Abstract
The physiological activity of the 50% ethanolic extract of Citrus aurantium flower before and after fermentation was investigated in this study. C. aurantium flowers grown in Taiwan were extracted using 100% methanol or 50% ethanol and then fermented by one of six microbes: four species of lactic acid bacteria (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus, and Lactobacillus brevis) anaerobically cultivated in MRS broth and two species of mold (Aspergillus oryzae and Aspergillus niger) aerobically cultivated in potato dextrose broth. The 50% ethanolic extract of C. aurantium flowers exhibited higher tyrosinase inhibition (IC50: 200.8 ± 11.6 mg/L) and antioxidative activity than did a 100% methanolic extract (IC50: 274.1 ± 15.7 mg/L). The 50% ethanolic extract fermented by L. brevis (L. brevis–fermented extract) exhibited the highest yield (86.2% ± 1.2%) and physiological activity. The L. brevis–fermented extract exhibited over 5.2-, 13.5-, 12.5-, 3.17-, and 4.29-fold higher antityrosinase activity, antioxidative activity, antibacterial activity, total flavonoid content, and antiwrinkle activity than did the unfermented extract. The L. brevis–fermented extract can be considered safe because it exerted no toxic effect on CCD-966SK or HEMn cells at concentrations of 400 and 200 mg/L, respectively. The fermented extract (40 mg/L) inhibited melanin formation, reducing it to 50.8% ± 2.3%. Furthermore, the L. brevis–fermented extract exhibited excellent antiaging and antiwrinkle activity, as determined from MMP-1, MMP-2, elastase, and collagenase activity. The improvement in physiological characteristics, especially the considerable formation of neohesperidin, is mainly attributable to biosynthesis or biotransformation by L. brevis during fermentation. In conclusion, the 50% ethanolic extract of C. aurantium flowers fermented with L. brevis can be used in cosmetics applications aiming for skin-whitening or antiwrinkle properties.
Keywords: Antioxidant, Antiwrinkle, Citrus aurantium, Fermentation, Melanin
Antioxidant; Antiwrinkle; Citrus aurantium; Fermentation; Melanin.
1. Introduction
Plant-based bioactives have nutraceutical potential and multifunctional properties and have attracted attention, particularly phenolic acids, flavonoids, and terpenoids. These natural compounds have low toxicity and few side effects; exert anticarcinogenic effects; and have health-promoting characteristics, including antiaging, antioxidative, and antimicrobial properties (Dosoky and Setzer, 2018; Değirmenci and Erkurt, 2020).
The skin and hair pigment melanin protects the skin from radiation. Melanogenesis occurs when melanin accumulates in the skin's epidermal layer and is related to skin aging. The production of melanin is facilitated by the enzyme tyrosinase (Mapunya et al., 2012). Overactivity of tyrosinase and its related proteins results in hyperpigmentation. Signs of aging, wrinkles in particular, are visible manifestations of proteolytic degradation of the extracellular matrix (ECM). Scholars have discovered significant associations of ECM degradation with wrinkly formation and dermal collagenase, elastase, interstitial collagenase [matrix metalloproteinase (MMP)-1], and 72-kDa gelatinase (MMP-2) activity (Maity et al., 2011).
The Rutaceae species Citrus aurantium L., or bitter orange is resistant to several viral diseases, is tolerant of cold, and can be used to improve the fruit quality of grafted plants (Sarrou et al., 2013). It also has numerous therapeutic properties. The entire immature fruit of this species is dried and used in traditional Chinese medicine (Stohs, 2017). Its peel, flowers, and leaves contain many bioactives—for example, essential oils, vitamins, flavonoids, and phenolics—and are thus used in the treatment of digestive and cardiovascular system disorders (Moraes et al., 2009; Haj Ammar et al., 2012). Phenolics and flavonoids are crucial because they are beneficial to health. Furthermore, the fruit, flowers, phytoconstituents, and essential oils of C. aurantium were discovered in a bioactivity study to exert antioxidative, anti-inflammatory, and antimicrobial effects (Suntar et al., 2018).
The physiological activity of biochemical products can be enhanced through their fermentation, which modifies their naturally occurring molecular constituents. Moreover, the cytotoxicity of herbal extracts can be reduced through fermentation by microorganisms (Wang et al., 2017). However, no study has investigated the physiological activity or characteristics of a fermented extract of C. aurantium flowers. We previously discovered that the bioactive and antioxidative activity of some herbal extracts were significantly improved when those extracts were fermented by probiotic bacteria, indicating that different bioactive compounds can be produced through various metabolic pathways during fermentation (Wang et al., 2016, 2017; Wu et al., 2018).
C. aurantium flowers were extracted with 100% methanol or 50% ethanol in this study. The extracts were fermented by various lactic acid bacteria or fungi. We evaluated whether the unfermented and fermented extracts had toxic effects on CCD-966SK cells and determined the extracts' tyrosinase inhibitory, antioxidative, antimicrobial, and antiwrinkle activity. The composition of unfermented and fermented extracts, including the levels of flavonoids and phenolics, was analyzed to elucidate the mechanisms underlying the extracts’ high physiological activity. Furthermore, to examine the skin-whitening potential and safety of the fermented extract, we investigated its toxic and tyrosinase inhibitory effects on human epidermal melanoma (HEMn) cells as well as its effect on the melanin content of these cells.
2. Material and methods
2.1. Plant material
C. aurantium cultivated in the mountainous area (at 500-m altitude) of Miaoli County, Taiwan, was obtained. C. aurantium flowers were collected in May 2020. The petals were carefully removed. Subsequently, the flowers were washed with distilled water, left to dry naturally, and then ground into a powder (particle size: 800 μm; modified from the method used by Razeghi et al., 2020). The dried powder was stored at 4 °C. Extraction was then performed with 100% methanol or 50% ethanol.
2.2. Microbial strains, cells, and tyrosinase
The C. aurantium flower extract was fermented by one of four lactic acid bacteria—namely, Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus, or Lactobacillus brevis [American Type Culture Collection (ATCC) codes 29521, 25741, 4356, and 8287, respectively)—or two fungi, namely, Aspergillus oryzae [Bioresource Collection and Research Center (BCRC) 32288] or A. niger (ATCC 42418). The activity of the fermented and unfermented extracts against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, and Aspergillus brasiliensis (ATCC codes 8739, 6538, 9027, 10231, and 16404, respectively) was evaluated on the basis of the USP 51-antimicrobial effectiveness test. These microbial strains were purchased from the BCRC (Hsinchu, Taiwan). HEMn cells from neonatal foreskin were obtained from Cascade Biologics (Cascade cat. C-102-5C, Portland, OR, USA) and cultured in human melanocyte growth supplement (HMGS)-supplemented Medium 254 (Cascade Biologics). For a comparison group, we obtained cells from the normal human skin fibroblast line CCD-966SK (ATCC CRL-1881) from the BCRC. We sourced mushroom tyrosinase and all other chemicals employed from Sigma-Aldrich (St. Louis, MO, USA) at analytical grade (purity >99%).
2.3. Microbial cultivation and extraction, and fermentation of C. aurantium flowers
The four lactic acid bacteria were anaerobically cultivated for 48 h in MRS broth at 37 °C. The mold species (A. oryzae, A. niger, and A. brasiliensis) were aerobically cultivated for 7 days in potato dextrose broth (PDB) at 25 °C while shaking at 300 rpm. C. albicans was aerobically cultivated for 3 days in a yeast mold broth at 24 °C while shaking at 300 rpm. Aerobic cultivation of the other bacteria (P. aeruginosa, S. aureus, and E. coli) was performed overnight in tryptic soy broth (TSB) at 37 °C with shaking at 350 rpm.
In the extraction, 1 g of dried C. aurantium flower powder was added to 90 mL of solution containing 10 mL of 6 M HCl with 80 mL of either 50% ethanol or 100% methanol, and the solution was then gently stirred for 30 min. The pH of the resulting mixtures ranged from 1.8 to 2.2 (modified from a method used by Karimi et al., 2012). Subsequently, the methanolic and ethanolic extracts were filtered, and a rotary vacuum evaporator operated for 2 h at 45 °C (methanolic extract) or 60 °C (ethanolic extract) was employed to concentrate them. Until used, the concentrated extracts were maintained at 4 °C.
In the fermentation, 200 mL of phosphate-buffered solution (PBS, pH 6) containing 2 g of C. aurantium flower extract was mixed with 2 × 107 cfu/mL lactic acid bacteria. Anaerobic incubation of the mixture (for 1–5 days) was then performed at 37 °C. In addition, 2 g of C. aurantium flower extract, dissolved in 200 mL of PBS (pH 5), was mixed with one of two fungi (A. oryzae and A. niger) at 2.0 × 107 spores/mL. Aerobic incubation of the mixtures (for 2–8 days) was then performed at 25 °C with shaking at 300 rpm (Wang et al., 2019). Curves of the lactic acid bacteria and fungi growth during fermentation are presented in Figures S1 and S2, respectively. Fermentation was conducted for a fixed period. After 10 min of centrifugation (8000 × g), the supernatant of the fermented solution was collected, filtered using a 0.45-μm filter, and concentrated with a rotary vacuum evaporator operated at 60 °C until a paste had formed. The fermented C. aurantium extracts were kept at 4 °C until further experiments were performed. We then compared the physiological characteristics of the unfermented extracts with those of the fermented extracts.
2.4. Antityrosinase activity and cellular melanin content
The method described by Zheng et al. (2012) was employed to analyze the antityrosinase activity of the fermented and unfermented C. aurantium flower extract. Dimethyl sulfoxide (DMSO, 1 g/L)-based solutions containing the extract at differing concentrations were prepared. Subsequently, 30 μL of the DMSO-based solutions was mixed with 970 μL of 0.05 mM PBS, after which the mixture was added to a solution containing 1 mL each of 350 unit/mL mushroom tyrosinase solution and 100 mg/L L-tyrosine. Under darkness, 3 mL of this reaction solution was mixed until homogenous. After the solution had been incubated for 20 min, the solution's absorbance at 490 nm was determined through ultraviolet–visible (UV–vis) spectrophotometry (UV-2600i, Shimadzu, Japan). The control comprised distilled water instead of an extract, and the positive controls employed were α-arbutin and kojic acid. The IC50 value was defined as the concentration of the extract when half the original tyrosinase activity was inhibited. The following equation, expressing the percentage of tyrosinase inhibition, was used to calculate an extract's antityrosinase activity (Wu et al., 2018):
| (1) |
where A, B, C, and D are the absorbance at 490 nm of no extract (control), no extract and enzyme (blank), extract and enzyme (experimental group), and extract but no enzyme (blank of C), respectively.
The method described by Wang et al. (2019) was employed to analyze the antityrosinase activity of the fermented extracts in HEMn cells. First, HEMn cells (2 × 106 cells/well) were incubated in Medium 254 supplemented with HMGS at 37 °C for 24 h and grown in 24-well plates. Various concentrations of the fermented extract were applied to the cells for 24 h, after which the cells were collected and washed in PBS before then being lysed with a solution containing 1% Triton X-100, 0.1 M PBS, and protease inhibitors. Lysates were obtained by sonicating the aforementioned solution for 5 min at 4 °C and then centrifuging it for 15 min at 8000 × g. In 96-well plates, 2.5 mM L-DOPA in 0.1 M PBS was reacted with the lysates for 60 min. A microplate in an Epoch ELISA reader (BioTek Instruments, USA) was used to read the absorbance at 475 nm.
To examine the melanin content of the HEMn cells, we used a slight modification of the approach reported by Liao et al. (2012). First, the 2 × 106 HEMn cells placed in each well of six-well culture plates and incubated for a period of 24 h. Subsequently, various concentrations of fermented extract were applied to the cells, which were subsequently incubated for another 72 h. Thereafter, trypsinization was performed to collect the cells, which were then centrifuged for 5 min at 2000 × g. After removal of the supernatant, we recovered the cell pellet and suspended it in 100 μL of 1 N NaOH containing 10% DMSO. After heating the suspension at 70 °C for 1.5 h, we used an ELISA plate reader to determine the suspension's absorbance at 405 nm. Finally, through comparison with a synthetic melanin standard, we determined the cells' melanin content.
2.5. Analysis of scavenging activity of 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
We employed a 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay to determine the antioxidative capacity of the fermented and unfermented C. aurantium flower extracts. The method described by Wu et al. (2018) was used to estimate levels of DPPH. First, 100 μM DPPH solution in 97% ethanol was prepared. Various concentrations (0.4 mL) of C. aurantium flower extract were added to 0.8 mL of methanol and 1 mL of the DPPH solution. After 1 h of dark incubation, a UV–vis spectrophotometer was employed to determine the absorbance at 517 nm of the extract solution and of a blank not containing the extract; the positive control was BHT. The scavenging activities of the DPPH radical of extracts were calculated as follows (Wu et al., 2018). The IC50 value of the DPPH scavenging activity of the extract was determined at 50% scavenging activity.
| (2) |
where A0 and A are the absorbance at 517 nm without and with the extract.
Scholars widely use the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical to examine the antioxidative activity of natural compounds. ABTS can interact with both hydrophilic and lipophilic systems. We employed a slightly modified version of the method presented by Merchán Arenas et al. (2011) to determine the ABTS scavenging activity of the unfermented and fermented extracts. First, 2.45 mM potassium persulfate was reacted with 7 mM ABTS solution (1:1) for 18 h in the dark to produce the ABTS radical cation. Before the extracts’ antioxidative activity was measured, the prepared ABTS solution was diluted to an optical density of 0.7 at 734 nm. Various concentrations of the fermented and unfermented extracts of C. aurantium flowers (50 μL) were mixed with 950 μL of the diluted ABTS solution for 30 min. The positive control was again BHT. The ABTS scavenging activity of the extracts was calculated as follows (Merchán Arenas et al., 2011). The IC50 value of the ABTS scavenging activity by the extract was expressed at 50% scavenging activity.
| (3) |
where A0 and A are the absorbance at 734 nm without and with the extract.
2.6. Total reducing power
The method of Wang et al. (2016) was used to quantify the total reducing power. We mixed the fermented and unfermented extracts of C. aurantium flowers (1 mL) at various concentrations with 2.5 mL of 0.2 M PBS and 2.5 mL of 1% potassium ferricyanide, and the ensuing reaction proceeded for 20 min in a water bath at the temperature 50 °C. After the solution had been left to cool, 2.5 mL of 10% trichloroacetic acid was employed to terminate the reaction (in approximately 5 min). After 15 min of centrifugation of the reaction solution at 3500 × g, we collected the supernatant and mixed 2 mL with 0.4 mL of 0.1% ferric chloride and 2 mL of deionized water. The consequent reaction proceeded for 10 min, and subsequently, the UV–vis spectrophotometer was used to measure the solution's absorbance at 700 nm. The positive controls were ascorbic acid and α-tocopherol. The IC50 value was defined as the concentration of the extracts at which the absorbance was 0.5.
2.7. Minimum inhibitory and fungicidal concentrations
The minimum inhibitory concentrations (MICs) of the fermented and unfermented extracts of C. aurantium flowers for P. aeruginosa, S. aureus, and E. coli were determined using a serial tube dilution method in accordance with Rahman et al. (2013). In brief, 2 mL of extract (the concentration of which was varied), 2 mL of TSB, and 1 mL of inoculum (5 × 106 cfu/mL) were combined in a test tube and incubated for 18 h at 37 °C. The lowest extract concentration for which the growth of E. coli, S. aureus, or P. aeruginosa was visibly prevented was considered the MIC.
We used the conventional plate count method to evaluate the extracts’ antifungal activity. In brief, 1 mL of extract (the concentration of which was varied), 1 mL of inoculum (2 × 107 cfu/mL), and 100 mL of specific broth (YM for C. albicans and PDB for A. brasiliensis) were combined in a conical flask and incubated for 5 days at 25 °C. The lowest concentration killing 99.9% of the inoculum (C. albicans or A. brasiliensis) was considered the minimum fungicidal concentration (MFC).
2.8. Total phenolic and flavonoid content
The approach of Kujala et al. (2000) was employed with minor modifications to quantify the total phenolic content (TPC) in terms of gallic acid equivalents (GAE) of the unfermented and fermented C. aurantium flower extracts. First, 0.5 mL of an extract was oxidized for 10 min with 1 mL of Folin–Ciocalteu phenol reagent (diluted 1/10); the mixture was then neutralized using 1 mL of 7.5% Na2CO3 solution for 3 h. This was followed by 15 min of centrifugation at 3000 × g. The UV–vis spectrophotometer was employed to measure the supernatant's absorbance at 760 nm. The TPC of each extract (mg-GAE/g-dried extract) was determined by consulting a calibration curve of gallic acid standard solutions. For absorbance y and gallic acid concentration x, the calibration curve could be expressed as y = 0.0525x + 0.0712 (R2 = 0.9912).
Total flavonoid content (TFC) was quantified through aluminum chloride colorimetry and is expressed in rutin equivalents (RE; Pourmorad et al., 2006). In brief, 1 mL of each extract was combined with 0.3 mL of 5% NaNO2, 0.3 mL of 10% AlCl3 solution, and 4 mL of distilled water, after which the mixture stood for 10 min. Subsequently, 2 mL of 1 M NaOH followed by 2.4 mL of distilled water was added. The 10-mL solution was gently stirred and stood for 30 min. The UV–vis spectrophotometer was employed to measure the mixture's absorbance at 510 nm. The TFC of the extract was calculated (mg-RE/g-dried extract) from a calibration curve of rutin standard solutions. For absorbance y versus rutin concentration x, the calibration curve could be expressed as y = 0.0721x + 0.0362 (R2 = 0.9825).
2.9. Quantification of phenolics and flavonoids
A high-performance liquid chromatography (HPLC) system (Hitachi, Tokyo, Japan) was used to determine the main phenolics and flavonoids in the C. aurantium flower extracts and the concentrations of these compounds. For HPLC, a flow rate of 0.6 mL/min, injection volume of 25 μL, and 5-μm and 4.6 × 150-mm2 Inertsil ODS-3 column (Gl Science, Tokyo, Japan) were used. Separation was performed using gradient elution (solution A: deionized water; solution B: acetonitrile) with the following settings: 85% A followed by 40% A at 25 min, 15% A at 50 min, 40% A at 75 min, and 85% A at 100 min. Trifluoroacetic acid was employed to adjust the pH to 2.5. For detecting various compounds, the wavelength was varied from 230 to 400 nm. The wavelength used for the detection of isoflavones, alcohols, and phenolic compounds was 280 nm, whereas that used for the detection of flavonoids was 350 nm (Crozier et al., 1997). A compound's retention time was compared with those of standard samples under the same conditions to determine the compound's identity.
2.10. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Yang et al., 2017) was used to assess the extracts' toxicity to CCD-966SK and HEMn cells. We cultivated the CCD-966SK and HEMn cells for 24 h in minimum essential medium containing 10% fetal bovine serum and HMGS-supplemented Medium 254, respectively, in a 96-well plate at 3 × 106 cells/well; subsequently, either fresh medium (control) or extract (the concentration of which was varied) was applied for 24, 48, or 72 h. Next, 0.02% MTT was added, and the cells were incubated in a 5% CO2 environment for 4 h at 37 °C. The solution was then removed, and 100 μL of DMSO was added. An Epoch ELISA reader was employed to measure the liquid's absorbance at 570 nm. The following formula was used to calculate the viability of the cells (Yang et al., 2017):
| (4) |
2.11. Antiwrinkle activity
Wrinkles are related to the inhibition of MMP-1, MMP-2, elastase, and collagenase activity (Azmi et al., 2014). Thus, we analyzed these four aging-related enzymes. The method proposed by Vandooren et al. (2011) was employed to measure collagenase activity by using a modified fluorogenic dye-quenched (DQ)-gelatin assay. First, extract (the concentration of which was varied) was added to a 96-well plate, with 1 U/mL collagenase (100 μL/well) and 15 μg/mL DQ-gelatin added to each well. The mixture then stood for 20 min. The absorbance at 485 and 530 nm (excitation and emission, respectively) was measured to determine the gelatin proteolysis rate. A colorimetric assay with N-succinyl-Ala-Ala-Ala-p-nitroanilide (Suc-Ala) as the substrate was employed to assay elastase activity (Karim et al., 2014); specifically, 50 μL of extract (the concentration of which was varied) was reacted with 125 μL of 7 mM Suc-Ala (pH 8.0; prepared in 0.1 M Tris–Cl buffer) for 15 min at 25 °C in a 96-well plate, after which 25 μL of 0.3 U/mL neutrophil elastase was added. The reaction proceeded for 15 min, and the Epoch ELISA reader was employed to determine the absorbance at 405 nm.
The MMP-1 activity in CCD-966SK cells was tested using a human MMP-1 ELISA kit (RayBiotech, Norcross, GA, USA) as described by Tsai et al. (2014). CCD-966SK cells were added to 96-well plates and left for 24 h under 5% CO2 at 37 °C. The media were then removed, and to each dish, extract (the concentration of which was varied) was added for 24 h. The ELISA kit was used for mixing, and a reaction occurred at 25 °C. After 2 h of incubation, the reacted mixture was spectrophotometrically analyzed at 420 nm. The MMP-2 activity in CCD-966SK cells was assayed using gelatin zymography (Toth et al., 2012). First, the cells underwent 24-h culturing in serum-free Dulbecco's modified eagle medium. After this 24 h, the supernatant from the culture was collected and reacted with a solution containing 10% polyacrylamide gel and 0.1% gelatin. Subsequently, 3% Triton X-100 was used to wash the gel, which was then incubated for 24 h with an activation buffer containing extract (the concentration of which was varied). Staining was performed to visualize MMP-2 activity. Coomassie Brilliant Blue R (0.1%) was used as the stain, and destaining was achieved using a solution containing 10% acetic acid and 30% methanol. In the staining images, clear bands indicating MMP-2 activity are shown against a blue background. Image J software was used to quantitate the digestion bands.
2.12. Statistical analysis
All experiments were performed in triplicate; we thus express the findings as means ± standard deviations. One-way analysis of variance followed by Duncan's multiple range test with P < 0.01 indicating statistical significance was employed. IBM SPSS version 26 (SPSS, Chicago, IL, USA) was used for all analyses. Nonlinear regression analysis, which was performed in the Origin software (Electronic Arts, Redwood City, CA, USA), was used to calculate the IC50 values.
3. Results and discussion
3.1. Solvent selection and yield
Fermentation by Aspergillus spp. and that by probiotic bacteria improve the physiological activity and reduce the cytotoxicity of herbal extracts (Wang et al., 2017, 2019). Thus, we investigated the physiological activity and cytotoxicity of the unfermented C. aurantium flower extract and extracts fermented by one of four lactic acid bacteria (L. brevis, L. acidophilus, B. lactis, and B. bifidum) or two fungi (A. oryzae and A. niger). Additionally, we determined the optimal fermentation time for various microbes to recovering the highest yield of fermented extract. During the extraction process, 50% ethanol (8.6% ± 0.4%) resulted in a higher extract yield than did 100% methanol (5.1% ± 0.7%; Table 1). The highest and lowest yields recovered from fermentation were achieved by the 50% ethanolic extract of C. aurantium flowers fermented by L. brevis (86.2% ± 1.2%) and that fermented by A. oryzae (70.2% ± 5.3%), respectively. The yield of the L. brevis–fermented extract relative to that of dried C. aurantium flower powder was 7.4%. The optimal fermentation time for lactic acid bacteria and fungi was discovered to be 3 and 5 d, respectively.
Table 1.
Yield of 100% methanolic and 50% ethanolic C. aurantium flower extracts and 50% ethanolic extracts fermented by one of four lactic acid bacteria or two fungi.
| 100% methanol | 50% ethanol | B. bifidum | B. lactis | L. acidophilus | L. brevis | A. oryzae | A. niger | |
|---|---|---|---|---|---|---|---|---|
| Extraction/Recover yield (%) | 5.1 ± 0.7%1 | 8.6 ± 0.4% | 78.2 ± 1.2%2,c | 76.5 ± 0.6%d | 83.1 ± 1.4%b | 86.2 ± 1.2%a | 70.2 ± 5.3%d | 73.5 ± 7.1%d |
| Optimal fermentation time (d) | – | – | 3 | 3 | 3 | 3 | 5 | 5 |
Yield1 = g of dried extract by solvent/g of dried C. aurantium flower powder.
Yield2 = g of dried fermented extract/g of dried 50% ethanolic extract.
In each row, letters (a–d) indicate P < 0.01.
3.2. Antityrosinase and antioxidative activity
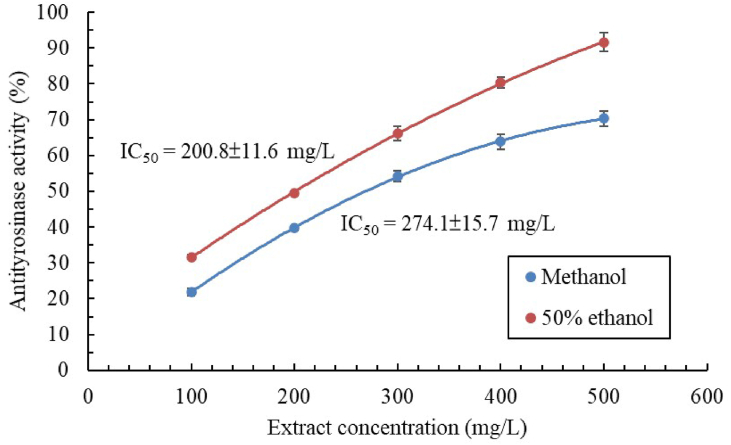
The antityrosinase activity of the extracts increased with their concentration (Figure 1). Such a dose-dependent increase was also discovered for the methanolic extract of pomegranate peel (Fawole et al., 2012). Nonlinear regression analysis indicated that the antityrosinase activity of the 50% ethanolic extract of C. aurantium flowers (IC50: 200.8 ± 11.6 mg/L) was higher than that of the 100% methanolic extract (IC50: 274.1 ± 15.7 mg/L). One study discovered that the methanolic extract of Nepalese C. aurantium exhibited inhibited tyrosinase activity more effectively (IC50: 48.2 mg/L) than did its Taiwanese counterpart (Adhikari et al., 2008). This finding indicates that the cultivation environment affects the physiological activity even when the same extraction technique is used. Because the antityrosinase activity of the 50% ethanolic extract was superior to that of the methanolic extract, we used the 50% ethanolic extract in the subsequent fermentation experiments. Table 2 lists the antityrosinase activity of the 50% ethanolic extracts of C. aurantium flowers fermented by various lactic acid bacteria or fungi. Among all the tested microbes, L. brevis resulted in the strongest tyrosinase inhibition, with the IC50 being 38.2 ± 0.9 mg/L. The fermented extracts had much higher antityrosinase activity (5.2 times higher) than the unfermented extracts (IC50: 200.8 ± 11.6 mg/L) had. However, after fermentation, physiological activity, increased nonsignificantly in some cases (e.g., after fermentation by B. lactis) or even decreased (e.g., after fermentation by A. oryzae and A. niger). Thus, selecting an appropriate microbe is vital. For the positive controls kojic acid and α-arbutin, the tyrosinase IC50 was 20.4 ± 1.2 and 276.1 ± 9.2 mg/L, respectively.
Figure 1.
Antityrosinase activity of extracts of C. aurantium flowers obtained using 100% methanol or 50% ethanol. Data are expressed as the mean ± standard deviation of three independent experiments.
Table 2.
Tyrosinase inhibition and DPPH radical scavenging (expressed as IC50, mg/L) of the 50% ethanolic extract of C. aurantium flowers fermented by various lactic acid bacteria or fungi.
| B. bifidum | B. lactis | L. acidophilus | L. brevis | A. oryzae | A. niger | |
|---|---|---|---|---|---|---|
| Tyrosinase inhibition | 120.8 ± 5.2c | 202.3 ± 8.8d | 48.2 ± 2.3b | 38.2 ± 0.9a | 536.3 ± 7.1e | 581.2 ± 4.6f |
| DPPH scavenging activity | 96.2 ± 3.8c | 275.8 ± 10.3d | 36.1 ± 1.2b | 18.6 ± 0.6a | 418.2 ± 6.5e | 462.3 ± 7.6f |
In each row, letters (a–f) indicate P < 0.01.
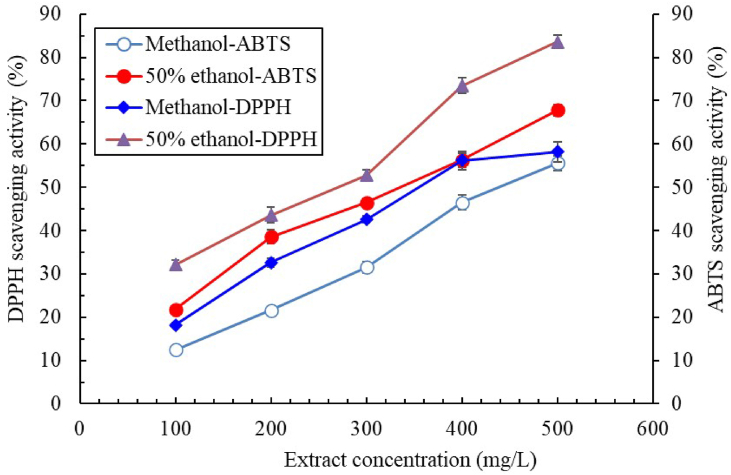
Figure 2 illustrates the DPPH and ABTS scavenging activity of the methanolic and ethanolic extracts of C. aurantium flowers. The scavenging activity increased with the extract concentration. The DPPH scavenging activity of the methanolic extract (IC50: 358.2 ± 16.1 mg/L) was lower than that of the 50% ethanolic extract (IC50: 272.4 ± 8.6 mg/L). In addition, the 50% ethanolic extract had higher ABTS scavenging activity (IC50: 345.8 ± 16.3 mg/L) than did the methanolic extract (IC50: 446.3 ± 17.1 mg/L). These results indicated that 50% ethanol was the optimal extraction solvent for C. aurantium flowers. Table 2 lists the DPPH scavenging activity of 50% ethanolic extracts fermented by various acid bacteria or fungi. The highest DPPH scavenging activity was achieved by the L. brevis–fermented extract (IC50: 18.6 ± 0.6 mg/L). The fermented extracts had much higher DPPH scavenging activity (14.6 times higher) than the unfermented extracts (IC50: 272.4 ± 8.6 mg/L). Hsouna et al. (2013) reported that the essential oil obtained from C. aurantium flowers exhibited excellent DPPH scavenging ability (IC50: 15.3 mg/L), and this finding is similar to our finding for the L. brevis–fermented extract. In the present study, the IC50 for DPPH of the positive control BHT was 49.6 ± 2.8 mg/L. The present results demonstrate the promise of fermented C. aurantium flower extract for use as an antioxidant. In conclusion the extract subjected to L. brevis fermentation achieved the highest antityrosinase and DPPH scavenging activity, compared with those fermented by other microbes. Thus, L. brevis was selected to ferment the 50% ethanolic extract in the subsequent experiments.
Figure 2.
DPPH and ABTS scavenging activity of C. aurantium flower extract obtained using 100% methanol or 50% ethanol. Data are expressed as the mean ± standard deviation of three independent experiments.
3.3. Antimicrobial activity
A previous study reported that antioxidative activity and antimicrobial activity are positively correlated (Wu et al., 2018). Because the L. brevis–fermented extract in that study exhibited high antioxidative activity, we examined our extracts’ antimicrobial activity against five of the microbial species mentioned in the USP 51-antimicrobial effectiveness test. The MIC and MFC of the 50% ethanolic extract of C. aurantium flowers were lower than those of the methanolic extract (Table 3); however, the antimicrobial activity of the 50% ethanolic extract was low (800–2500 mg/L). The antibacterial activity (MIC) of the L. brevis–fermented extract against E. coli, S. aureus, and P. aeruginosa significantly increased by 15, 12.5, and 16 times, respectively, after fermentation compared with before fermentation. Moreover, the antifungal activity (MFC) of the L. brevis–fermented extract against C. albicans and A. brasiliensis increased by 6.7 and 13.9 times after fermentation compared with before fermentation (50% ethanolic extract). The fermented extracts were most effective against P. aeruginosa (50 mg/L), followed by E. coli (80 mg/L), S. aureus (120 mg/L), C. albicans (150 mg/L), and A. brasiliensis (180 mg/L), in that order. Similar antimicrobial activity was reported for the essential oil of C. aurantium; however, this oil was discovered to be ineffective against S. aureus (Ammar et al., 2012).
Table 3.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of 100% methanolic and 50% ethanolic C. aurantium flower extracts and 50% ethanolic C. aurantium flower extracts fermented by L. brevis against various bacteria and fungi.
| MIC (mg/L) |
MFC (mg/L) |
||||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. albicans | A. brasiliensis | |
| Methanolic extract | 2,000a | 2,000a | 1,500a | 3,000a | 5,000a |
| 50% ethanolic extract | 1,500b | 1,200b | 800b | 1,000b | 2,500b |
| L. brevis–fermented extract | 120c | 80c | 50c | 150c | 180c |
In each column, letters (a–c) indicate P < 0.01.
Hsouna et al. (2013) reported the MICs of the essential oil of C. aurantium against S. aureus and P. aeruginosa were 312 and 2500 mg/L, respectively. Teneva et al. (2019) reported that an essential oil obtained from C. aurantium through steam distillation was relatively ineffective against Gram-negative bacteria (E. coli, P. aeruginosa, and Salmonella sp.), with an MIC of >600 mg/L, but effective against Gram-positive bacteria (S. aureus and B. subtilis), with an MIC of 60 mg/L. Compared with the essential oils extracted in these previous studies, the L. brevis–fermented extract obtained in our study was more effective, with an MIC of ≤120 mg/L, against the tested microbes.
3.4. Analysis of antioxidative characteristics
Wen et al. (2013) reported that fermented products have higher TPC and antioxidative and antibacterial activity than do unfermented extracts. We determined the possible sources of and mechanisms underlying the antityrosinase, antioxidative, and antimicrobial activity of our extracts. Table 4 lists the antioxidative activity of the 50% ethanolic extracts, unfermented or fermented by L. brevis. According to our results, the ABTS scavenging activity, total reducing power, TPC, and TFC of the fermented 50% ethanolic extract were 13.5, 2.06, 1.79, and 3.17 times higher, respectively, than those of the unfermented extract. Thus, the L. brevis–fermented extract appeared to have high antioxidative ability. The difference in TFC between the fermented versus unfermented extracts was greater than that in their TPC. Thus, the high physiological activity of the L. brevis–fermented extract may be attributable to the high concentration of flavonoids. The IC50 of the L. brevis–fermented extract in the ABTS assay was 25.6 ± 1.4 mg/L; this value was superior to the IC50 (672 mg/L) of a C. aurantium extract obtained using boiled distilled water (Ammar et al., 2012) and that of the positive control, BHT (52.3 ± 3.7 mg/L). The TPC and TFC of the L. brevis–fermented extract were 134.8 ± 20.6 mg-GAE/g-dried extract and 102.8 ± 10.8 mg-RE/g-dried extract, respectively; these values are higher than those of an 80% ethanolic extract of C. aurantium flowers reported previously (4.55 mg-GAE/g-dried extract and 3.83 mg-RE/g-dried extract; Karimi et al., 2012). This finding indicates the superiority and therapeutic potential of the extraction–fermentation process developed in the present study.
Table 4.
Antioxidative characteristics of 50% ethanolic C. aurantium flower extract unfermented and fermented by L. brevis.
| ABTS scavenging activity (IC50, mg/L) | Total reducing power (IC50, mg/L) | Total phenolic content (mg-GAE/g-dried extract) | Total flavonoid content (mg-RE/g-dried extract) | |
|---|---|---|---|---|
| 50% ethanolic extract | 345.8 ± 2.5a | 325.8 ± 20.6a | 75.3 ± 2.7a | 32.4 ± 6.2a |
| L. brevis–fermented extract | 25.6 ± 1.4b | 158.1 ± 16.3b | 134.8 ± 20.6b | 102.8 ± 10.8b |
In each column, letters (a–b) indicate P < 0.01.
3.5. Identification of phenolic and flavonoid compounds
Wang et al. (2016) discovered high TPC in herb extracts after fermentation and indicated that the high TPC enhanced the extracts' tyrosinase inhibition and DPPH scavenging activity. The high physiological activity of the L. brevis–fermented extract may be attributable to the high flavonoid concentration after fermentation (results of section 3.4). The phenolics and flavonoids in the unfermented and fermented extracts were analyzed through HPLC to determine their phenolic and flavonoid compositions (Table 5). In the unfermented extract, the major phenolic compounds were ferulic acid, pyrogallol, syringic acid, and gallic acid and the major flavonoid compounds were rutin, naringin, hesperidin, and neohesperidin. The major phenolic compounds in the L. brevis–fermented extract were pyrogallol, syringic acid, and gallic acid, and the principal flavonoid compounds were rutin, naringin, and neohesperidin. The extract's rosmarinic acid content, ferulic acid content, and hesperidin content were lower after fermentation. The hesperidin content significantly decreased to 14.6% after fermentation. The caffeic acid, rutin, apigenin, luteolin, and quercetin content differed only slightly after fermentation. Conversely the pyrogallol, syringic acid, gallic acid, naringin, rhoifolin, eriocitrin, and neohesperidin content increased with fermentation.
Table 5.
Compositions and contents of phenolics and flavonoids in 50% ethanolic C. aurantium flower extract, unfermented and fermented by L. brevis.
| 50% ethanolic extract | L. brevis–fermented extract | |
|---|---|---|
| Phenolics (μg/g-dried extract) | ||
| caffeic acid | 224 ± 2.31a | 206 ± 1.28b |
| rosmarinic acid | 75.4 ± 0.82a | 36.1 ± 1.12b |
| ferulic acid | 235 ± 1.89a | 52.3 ± 3.26b |
| pyrogallol | 526 ± 6.23b | 806 ± 8.61a |
| syringic acid | 278 ± 1.56b | 625 ± 9.33a |
| gallic acid | 206 ± 3.26b | 513 ± 7.21a |
| Flavonoids (μg/g-dried extract) | ||
| rutin | 405 ± 2.65a | 415 ± 2.14a |
| apigenin | 73.6 ± 0.63b | 82.3 ± 0.51a |
| luteolin | 86.3 ± 1.25b | 92.6 ± 0.95a |
| naringin | 361 ± 2.41b | 754 ± 5.83a |
| quercetin | 126 ± 0.95b | 138 ± 1.21a |
| rhoifolin | 21.6 ± 0.35b | 56.3 ± 0.84a |
| eriocitrin | 32.6 ± 1.45b | 47.2 ± 1.08a |
| hesperidin | 251 ± 3.48a | 36.7 ± 0.84b |
| neohesperidin | 658 ± 7.81b | 1,036 ± 12.3a |
In each row, letters (a–b) indicate P < 0.01.
In particular, the content of neohesperidin significantly increased from 658 ± 7.81 to 1036 ± 12.3 μg/g-dried extract after fermentation. The results indicated that hesperidin biotransformed into neohesperidin during the fermentation process (Frydman et al., 2005). We also discovered that the major phenolics and flavonoids were more abundant in the fermented extract than the unfermented extract. Enzymes released from the cell wall of C. aurantium were inactive during extraction. Thus, the generation of phenolic and flavonoid compounds was attributed to fermentation and considered to result from modification of naturally occurring molecular constituents (Wang et al., 2016, 2017; Wu et al., 2018). Li (2012) reported that phenolic compounds namely pyrogallol, caffeic acid, and gallic acid, present in C. aurantium flowers extracts were favorable antioxidants. Flavonoid compounds—naringin, eriocitrin, and neohesperidin—exhibit high antioxidative and radical scavenging activity (Sicari et al., 2016). The high antioxidative activity of the L. brevis–fermented extract can thus be explained by the presence of these compounds. Similar flavonoid components—including rutin, naringenin, quercetin, rhoifolin, eriocitrin, hesperidin, and neohesperidin—were found in a Chinese C. aurantium extract (Shen et al., 2017), and common flavonoid compounds including apigenin, luteolin, naringenin, and hesperidin were discovered in a Korean C. aurantium extract (Suntar et al., 2018). In a study of methanolic C. aurantium flower extracts, pyrogallol was found to be most abundant (541 μg/g-dry weight) phenolic compound, followed by syringic acid (269 μg/g-dry weight), caffeic acid (249 μg/g-dry weight), and gallic acid (212 μg/g-dry weight), whereas naringin was the most abundant flavonoid compound (688.1 μg/g-dry weight), followed by rutin (362 μg/g-dry weight) and quercetin (185 μg/g-dry weight; Karimi et al., 2012). The differences between the results of previous studies and ours are mainly attributable to the extraction solvents used.
3.6. Analysis of cell viability, antityrosinase activity, and melanin content
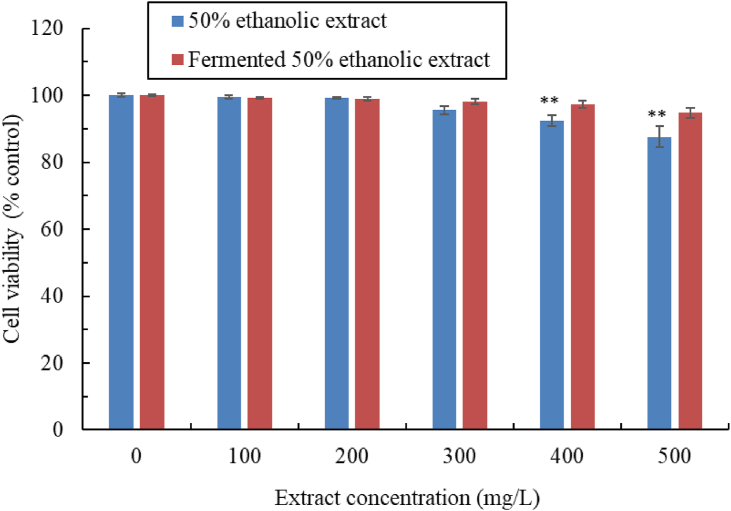
Safety is a more important consideration than pharmacological efficacy in the adoption of a new therapeutic substance. The toxicity to CCD-966SK cells of various concentrations of the unfermented and fermented extracts was examined using the MTT method (Figure 3). The cell viability of CCD-966SK cells was determined after 24, 48, and 72 h of treatment. When the treatment duration was 24 or 48 h, the C. aurantium flower extracts (both unfermented and fermented) were found to not have influenced cell viability. After 72 h of treatment, we discovered cell viability of >95.6% and found that the cytotoxicity of the 50% ethanolic extract did not significantly differ from the cytotoxicity of the control when the concentration was low (≤300 mg/L). Significantly lower cell viability compared with the control (P < 0.01) was discovered when the concentration of the unfermented extract was ≥400 mg/L (viability of 92.3%–87.6%). However, the L. brevis–fermented extract nonsignificantly inhibited cell viability, even at 500 mg/L, suggesting that the L. brevis–fermented extract had low cytotoxicity; this finding is attributable to the biotransformation that occurred during fermentation (Wang et al., 2019).
Figure 3.
Viability of CCD-966SK cells subjected to 72 h of treatment with various concentrations of 50% ethanolic C. aurantium flower extract, unfermented or fermented by L. brevis. Data are expressed as the mean ± standard deviation of three independent experiments (∗∗P < 0.01 vs. blank control).
The IC50 was 38.2 mg/L for tyrosinase inhibition and 18.6 mg/L for DPPH scavenging activity (Table 2). The MIC value was 50–120 mg/L for bacterial inhibition, whereas the MFC value was 150–180 mg/L for fungal inhibition (Table 3) for the L. brevis–fermented extract. These concentrations had no negative effect on the viability of the investigated cells. According to the present findings, the L. brevis–fermented extract is relatively safe and can potentially be applied in the cosmetics industry.
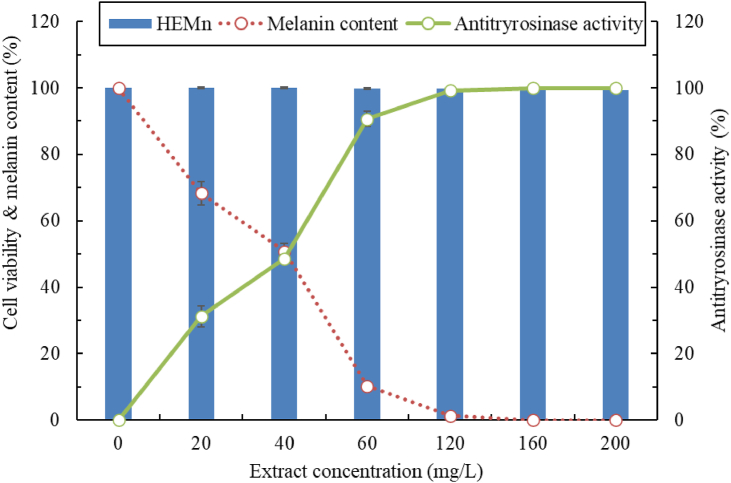
We varied the concentration of the fermented extract and investigated its effect on the viability, antityrosinase activity, and melanin content of HEMn cells (Figure 4). When the concentration was 0–200 mg/L, the fermented extract nonsignificantly inhibited (P < 0.01) HEMn cell viability (≥99.2%). Moreover, as the concentration was increased, the melanin content was discovered to gradually decrease, and the antityrosinase activity to markedly increase. Melanin content and antityrosinase activity exhibited a high degree of synchronization; thus, the L. brevis–fermented extract exerts a whitening effect, possibly through inhibition of tyrosinase activity (Harir et al., 2021). The fermented extract inhibited melanin formation, with the melanin content being 50.8% ± 2.3% at the extract concentration of 40 mg/L. When the concentration was higher than 160 mg/L, melanin was undetectable in HEMn cells. Tyrosinase activity was substantially inhibited, but the HEMn cells were not killed by the extract. In a previous study, 5-hydroxy-6,7,3′,4′-tetramethoxyflavone and limonexic acid, which were isolated from C. aurantium var. amara, significantly inhibited B16 cells at 12.5 mg/L (Zhao et al., 2012). The IC50 value of antityrosinase activity in the in vivo HEMn cells in our study was 40.3 mg/L, which was slightly lower than the corresponding value for in vitro tyrosinase activity (IC50: 38.2 mg/L). The slight difference between these two values indicates that the whitening mechanism may be related to the tyrosinase itself and unaffected by other enzymes and messenger pathways.
Figure 4.
HEMn cells' viability, melanin content, and antityrosinase activity after treatment with 50% ethanolic C. aurantium flower extract fermented by L. brevis. Data are expressed as the mean ± standard deviation of three independent experiments.
3.7. Effect of L. brevis–fermented extract on antiwrinkle activity
Although studies have reported the high physiological activity and safety of L. brevis–fermented extracts, no study has focused on the antiwrinkle effects of C. aurantium flowers. The breakdown of collagen in the skin can be prevented by inhibiting collagenase activity. Furthermore, elastase activity can be inhibited to prevent skin aging (Karim et al., 2014). Collagen and gelatin are respectively digested by the MMP-1 and MMP-2 that skin fibroblasts secrete (Limtrakul et al., 2016); thus, inhibiting MMP-1 and MMP-2 may lead to antiwrinkle activity. Analysis of collagenase, elastase, MMP-1, and MMP-2 (antiwrinkle-related enzymes) inhibitory activity is crucial to evaluating antiaging and antiwrinkle activity. We discovered that treatment with the L. brevis–fermented extract resulted in considerably lower collagenase, elastase, MMP-1, and MMP-2 activity than did treatment with the unfermented extract (Table 6). The inhibitory activity of these four antiwrinkle-related enzymes was 4.29–5.90 times higher when the extract had been fermented. Maity et al. (2011) reported that syringic acid effectively inhibited elastase and MMP-1 activity. In addition, gallic acid is an effective inhibitor of collagenase, elastase, and MMP-2 activity (Wittenauer et al., 2015; Poomanee et al., 2021). Caffeic acid has been found to be an effective inhibitor of collagenase and MMP-1 activity (Shin et al., 2019; Saechan et al., 2021), and neohesperidin reduces MMP-1 activity (Karim et al., 2021). Naringin has been discovered to significantly inhibit the production of elastase or downregulate MMP-2 expression (Annapoorani et al., 2012; Aroui et al., 2016). Rutin was previously determined to be an effective inhibitor of collagenase activity or MMP-1 production (Hwang et al., 2014; Mota et al., 2020). The quantities of these phenolic and flavonoid compounds in our extracts were considerably higher or almost the same when fermentation was applied (Table 3), which explains the considerably higher antiwrinkle activity of the extract after fermentation. Wu et al. (2018) reported that antiwrinkle-related enzyme activity could be attenuated using a fermented extract. Thus, our results suggest that the L. brevis–fermented extract has potential for use in the cosmetics industry in formulations designed to slow the aging of the skin aging and prevent the development of wrinkles.
Table 6.
Antiwrinkle activity (IC50 values) of 50% ethanolic C. aurantium flower extract, unfermented and fermented by L. brevis.
| Collagenase activity (mg/L) | Elastase activity (mg/L) | MMP-1 activity (mg/L) | MMP-2 activity (mg/L) | |
|---|---|---|---|---|
| 50% ethanolic extract | 315.6 ± 27.4b | 502.8 ± 32b | 864.3 ± 36.8b | 912.0 ± 52.3b |
| L. brevis–fermented extract | 73.5 ± 11.3a | 85.2 ± 7.2a | 152.4 ± 14.8a | 175.2 ± 16.2a |
In each column, letters (a–b) indicate P < 0.01.
4. Conclusion
The phenolic and flavonoid compounds in C. aurantium flower extracts undergo biosynthesis or biotransformation during fermentation; this increases their TPC and TFC, in turn increasing the physiological activity of the product. Our fermented C. aurantium flower extract exerted a nontoxic effect on skin cells. Our study is the first to examine the antiaging and antiwrinkle activity of C. aurantium flowers. The results revealed that wrinkle-related enzymes are significantly inhibited by the C. aurantium flower extract after L. brevis fermentation. In addition, the extract exerts a nonsignificant effect on CCD-966SK cell viability, even at high concentrations. In this article, we report for the first time the suitability of L. brevis–fermented extract as a safe ingredient for inclusion in cosmetic formulations designed to have skin-whitening or antiwrinkle effects.
Declarations
Author contribution statement
Chih-Yu Chen: Conceived and designed the experiments; Wrote the paper.
Chi-Yao Hu: Performed the experiments; Analyzed and interpreted the data.
Ssu-Hsuan, Chen; Ya-Ting, Li: Performed the experiments.
Ying-Chien Chung: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Professor Ying-Chien Chung was supported by Ministry of Science and Technology, Taiwan [109-2622-E-157-001 & 110-2313-B-157-001-MY2].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adhikari A., Devkota H.P., Takano A., Masuda K., Nakane T., Basnet P., Skalko-Basnet N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: in vitro tyrosinase inhibition. Int. J. Cosmet. Sci. 2008;30:353–360. doi: 10.1111/j.1468-2494.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Ammar A.H., Bouajila J., Lebrihi A., Mathieu F., Romdhane M., Zagrouba F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (Neroli oil) Pakistan J. Biol. Sci. 2012;15:1034–1040. doi: 10.3923/pjbs.2012.1034.1040. [DOI] [PubMed] [Google Scholar]
- Annapoorani A., Umamageswaran V., Parameswari R., Pandian S.K., Ravi A.V. Computational discovery of putative quorum sensing inhibitors against LasR and RhlR receptor proteins of Pseudomonas aeruginosa. J. Comput. Aided Mol. Des. 2012;26:1067–1077. doi: 10.1007/s10822-012-9599-1. [DOI] [PubMed] [Google Scholar]
- Aroui S., Aouey B., Chtourou Y., Meunier A.C., Fetoui H., Kenani A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem. Biol. Interact. 2016;244:195–203. doi: 10.1016/j.cbi.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Azmi N., Hashim P., Hashim D.M., Halimoon N., Majid N.M.N. Anti–elastase, anti–tyrosinase and matrix metalloproteinase–1 inhibitory activity of earthworm extracts as potential new anti–aging agent. Asian Pac. J. Trop. Biomed. 2014;4:S348–S352. doi: 10.12980/APJTB.4.2014C1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A., Jensen E., Lean M.E.J., McDonald M.S. Quantitative analysis of flavonoids by reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 1997;761:315–321. [Google Scholar]
- Değirmenci H., Erkurt H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J Infect. Public Health. 2020;13:58–67. doi: 10.1016/j.jiph.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Dosoky N.S., Setzer W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018;19:1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawole O.A., Makunga N.P., Opara U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Compl. Alternative Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman A., Weisshaus O., Huhman D.V., Sumner L.W., Bar-Peled M., Lewinsohn E., Fluhr R., Gressel J., Eyal Y. Metabolic engineering of plant cells for biotransformation of hesperedin into neohesperidin, a substrate for production of the low-calorie sweetener and flavor enhancer NHDC. J. Agric. Food Chem. 2005;53:9708–9712. doi: 10.1021/jf051509m. [DOI] [PubMed] [Google Scholar]
- Haj Ammar A., Bouajila J., Lebrihi A., Mathieu F., Romdhane M., Zagrouba F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (Neroli oil) Pakistan J. Biol. Sci. 2012;15:1034–1040. doi: 10.3923/pjbs.2012.1034.1040. [DOI] [PubMed] [Google Scholar]
- Harir i R., Saeedi M., Akbarzadeh T. Naturally occurring and synthetic peptides: efficient tyrosinase inhibitors. J. Pept. Sci. 2021;27:e3329. doi: 10.1002/psc.3329. [DOI] [PubMed] [Google Scholar]
- Hsouna A.B., Hamdi N., Halima N.B., Abdelkafi S. Characterization of essential oil from Citrus aurantium L. flowers: antimicrobial and antioxidant activities. J. Oleo Sci. 2013;62:763–772. doi: 10.5650/jos.62.763. [DOI] [PubMed] [Google Scholar]
- Hwang E., Park S.Y., Lee H.J., Sun Z.W., Lee T.Y., Song H.G., Shin H.S., Yi T.H. Vigna angularis water extracts protect against ultraviolet b-exposed skin aging in vitro and in vivo. J. Med. Food. 2014;12:1339–1349. doi: 10.1089/jmf.2013.3017. [DOI] [PubMed] [Google Scholar]
- Karim A.A., Azlan A., Ismail A., Hashim P., Gani S.S.A., Zainudin B.H., Abdullah N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014;14:381. doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi E., Oskoueian E., Hendra R., Oskoueian A., Jaafar H.Z.E. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules. 2012;17:1203–1218. doi: 10.3390/molecules17021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim N., Shishir M.R.I., Rashwan A.K., Ke H., Chen W. Suppression of palmitic acid-induced hepatic oxidative injury by neohesperidin-loaded pectin-chitosan decorated nanoliposomes. Int. J. Biol. Macromol. 2021;183:908–917. doi: 10.1016/j.ijbiomac.2021.05.010. [DOI] [PubMed] [Google Scholar]
- Kujala T.S., Loponen J.M., Klika K.D., Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000;48:5338–5342. doi: 10.1021/jf000523q. [DOI] [PubMed] [Google Scholar]
- Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012;60:6418–6424. doi: 10.1021/jf204970r. [DOI] [PubMed] [Google Scholar]
- Liao W.T., Huang T.S., Chiu C.C., Pan J.L., Liang S.S., Chen B.H., Chen S.H., Liu P.L., Wang H.C., Wen Z.H., Wang H.M., Hsiao S.W. Biological properties of acidic cosmetic water from seawater. Int. J. Mol. Sci. 2012;13:5952–5971. doi: 10.3390/ijms13055952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limtrakul P., Yodkeeree S., Thippraphan P., Punfa W., Srisomboon J. Anti-aging and tyrosinase inhibition effects of Cassia fistula flower butanolic extract. BMC Compl. Alternative Med. 2016;16:497–503. doi: 10.1186/s12906-016-1484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity N., Nema N.K., Abedy M.K., Sarkar B.K., Mukherjee P.K. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J. Ethnopharmacol. 2011;137:1300–1305. doi: 10.1016/j.jep.2011.07.064. [DOI] [PubMed] [Google Scholar]
- Mapunya M.B., Nikolova R.V., Lall N. Melanogenesis and antityrosinase activity of selected South African plants. Evid. Based. Complementary Altern. Med. 2012 doi: 10.1155/2012/374017. Article ID 374017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchán Arenas D.R., Muñoz Acevedo A., Vargas Méndez L.Y., Kouznetsov V.V. Scavenger activity evaluation of the clove bud essential oil (Eugenia caryophyllus) and eugenol derivatives employing ABTS+• decolorization. Sci. Pharm. 2011;79:779–792. doi: 10.3797/scipharm.1109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes T.M., Kushima H., Moleiro F.C., Santos R.C., Machado Rocha L.R., Marques M.O., Vilegas W., Hiruma-Lima C.A. Effects of limonene and essential oil from (Citrus aurantium) on gastric mucosa: role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009;180:499–505. doi: 10.1016/j.cbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Mota A.H., Duarte N., Serra A.T., Ferreira A., Bronze M.R., Custódio L., Gaspar M.M., Simões S., Rijo P., Ascensão L., Faísca P., Viana A.S., Pinto R., Kumar P., Almeida A.J., Reis C.P. Further evidence of possible therapeutic uses of Sambucus nigra L. extracts by the assessment of the in vitro and in vivo anti-inflammatory properties of its PLGA and PCL-based nanoformulations. Pharmaceutics. 2020;12:1181. doi: 10.3390/pharmaceutics12121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poomanee W., Khunkitti W., Chaiyana W., Intasai N., Lin W.C., Lue S.C., Leelapornpisid P. Multifunctional biological properties and phytochemical constituents of Mangifera indica L. seed kernel extract for preventing skin aging. Toxicol. Res. 2021;37:459–472. doi: 10.1007/s43188-020-00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmorad F., Hosseinimehr S.J., Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006;5:1142–1145. [Google Scholar]
- Rahman M.A., Imran T.B., Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013;20:213–225. doi: 10.1016/j.sjbs.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razeghi F., Haghi E., Ahmadi A., Kamkar A. Antioxidant characterization of cinnamon, Citrus aurantium and green tea leaves. J. Human Heal. Halal Metrics. 2020;1:57–65. [Google Scholar]
- Saechan C., Nguyen U.H., Wang Z., Sugimoto S., Yamano Y., Matsunami K., Otsuka H., Phan G.M., Pham V.H., Tipmanee V., Kaewsrichan J. Potency of bisresorcinol from Heliciopsis terminalis on skin aging: in vitro bioactivities and molecular interactions. Peer J. 2021;9 doi: 10.7717/peerj.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrou E., Chatzopoulou P., Dimassi-Theriou K., Therios I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules. 2013;18:10639–10647. doi: 10.3390/molecules180910639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.Y., Jiang J.G., Huang C.L., Zhu W., Zheng C.Y. Polyphenols from blossoms of Citrus aurantium L. var. amara Engl. show significant anti-complement and anti-inflammatory effects. J. Agric. Food Chem. 2017;65:9061–9068. doi: 10.1021/acs.jafc.7b03759. [DOI] [PubMed] [Google Scholar]
- Shin E.J., Jo S., Choi H.K., Choi S., Byun S., Lim T.G. Caffeic acid phenethyl ester inhibits UV-induced MMP-1 expression by targeting histone acetyltransferases in human skin. Int. J. Mol. Sci. 2019;20:3055. doi: 10.3390/ijms20123055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari V., Loizzo M.R., Branca V., Pellicanò T.M. Bioactive and antioxidant activity from Citrus Bergamia Risso (Bergamot) juice collected in different areas of Reggio Calabria province, Italy. Int. J. Food Prop. 2016;19:1962–1971. [Google Scholar]
- Stohs S.J. Safety, efficacy, and mechanistic studies regarding Citrus aurantium (bitter orange) extract and p-synephrine. Phytother Res. 2017;31:1463–1474. doi: 10.1002/ptr.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntar I., Khan H., Patel S., Celano R., Rastrelli L. An overview on Citrus aurantium L.: its functions as food ingredient and therapeutic agent. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/7864269. Article ID 7864269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneva D., Denkova-Kostova R., Goranov B., Hristova-Ivanova Y., Slavchev A., Denkova Z., Kostov G. Chemical composition, antioxidant activity and antimicrobial activity of essential oil from Citrus aurantium L zest against some pathogenic microorganisms. Z. Naturforsch. C Biosci. 2019;74:105–111. doi: 10.1515/znc-2018-0062. [DOI] [PubMed] [Google Scholar]
- Toth M., Sohail A., Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 2012;878:121–135. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- Tsai M.L., Huang H.P., Hsu J.D., Lai Y.R., Hsiao Y.P., Lu F.J., Chang H.R. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 pathway. Int. J. Mol. Sci. 2014;15:7563–7578. doi: 10.3390/ijms15057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandooren J., Geurts N., Martens E., Van den Steen P., Jonghe. S.D., Herdewijn P., Opdenakker G. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J. Biol. Chem. 2011;2:14–24. doi: 10.4331/wjbc.v2.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.H., Chen C.Y., Lin C.P., Huang C.L., Lin C.H., Cheng C.Y., Chung Y.C. Tyrosinase inhibitory and antioxidant activities of three Bifidobacterium bifidum-fermented herb extracts. Ind. Crop. Prod. 2016;89:376–382. [Google Scholar]
- Wang G.H., Chen C.Y., Tsai T.H., Chen C.K., Cheng C.Y., Huang Y.H., Hsieh M.C., Chung Y.C. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J. Biosci. Bioeng. 2017;123:679–684. doi: 10.1016/j.jbiosc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Wang G.H., Lin Y.M., Kuo J.T., Lin C.P., Chang C.F., Hsieh M.C., Cheng C.Y., Chung Y.C. Comparison of biofunctional activity of Asparagus cochinchinensis (Lour.) Merr. extract before and after fermentation with Aspergillus oryzae. J. Biosci. Bioeng. 2019;127:59–65. doi: 10.1016/j.jbiosc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Wen Y.L., Yan L.P., Chen C.S. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J. Food Drug Anal. 2013;21:219–226. [Google Scholar]
- Wittenauer J., Mäckle S., Sußmann D., Schweiggert-Weisz U., Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179–187. doi: 10.1016/j.fitote.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Wu L.C., Chen C.Y., Cheng C.Y., Dai H., Ai Y.H., Lin C.H., Chung Y.C. Evaluation of tyrosinase inhibitory, antioxidant, antimicrobial, and antiaging activities of Magnolia officinalis extracts after Aspergillus niger fermentation. BioMed Res. Int. 2018 doi: 10.1155/2018/5201786. Article ID 5201786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.H., Tsatsakis A.M., Tzanakakis G., Kim H.S., Le B., Sifaki M., Spandidos D.A., Tsukamoto C., Golokhvast K.S., Izotov B.N., Chung G. Soyasaponin Ag inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells via the downregulation of TRP-2. Int. J. Mol. Med. 2017;40:631–636. doi: 10.3892/ijmm.2017.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.Y., Yang L., Wei J., Huang M., Jiang J.G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. Food Chem. 2012;135:2175–2181. doi: 10.1016/j.foodchem.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Zheng Z.P., Tan H.Y., Wang M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia. 2012;83:1008–1013. doi: 10.1016/j.fitote.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.