Abstract
Background
Hepatoblastoma (HB) is the most common primary malignant liver tumor in children. The prognosis of HB metastasis is poor, despite the increasing diversity of treatment. Piezo, a ubiquitously expressed membrane mechano-transduction protein, is involved in the process of tumor cell migration. Under the gene expression profiling interactive analysis (GEPIA) database, Piezo1 was highly expressed in HB and negatively correlated with the overall survival time.
Methods
Firstly, the expression of Piezo1 in both paracancerous and HB tissues (n = 7) was detected, and the prognostic value of Piezo1 was assessed in HB (n = 160) patients. Secondly, the inhibition and overexpression of Piezo1were executed in two HB cell lines, HepG2 and Huh 6. Methyl thiazolyl tetrazolium (MTT), wound healing and trans-well assays were performed to identify the effect of Piezo1 on the proliferation and metastasis of HB cells, respectively. In addition, a co-immunoprecipitation assay was performed to determine whether Piezo1 has an interaction with HIF-1α. Finally, the expressions level of Piezo1, HIF-1α, and VEGF by overexpression/inhibition each other were detected by RT-qPCR and western blots to find a possible signaling channel in HB metastasis.
Results
We found that Piezo1 was highly expressed in HB tissues and associated with poor prognosis of patients. Piezo1 was related to cell proliferation in HepG2 and Huh 6 cells. We also found that Piezo1 stimulated HIF-1α expression. Meanwhile, overexpression of Piezo1 promoted the migration and invasion of HB cells, while the promotion was not detected when HIF-1α was suppressed. Additionally, the silencing of HIF-1α inhibited the expression of VEGF, but showed no effect on Piezo1 expression.
Conclusion
In this study, we identified that Piezo1 was involved in HB metastasis, and the Piezo1-HIF-1α-VEGF axis could be a possible signaling pathway in HB metastasis.
Keywords: Hepatoblastoma, Metastasis, Pioze1, HIF-1α, VEGF
Hepatoblastoma, Metastasis, Pioze1, HIF-1α, VEGF.
1. Introduction
Hepatoblastoma (HB) is the most familiar pediatric hepatic malignancy, which accounts for ∼50% of pediatric liver cancers [1]. It is unfortunate that, around 20% of those children's patients already presented detectable metastases at the first diagnosis [2]. Although the applications of chemotherapy, surgical resection and liver transplantation have improved the local control of HB, the prognosis for advanced HB patients still remains very poor [3, 4]. Coincidentally, the presence of metastatic foci at HB diagnosis is a well-recognized factor for poor prognosis [5]. Hence, the search for an agent to block metastasis in HB is a hope to sustain life. The increased migratory and invasive potentials of tumor cells are the major pathophysiological properties of malignant tumor metastasis. Cells' motility and migration are achieved through a series of strictly regulated events and reactions, including abundant signal cascades, ionic channels, and transporters synergistic effect [6]. It is well known that mechanosensitive calcium-permeable ion channels are among the main actors participating in the processes of cellular motility events [7]. Mechano-sensitive channels can provide local calcium influx and regulate the cascade of critical calcium-dependent signals associated with cell migration [8]. Piezo proteins, including two members piezo1 and piezo2, are ubiquitously expressed in both cancer cells and epithelial cells, and have been reported to be implicated in membrane mechano-transduction in different processes [9, 10]. In the early stage of neoplasm, following the epithelial cells morphological change and cell homeostasis disrupt, piezo1 could promote the migration of vascular endothelial cells (ECs) by activating the calcium-ions channels, thereby driving embryonic angiogenesis [11]. It was reported that the hypoxia inducible factor-1α (HIF-1α) level was notably elevated in anoxic tumor cells [12]. As a calcium-ions sensitive factor, HIF-1α also has been confirmed to participate in tumor cell metastasis by promoting epithelial-mesenchymal transition (EMT) and angiogenesis [13]. Overall, Piezo1 has been certainly identified as a mechanical inductor, while the function of this protein is still unclear in HB environment. Therefore, in our study, we investigated the potential function of Piezo1 in regulating the migration and invasion of HB. Meanwhile, high-metastatic HB cell lines HepG2 and Huh 6 were applied to explore the association between Piezo1and HIF-1α in regulating HB metastasis.
2. Results
2.1. Piezo1 up-regulated in HB tissue and correlated with the prognosis
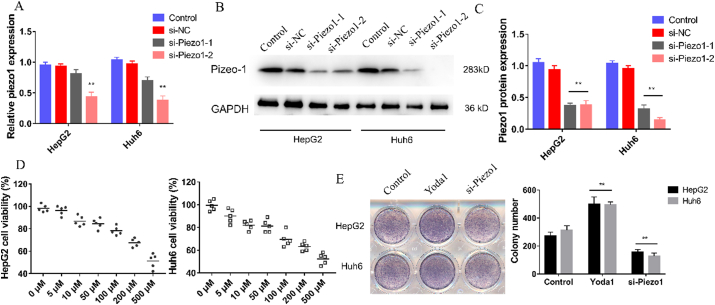
Based on the gene expression profiling interactive analysis (GEPIA) database (http://gepia.cancer-pku.cn), the expression of Piezo1 in liver hepatocellular carcinoma (LIHC) tissues was remarkably higher than that in normal tissues (Figure 1A). A total of 364 patients were recruited in our research, who were divided into two groups, high Piezo1 expression group and low Piezo1 expression group. The survival rate analysis revealed that Piezo1 was negatively correlated with the overall survival time of LIHC patients (Figure 1B). To explore the correlation between Piezo1 expression and clinical relevance of HB patients, seven pairs of HB and paracarcinoma tissues were collected. The expression of Piezo1 was distinctly higher in HB tissues than that in para-carcinoma tissues in both mRNA and protein levels (Fig 1C-E). Furthermore, immunohistochemistry was performed to detect the expression of Piezo1 in HB tissues. As shown in Figure 1F, Piezo1 was expressed in the cytoplasm of cells, and the expression trend was consistent with the protein results. The differences of Piezo1 expression in HB and para-carcinoma tissues indicated that Piezo1 may play a distinct role in HB development.
Figure 1.
High Piezo1 expression in HB tissues was associated with clinical prognosis. A, Piezo1 expression of LIHC tissues by GEPIA database, red represents cancer samples and grey represents normal samples. B, Overall survival analysis of LIHC with high Piezo1 expression by GEPIA database. C-E, Western blot and RT-qPCR analysis of Piezo1 expression in 7 pairs of paracancerous and HB tissues. F, Representative immunohistochemistry images of paracancerous and HB tissues (scale bar = 200 μm). ∗∗P < 0.01 vs Paracancerous, Data were shown as mean ± SD, statistical significance was calculated by student's t-test, one-way ANOVA and two-way ANOVA, respectively.
2.2. Piezo1 facilitated cell proliferation in HB cells
We further investigated the influences of Piezo1 inhibition and overexpression on the biological functions of HB cells (HepG2 and Huh 6). Two specific siRNA sequences were selected for silencing Piezo1. WB and RT-qPCR analyses were performed to detect the Piezo1 inhibition efficiency in two cell lines. The results revealed that si-Piezo1-2 remarkably and stably inhibited Piezo1 expression, which was used for subsequent assays (Fig 2A-C). Yoda 1, which has been confirmed as a specific reagent to activate Piezo1 channel in many types of cells, was used as a Piezo1 channel activator [14]. The MTT and colony formation assays were used to evaluate the viability of HepG2 and Huh6 cells treated with different concentrations of Yoda1. As shown in Figure 2D, the cell viability was restrained by Yoda1 in a dose-dependent manner, and the optimal concentration of 50 μM was determined for subsequent experiments. In colony formation assay, the proliferation ability of HB cells was remarkably increased after stimulation with Yoda1 and significantly reduced after transfection with si-Pizeo1 (Figure 2E). These results suggested that the proliferation ability of HB cells was improved after Piezo1 activation.
Figure 2.
Piezo1 induced HB cells proliferation. A-C, Western blot and RT-qPCR analysis of Piezo1 expression after transfected with two si-Piezo1. D, MTT assay of two HB cells with different concentrations of Yoda 1. E, Representative images and the statistical result of colony formation in two HB cells after transfected with si-Piezo1 and Yoda1. ∗∗P < 0.01 vs control, Data were shown as mean ± SD, statistical significance was calculated by one-way ANOVA.
2.3. Piezo1 promoted HB cells metastasis
Migration and invasion are two important processes for cancer cells to metastasize to distal normal tissues. Therefore, wound-healing and trans-well assays were performed to investigate the function of Piezo1 in HB metastasis. As shown in Fig 3A-B, compared with the control group, the wound closure was slower in si-Piezo1 groups. However, the Piezo1 overexpressing cells migrated faster to the middle area than cells in control group. Furthermore, trans-well assay results suggested that there were slightly fewer cells passing through the membrane in si-Piezo1 groups, but the cell number was increased in Yoda 1 treated groups (Fig 3C-D). The migration and invasion abilities of both cell lines overexpressing Piezo1 were significantly higher than the control group. These results indicated that Piezo1 promoted HB cells metastasis in vitro.
Figure 3.
The effect of Piezo1 in HB cells metastasis. A-B, Wound-healing representative images and statistical results of Piezo1 in two HB cells (scale bar = 200 μm). C-D, Transwell representative images and statistical results of Piezo1 on cell invasion (scale bar = 100 μm). ∗∗P < 0.01 vs control, Data were shown as mean ± SD, statistical significance was calculated by one-way ANOVA.
2.4. Piezo promoted HB cells metastasis through HIF-1α
In HepG2 and Huh 6 HB cell lines, the mRNA expression levels of HIF-1α were remarkedly elevated in Yoda 1 treated group, which were suppressed after transfection with si-Piezo1 (Figure 4A). The proteins expressions of HIF-1α, Yoda1 and Piezo1 were also examined by WB (Figure 4B). As expected, Yoda1 treatment remarkably elevated the expressions of Piezo1 and HIF-1α. However, expression levels of these two proteins were decreased following transfection with si-Piezo1. To investigate the relationship between HIF-1α and Piezo1, HIF-1α was successfully silenced by stably transfection of GFP- HIF-1α into both HB cell lines. WB and RT-qPCR results confirmed that HIF-1α was efficiently suppressed in two HB cells (Fig 4C-D). Trans-well assay results confirmed that the invasion ability of HB cells was significantly inhibited by si-Piezo1 and facilitated by Yoda1. Moreover, GFP- HIF-1α transfection group also inhibited HB cell invasion. However, when co-transfected with GFP–HIF–1α and Yoda1, HB cells invasion was remarkably decreased compared with Yoda1 transfection group, indicating that silenced HIF-1α could reverse the promotion effect of Piezo1 overexpression on HB cells invasion (Figure 4E). We got the same conclusion of migration from the wound healing assays. Similarly, Piezo1 knockdown prominently suppressed while Yoda1 significantly accelerated the migration of HB cells. Nevertheless, after HIF-1α silencing, the promotion of Piezo1 overexpression on the migration was remarkably weakened in HB cells (Fig 3A-B). These results indicated that HIF-1α could be a downstream of Piezo1, and Piezo1 might promote cell migration and invasion via regulation of HIF-1α in HB cells. To investigate the regulatory mechanism underlying Piezo1 mediated selective expression of HIF-1α, the co-immunoprecipitation assay was conducted. As shown in Figure 4F, anti-Piezo1 successfully precipitated the complex of Piezo1 and HIF-1α. Notably, compared with Piezo1 silencing, Piezo1 overexpression increased the interaction between HIF-1α and Piezo1 proteins. Hence, the results indicated that Piezo1 could cooperate with HIF-1α.
Figure 4.
Piezo1 promoted HB cells invasion via up-regulation of HIF-1α. A-B, Western blot, and RT-qPCR analysis of Piezo1 and HIF-1α expression in two HB cells after Piezo1 overexpression and inhibition. C-D, Western blot, and RT-qPCR analysis of the inhibition efficiency of GFP-PURO–HIF–1α in two HB cells. E, Trans-well representative images and statistical results of two HB cells after being transfected with si-Piezo1, Yoda 1, GFP-PURO–HIF–1α, and Yoda1+ GFP-PURO–HIF–1α (scale bar = 200 μm). F, co-immunoprecipitation images of HIF-1α and Piezo1. ∗P < 0.05, ∗∗P < 0.01 vs control, #P < 0.05 vs GFP-PURO-control, &&P < 0.01 vs Yoda1, Data were shown as mean ± SD, statistical significance was calculated by one-way ANOVA.
2.5. Piezo1-HIF-1α-VEGF: a possible signaling pathway in HB metastasis
To verify the Piezo1-HIF-1α relative signal pathway in HB metastasis, the interaction of Piezo1 and HIF-1α was primarily investigated in HepG2 and Huh 6 cells. The results showed that the Yoda 1 significantly promoted the expressions of Piezo1 and HIF-1α. After silencing of Piezo1, HIF-1α expression was significantly inhibited. In addition, the expression level of HIF-1α was detected after co-transfection of si-Pioze1 and Yoda1. As shown in Fig 5A-B, the combined treatment significantly reversed the ascent of HIF-1α induced by Yoda1, which indicated that Yoda1 promoted HIF-1α expression through activating Piezo1. VEGF is a common migration, invasion, and metastasis participator of tumor cells, which has been confirmed as a downstream target activated of HIF-1α [15, 16]. In our study, HIF-1α and VEGF expressions were both restrained after Piezo1 inhibition. When HIF-1α was silenced, the VEGF expression was still reduced although there is no significant change with Piezo1 at either mRNA or protein level (Fig 5C-D). Intriguingly, after silenced the HIF-1α and Piezo1, the expression of VEGF exhibited slightly downtrend (Fig 5E-F). Taken together, the results indicated that VEGF was a downstream target of HIF-1α, and the Piezo1-HIF-1α-VEGF axis could be a possible signaling pathway in HB tumor metastasis. Nevertheless, the solid specific mechanism still needs further exploration.
Figure 5.
The expression of Piezo1, HIF-1α, and VEGF in two HB cells after transfected with Yoda 1, si-Piezo1 and GFP-PURO–HIF–1α. A-B, Western blot and RT-qPCR analysis of Piezo1 and HIF-1α expression after Piezo1 overexpression and inhibition in two HB cells. C-D, Western blot and RT-qPCR analysis of Piezo1, HIF-1α and VEGF after transfected with si-Piezo1 or GFP-PURO–HIF–1α in two HB cells. E-F, Western blot and RT-qPCR analysis of Piezo1, HIF-1α and VEGF expression after transfected with si-Piezo1 and GFP-PURO–HIF–1α in two HB cells. ∗P < 0.05, ∗∗P < 0.01 vs control, Data were shown as mean ± SD, statistical significance was calculated by one-way ANOVA.
3. Discussion
Piezo1 has been proved to be associated with the prognoses of many cancers such as breast cancer [9], gastric cancer [17], non-small cell lung cancer [18], and glioma [19]. However, the Piezo channel might reveal opposite function. For example, the inhibition of Piezo by a toxic peptide GsMTx-4 suppressed the motility of breast cancer cell line MCF-7 [9]. Higher expression of Piezo was detected in human and mouse bladder cancer than normal tissues [20]. In the contrary, the depletion of Piezo1 could facilitate the migratory properties and cause switch to an integrin independent type of cell migration in small lung cancer [21]. The contradictory in phenotypes may be attributed to the pleiotropic effects of Piezo and the difference in tumor types. Herein, this study demonstrated the potential role of Piezo1 in HB. Taken together, our data indicated that Piezo1 showed an ectopic overexpression in HB tissues and was associated with shortened survival time in HB patients. Then, two typical HB cell lines HepG2 and Huh 6 were transfected with si-Piezo1 to suppress Piezo1 expression, and with Yoda 1 to activate Pioze1 channel. Overexpression of Piezo1 was negatively associated with HB cells viability, while Piezo1 silencing could suppress HB cells proliferation. In addition, overexpression of Piezo1 promoted HB cells migration and invasion. Thereby, the above results suggested that Piezo1 was associated with the viability and metastasis of HB cells.
Subsequently, we revealed that HIF-1α was targeted by Pioze1 in HB cells metastasis. Previous researches have elucidated that the hypoxia tumor environment caused by HIF-1α high expression was one of the most important mechanisms to promote metastasis [22]. For instance, Sun et al. demonstrated that Piezo1 promoted the expression of HIF-1α in colon cancer cells [23]. Wang et al suggested that Piezo1 was a key component during gastric cancer omenta metastasis, which could be related to up-regulation of HIF-1α [16]. In line with these findings, our study demonstrated that the expression of HIF-1α was significantly elevated in cells treated with Yoda 1, whereas was significantly restrained by Piezo1 knockdown. Similarly, HIF-1α knockdown reversed the elevated invasion caused by Yoda1-mediated Piezo1 activation. In addition, the interaction between Piezo1 and HIF-1α was confirmed by co-immunoprecipitation in HB cells. Thus, our findings indicated that HIF-1α could be a downstream target of Piezo1, and Piezo1 might induce HB cells to facilitated metastasis by up-regulation of HIF-1α [24, 25, 26].
It has been reported that HIF-1α was involved in tumor cell metastasis by promoting angiogenesis [13]. Meanwhile, VEGF is an angiogenesis-related regulatory factor in tumors, which is an important downstream factor of HIF-1α [35669101] [27]. Li et al found that knockdown of Piezo1 attenuated migration of human umbilical vein endothelial cells towards VEGF [28]. Hence, we further explored the relationship between Piezo1/HIF-1α and VEGF in HB metastasis. Our results revealed that VEGF expression was inhibited not only by the silencing of Piezo1 but also by the restraint of HIF-1α. Thereby, VEGF could be a downstream molecule of Piezo1-HIF-1α. Moreover, HIF-1α silencing could not exhibit the promotion behavior of Yoda 1 on VEGF. Thus, we proposed a potential Piezo1 involved signal pathway, the Piezo1-HIF-1α-VEGF axis, which might mediate HB cells metastasis.
In summary, our results indicated that Piezo1 overexpression could promote HIF-1α expression, and Piezo1 silencing could impair the expression of HIF-1α and further restrain HB cells metastasis, thereby effectively inhibited the VEGF expression. Thus, Piezo1 could be a potential treatment target for HB metastasis. This research still had several limitations. Firstly, the Pioze1 expression in HB metastasis lesions was not examined due to restrictions that not all lesions can be resected in clinical practice. Secondly, other significant downstream targets of Piezo1 in HB metastasis need to be investigated in the future. With the in-depth studies on Piezo1, we believed that it could be a promising target for HB therapy.
4. Conclusion
Our results suggested that Piezo1 had an ectopic overexpression in HB tissue and associated with shortened survival time in HB patients. Meanwhile, a potential signal transduction mechanism involving Piezo1-HIF-1α-VEGF was proposed for the proliferation and metastasis of HB cells.
5. Materials and methods
5.1. Human tissues and cell lines
Human HB and adjacent nontumor liver tissues were collected from patients treated at Shenzhen Children's Hospital (Shenzhen, China). Tumors were diagnosed as HB by the department of pathology. Every patient signed an informed consent form. Our study was ethically approved by Shenzhen Children's Hospital Ethics Committee. HepG2 and Huh 6 cell lines were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China) and cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin (100 U/mL) and streptomycin (100 U/mL) in cell incubator (37 °C, 5% CO2).
5.2. Materials
Trypsin-EDTA was obtained from Gibco Ltd (Grand Island, USA). The Yoda 1 (S6678) was purchased from SelleckChem (Houston, USA).
5.3. Cell proliferation and viability assays
HepG2 and Huh 6 cells were treated with different concentrations of Yoda 1 (5, 10, 50, 100, 200, 500 μmol/L) for 48 h. Cell proliferation was detected using MTT kits (Beyotime, ST316, Shanghai, China). Briefly, the cells were seeded into 96-wells plates as 1000 cells per well for adherence. After 48 h of Yoda1 incubation, 50 μL MTT (5 mg/mL in PBS) was added to each well, and the cells were incubated for 4 h at 37 °C. The absorbance was measured at 570 nm with a microplate reader (Synergy HT, Bio-Tek, USA) and expressed as a percentage of the absorbance of non-transfected cells. Data were presented as mean ± SD.
5.4. Transfection
The small interfering RNA (siRNA) for Piezo1 were obtained from Sangon Biotech (Shanghai, China) and the sense and antisense sequence were shown in Table 1. Briefly, the cells were seeded in 24-well plates as 1×104 cells per well for adherence. Transfection was performed using Lipofectamine 2000 Transfection Reagent (Invitrogen, USA).
Table 1.
Piezo1 siRNA sequence.
| gene | Sequence (5′-3′) | |
|---|---|---|
| Piezo1 siRNA-1 | sense | CCAAGACGUACAAUCAUCAUAUCA |
| antisense | ACAUGAUCGUACUUCUUAGTG | |
| Piezo1 -2siRNA | sense | GACGACUUCCUGUUUGAGUAC |
| antisense | ACUCGAACAGGAAUUAGUCGC |
5.5. Reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNAs of cells were isolated with E. Z.N.A.® Total RNA Kit II (Omega Bio-tek, Inc. Norcross, GA). The cDNAs were synthesized using HiScript II Q RT SuperMix (Vazyme Biotech, China) with random hexamers. RT-qPCR analyses were carried out using SYBR Green Master Mix (Vazyme Biotech, China) and gene-specific primers on a CFX96 Touch real-time PCR system (Bio-Rad, Hercules, CA). GAPDH was used as an internal control for mRNA levels respectively. Cells were treated in triplicate and assayed separately. The comparative threshold cycle (Ct) method with formula 2−ΔΔCt was used to calculate the relative gene expression. The primer sequences were shown in Table 2.
Table 2.
Primer sequence.
| hum-Piezo1-F | 5′- GGACTCTCGCTGGTCTATTC -3′ |
| hum-Piezo1-R | 5′- GGGCACAATATGCAGGCTCT -3′ |
| hum-GAPDH-F | 5′- CCTCTGACTTCAACAGCGAC -3′, |
| hum-GAPDH-R | 5′-TCCTCTTGTGCTCTTGCT GG -3′ |
| hum–HIF–1α-F | 5′- CTTAGCGCAAGTCTTCAATC -3′ |
| hum–HIF–1α-R | 5′- ACCGTAGGAGATGGAGATCG -3′ |
| hum-VEGF-F | 5′- CTCGCAGTCGCGGAGA -3′ |
| hum-VEGF-R | 5′- GCAGCCTGGACCCTTGGC -3′ |
5.6. Western blotting and Co-immunoprecipitation
Cells were harvested and washed twice with cold PBS. Cell lysates were prepared by RIPA buffer (Beyotime, Shanghai, China) supplemented with 1% Protease inhibitor Cocktail Set III (Valbiochem, Darmstadt, Germany) on ice. Protein concentrations were determined using a BCA Protein Assay Kit (Thermo Fisher Scientific, USA). For co-immunoprecipitation, cell lysates were extracted and incubated with 2 μg specific antibody for 12 h at 4 °C. Then, the samples were incubated with 50 μL protein G agarose beads for 3 h. After adequate washing, the precipitated proteins were resuspended in 20 μL SDS buffer and boiled at 95 °C for 10 min. Whole-cell proteins (50 μg/lane) were loaded and resolved on a 10% SDS-PAGE, then transferred onto PVDF membranes. The membranes were blocked for 1 h in 5% non-fat milk in TBST buffer, then immunoblot was performed by incubating the membranes with the anti-Piezo1 (Proteintech, 28511-1-AP, 1:1000), anti–HIF–1α (Abclonal, A7684, 1:1000), anti-VEGF (Abclonal, A12303, 1:1000) or anti-GAPDH (Abclonal, A19056, 1:2000) rabbit antibody. Then the membranes were incubated with secondary antibodies (goat anti-rabbit IgG, Zhuangzhi) conjugated with horseradish peroxidase for 3 h at room temperature. After being incubated with Clarity Western ECL substrates (Bio-Rad), the protein bands were detected by Tanon 4600 S F gel imaging system (Tanon Science & Technology CO., Ltd, Shanghai, China).
5.7. Immunohistochemistry
For immunohistochemistry staining, formalin-fixed and paraffin-embedded specimens were incubated with primary antibodies (anti-Piezo1, 1:200) at 4 °C overnight, and followed by second antibody at room temperature for 2 h. After adding DAB solution, the sections were stained with hematoxylin for 2 min.
5.8. Cell invasion assays
Cell invasion assay used 24-well plates with 8 μm micropore inserts, 1×105 cells were seeded into upper wells, which were coated with 50 μL matrigel (BD Transduction Laboratories, diluted at 1:25 with DMEM) without FBS for 24 h. While the complete growth medium (10% FBS) was placed in the lower chamber. The cells on the upper surface of filter were wiped with a cotton swab, and the cells on the lower surface of filters were fixed for 15 min with 4% paraformaldehyde and stained with 0.1% crystal violet for another 15 min. Finally, the lower surface of the filter was photographed by microscopy.
5.9. Wound healing assay
Cells were seeded into 6-well plates as 1×106 cells per well. When the confluence reached 80%, the 200 μL pipette tip was used to scratch 1cm wide gap. Then the cells were incubated in a serum-free medium for 48 h and the images were observed under the microscope.
5.10. Statistical assessment
SPSS13.0 software was used to analyze the data. Results were expressed as mean ± SD. For all experimentation standard deviation indicates SD. The independent-sample t test was used for comparison between the two groups. The one-way ANOVA was used for comparison among three or more group. The two-way ANOVA was used for comparisons two-factor between two groups. P < 0.05 was considered statistically significant (GraphPad).
Declarations
Author contribution statement
Xiaoshuo Ye: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Yongjie Xia; Wei Chen: Analyzed and interpreted the data.
Yuelan Zheng: Contributed reagents, materials, analysis tools or data.
Zimin Chen; Zhen Cheng: Performed the experiments.
Bin Wang: Conceived and designed the experiments; Wrote the paper.
Funding statement
Professor Bin Wang was supported by Innovative Research Group Project of the National Natural Science Foundation of China [81770512], Third Health Programme [SZSM201812055].
Data availability statement
Data associated with this study has been deposited at gene expression profiling interactive analysis (GEPIA) database (http://gepia.cancer-pku.cn)
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank 51runse (www.51runse.cn) for the English language editing during the preparation of this manuscript. Xiaoshuo Ye: Conceived and designed the experiments, performed the experiments, and wrote the paper; Yongjie Xia: Analyzed and interpreted the data; Yuelan Zheng: Contributed reagents, materials, analysis tools or data; Wei Chen: Analyzed and interpreted the data; Zimin Chen: Performed the experiments; Zhen Cheng: Performed the experiments; Bin Wang: Conceived and designed the experiments, and wrote the paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
FIG4B-Pizeo.tif.

Fig5F-HIF-2.tif.
Fig5B-HIF1-1.tif.
Fig5F-VEGF-1.tif.
FIG4F-HIF1-2.tif.
Fig1C-GAPDH.tif.
FIG4F-Pizeo-1.tif.
Fig5F-HIF-1.tif.
Fig4F-Pizeo-3.tif.
Fig4D-HIF1.tif.
Fig-2B-GAPDH.tif.
Fig5F-VEGF-2.tif.
Fig5B-GAPDH-2.tif.
FIG4F-Pizeo-2.tif.
Fig5D-VEGF.tif.
Fig5D-HIF-2.tif.
Fig5B-HIF-2.tif.
Fig5D-HIF-1.tif.
Fig5F-Pizeo-1.tif.
fIG5B-Pizeo-1.tif.
Fig1C-Pizeo-1.tif.
FIG4B-HIF1.tif.
Fig4D-GAPDH.tif.
Fig5D-Pizeo-1.tif.
Fig2B-piezo-1.tif.
FIG4F-HIF1.tif.
Fig-5B-Pizeo-2.tif.
Fig5D-GAPDH-2.tif.
Fig5D-Pizeo-2.tif
Fig5F-GAPDH.tif.
FIG4F-GAPDH.tif.
Fig5B-GAPDH-1.tif.
Fig5D-GAPDH-1.tif.
Fig4B-GAPDH.tif.
References
- 1.Cabello-Laureano I. Fernandez-Pineda.R. Differential diagnosis and management of liver tumors in infants. World J. Hepatol. 2014;6:486–495. doi: 10.4254/wjh.v6.i7.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch F.X., Ribes J., Diaz M., Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Trobaugh-Lotrario A.D., Meyers R.L., O'Neill A.F., Feusner J.H. Unresectable hepatoblastoma: current perspectives. Hepat Med. 2017;9:1–6. doi: 10.2147/HMER.S89997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson D.C., Czauderna P., Maibach R., Perilongo G., Morland B. The treatment of hepatoblastoma: its evolution and the current status as per the SIOPEL trials. J. Indian Assoc. Pediatr. Surg. 2014;19:201–207. doi: 10.4103/0971-9261.142001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers R.L., Maibach R., Hiyama E., Haberle B., Krailo M., Rangaswami A., Aronson D.C., Malogolowkin M.H., Perilongo G., von Schweinitz D., Ansari M., Lopez-Terrada D., Tanaka Y., Alaggio R., Leuschner I., Hishiki T., Schmid I., Watanabe K., Yoshimura K., Feng Y., Rinaldi E., Saraceno D., Derosa M., Czauderna P. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children's Hepatic tumors International Collaboration. Lancet Oncol. 2017;18:122–131. doi: 10.1016/S1470-2045(16)30598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayasu T., Kurisu K., Esquenazi Y., Ballester L.Y. Ion channels and their role in the pathophysiology of gliomas. Mol. Cancer Therapeut. 2020;19:1959–1969. doi: 10.1158/1535-7163.MCT-19-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroto R., Hamill O.P. MscCa regulation of tumor cell migration and metastasis. Curr. Top. Membr. 2007;59:485–509. doi: 10.1016/S1063-5823(06)59019-2. [DOI] [PubMed] [Google Scholar]
- 8.Chubinskiy-Nadezhdin V.I., Vasileva V.Y., Vassilieva I.O., Sudarikova A.V., Morachevskaya E.A., Negulyaev Y.A. Agonist-induced Piezo1 activation suppresses migration of transformed fibroblasts. Biochem. Biophys. Res. Commun. 2019;514:173–179. doi: 10.1016/j.bbrc.2019.04.139. [DOI] [PubMed] [Google Scholar]
- 9.Li C., Rezania S., Kammerer S., Sokolowski A., Devaney T., Gorischek A., Jahn S., Hackl H., Groschner K., Windpassinger C., Malle E., Bauernhofer T., Schreibmayer W. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci. Rep. 2015;5:8364. doi: 10.1038/srep08364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T., Muraki Y., Hatano N., Suzuki H., Muraki K. PIEZO1 channel is a potential regulator of synovial sarcoma cell-viability. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19051452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Zhou H., Zhang M., Liu W., Deng T., Zhao Q., Li Y., Lei J., Li X., Xiao B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573:225–229. doi: 10.1038/s41586-019-1505-8. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Jin X., Huang Y., Dong J., Wang H., Wang X., Cao X. Inhibition of peritoneal metastasis of human gastric cancer cells by dextran sulphate through the reduction in HIF-1 alpha and ITGbeta1 expression. Oncol. Rep. 2016;35:2624–2634. doi: 10.3892/or.2016.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Zhu Y., Dong Y., Zhang P., Han X., Jin J., Ma X. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1 alpha-Twist signaling pathway in colon cancer. Clin. Sci. (Lond.) 2017;131:2439–2450. doi: 10.1042/CS20171069. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya M., Hara Y., Okuda M., Itoh K., Nishioka R., Shiomi A., Nagao K., Mori M., Mori Y., Ikenouchi J., Suzuki R., Tanaka M., Ohwada T., Aoki J., Kanagawa M., Toda T., Nagata Y., Matsuda R., Takayama Y., Tominaga M., Umeda M. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat. Commun. 2018;9:2049. doi: 10.1038/s41467-018-04436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.Y., Kim H.J., Oh S.C., Lee D.H. Genipin inhibits the invasion and migration of colon cancer cells by the suppression of HIF-1 alpha accumulation and VEGF expression. Food Chem. Toxicol. 2018;116:70–76. doi: 10.1016/j.fct.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Cheng G., Miao Y., Qiu F., Bai L., Gao Z., Huang Y., Dong L., Niu X., Wang X., Li Y., Tang H., Xu Y., Song X. Piezo type mechanosensitive ion channel component 1 facilitates gastric cancer omentum metastasis. J. Cell Mol. Med. 2021;25:2238–2253. doi: 10.1111/jcmm.16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Zhou Y., Huang T., Wu F., Liu L., Kwan J.S.H., Cheng A.S.L., Yu J., To K.F., Kang W. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol. Carcinog. 2018;57:1144–1155. doi: 10.1002/mc.22831. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z., Sun Z., Zhang X., Niu K., Wang Y., Zheng J., Li H., Liu Y. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Wanggou S., Bodalia A., Zhu M., Dong W., Fan J.J., Yin W.C., Min H.K., Hu M., Draghici D., Dou W., Li F., Coutinho F.J., Whetstone H., Kushida M.M., Dirks P.B., Song Y., Hui C.C., Sun Y., Wang L.Y., Li X., Huang X. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron. 2018;100:799–815 e7. doi: 10.1016/j.neuron.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Etem E.O., Ceylan G.G., Ozaydin S., Ceylan C., Ozercan I., Kuloglu T. The increased expression of Piezo1 and Piezo2 ion channels in human and mouse bladder carcinoma. Adv. Clin. Exp. Med. 2018;27:1025–1031. doi: 10.17219/acem/71080. [DOI] [PubMed] [Google Scholar]
- 21.McHugh B.J., Murdoch A., Haslett C., Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M.H., Wu K.J. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y., Li M., Liu G., Zhang X., Zhi L., Zhao J., Wang G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020;146:1139–1152. doi: 10.1007/s00432-020-03179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X., Huang R., Zhong Y., Cui N., Wang Y., Weng J., Chen L., Zang M. CTHRC1 promotes gastric cancer metastasis via HIF-1 alpha/CXCR4 signaling pathway. Biomed. Pharmacother. 2020;123 doi: 10.1016/j.biopha.2019.109742. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y., Xu Q., Shang J., Lu L., Chen G. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1 alpha signaling. J. Cell. Physiol. 2019;234:17876–17885. doi: 10.1002/jcp.28418. [DOI] [PubMed] [Google Scholar]
- 26.Gan L., Meng J., Xu M., Liu M., Qi Y., Tan C., Wang Y., Zhang P., Weng W., Sheng W., Huang M., Wang Z. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin beta 4/FAK/SOX2/HIF-1 alpha signaling pathway in gastric cancer. Oncogene. 2018;37:744–755. doi: 10.1038/onc.2017.363. [DOI] [PubMed] [Google Scholar]
- 27.Pang L., Wang J., Fan Y., Xu R., Bai Y., Bai L. Correlations of TNM staging and lymph node metastasis of gastric cancer with MRI features and VEGF expression. Cancer Biomarkers. 2018;23:53–59. doi: 10.3233/CBM-181287. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Hou B., Tumova S., Muraki K., Bruns A., Ludlow M.J., Sedo A., Hyman A.J., McKeown L., Young R.S., Yuldasheva N.Y., Majeed Y., Wilson L.A., Rode B., Bailey M.A., Kim H.R., Fu Z., Carter D.A., Bilton J., Imrie H., Ajuh P., Dear T.N., Cubbon R.M., Kearney M.T., Prasad R.K., Evans P.C., Ainscough J.F., Beech D.J. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at gene expression profiling interactive analysis (GEPIA) database (http://gepia.cancer-pku.cn)