Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and affects about 25% of the population globally. Obesity and diabetes are the main causes of the disease characterized by excessive accumulation of lipids in the liver. There is currently no direct pharmacological treatments for NAFLD. Dietary intervention and lifestyle modification are the key strategies in the prevention and treatment of the disease. Soy consumption is associated with many health benefits such as decreased incidence of coronary heart disease, type-2 diabetes, atherosclerosis and obesity. The hypolipidemic functions of soy components have been shown in both animal studies and human clinical trials. Dietary soy proteins and associated isoflavones suppressed the formation and accumulation of lipid droplets in the liver and improved NAFLD-associated metabolic syndrome. The molecular mechanism(s) underlying the effects of soy components are mainly through modulation of transcription factors, sterol regulatory element-binding protein-1 and peroxisome proliferator-activated receptor-γ2, and expressions of their target genes involved in lipogenesis and lipolysis as well as lipid droplet-promoting protein, fat-specific protein-27. Inclusion of appropriate amounts of soy protein and isoflavones in the diets might be a useful approach to decrease the prevalence of NAFLD and mitigate disease burden.
Keywords: Soy, Isoflavones, Hypolipidemic, Non-alcoholic fatty liver disease, Human and animal studies
Introduction
Soy foods have been consumed for centuries in Asian countries. Soy protein is one of the major sources of plant-based protein for human consumption. In addition to its nutritional roles as a rich source of indispensable amino acids, soy protein and its associated isoflavones have been extensively studied for their functional properties in various aspects.
Epidemiological investigations have shown that consumption of soy foods is associated with various health benefits such as lower incidences of coronary heart disease and associated mortality [1], obesity, type-2 diabetes, and atherosclerosis [2–4]. Soy protein and isoflavones have been shown to play major roles in modulation of lipid and glucose metabolism in human clinical trials [5, 6], animal studies [7, 8] and cultured cells [9]. Inclusion of soy protein or soy isoflavones in the diet improved hepatic and blood lipid profiles by reducing triglycerides, total and low density lipoprotein (LDL) cholesterol levels and increasing the ratio of high density lipoprotein (HDL)/LDL cholesterol in both human [6,10] and animal studies [2,11,12]. As a result of these benefits, health claims associated with hypocholesterolemic effects of soy protein have been approved in a dozen countries [13].
Increasing evidence has shown that soy intake had beneficial effects in patients with non-alcoholic fatty liver disease (NAFLD) [14–18], and reduced the formation and accumulation of hepatic lipid droplets and ameliorated liver steatosis in animal models of NAFLD [19–22]. Dietary soy improved glucose and lipid metabolism via modulation of insulin secretion and sensitivity in diabetic animal models with NAFLD [7, 8, 23]. NAFLD prevalence is rapidly increasing worldwide, especially in developed countries. Direct pharmacological treatments for NAFLD are not available. Dietary intervention and lifestyle changes are the major strategies for prevention and treatment of NAFLD. This review focuses on the effects of soy proteins and associated isoflavones on the metabolic syndrome of NAFLD in both human and animal studies and current understanding of the molecular event(s) involved in the hypolipidemic actions and NAFLD prevention of soy components.
NAFLD
NAFLD is the most common chronic liver disease [24]. The typical features of NAFLD are accumulation of high levels of lipids in hepatocytes, usually greater than 5% of liver weight [25], and formation of excessive hepatic lipid droplets [26]. With the progression of NAFLD, histological changes in the liver can range from hepatic steatosis to steatohepatitis, fibrosis, cirrhosis and even hepatocellular carcinoma. Obesity and diabetes are the main causes of the diseases. NAFLD is associated with metabolic syndrome and changes in biomarkers such as increased insulin resistance, hypertension, hyperlipidemia, elevated oxidative stress and increased plasma fibrinogen, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels [16].
The global prevalence of NAFLD is approximately 25% [24,27], and affecting about one-third of the population in Western countries [28]. Around 80% of the obese population and 50% of individuals with diabetes have fatty livers [29]. It is predicted that the incidences of NAFLD, non-alcoholic steatohepatitis (NASH), decompensated cirrhosis, hepatocellular carcinoma and liver deaths in different countries or regions will rise by up to 48, 96, 273, 199 and 295%, respectively, by 2030 (Table 1) [30–35]. Thus, strategies to slow the increase in NAFLD prevalence and therapeutic options are urgently needed to mitigate disease burden [30]. Increasing evidence suggests that dietary intervention and lifestyle changes might play a role in preventing and managing NAFLD [36, 37].
Table 1.
Projected burden of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatictis (NASH) by the year of 2030 in different countries and regions based on modelling studies
| NAFLD | Percentage increase by the year of 2030 (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Country or region | Year | Overall rate (%) | NAFLD | NASH | D. cirrhosis* | HCC* | Liver death | References |
| Australia | 2019 | 22 | 25 | 40 | 85 | 75 | 90 | [32] |
| Canada | 2019 | 21 | 20 | 35 | 97 | 80 | 107 | [33] |
| Hong Kong | 2019 | 22 | 11 | 20 | 66 | 67 | 74 | [34] |
| Saudi Arabia | 2017 | 26 | 48 | 96 | 273 | 199 | 295 | [31] |
| Singapore | 2019 | 26 | 20 | 36 | 108 | 78 | 113 | [34] |
| South Korea | 2019 | 21 | 6 | 21 | 87 | 79 | 92 | [34] |
| Switzerland | 2018 | 24 | 18 | 35 | 52 | 46 | 41 | [35] |
| Taiwan | 2019 | 22 | 8 | 24 | 108 | 84 | 106 | [34] |
| United Arab Emirates | 2017 | 25 | 46 | 87 | 241 | 178 | 27 | [31] |
| United States | 2015 | 34 | 21 | 63 | 168 | 137 | 178 | [30] |
*D. cirrhosis, Decompensated cirrhosis; HCC, hepatocellualr carcinoma
Major Nutrient Composition of Soybean
Soybean (Glycine max) is one of major dietary sources of plant-based protein and soy protein contains all essential amino acids required by human body, which makes soy products almost equivalent to the foods of animal sources in protein quality [38]. For example, the protein digestibility-corrected amino acid scores, a measurement of protein quality, for soy, beef, cow’s milk, and egg are 1, 0.92, 1, and 1, respectively [39–41].
Dry soybean contains 40% protein, 22% fat, 33% carbohydrate including 10.2% dietary fiber, 5% minerals and vitamins [42]. Soy protein is mainly comprised of two storage globulins, 7S β-conglycinin and 11S glycinin [43] (Table 2). β-conglycinin has α’, α, and β subunits, and accounting for ~25% of the total protein, while glycinin has acidic (A) and basic (B) polypeptides to form 5 subunits, A1aB2, A2B1a, A3B4, A1bB1b and A5A4B3, and accounting for ~40% of the total protein. The other minor proteins include 2S, 9S, and 15S storage proteins, lectin, Kunitz and Bowman-Birk protease inhibitors, β-amylases, lipoxygenases, and urease [43]. Depending on processing, the protein content in soy foods/products can reach over 90% as in the soy protein isolate (SPI) that is usually used in soy-based infant formulas.
Table 2.
Nutrient content of dry soybean seeds (per 100 g dry weight)
| Nutrients | Content | |
|---|---|---|
| Protein (g) | 40 | |
| β-conglycinin (7S) (α’, α, β subunits) | ||
| Glycinin (11S) [Acidic (A), Basic (B) polypeptides] | ||
| Other minor proteins | ||
| 2S, 9S, 15S storage proteins | ||
| Lectin, β-amylases, lipoxygenases, urease | ||
| Kunitz and Bowman-Birk protease inhibitors | ||
| Fat (g) | 22 | |
| Carbohydrate (g) | 33 | |
| Dietary fiber | 10.2 | |
| Sugars | 8 | |
| Minerals and vitamins (g) | 5 | |
| Isoflavones (mg/g protein) | 3–5.1 | |
| Genistin:Daidzin:Glycitein ≈ 1:1:0.1 | ||
Isoflavones are one of the most studied bioactive compounds in soybeans, and are closely associated with proteins. Soy foods and soy-based infant formulas are rich sources of isoflavones, and contain about 3–5.1 mg/g protein [44]. Isoflavones are the major soy phytoestrogens, including genistin, daidzin and glycitein. Both genistin and daidzin are conjugated to sugars and present as glycosides in soybeans and most of the soy foods in Western diets. Glycoside isoflavones cannot be absorbed in the body unless hydrolyzed and converted to aglycones, genistein and daidzein by intestinal microflora or in vitro fermentation [45]. In addition, Daidzin or daidzein can be metabolized to equol by certain strains of intestinal microflora in the gastrointestinal tract. However, only 30–50% of the adult population can produce and excrete equol in the urine after daily ingestion of soy foods [46].
Effects of Soy Intake on NAFLD
The hypolipidemic properties of soy components have been shown in human and animal studies as well as in cultured cells [3, 19, 47, 48], and are critical in reducing the risk for certain chronic diseases. The potential impact of soy intake on metabolic syndrome and biomarkers of NAFLD have been investigated in both human and different animal models of NAFLD. The results suggest that soy protein and associated isoflavones might be promising dietary supplements for prevention or treatment of NAFLD.
Human Clinical Trials
Inclusion of soy protein in diets may improve metabolic syndrome and risk factors associated with NAFLD. Consumption of 30 g soy nuts that contain 11.3 g protein and 102 mg total isoflavones in replacement of the same amount of red meat for eight weeks significantly lowered blood markers for NAFLD including ALT and AST, malondialdehyde (MDA) and fibrinogen levels compared to other non-soy groups in patients with NAFLD (n = 45) [18]. Moreover, the fasting blood sugar, serum insulin, high-sensitivity C-reactive protein (hs-CRP) levels, and systolic and diastolic blood pressure in the soy group were lower than in the non-soy groups [17]. The study was conducted in the patients with NAFLD and no other specific disorders, which should have good external validity. All patients completed the study and detailed data of their dietary intake was collected. The weaknesses of this parallel clinical trial include that the adherence of the patients to the designed diets could only be assessed through patients’ self-reported food records instead of measuring plasma or urine isoflavone levels [18]. In another parallel randomized clinical trial, daily drinking of 240 mL soy milk as a part of low-calorie diet for eight weeks significantly reduced serum ALT, hs-CRP [14], and insulin, and improved insulin resistance, and systolic and diastolic blood pressure in the NAFLD patients (n = 70) [16]. However, no changes were observed in fatty liver grade and other liver enzymes including AST, alkaline phosphatase, γ-glutamyl transferase, as well as lipid profile and anthropometric indices. The limitations of this study include that the types of dietary interventions were not blinding, and that the interpretations of ultrasound imaging for evaluation of liver steatosis were subjective, and that serum or urine isoflavones could not be determined as markers for the adherence to the intervention, and the study duration was relatively short [14].
The patients with NASH (n = 22, 9 women and 13 men) taking meal replacements containing 44% soy protein and 9% milk protein for 24 weeks had significant reduction in body weight, body mass index (BMI), body and liver fat content, serum ALT and AST, and improved glycemic control and lipid profile. The decrease in ALT was strongly correlated with the reduction in abdominal fat, subcutaneous fat, internal fat and AST. One of the weaknesses of the study was the use of 1H-magnetic resonance imaging analysis method in the quantification of hepatic steatosis that was not able to assess inflammation or fibrosis. Additionally, the specific effect of soy on the composition of liver fat could not be differentiated from the impact of caloric restriction. Small sample size limited further analysis of the participant subgroups (i.e., sex and age groups) [15]. In a randomized double-blind controlled trial, patients with NAFLD received either a daily supplement of 250 mg genistein (n = 41) or placebo (n = 41) for 8 weeks. The genistein group had significantly lower levels of serum insulin, MDA, TNF-α, IL-6, and improved insulin resistance, as well as reduced waist to hip ratio, body fat percentage and triglyceride compared to the placebo. However, BMI, fasting blood glucose, ALT and AST were not different between the two groups [49].
Akahane et al. [50] recently reported that progression of NAFLD and NASH was strongly associated with the production of equol. A clinical study conducted in 38 NAFLD patients (13 men and 25 women) showed that the degree of hepatic fibrosis and ballooning was markedly higher in the equol non-producers than in the producers in women. The percentage of non-producers (n = 8) with NAFLD activity score (NAS, including four histological features: steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis) ≥5 was significantly higher than that of the producers (n = 17) in women. However, these associations were not observed in men. Limitations of the study include small sample size, a single-center study, unknown exact amounts of soy products consumed, liver fibrosis assessed by less ideal method, and inconclusive causal relationship [50]. The sex-dependent effect of equol may be attributed to the difference in the endogenous estrogen levels, abundance of hepatic estrogen receptor (ER) [51] and responsiveness to equol between males and females. Like the other soy isoflavones, equol is estrogenic and can bind both ERα and ERβ [52]. It was shown that the female liver has higher ER concentration than the male liver [51], and that female liver is more responsive to estrogen exposure than the male liver due to the more efficient nuclear uptake of cytosolic receptor ligand complexes in females than in males [53].
Animal Studies
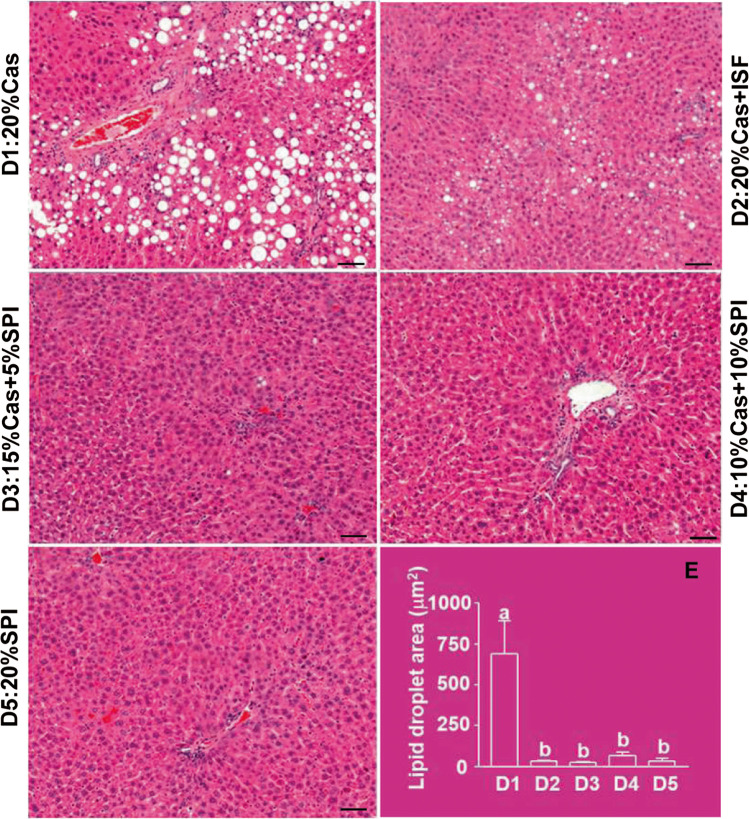
The hypolipidemic functions and preventive effects of soy on NAFLD have also been shown in genetically obese or high-fat/high-sugar induced obese rodent models. Feeding soy protein-containing diet attenuated hepatic lipid depots of triacylglycerols and cholesterol, decreased plasma lipid peroxides and body fat accumulation in Sprague-Dawley (SD) rats with high-fat induced NASH [20]. Dietary SPI reduced high-fat induced steatosis in the liver of SD rats [19], and decreased hepatic steatosis and diacylglycerols, changed microbiota populations, bile acid signaling and cholesterol homeostasis in Otsuka Long-Evans Tokushima fatty rats [54]. Feeding a diet containing soy protein concentrate enriched with isoflavones reduced fatty liver, and decreased plasma ALT, AST and triacylglycerol levels [21, 22], and increased activities of mitochondrial and peroxisomal β-oxidation, acetyl-CoA carboxylase, fatty acid synthase (FAS) and glycerol-3-phosphate acyltransferase in the liver of the obese Zucker rats [21]. Partial or full replacements of dietary casein by alcohol-washed SPI (devoid of isoflavones) or supplementation with soy isoflavones in the casein-based diet could effectively prevent the accumulation of lipid droplets in the liver of non-obese SD rats (Fig. 1) [55].
Fig. 1.
Liver histology of the female Sprague Dawley rats fed diets containing 20% casein in the absence (D1) or presence (D2) of supplemental isoflavones (ISF, 50 mg/kg diet) or increasing amounts of alcohol-washed soy protein isolate (SPI), 5% (D3), 10% (D4) or 20% (D5) in replacement of the same amounts of casein for 90 days. For the assessment of hepatic lipid droplet (HLD) formation and accumulation, the sections were stained with hematoxylin and eosin. The circumference of 100 randomly selected fat droplets in five fields of each section at 20× was measured under microscope using the software Northern eclipse version 7.0. The scale bars represent 10 μm, and the total areas of HLD were presented (E), and the means in (E) with different letters (a, b) differ. (Adapted and reformatted from Xiao et al. [55])
Functional Components in the Soy
The bioactive components in soy that play major roles in mediating the hypolipidemic actions and improvement of NAFLD are not fully understood, and inconsistency exist in the literature. For example, soy protein markedly lowered serum triglycerides and cholesterol levels, and regulated gene expression involved in the synthesis of fatty acids in the liver of rats compared to casein protein. Supplementation of soy isoflavones had little effect on liver lipogenesis [56]. Simmen et al. [57] showed that suppression of fat droplet formation and accumulation in the liver of non-obese rats fed soy diets was independent of genistein [57]. Our study in SD rats also showed that intake of 20% alcohol-washed SPI with or without added isoflavones markedly lowered plasma triglycerides levels compared to a casein diet, however the added isoflavones had no additional effects [58].
β-conglycinin, one of the major storage proteins in soybean, was shown to reduce serum triglycerides, glucose and insulin levels [12], and prevented high-fat induced fatty liver in mice [59], and increased blood adiponectin level and insulin sensitivity in Wistar rats [23]. Our studies, using soy proteins with depletion of different subunits, further demonstrated that α’ subunit of β-conglycinin and all acidic polypeptides (A1 to A5) in glycinin were not required for the lipid-lowering effects and fatty liver reduction of soy proteins [60, 61]. This indicates that the other subunits of β-conglycinin and glycinin may play major roles in this regard.
Nevertheless, soy isoflavone extract markedly alleviated the high-fat induced hepatic steatosis and altered related gene expressions in an ovariectomized Wistar rat model for postmenopausal women [62]. Genistein and daidzein regulated hepatic lipogenesis, insulin resistance or adiposity and adipocytokines involved in hepatic steatosis [8, 63]. Administration of genistein reduced lipid accumulation in the livers and ameliorated fatty liver, improved insulin sensitivity, lipid profiles, liver injury, histological abnormalities and activated the antioxidant profiles, decreased the pre-inflammatory cytokines, IL-6 and TNF-α, and prevented oxidative damage in the high-fructose induced insulin-resistant rats [7]. Soy genistein and daidzein could inhibit oleic acid-induced intracellular lipid accumulation in human HepG2 liver cell lines [64]. In general, both soy protein and isoflavones appear to be effective in lowering liver and blood lipids, improving glucose tolerance and insulin sensitivity and reducing liver steatosis although some inconsistencies exist in the effects of isoflavones.
Potential Molecular Mechanism(S) Involved
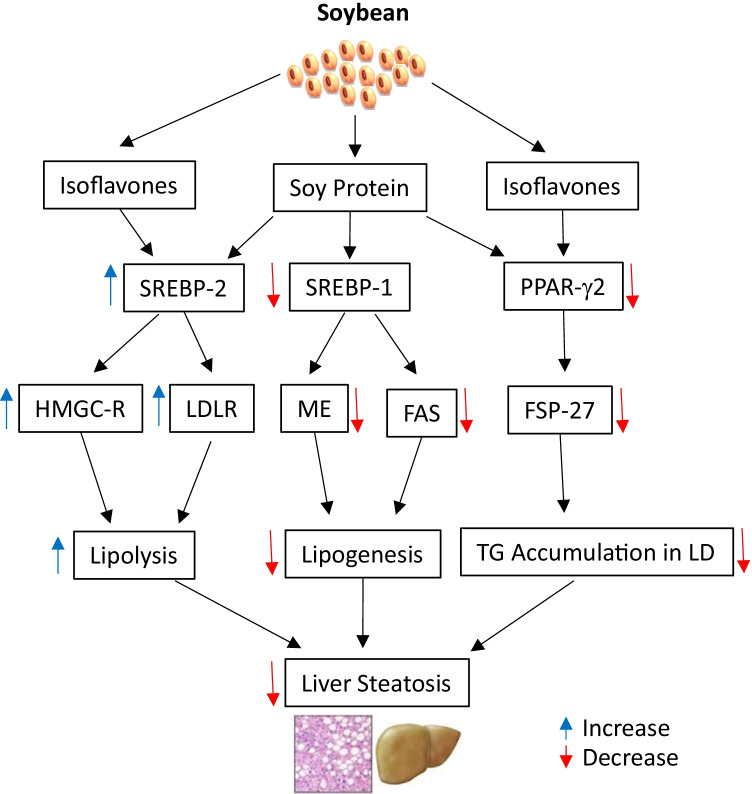
Although soy intake suppresses the formation and accumulation of liver lipid droplets and reduces triglyceride content in both obese [21, 22] and non-obese animal models [57], the mechanism(s) involved in the hypolipidemic actions and NAFLD prevention of soy are different in the two models. In non-obese rats (Fig. 2), dietary soy proteins down-regulated expression of hepatic genes for lipogenesis such as sterol regulatory element-binding protein-1 (SREBP-1), malic enzyme and FAS, while up-regulated expression of the genes for lipolysis such as SREBP-2, 3-hydroxy-3-methyl-glutaryl-CoA (HMGC) reductase, HMGC synthase and LDL receptor [65].
Fig. 2.
Potential molecular mechanism(s) involved in the soy effects on NAFLD in the non-obese models. Soy protein and isoflavones speed up hepatic lipolysis through activation of SREBP-2 and up-regulation of the downstream gene (HMGC-R and LDLR) expression, while soy protein suppresses hepatic lipogenesis by down-regulation of SREBP-1 and its target genes (such as ME and FAS). Moreover, both soy protein and isoflavones inhibit formation and accumulation of lipid droplets in liver via suppression of PPAR-γ2 and FSP-27 expression. FAS, fatty acid synthase; FSP-27, fat-specific protein 27; HMGC-R, 3-hydroxyl-3-methyl-glutaryl-CoA reductase; LD, lipid droplets; LDLR, low-density lipoprotein receptor; ME, malic enzyme; PPAR-γ2, peroxisome proliferation-activated receptor γ2; SREBP-1, sterol regulatory element-binding protein-1; TG, triglycerides
This is consistent with our results from proteomic analysis of liver proteins in the non-obese SD rats fed SPI or casein diets. Soy protein attenuated the abundance of the proteins involved in the lipogenesis and enhanced the proteins or enzymes in the lipolysis. In addition, both soy protein and supplemental isoflavones markedly reduced peroxisome proliferator-activated receptor-γ2 (PPARγ2) and its target gene, fat-specific protein 27 (FSP27), in the liver, which was associated with decreased accumulation of hepatic lipid droplets [55]. FSP27 is a lipid droplet-associated protein, and promotes the formation of hepatic lipid droplets by enhancing triglyceride accumulation within lipid droplets and regulating fat storage [66]. Overexpression of FSP27 in the liver of the leptin-deficient (ob/ob) mice increased hepatic triglyceride content [67]. Our results suggested that prevention of hepatic lipid droplet accumulation by supplemented isoflavones was mainly mediated by suppression of hepatic FSP27 and that soy proteins reduced the abundance of FSP27 and hepatic triglyceride content, thereby preventing fatty liver [55]. This is in line with the effects of β-conglycinin that attenuated PPARγ2 protein and FSP27 mRNA expression in mice [59].
However, in the obese rats, soy proteins enhanced hepatic lipogenesis and increased activities of hepatic FAS and plasma triacylglycerol levels [21], which might be due to increased blood insulin levels [68]. SPI could restore the suppressed β-catenin signaling pathway in the Zucker obese rats compared to their lean mates, and thereby attenuating hepatic fat accumulation, liver damage and hepatocellular vacuolation [69].
β-conglycinin is one of the most bioactive globulins in soy and modulated genes and proteins associated with hypolipidemic functions and preventive effects on NAFLD. β-conglycinin effectively prevented high-sucrose induced fatty liver through suppression of SREBP-1c and carbohydrate response element-binding protein mRNA [59], and lowered hepatic triglycerides, serum insulin and leptin concentrations and prevented high-fat induced fatty liver via suppression of liver PPARγ2 and/or SREBP-1c protein in mice [59, 70]. β-conglycinin and β-conglycinin-derived peptides reduced liver weight and lipid content, and inhibited lipogenic gene expression and enzymatic activity and increased lipolytic enzyme activity in the rat models of NAFLD [71, 72]. It was proposed that the hypolipidemic effects of β-conglycinin might be due to increased insulin sensitivity of the liver, down-regulation of hepatic SREBP-1 [23] and PPARγ2 gene expressions [59], as well as acceleration of β-oxidation of fatty acids and suppression of FAS and/or increased triglycerides fecal excretion.
Hashidume et al. (2016) [73] showed that some of β-conglycinin effects were mediated through induction of hepatic fibroblast growth factor 21 (FGF21) expression and circulating FGF21 levels in a mouse model, which was regulated by activating transcription factor 4 (ATF4). It was further revealed that β-conglycinin ingestion resulted in methionine imbalance in portal blood as methionine content in β-conglycinin is only 1% compared to 2.2% in glycinin [74] and 3% in casein [73], which played a critical role in activation of the ATF4-FGF21 signaling axis and stimulation of the metabolic responses in hepatocytes [73]. The score of the sulfur-containing amino acids (the sum of methionine and cysteine) in β-conglycinin calculated against the requirement of rodents is 0.39, compared to 1.04 in casein (Table 3). Methionine is the first limiting amino acid in β-conglycinin for rodents. However, when the β-conglycinin diet was supplemented with enough methionine, the β-conglycinin effect on ATF4-FGF21 signaling was almost completely eliminated [73]. It was verified that all the other studies on β-conglycinin in rodents cited in this paper were supplemented with enough methionine or cysteine. Thus, methionine imbalance-induced mechanism might be not involved in the functions of β-conglycinin observed in those studies.
Table 3.
Comparison of indispensable amino acid (IAA) content and ratios in casein and β-conglycinin
| Caseinb | β-conglycininb | β-conglycinin+Metb,c | |||||
|---|---|---|---|---|---|---|---|
| Indispensable Amino Acid (IAA) | IAAa Req. (mg/g) | IAA (mg/g) | IAA ratio | IAA (mg/g) | IAA ratio | IAA (mg/g) | IAA ratio |
| Arginine | 36 | 36 | 0.99 | 91 | 2.53 | 88.8 | 2.48 |
| Histidine | 26 | 30 | 1.16 | 21 | 0.82 | 20.6 | 0.80 |
| Isoleucine | 48 | 53 | 1.10 | 50 | 1.05 | 49.1 | 1.03 |
| Leucine | 86 | 92 | 1.06 | 80 | 0.93 | 78.5 | 0.91 |
| Lysine | 73 | 98 | 1.34 | 72 | 0.99 | 70.7 | 0.97 |
| Met + Cysd | 46 | 49 | 1.04 | 18 | 0.39 | 36.3 | 0.78 |
| Phed + Tyrd | 101 | 106 | 1.04 | 101 | 0.99 | 98.6 | 0.97 |
| Threonine | 38 | 40 | 1.07 | 25 | 0.67 | 24.5 | 0.65 |
| Tryptophan | 12 | 13 | 1.06 | 6 | 0.51 | 5.9 | 0.50 |
| Valine | 56 | 67 | 1.19 | 42 | 0.74 | 40.7 | 0.73 |
aBased on AIN-93G indispensable amino acid requirements for growing rodents
bIAA content were from Hashidume et al. [73]
c,dMet, methionine; Cys, cysteine; Phe, phenylalanine; Tyr, tyrosine
The other promising genes and proteins that may play important roles in mediating the reduction of liver steatosis by soy protein include hepatic Neuregulin 1 (NRG1), Erb-B2 Receptor Tyrosine Kinase 3 (ERBB3) and mitogen-activated protein kinase interacting serine/threonine kinase 1 (MKNK1). NRG1 and ERBB3 are membrane-bound proteins. ERBB3 could be modulated through phosphorylation by NRG1 to alleviate liver steatosis [75]. MKNK1 gene knocked-out mice were protected against a high-fat diet-induced obesity and detrimental effects such as impaired glucose tolerance, increased body weight gain and inflammatory biomarkers [76, 77]. A shotgun proteomics analysis showed that NRG1 and ERBB3 were the top activated proteins and MKNK1 was the top inhibited protein in the liver of the obese Zucker rats fed SPI [78]. This suggests that modulation of these molecules might be important cellular events by which soy protein exerts its hypolipidemic actions and alleviation of liver steatosis. However, this needs further investigation.
Conclusions
Both soy protein and associated isoflavones have been shown to be hypolipidemic and play a role in reduction of liver steatosis and improving NAFLD-related syndrome in both human and animal studies. The molecular mechanism(s) involved are mainly through inhibiting lipogenesis by down-regulation of the transcription factors, SREBP-1c and PPARγ2, and their target genes, and enhancing lipolysis via up-regulation of SREBP-2 and its downstream genes in the non-obese models, while improving insulin resistance and restoring the suppressed β-catenin signaling pathway in the genetically obese models. The other benefits of soy components include protection of liver against oxidative damage and inflammation. Some effects of soy isoflavones and equol are sex-dependent, and the mechanism(s) involved remain unclear. Since most of the mechanism studies on soy actions were conducted in either cultured cells or rodent models that are known to differ in protein and indispensable amino acid requirements, whether the same mechanisms are shared in human remains to be determined. Overall, consumption of soy foods or supplements might be a useful strategy to mitigate the disease burden and prevalence of NAFLD, which is consistent with the new Canada’s Food Guide that recommends consumption of more plant-based protein foods [79].
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ATF4
Activating transcription factor 4
- BMI
Body mass index
- ER
Estrogen receptor
- ERBB3
Erb-B2 receptor tyrosine kinase 3
- FAS
Fatty acid synthase
- FGF21
Fibroblast growth factor 21
- FSP27
Fat-specific protein 27
- hs-CRP
High-sensitivity C-reactive protein
- IAA
Indispensable amino acid
- LDL
Low density lipoprotein
- MDA
malondialdehyde
- MKNK1
MAPK interacting serine/threonine kinase 1
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NRG1
Neuregulin 1
- PPARγ2
Peroxisome proliferator-activated receptor γ2
- SD
Sprague-Dawley
- SPI
Soy protein isolate
- SREBP-1
Sterol regulatory element-binding protein-1
Authors’ Contributions
CWX initiated the idea of this work, and conducted literature and data collection, and drafted the manuscript. AH conducted literature search and provided critical revision. All authors read and approved the final version of the manuscript.
Funding
Open Access provided by Health Canada. This work was partially supported by Health Canada A-Base Fund.
Data Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagarajan S. Mechanisms of anti-atherosclerotic functions of soy-based diets. J Nutr Biochem. 2010;21:255–260. doi: 10.1016/j.jnutbio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Ascencio C, Torres N, Isoard-Acosta F, et al. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr. 2004;134:522–529. doi: 10.1093/jn/134.3.522. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins DJ, Kendall CW, Jackson CJ, et al. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–372. doi: 10.1093/ajcn/76.2.365. [DOI] [PubMed] [Google Scholar]
- 4.Rebholz CM, Reynolds K, Wofford MR, et al. Effect of soybean protein on novel cardiovascular disease risk factors: a randomized controlled trial. Eur J Clin Nutr. 2013;67:58–63. doi: 10.1038/ejcn.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squadrito F, Marini H, Bitto A, et al. Genistein in the metabolic syndrome: results of a randomized clinical trial. J Clin Endocrinol Metab. 2013;98:3366–3374. doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- 6.Moradi M, Daneshzad E, Azadbakht L. The effects of isolated soy protein, isolated soy isoflavones and soy protein containing isoflavones on serum lipids in postmenopausal women: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60:3414–3428. doi: 10.1080/10408398.2019.1689097. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed SS, Nallasamy P, Muniyandi P, et al. Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J Diabetes. 2009;1:278–287. doi: 10.1111/j.1753-0407.2009.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim MH, Kang KS, Lee YS. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br J Nutr. 2010;104:1333–1342. doi: 10.1017/S0007114510002266. [DOI] [PubMed] [Google Scholar]
- 9.Manzoni C, Duranti M, Eberini I, et al. Subcellular localization of soybean 7S globulin in HepG2 cells and LDL receptor up-regulation by its alpha' constituent subunit. J Nutr. 2003;133:2149–2155. doi: 10.1093/jn/133.7.2149. [DOI] [PubMed] [Google Scholar]
- 10.Blanco MS, Messina M, Li SS, et al. A Meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and Total cholesterol concentrations in adults. J Nutr. 2019;149:968–981. doi: 10.1093/jn/nxz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Meijer GW, Vermeer MA, et al. Soy protein enhances the cholesterol-lowering effect of plant sterol esters in cholesterol-fed hamsters. J Nutr. 2004;134:143–148. doi: 10.1093/jn/134.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Moriyama T, Kishimoto K, Nagai K, et al. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotechnol Biochem. 2004;68:352–359. doi: 10.1271/bbb.68.352. [DOI] [PubMed] [Google Scholar]
- 13.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 14.Eslami O, Shidfar F, Maleki Z, et al. Effect of soy Milk on metabolic status of patients with nonalcoholic fatty liver disease: a randomized clinical trial. J Am Coll Nutr. 2019;38:51–58. doi: 10.1080/07315724.2018.1479990. [DOI] [PubMed] [Google Scholar]
- 15.Deibert P, Lazaro A, Schaffner D, et al. Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis. World J Gastroenterol. 2019;25:1116–1131. doi: 10.3748/wjg.v25.i9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleki Z, Jazayeri S, Eslami O, et al. Effect of soy milk consumption on glycemic status, blood pressure, fibrinogen and malondialdehyde in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Complement Ther Med. 2019;44:44–50. doi: 10.1016/j.ctim.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Kani AH, Alavian SM, Esmaillzadeh A, et al. Effects of a low-calorie, low-carbohydrate soy containing diet on systemic inflammation among patients with nonalcoholic fatty liver disease: a parallel randomized clinical trial. Horm Metab Res. 2017;49:687–692. doi: 10.1055/s-0042-118707. [DOI] [PubMed] [Google Scholar]
- 18.Kani AH, Alavian SM, Esmaillzadeh A, et al. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition. 2014;30:814–821. doi: 10.1016/j.nut.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Badger TM, Ronis MJ, Wolff G, et al. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood ) 2008;233:1242–1254. doi: 10.3181/0802-RM-60. [DOI] [PubMed] [Google Scholar]
- 20.Yang HY, Tzeng YH, Chai CY, et al. Soy protein retards the progression of non-alcoholic steatohepatitis via improvement of insulin resistance and steatosis. Nutrition. 2011;27:943–948. doi: 10.1016/j.nut.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Gudbrandsen OA, Wergedahl H, Mørk S, et al. Dietary soya protein concentrate enriched with isoflavones reduced fatty liver, increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Br J Nutr. 2006;96:249–257. doi: 10.1079/bjn20061837. [DOI] [PubMed] [Google Scholar]
- 22.Hakkak R, Gauss CH, Bell A, et al. Short-term soy protein isolate feeding prevents liver steatosis and reduces serum ALT and AST levels in obese female Zucker rats. Biomedicines. 2018;6:55. doi: 10.3390/biomedicines6020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachibana N, Iwaoka Y, Hirotsuka M, et al. Beta-conglycinin lowers very-low-density lipoprotein-triglyceride levels by increasing adiponectin and insulin sensitivity in rats. Biosci Biotechnol Biochem. 2010;74:1250–1255. doi: 10.1271/bbb.100088. [DOI] [PubMed] [Google Scholar]
- 24.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 25.Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9:715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 27.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 28.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 29.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alswat K, Aljumah AA, Sanai FM, et al. Nonalcoholic fatty liver disease burden - Saudi Arabia and United Arab Emirates, 2017-2030. Saudi J Gastroenterol. 2018;24:211–219. doi: 10.4103/sjg.SJG_122_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams LA, Roberts SK, Strasser SI, et al. Nonalcoholic fatty liver disease burden: Australia, 2019-2030. J Gastroenterol Hepatol. 2020;35:1628–1635. doi: 10.1111/jgh.15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain MG, Ramji A, Patel K, et al. Burden of nonalcoholic fatty liver disease in Canada, 2019-2030: a modelling study. CMAJ Open. 2020;8:E429–E436. doi: 10.9778/cmajo.20190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estes C, Chan HLY, Chien RN, et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther. 2020;51:801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goossens N, Bellentani S, Cerny A, et al. Nonalcoholic fatty liver disease burden - Switzerland 2018-2030. Swiss Med Wkly. 2019;149:w20152. doi: 10.4414/smw.2019.20152. [DOI] [PubMed] [Google Scholar]
- 36.Carvalhana S, Machado MV, Cortez-Pinto H. Improving dietary patterns in patients with nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:468–473. doi: 10.1097/MCO.0b013e3283566614. [DOI] [PubMed] [Google Scholar]
- 37.Alferink LJM, Erler NS, de Knegt RJ, et al. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur J Epidemiol. 2020;35:1069–1085. doi: 10.1007/s10654-020-00627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young VR. Soy protein in relation to human protein and amino acid nutrition. J Am Diet Assoc. 1991;91:828–835. [PubMed] [Google Scholar]
- 39.Hughes GJ, Ryan DJ, Mukherjea R, et al. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: criteria for evaluation. J Agric Food Chem. 2011;59:12707–12712. doi: 10.1021/jf203220v. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman JR, Falvo MJ. Protein - Which is Best? J Sports Sci Med. 2004;3:118–130. [PMC free article] [PubMed] [Google Scholar]
- 41.Schaafsma G. The protein digestibility-corrected amino acid score. J Nutr. 2000;130:1865S–1867S. doi: 10.1093/jn/130.7.1865S. [DOI] [PubMed] [Google Scholar]
- 42.USDA FoodData Central (2019) Nutrients of mature soybean seeds https://fdc.nal.usda.gov/fdc-app.html#/food-details/174270/nutrients (Accessed 1 April 2019)
- 43.Zarkadas CG, Gagnon C, Poysa V, et al. Protein quality and identification of the storage protein subunits of tofu and null soybean genotype, using amino acid analysis, one- and two-dimensional gel electrophoresis, and tandem mass spectrometry. Food Res Int. 2007;40:111–128. [Google Scholar]
- 44.Xiao CW, Wood CM, Robertson P, et al. Protease inhibitor activities and isoflavone content in commercial soymilks and soy-based infant formulas sold in Ottawa, Canada. J Food Comp Anal. 2012;25:130–136. [Google Scholar]
- 45.Miniello VL, Moro GE, Tarantino M, et al. Soy-based formulas and phyto-oestrogens: a safety profile. Acta Paediatr Suppl. 2003;91:93–100. doi: 10.1111/j.1651-2227.2003.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 46.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 47.Frigolet ME, Torres N, Uribe-Figueroa L, et al. White adipose tissue genome wide-expression profiling and adipocyte metabolic functions after soy protein consumption in rats. J Nutr Biochem. 2011;22:118–129. doi: 10.1016/j.jnutbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JW, Bush HM. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr. 2011;30:79–91. doi: 10.1080/07315724.2011.10719947. [DOI] [PubMed] [Google Scholar]
- 49.Amanat S, Eftekhari MH, Fararouei M, et al. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: a randomized, controlled trial. Clin Nutr. 2018;37:1210–1215. doi: 10.1016/j.clnu.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Akahane T, Kaya D, Noguchi R, et al. Association between Equol production status and nonalcoholic steatohepatitis. Int J Mol Sci. 2021;22:11904. doi: 10.3390/ijms222111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coldham NG, Sauer MJ. Pharmacokinetics of [(14)C]Genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol. 2000;164:206–215. doi: 10.1006/taap.2000.8902. [DOI] [PubMed] [Google Scholar]
- 52.Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Thompson C, Lucier GW. Hepatic estrogen responsiveness. Possible mechanisms for sexual dimorphism. Mol Pharmacol. 1983;24:69–76. [PubMed] [Google Scholar]
- 54.Panasevich MR, Schuster CM, Phillips KE, et al. Soy compared with milk protein in a Western diet changes fecal microbiota and decreases hepatic steatosis in obese OLETF rats. J Nutr Biochem. 2017;46:125–136. doi: 10.1016/j.jnutbio.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao CW, Wood CM, Weber D, et al. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr. 2014;9:373. doi: 10.1007/s12263-013-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi Y, Konishi T. Tofu (soybean curd) lowers serum lipid levels and modulates hepatic gene expression involved in lipogenesis primarily through its protein, not isoflavone, component in rats. J Agric Food Chem. 2011;59:8976–8984. doi: 10.1021/jf201403u. [DOI] [PubMed] [Google Scholar]
- 57.Simmen FA, Mercado CP, Zavacki AM, et al. Soy protein diet alters expression of hepatic genes regulating fatty acid and thyroid hormone metabolism in the male rat. J Nutr Biochem. 2010;21:1106–1113. doi: 10.1016/j.jnutbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Xiao CW, Wood C, Huang W, et al. Tissue-specific regulation of acetyl-CoA carboxylase gene expression by dietary soya protein isolate in rats. Br J Nutr. 2006;95:1048–1054. doi: 10.1079/bjn20061776. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki T, Kishimoto K, Miura S, et al. Dietary beta-conglycinin prevents fatty liver induced by a high-fat diet by a decrease in peroxisome proliferator-activated receptor gamma2 protein. J Nutr Biochem. 2012;23:123–132. doi: 10.1016/j.jnutbio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Chen Q, Wood C, Gagnon C, et al. The alpha' subunit of beta-conglycinin and the A1-5 subunits of glycinin are not essential for many hypolipidemic actions of dietary soy proteins in rats. Eur J Nutr. 2014;53:1195–1207. doi: 10.1007/s00394-013-0620-9. [DOI] [PubMed] [Google Scholar]
- 61.Chatterjee C, Liu J, Wood C, et al. The alpha' subunit of beta-conglycinin and various glycinin subunits of soy are not required to modulate hepatic lipid metabolism in rats. Eur J Nutr. 2018;57:1157–1168. doi: 10.1007/s00394-017-1399-x. [DOI] [PubMed] [Google Scholar]
- 62.Panneerselvam S, Packirisamy RM, Bobby Z, et al. Soy isoflavones (Glycine max) ameliorate hypertriglyceridemia and hepatic steatosis in high fat-fed ovariectomized Wistar rats (an experimental model of postmenopausal obesity) J Nutr Biochem. 2016;38:57–69. doi: 10.1016/j.jnutbio.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Kim MH, Park JS, Jung JW, et al. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int J Obes. 2011;35:1019–1030. doi: 10.1038/ijo.2010.256. [DOI] [PubMed] [Google Scholar]
- 64.Huang C, Pang D, Luo Q et al (2016) Soy Isoflavones regulate lipid metabolism through an AKT/mTORC1 pathway in diet-induced obesity (DIO) male rats. Molecules 21:586 [DOI] [PMC free article] [PubMed]
- 65.Tovar AR, Murguia F, Cruz C, et al. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J Nutr. 2002;132:2562–2569. doi: 10.1093/jn/132.9.2562. [DOI] [PubMed] [Google Scholar]
- 66.Puri V, Konda S, Ranjit S, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 67.Matsusue K, Kusakabe T, Noguchi T, et al. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hakkak R, Al-Dwairi A, Fuchs GJ, et al. Dietary soy protein induces hepatic lipogenic enzyme gene expression while suppressing hepatosteatosis in obese female Zucker rats bearing DMBA-initiated mammary tumors. Genes Nutr. 2012;7:549–558. doi: 10.1007/s12263-012-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou D, Lezmi S, Wang H, et al. Fat accumulation in the liver of obese rats is alleviated by soy protein isolate through beta-catenin signaling. Obesity (Silver Spring) 2014;22:151–158. doi: 10.1002/oby.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D, Ikaga R, Yamazaki T. Soya protein beta-conglycinin ameliorates fatty liver and obesity in diet-induced obese mice through the down-regulation of PPARgamma. Br J Nutr. 2018;119:1220–1232. doi: 10.1017/S0007114518000739. [DOI] [PubMed] [Google Scholar]
- 71.Wanezaki S, Tachibana N, Nagata M, et al. Soy beta-conglycinin improves obesity-induced metabolic abnormalities in a rat model of nonalcoholic fatty liver disease. Obes Res Clin Pract. 2015;9:168–174. doi: 10.1016/j.orcp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Wanezaki S, Saito S, Inoue N, et al. Soy beta-Conglycinin peptide attenuates obesity and lipid abnormalities in obese model OLETF rats. J Oleo Sci. 2020;69:495–502. doi: 10.5650/jos.ess20010. [DOI] [PubMed] [Google Scholar]
- 73.Hashidume T, Kato A, Tanaka T, et al. Single ingestion of soy beta-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci Rep. 2016;6:28183. doi: 10.1038/srep28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nielsen NC, Dickinson CD, Cho TJ, et al. Characterization of the glycinin gene family in soybean. Plant Cell. 1989;1:313–328. doi: 10.1105/tpc.1.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng D, Pan H, Chen Y, et al. Roles and mechanisms of NRG1 in modulating the pathogenesis of NAFLD through ErbB3 signaling in hepatocytes (NRG1 modulates NAFLD through ErbB3 signaling) Obes Res Clin Pract. 2021;15:145–151. doi: 10.1016/j.orcp.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Moore CE, Pickford J, Cagampang FR, et al. MNK1 and MNK2 mediate adverse effects of high-fat feeding in distinct ways. Sci Rep. 2016;6:23476. doi: 10.1038/srep23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sandeman LY, Kang WX, Wang X, et al. Disabling MNK protein kinases promotes oxidative metabolism and protects against diet-induced obesity. Mol Metab. 2020;42:101054. doi: 10.1016/j.molmet.2020.101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozaczek M, Kong B, Bottje W, et al. Hepatic proteomics analysis of nonalcoholic fatty liver disease obese rat model after short- and long-term soy protein isolate feeding. J Med Food. 2021;25:293–302. doi: 10.1089/jmf.2021.0088. [DOI] [PubMed] [Google Scholar]
- 79.Health Canada (2019) Canada's Food Guide. https://food-guide.canada.ca/en/ (Accessed 28 March 2019)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.