbiotics as a valuable tool for the poultry industry (Fancher et al. 2020).
In conclusion, this study showed that the used postbiotic (Culbac®) either in a dry or an aqueous form was effective in amelioration of colisepticaemia in broiler chickens. Moreover, the synergistic beneficial action of both treatment regimens when supplemented together was evident. Combined feed and water treatment with the postbiotic was even better than a separate treatment. Under the present experimental conditions, reduction of the clinical disease picture, improvement of performance parameters, modulation of the humoral immune response to NDv, HPAIv, and IBDv vaccines, and decreasing in intestinal coliform count were proven after postbiotic treatment of E. coli-challenged chickens. This may indicate the growth promoting, immunomodulatory, and protective activities against pathogenic bacteria with the tested natural compound (Culbac®) which may be used in the near future for opposing this serious devastating infections in poultry. With the current climate of “no antibiotics ever,” the poultry industry is looking for options, and this work provides a new option for producers.
Acknowledgements
The authors acknowledge Promovet Egypt Trade, Egypt and Transagra International Inc., USA for the funding acquisition.
Author contribution
The idea for the paper was conceived by WAA and FHE. The experiments were designed by WAA and FHE. The experiments were performed by WAA. The data were analyzed by WAA. The paper was written by WAA. The paper was revised by FHE. All authors read and approved the manuscript.
Funding
This study is partially funded by the Promovet Egypt Trade, Egypt and Transagra International Inc., USA for the funding acquisition. Payment or honoraria for lectures, oral presentations, poster presentations, manuscript writing, and educational events as well as support for author’s time and efforts has been provided to support dissemination of study results.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
This study was conducted according to the ethical standards applied to animal research and approved by Institutional Animal Welfare and Institutional Animal Ethical Committee.
Consent for publication
All authors hereby declare that they participated in the development of the manuscript titled (Assessment of the capacity of stabilized non-viable lactobacillus to counteract colisepticaemia in broiler chickens). They have read the final version and give the consent for the article to be published in Tropical Animal Health and Production.
Conflict of interest
The author, Wafaa A. Abd El-Ghany, had no conflict of interest during experimental design, planning, execution, nor data collection for this experimental protocol. This study was not initially financially supported by TransAgra International Inc. Company provided a product for study prior to initiation of study. Authors Quesnell and Sakai are employed by TransAgra International Inc., and Hosny is employed by Promovet Egypt Trade in Egypt. Product was provided for use in the study with directions for common usage rates in poultry.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abalaka S, Sani N, Idoko I, Tenuche O, Oyelowo F, Ejeh S, Enem S. Pathological changes associated with an outbreak of colibacillosis in a commercial broiler flock. Sokoto Journal of Veterinary Sciences. 2017;15:95–102. doi: 10.4314/sokjvs.v15i3.14. [DOI] [Google Scholar]
- Abd El-Ghany WA. Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Veterinary Journal. 2020;10:323–330. doi: 10.4314/ovj.v10i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CA. The probiotic paradox: Live and dead cells are biological response modifiers. Nutrition Research Reviews. 2010;23:37–46. doi: 10.1017/S0954422410000090. [DOI] [PubMed] [Google Scholar]
- Aguilar-Toalá J, Garcia-Varela R, Garcia H, Mata-Haro V, González-Córdova A, Vallejo-Cordoba B, Hernández-Mendoza A. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- Ali, A.I., Abd El-Mawgoud, A.M., Dahshan, A.A., El-Sawah, A.A. and Nasef, S., 2019. Escherichia coli in broiler chickens in Egypt, its virulence traits and vaccination as an intervention strategy. Novel Research in Microbiology Journal 3, 415–427. 10.21608/NRMJ.2019.44950
- Bancroft JD, Gamble M. Theory and practice of histopathological techniques. 5. London, UK: Churchill Livingstone; 2007. pp. 125–138. [Google Scholar]
- Belanger L, Garenaux A, Harel J, Boulianne M, Nadeau E, Dozois CM. Escherichia coli from animal reservoirs as a potential source of human extra intestinal pathogenic E. coli. FEMS Immunology & Medical Microbiology. 2011;62:1–10. doi: 10.1111/j.1574-695X.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- Blomberg L, Henriksson A, Conway PL. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Applied and Environmental Microbiology. 1993;59:34–39. doi: 10.1128/aem.59.1.34-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JM. Abiotic, their fermentates have advantages for host. Feedstuffs. 2016;88:1–3. [Google Scholar]
- Chen X, Ishfaq M, Wang J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune responses and Salmonella Pullorum infection resistance of broilers challenged with Aflatoxin B1. Poultry Science, 101, 101651. 10.1016/j.psj.2021.101651 [DOI] [PMC free article] [PubMed]
- Choe DW, Loh TC, Foo HL, Hair-Bejo M, Awis QS. Egg production, faecal pH and microbial population, small intestine morphology, and plasma and yolk cholesterol in laying hens given liquid metabolites produced by Lactobacillus plantarum strains. British Poultry Science. 2012;53:106–115. doi: 10.1080/00071668.2012.659653. [DOI] [PubMed] [Google Scholar]
- Choe, D.W., Foo, H.L., Loh, T.C., Hair-Bejo, M. and Awis, Q.S., 2013. Inhibitory property of metabolite combinations produced from Lactobacillus plantarum strains. Pertanika Journal of Tropical Agricultural Science, 36, 79–88. http://psasir.upm.edu.my/id/eprint/21388
- Cicenia, A., Santangelo, F., Gambardella, L., Pallotta, L., Iebba, V., Scirocco, A., Marignani, M., Tellan, G., Carabotti, M., Corazziari, E.S., Schippa, S. and Severi, C., 2016. Protective role of postbiotic mediators secreted by Lactobacillus Rhamnosus GG versus lipopolysaccharide-induced damage in human colonic smooth muscle cells. Journal of Clinical Gastroenterology, 50, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015: S140– S144. 10.1097/mcg.0000000000000681 [DOI] [PubMed]
- Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C, Kiely B, Quigley EM, O’Sullivan GC, Shanahan F, Collins JK. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. International Journal of General and Molecular Microbiology. 1999;76:279–292. [PubMed] [Google Scholar]
- Ebrahimi-Nik, H., Bassami, M.R., Mohri, M., Rad, M. and Khan, M.I., 2018. Bacterial ghost of avian pathogenic E. coli (APEC) serotype O78:K80 as a homologous vaccine against avian colibacillosis. PLoS One, 13, e0194888. 10.1371/journal.pone.0194888 [DOI] [PMC free article] [PubMed]
- Fancher CA, Zhang L, Kiess AS, Adhikari PA, Dinh TTN, Sukumaran AT. Avian pathogenic Escherichia coli and Clostridium perfringens: Challenges in no antibiotics ever broiler production and potential solutions. Microorganisms. 2020;8:1533. doi: 10.3390/microorganisms8101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Lara C, Loste A, Marca MC. Efficacy of calcium fosfomycin for the treatment of experimental infection of broiler chickens with Escherichia coli O78:K80. Veterinary Research Communications. 2002;26:427–436. doi: 10.1023/A:1020582207129. [DOI] [PubMed] [Google Scholar]
- Hegazy M, Abd-El Samie LK, El Sayed EM. The immunosuppresive effect of E. coli in chickens vaccinated with Infectious Bronchitis (IB) or Infectious Bursal Disease (IBD) Journal of the American Science. 2010;6:762–767. [Google Scholar]
- Huang L, Luo L, Zhang Y, Wang Z, Xia Z. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics and Antimicrobial Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humam AM, Loh TC, Foo HL, Samsudin AA, Mustapha NM, Zulkifli I, Izuddin WI. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals (basel) 2019;9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi MF, Altaher YW, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, Tufarelli V, Liang JB. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. International Journal of Biometeorology. 2016;60:1099–1110. doi: 10.1007/s00484-015-1103-x. [DOI] [PubMed] [Google Scholar]
- Johnson, C.N., Kogut, M.H., Genovese, K., He, H., Kazemi, S. and Arsenault, R.J., 2019. Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms, 7, 268. 10.3390/2Fmicroorganisms7080268 [DOI] [PMC free article] [PubMed]
- Kalavathy R, Abdullah N, Jalaludin S, Ho YW. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. British Poultry Science. 2003;44:139–144. doi: 10.1080/0007166031000085445. [DOI] [PubMed] [Google Scholar]
- Kant R, deVos WM, Palva A, Satokari R. Imuunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. Journal of Medical Microbiology. 2014;63:293–308. doi: 10.1099/jmm.0.064220-0. [DOI] [PubMed] [Google Scholar]
- Kareem KY, Ling FH, Chwen LT, Foong OM, Asmara SA. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathogen. 2014;6:23. doi: 10.1186/1757-4749-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem KY, Loh TC, Foo HL, Akit H, Samsudin AA. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Veterinary Research. 2016;12:163. doi: 10.1186/s12917-016-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem KY, Loh TC, Foo HL, Asmara SA, Akit H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poultry Science. 2017;96:966–975. doi: 10.3382/ps/pew362. [DOI] [PubMed] [Google Scholar]
- Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Current Opinion in Biotechnology. 2014;26:85–90. doi: 10.1016/j.copbio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Koutsianos, D., Athanasiou, L., Mossialos, D. and Koutoulis, K., 2021. Colibacillosis in poultry: A disease overview and the new perspectives for its control and prevention. Journal of the Hellenic Veterinary Medical Society, 71, 2425–2436. 10.12681/jhvms.25915
- Loh TC, Thanh NT, Foo HL, Hair-Bejo M, Azhar BK. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Animal Science Journal. 2010;81:205–214. doi: 10.1111/j.1740-0929.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Loh TC, Choe DW, Foo HL, Sazili AQ, Bejo MH. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Veterinary Research. 2014;10:149. doi: 10.1186/1746-6148-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutful Kabir, S.M., 2010. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. International Journal of Environmental Research and Public Health, 7, 89–114. 10.3390/2Fijerph7010089 [DOI] [PMC free article] [PubMed]
- Ma Z, Lee S, Jeong KC. Mitigating antibiotic resistance at the livestock-environment interface: A review. Journal of Microbiology and Biotechnology. 2019;29:1683–1692. doi: 10.4014/jmb.1909.09030. [DOI] [PubMed] [Google Scholar]
- Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules. 2018;23:795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGruder ED, Moore GM. Use of lipopolysaccharide (LPS) as a positive control for the evaluation of immunopotentiating drug candidates in experimental avian colibacillosis models. Research in Veterinary Science. 1998;66:33–37. doi: 10.1053/rvsc.1998.0237. [DOI] [PubMed] [Google Scholar]
- Mohamed HMA, Younis W. Trials on the role of prebiotics and probiotics in colonization and immune response of broiler chickens challenged with Escherichia coli K88. Alexandria Journal of Veterinary Sciences. 2018;58:48–56. doi: 10.5455/ajvs.297887. [DOI] [Google Scholar]
- Nakamura K, Yuasa N, Abe H, Narita M. Effect of Infectious bursal disease virus on infections produced by Escherichia coli of high and low virulence in chickens. Avian Pathology. 1990;19:713–721. doi: 10.1080/03079459008418726. [DOI] [PubMed] [Google Scholar]
- National Research Council, (NRC) 1994. Nutrient Requirement of Poultry. 9th revised edition. National Academic Press, Washington, DC, USA.
- OIE. 2002. Office International Des Epizooties. Manual of Standards for Diagnostic Tests and Vaccines. 4th Eds., Paris, France.
- Pollman, D.S., Kennedy, G.A., Koch, B.A. and Allee, G.L., 1982. Influence of nonviable Lactobacillus fermentation product in artificially reared pigs challenged with E. coli. Conference Paper, Swine Day, Manhattan, Kan., November, 11, 1982. Kansas State University, pp. 86–91.
- Radwan, I.A., Abd El-Halim, M.W. and Abed, A.H., 2021. Molecular characterization of antimicrobial resistant Escherichia coli isolated from broiler chickens. Journal of Veterinary Medical Research, 27, 128–142. 10.21608/JVMR.2020.31870.1009
- Rosyidah M, Loh T, Foo H, Cheng X, Bejo M. Effect of feeding metabolites and acidifier on growth performance, faecal characteristics and microflora in broiler chickens. Journal of Animal and Veterinary Advances. 2011;10:2758–2764. doi: 10.3923/javaa.2011.2758.2764. [DOI] [Google Scholar]
- Roth, N., Käsbohrer, A., Mayrhofer, S., Zitz, U., Hofacre, C. and Domig, K.J., 2019. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poultry Science, 98, 1791–1804. 10.3382/2Fps/2Fpey539 [DOI] [PMC free article] [PubMed]
- Sainsbury, D., 1984. Systems of management. Ch.9 P.102. In Poultry Health and Management. 2nd Ed. By Sainsbury. Granada Publishing LTD. 8 Grafton Street, London WIX3 LA.
- Seal BS, Lillehoj HS, Donovan DM, Gay CG. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim. Health Research Review. 2013;14:78–87. doi: 10.1017/s1466252313000030. [DOI] [PubMed] [Google Scholar]
- Sharma P, Tomar SK, Goswami P, Sangwan V, Singh R. Antibiotic resistance among commercially available probiotics. Food Research International. 2014;57:176–195. doi: 10.1016/j.foodres.2014.01.025. [DOI] [Google Scholar]
- Shazali N, Foo HL, Loh TC, Choe DW, Rahim RA. Prevalence of antibiotic resistance in lactic acid bacteria isolated from the faeces of broiler chicken in Malaysia. Gut Pathogens. 2014;6:1. doi: 10.1186/1757-4749-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. 7. Ames, IA: Iowa State College Press; 1980. pp. 39–63. [Google Scholar]
- Snyder DB, Marquardt WW, Mallinson ET, Savage PK, Allen DC. Rapid serological profiling by enzyme-linked immunosorbent assay. III. Simultaneous measurements of antibody titers to infectious bronchitis, infectious bursal disease, and Newcastle disease viruses in a single serum dilution. Avian Diseases. 1984;28:12–24. doi: 10.2307/1590125. [DOI] [PubMed] [Google Scholar]
- Thanh NT, Loh TC, Foo HL, Hair-Bejo M, Azhar BK. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. British Poultry Science. 2009;50:298–306. doi: 10.1080/00071660902873947. [DOI] [PubMed] [Google Scholar]
- Thu, T.V., Loh, T.C., Foo, H.L., Yaakub, H. and Bejo, M.H., 2011. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Tropical Animal Health and Production, 43, 69–75. 10.1007/2Fs11250-010-9655-6 [DOI] [PMC free article] [PubMed]
- Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, Aindelis G, Tompoulidou E, Lamprianidou EE, Saxami G, Ypsilantis P, Lampri ES, Simopoulos C, Kotsianidis I, Galanis A, Kourkoutas Y, Dimitrellou D, Chlichlia K. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One. 2016;11:e0147960. doi: 10.1371/journal.pone.0147960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, Rescigno M. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut. 2012;61:1007–1015. doi: 10.1136/gutjnl-2011-300971. [DOI] [PubMed] [Google Scholar]
- Valiakos G, Kapna I. Colistin resistant mcr genes prevalence in livestock animals (swine, bovine, poultry) from a multinational Perspective. A Systematic Review. Veterinary Sciences. 2021;8:265. doi: 10.3390/vetsci8110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Thu T, Foo HL, Loh TC, Bejo MH. Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. African Journal of Biotechnology. 2011;10:1359–1363. [Google Scholar]
- Veterinary Feed Directive. 2019. Available online: https://www.federalregister.gov/documents/2015/06/03/2015-13393/veterinary-feed-directive (accessed on 12 June 2019).
- Vidal K, Donnet-Hughes A, Granato D. Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and Gram-negative bacteria. Infection and Immunity. 2002;70:2057–2064. doi: 10.1128/iai.70.4.2057-2064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Not applicable.