Abstract
Introduction
Patients with variants in the GNAO1 gene may present with life-threatening dystonic storm. There is little experience using pallidal deep brain stimulation (DBS) as an emergency treatment in such cases.
Case description
We report on a 16-year-old girl with a variant in the GNAO1 gene (c.626G > T; p.(Arg209Leu)) who was admitted to the intensive care unit with medically refractory dystonic storm with secondary complications inducing rhabdomyolysis and acute colitis. Emergency pallidal DBS resulted in rapid improvement of dystonic storm and the subsidence of rhabdomyolysis and colitis. There were no further episodes of dystonic storm during follow-up of 2 years.
Conclusion
Pallidal DBS is a useful treatment option for GNAO1-related dystonic storm with secondary complications which can be performed as an emergency surgery.
Keywords: Deep brain stimulation, Dystonic storm, DYT-GNAO1, Globus pallidus internus, GNAO1 gene
Introduction
Dystonic storm can present as a life-threatening condition with a high risk of mortality and morbidity due to respiratory and metabolic complications [1–3]. The management of status dystonicus includes administration of high doses of sedatives, antispasmodic drugs, and treatment of triggering conditions such as infection [2, 4, 5]. In patients who do not respond well to medical treatment, both deep brain stimulation (DBS) and pallidal radiofrequency lesioning have been shown to provide rapid and effective relief [1, 3, 6, 7].
Variants in the GNAO1 gene (guanine-nucleotide-binding protein) have been identified in few patients with dystonic storm [7]. Such patients have been described to respond well to pallidal DBS [7–17]. Here, we report on the use of pallidal DBS as an emergency treatment in a 16-year-old girl with a variant in the GNAO1 gene who presented with a medically refractory dystonic storm complicated by severe rhabdomyolysis and acute colitis.
Case report
This 16-year-old girl was admitted to the pediatric intensive care unit (ICU) with severe dystonic storm which had started after a tooth extraction under general anesthesia. She had a history of markedly delayed development since infancy. Seizures started during the first months of life, and she also developed episodes of varying intensity of dystonic movements of her arms and legs and later involving also the trunk. Initially, she had a diagnosis of cerebral palsy. She never learned to speak, but developed understanding of language, and she was later able to communicate with a talking device. She could not walk independently and was mobilized with a wheelchair and a walking frame. Further symptoms included hypersalivation and multiple contractures and luxations of her extremities which needed surgical correction.
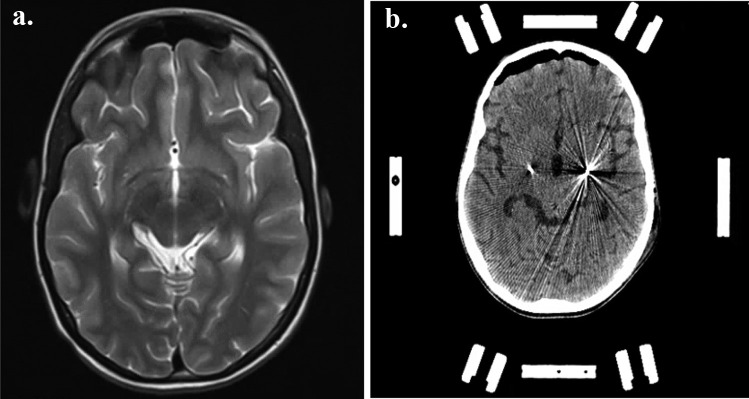
Over the years, she had several episodes of dystonic storm, occasionally associated with rhabdomyolysis and renal impairment which were managed conservatively. Genetic analysis revealed a de novo heterozygous variant in the GNAO1 gene (c.626G > T; p.(Arg209Leu)). MR imaging of her brain was unremarkable (Fig. 1a).
Fig. 1.
a MRI of the brain: T2-weighted axial image shows no abnormalities. b Postoperative stereotactic CT shows the positioning of the electrodes in the posteroventral lateral globus pallidus internus
After admission to the ICU at age 16, treatment was started with infusions of clonidine, midazolam, and morphine. Thereafter, continuous infusions of hydromorphone (6 µg/kg/h) and clonidine (1.2 µg/kg/h) were given which yielded little improvement of the dystonic storm. Additional treatment with clobazam, tetrabenazine, and gabapentin was ineffective.
Subsequently, hyperkalemia (6.9 mmol/l) and rhabdomyolysis (creatine kinase > 100.00 U/l) developed. Sedation with chloral hydrate resulted in improvement of dystonic storm; however, upon reduction of medication, dystonic storm reemerged over a period of 3 weeks. In the following, her state was further complicated by the development of acute colitis and pneumatosis hepatis.
With regard to her deteriorating condition, she was scheduled for emergency DBS surgery under general anesthesia. Quadripolar DBS electrodes (Vercise Cartesia Directional, Boston Scientific) were implanted bilaterally into the posteroventral lateral globus pallidus internus with CT stereotactic guidance supplemented by preoperative MR imaging and microelectrode recording as described in detail elsewhere [18, 19].
Subsequently, the electrodes were connected to an implantable pulse generator (Vercise PC, Boston Scientific). Postoperative stereotactic CT-imaging demonstrated appropriate electrode placement in the target bilaterally (Fig. 1b). Pallidal stimulation was started directly after completion of the surgery with the following settings: amplitude 2 mA, frequency 130 Hz, and triple monopolar electrode montage on both sides.
The dystonic movements improved rapidly after weaning from general anesthesia. On the second day after implantation of the electrodes, the medication with midazolam, clonidine, and hydromorphone could be reduced and was completely stopped over the next few days. The stimulation amplitude was increased in parallel to 2.9 mA. The rhabdomyolysis ceased, and the colitis subsided. The patient was discharged from the hospital 17 days after DBS surgery.
At 3-month follow-up, there was sustained marked improvement. No further episodes of dystonic storm had occurred. The patient could participate in daily activities again. She lived at home with her parents who were very satisfied with the result. At 2-year follow-up, she was in a stable condition.
Discussion
Mutations in the GNAO1 gene were first identified in patients with epileptic encephalopathy. Subsequently, various phenotypes were defined characterized by severe developmental delay and hyperkinetic movement disorders [20]. GNOA1 is located on the long arm of chromosome 16 (16q12.2). It encodes the Gαo subunit of the guanine-nucleotide-binding protein, which has an important function in modulating transmembrane systems. Loss of function variants in the GNAO1 gene are associated with epilepsy, whereas gain of function variants are reported in patients with movement disorders [7, 14, 21]. In particular, disruption of the G-protein-cAMP pathway axis may be a key contributor to the pathophysiology of dystonia in these patients [7].
Pallidal DBS has become an accepted treatment option for various forms of dystonia [9, 22, 23]. In particular, inherited forms of dystonia tend to respond well to chronic pallidal stimulation [9]. While the benefit of DBS is often seen only with a delay of weeks and months in patients with dystonia [24], in some forms of dystonia, in particular in the case of dystonic storm, improvement may be observed within hours or days [9, 13, 15].
There is a limited experience with DBS in patients with GNAO1-related dystonia. According to the limited experience published thus far, these patients tend to respond well to pallidal DBS in the context of dystonic storm [7–17]. For instance, pallidal DBS has been used as an emergency measure to abate the severe dyskinesias and to restore normal daily functioning including feeding and sleeping [7, 13].
Our report highlights that pallidal DBS may be used as a life-saving treatment in an emergency context in patients with GNAO1-related dystonic storm abating also subsequent severe complications such as rhabdomyolysis and the rare occurrence of acute colitis.
Author contribution
HC, JCSB, and JKK prepared the first version of the manuscript. All authors were involved in taking care and coordinating treatment. All authors critically reviewed, worked on the manuscript, and consented with its final version.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Upon reasonable request.
Declarations
Ethics approval and consent to participate
This case report complies with the established ethics rules at Hannover Medical School. The parents gave informed consent to perform surgery.
Consent for publication
Since this case report does not contain any identifying information, consent for publication is not needed.
Conflict of interest
JKK is a consultant for Medtronic and Boston Scientific. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allen NM, Lin JP, Lynch T, King MD. Status dystonicus: a practice guide. Dev Med Child Neurol. 2014;56(2):105–112. doi: 10.1111/dmcn.12339. [DOI] [PubMed] [Google Scholar]
- 2.Termsarasab P. Frucht SJ (2017) Dystonic storm: a practical clinical and video review. Journal of Clinical Movement Disorders. 2017;4:10. doi: 10.1186/s40734-017-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walcott BP, Nahed BV, Kahle KT, Duhaime AC, Sharma N, Eskandar EN. Deep brain stimulation for medically refractory life-threatening status dystonicus in children. J Neurosurg Pediatr. 2012;9(1):99–102. doi: 10.3171/2011.10.PEDS11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garone G, Graziola F, Nicita F, Frascarelli F, Randi F, Zazza M, Cantonetti L, Cossu S, Marras CE, Capuano A. Prestatus and status dystonicus in children and adolescents. Dev Med Child Neurol. 2020;62(6):742–749. doi: 10.1111/dmcn.14425. [DOI] [PubMed] [Google Scholar]
- 5.Iodice A, Pisani F. Status dystonicus: management and prevention in children at high risk. Acta Biomed. 2019;90(3):207–212. doi: 10.23750/abm.v90i3.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi V, Zorzi G, Messina G, Romito L, Tramacere I, Dones I, Nardocci N, Franzini A. Deep brain stimulation versus pallidotomy for status dystonicus: a single-center case series. J Neurosurg. 2019;134:197–207. doi: 10.3171/2019.10.JNS191691. [DOI] [PubMed] [Google Scholar]
- 7.Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, Parrini E, Bianchini C, McShane T, Dale RC, Mohammad SS, Shah U, Mahant U, Ng J, McTague A, Samanta R, Vadlamani G, Valente EM, Leuzzi V, Kurian MA, Guerrini R. GNAO1 encephalopathy: broadening the phenotype and evaluating treatment and outcome. Neurology Genetics. 2017;3(2):e143. doi: 10.1212/NXG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisch S, Kumar KR. Pallidal deep brain stimulation for monogenic dystonia: the effect of gene on outcome. Front Neurol. 2021;11:630391. doi: 10.3389/fneur.2020.630391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honey CM, Malhotra AK, Tarailo-Graovac M, van Karnebeek CDM, Horvath G, Sulistyanto A. GNAO1 mutation–induced pediatric dystonic storm rescue with pallidal deep brain stimulation. J Child Neurol. 2018;33(6):413–416. doi: 10.1177/0883073818756134. [DOI] [PubMed] [Google Scholar]
- 10.Benato A, Carecchio M, Burlina A, Paoloni F, Sartori S, Nosadini M, d'Avella D, Landi A, Antonini A. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm (Vienna) 2019;126(6):739–757. doi: 10.1007/s00702-019-02010-2. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni N, Tang S, Bhardwaj R. Progressive movement disorder in brothers carrying a GNAO1 mutation responsive to deep brain stimulation. J Child Neurol. 2016;31(2):211–214. doi: 10.1177/0883073815587945. [DOI] [PubMed] [Google Scholar]
- 12.Koy A, Cirak S, Gonzalez V, Becker K, Roujeau T, Milesi C, Baleine J, Cambonie G, Boularan A, Greco F, Perrigault PF, Cances C, Dorison N, Doummar D, Roubertie A, Beroud C, Körber F, Stüve B, Waltz S, Mignot C, Nava C, Maarouf M, Coubes P, Cif L. Deep brain stimulation is effective in pediatric patients with GNAO1 associated severe hyperkinesia. Journal of the Neurological Science. 2018;391:31–39. doi: 10.1016/j.jns.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Danhofer P, Balintova Z, Balaz M, Jech R, Oslejskova H. Brittle biballism-dystonia in a pediatric patient with GNAO1 mutation managed using pallidal deep brain stimulation. Movement Disorders Clinical Practice. 2021;8:153–155. doi: 10.1002/mdc3.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita Y, Ogawa T, Ogaki K, Kamo H, Sukigara T, Kitahara E, Izawa N, Iwamuro H, Oyama G, Kamagata K, Hatano T, Umemura A, Kosaki R, Kubota M, Shimo Y, Hattori N. Neuroimaging evaluation and successful treatment by using directional deep brain stimulation and levodopa in a patient with GNAO1-associated movement disorder: a case report. Journal of the Neurological Science. 2020;411:116710. doi: 10.1016/j.jns.2020.116710. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz S, Turhan T, Ceylaner S, Gökben S, Tekgul H, Serdaroglu G. Excellent response to deep brain stimulation in a young girl with GNAO1-related progressive choreoathetosis. Childs Nerv Syst. 2016;32(9):1567–1568. doi: 10.1007/s00381-016-3139-6. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Nakashima M, Fukumura S, Kumada S, Saitsu H. An intronic GNAO1 variant leading to in-frame insertion cause movement disorder controlled by deep brain stimulation. Neurogenetics. 2022;23(2):129–135. doi: 10.1007/s10048-022-00686-5. [DOI] [PubMed] [Google Scholar]
- 17.Waak M, Mohammad SS, Coman D, Sinclair K, Copeland L, Silburn P, Coyne T, McGill J, O’Regan M, Selway R, Symonds J, Grattan-Smith P, Lin JP, Dale RC, Malone S. GNAO1-related movement disorder with life-threatening exacerbations: movement phenomenology and response to DBS. J Neurol Neurosurg Psychiatry. 2018;89(2):221–222. doi: 10.1136/jnnp-2017-315653. [DOI] [PubMed] [Google Scholar]
- 18.Alam M, Sanghera MK, Schwabe K, Lütjens G, Jin X, Song J, von Wrangel C, Stewart RM, Jankovic J, Grossman RG, Darbin O, Krauss JK. Globus pallidus internus neuronal activity: a comparative study of linear and non-linear features in patients with dystonia or Parkinson’s disease. J Neural Transm (Vienna) 2016;123(3):231–240. doi: 10.1007/s00702-015-1484-3. [DOI] [PubMed] [Google Scholar]
- 19.Runge J, Nagel JM, Cassini L et al (2022) Are transventricular approaches associated with increased hemorrhage? A comparative study in a series of 624 deep brain stimulation surgeries. Oper Neurosurg in press [DOI] [PubMed]
- 20.Nakamura K, Kodera H, Akita T, Shiina M, Kato M, Hoshino H, Terashima H, Osaka H, Nakamura S, Tohyama J, Kumada T, Furukawa T, Iwata S, Shiihara T, Kubota M, Miyatake S, Koshimizu E, Nishiyama K, Nakashima M, Tsurusaki Y, Miyake N, Hayasaka K, Ogata K, Fukuda A, Matsumoto N, Saitsu H. De novo mutations in GNAO1, encoding a Gao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. The American Journal of Human Genetics. 2013;93(3):496–505. doi: 10.1016/j.ajhg.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arya R, Spaeth C, Gilbert DL, Leach JL, Holland KD. GNAO1-associated epileptic encephalopathy and movement disorders c607G>A variant represents a probable mutation hotspot with a distinct phenotype. Epileptic Disord. 2017;19(1):67–75. doi: 10.1684/epd.2017.0888. [DOI] [PubMed] [Google Scholar]
- 22.Moro E, Gross RE, Krauss JK. What's new in surgical treatment for dystonia? Mov Disord. 2013;28(7):1013–1020. doi: 10.1002/mds.25550. [DOI] [PubMed] [Google Scholar]
- 23.Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y, Volkmann J, Krauss JK. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese R, Volkmann J. Deep brain stimulation for the dystonias: evidence, knowledge gaps, and practical considerations. Movement Disorders Clinical Practice. 2017;4(4):486–494. doi: 10.1002/mdc3.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request.