Abstract
Purpose of Review
Achilles tendon ruptures (ATR) are detrimental to sports performance, and optimal treatment strategy and guidelines on return to play (RTP) remain controversial. This current review investigates the recent literature surrounding nonoperative versus operative management of ATR, clinical outcomes, and operative techniques to allow the athlete a successful return to their respective sport.
Recent Findings
The Achilles tendon (AT) is crucial to the athlete, as it is essential for explosive activities such as running and jumping. Athletes that sustain an ATR play in fewer games and perform at a lower level of play compared to age-matched controls. Recent studies also theorize that ATRs occur due to elongation of the tendon with fatigue failure. Biomechanical studies have focused on comparing modes of fixation under dynamic loading to recreate this mechanism.
Summary
ATRs can be career-ending injuries. Fortunately, the recent incorporation of early weight-bearing and functional rehabilitation programming for non-operative and operative patients alike proves to be beneficial. Especially for those treated nonoperatively, with the incorporation of functional rehabilitation, the risk of re-rupture among non-operative patients is beginning to approach the historical lower risk of re-rupture observed among patients treated operatively. Despite this progress in decreasing risk of re-rupture particularly among non-operative patients, operative managements are associated with unique benefits that may be of particular interest for athletes and active individuals. Recent studies demonstrate that operative intervention improves strength and functional outcomes with more efficacy compared to nonoperative management with rehabilitation. The current literature supports operative intervention in elite athletes to improve performance and shorten the duration to RTP. However, we acknowledge that surgical intervention does have inherent risks. Ultimately, most if not all young and/or high-level athletes with an ATR benefit from surgical repair, but it is crucial to take a stepwise algorithmic approach and consider other factors, which may lead towards nonoperative intervention. These factors include age, chronicity of injury, gap of ATR, social factors, and medical history amongst others in this review.
Keywords: Achilles tendon, Achilles tendon rupture, Athlete injury, Return to play
Achilles tendon ruptures (ATRs) are one of the most common tendon injuries to plague athletes [1]. The overall incidence of ATRs is increasing, with sports-related injury being the most common cause [1–4]. In the general population, ATRs affect between 8 and 18 per 100,000 persons [5].
Anatomy and Biomechanics
The AT is the strongest and thickest tendon in the human body and is also the primary plantar flexor of the ankle [6]. It is subjected to immense loads, with 2–3 times a person’s body weight with walking, and up to ten times a person’s body weight with high-impact activities [5]. The AT is comprised of the medial and lateral gastrocnemius and soleus muscles, which course distally and rotate 90° internally before inserting on the posterior calcaneal tuberosity [6].
The histological properties of the AT are unique and allow the AT to exhibit specific properties. The AT lacks a synovial sheath unlike most tendons. Rather, the AT is surrounded by a single layer of paratenon, a thin membrane-like structure composed of loose connective tissue, and specialized cells (tenocytes), among other cell types [3, 7, 8•]. The paratenon sheath allows the AT to slide and allows the blood vessels to enter and supply the AT [7]. The tenocytes, within the paratenon, are specialized cells formed from the tenoblasts which produce collagen cell types and allow for the regeneration of type III collagen after AT rupture. With increased production of type III collagen in contrast to type I, this causes the AT to become less resistant to tensile forces [3, 5, 9]. However, overall collagen contributes to AT’s high tensile strength with 90% being type I collagen and the remainder being composed of type III, which can also be found in the fibrocartilage of the AT [3, 9].
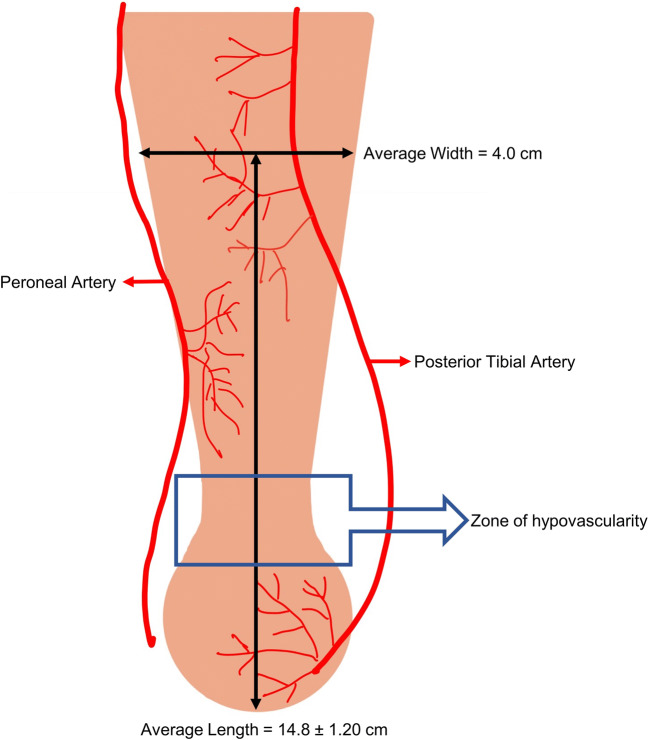
There are several anatomical factors that contribute to AT ruptures. Based on cadaveric studies, the average length of the AT is 15 cm, with the narrowest point being 2–6 cm proximal to the calcaneal insertion [6, 9]. Chen et al.’s cadaveric study depicts the average width of the tendon at different distances from the calcaneal insertion point, with distances ranging from 1 to 14.8 cm [10]. This variability in muscle fiber length and thickness, predisposes the AT to rupture. The blood supply to the AT has also been studied, with the posterior tibial artery supplying the proximal and distal sections, and the peroneal artery supplying the mid-section [10]. Furthermore, the nature of the AT spiraling 90° as it inserts into the calcaneus and the hypovascular region of the AT contributes to a propensity for rupture in the AT [11]. Figure 1 depicts the region 2–6 cm proximal to the insertion point, having a vascular watershed area, making this region prone to pathology [10].
Fig. 1.
Achilles tendon vascular supply
Biomechanics of Achilles Tendon Ruptures
The AT plays a major role in the absorption of energy caused by the mechanical shock of ground reaction forces. Although athletes often suffer ruptures due to acute loading, and approximately 90% occur during acceleration or deceleration during running, there is often evidence of prior degenerative changes [12–17]. The prevalence of ruptures during these explosive activities is due to the short relaxed tendon rapidly being lengthened with an oblique load [14, 15]. However, no evidence suggests that atypical kinematics are the cause of this, leading most to believe that fatigue failure may play a role. Despite the size and strength of the AT, the repetitive high energy loads experienced during sports accelerate degenerative changes causing elongation and fatigue failure. Finite element models have determined the AT tensile force to be 75% of the weight applied to the center of pressure in a balanced stance phase and has been recorded to be upwards of 10 times bodyweight during some activities [18–20]. Along with specific activities such as lateral jumping and hopping, different striking patterns during gait or running may further increase these loads [21, 22]. These activities incidentally change the moment arm of the AT through an increased shortening velocity or muscle fiber length during force generation [23]. Recent studies have focused on the AT moment arm and its importance during plantar flexion and the push-off phase of gait [24, 25]. In vivo methodologies involving both ultrasonography (US) and magnetic resonance imaging (MRI) have been employed to estimate the AT moment arm and to evaluate its function under loading [23, 24, 26, 27].
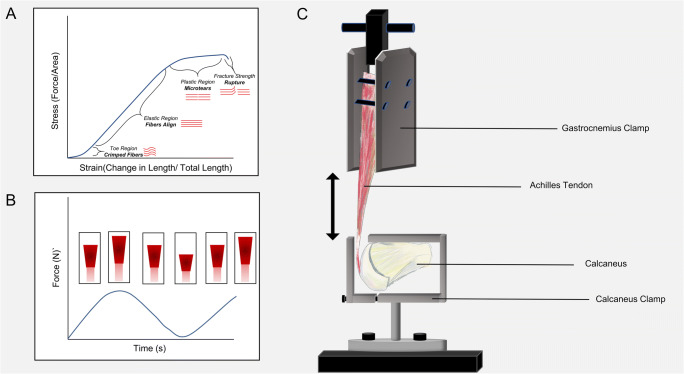
Post-operative analyses, various imaging techniques, computational models, and cadaveric studies have been performed to evaluate different fixations, postoperative weight-bearing protocols, and recovery. ATRs often stem from loading to fatigue amidst elongation of the tendon, correlating to outcomes involving re-rupture rate [28, 29]. Early rehabilitation protocols have been biomechanically modeled using stepwise increases in cyclic loading rather than ultimate load to failure conditions in order to simulate progressive and repetitive loading [30–33]. Dynamic loading, such as cyclic loading, at higher loads may be more representative of an AT injury during sport (Fig. 2).
Fig. 2.
A Representative stress-strain curve for AT under monotonic loading. B Example of cyclic loading plot with corresponding lengthening of the Achilles tendon. C Image of general biomechanical setup for Achilles tensile test
Patients that experience an ATR have a 30% difference in heel-rise height between the previously injured extremity and the contralateral side after 1 year and demonstrate significant deficits in ankle kinetics when performing activities such as jumping and running. The heel-rise height has been found to correlate to the long-term ability to regain normal biomechanics [34]. Although typically recommended for athletes, surgical intervention has been found to significantly increase resting tendon length and stiffness while significantly decreasing elongation leading to asymmetrical gait and movements affecting sport performance [35]. While randomized controlled-trials have not found significant outcomes between loading protocols, loading of the tendon has been found to stimulates the cells required for healing [36, 37]. Restoring AT tensile strength, elasticity, and fatigue strength are critical in reintroducing an athlete to the super-physiologic loads that are experienced during sport.
Treatment and Outcomes
With no validated guidelines, RTP decisions largely rest on the clinical judgment and collaboration between the clinician, physical therapist, athletic trainer, and athlete [38••]. RTP can be discussed in the terms of a continuum comprising return to participation, return to sport, and return to performance [39]. With this in mind, recommendations for RTP should be made in the context of the sport and when possible objective criteria should be assessed. There is currently no clear consensus on which treatment, either conservative management or surgical repair, is the best for ATR [40–42]. An aspect of treatment that is evident in lowering the rerupture rate among patients, whether or not they receive nonoperative or operative treatments, is the implementation of functional rehabilitation and should be included in the post-operative rehabilitation plan of care [43].
Despite the inherent risks of surgery, including postoperative wound infection, adhesions, and sural nerve injury, surgical intervention when compared with nonoperative conservative management has been noted to provide superior improvements in functional outcomes [44, 45]. The superior jumping and muscular endurance observed across surgery patients may be particularly important to elite athletes hoping to RTP, with the understanding that ATR can be a career-ending injury for many [46–48]. Surgical patients demonstrate superior early and late functional outcomes (in jumping tests and muscular endurance heel-rise work test), which may be impacted by the fact that surgical patients progress through the rehabilitation protocol more quickly [41, 44]. Surgery restores the calf muscle strength earlier over the entire range of motion of the ankle joint, such that patients who undergo surgery demonstrate a strength increase of 10 to 18% and peak torque increase of 14% over what is observed in patients undergoing nonoperative functional rehabilitation at an 18-month follow-up [48]. In turn, surgical interventions may be recommended to elite athletes, who experience unique benefits from these improved physical functional outcomes as well as decreased risk of re-rupture, which are well-aligned with their desire to RTP at a faster rate [41, 42, 46–48].
Despite the superior functional outcomes and reduced risk of re-rupture observed among patients with ATRs treated surgically, outcomes for patients treated nonoperatively have markedly improved with the recent incorporation of early weight-bearing and functional rehabilitation programming [41, 42, 44, 46, 47]. With the introduction of early weight-bearing and functional bracing, patients with ATR treated nonoperatively in comparison to those treated operatively demonstrate similar efficacy (rate of re-rupture and patient reported outcomes), without the risk of surgical complications [41, 42, 44, 46, 47]. A randomized control trial comparing the efficacy of conservative treatment, open surgery, and percutaneous surgery, demonstrate comparable pain scores, ability to stand heel rise mono- and bi-podally for 3 s, and return to previous activity or sport among patients across the intervention at 1-year follow-up [42•]. However, with very few patients performing sports at a high-intensity in this sample, it is unclear to what extent these findings may be generalizable to all athletes. In Lerch et al.’s retrospective observational study, the RTP rate among patients undergoing nonoperative management with functional rehabilitation was significantly higher for patients with lower activity levels (91%) in contrast to those with higher activity levels (67%) at 5-year follow-up [49].
Findings from recent research suggest, US may be a useful imaging modality to incorporate into clinical decision making regarding ATRs. More specifically, in their 45-patient study from a cohort of 97 patients participating in a randomized controlled trial comparing surgical and nonsurgical treatments, Westin et al. provides evidence that using US to measure tendon diastasis is a useful tool to incorporate into a clinical treatment algorithm [50]. Westin et al., using US to measure the diastasis of AT ends, demonstrated that patients in the nonsurgical groups with diastasis of >5 mm fare significantly worse with a greater (p=0.004) degree of symptoms (AT total rupture score) and significantly lower (p=0.048) heel-rise height at 12 months [50–52]. This is consistent with prior research by Thermann and Zwipp and Kotnis et al., both suggesting that an initial AT diastasis of more than 5 mm adversely affects functional outcomes for nonsurgical management. Moreover, Westin et al.’s study indicates patients with a diastasis of >10 mm who were treated without surgery had a higher degree of re-rupture, with three of four patients suffering re-rupture (p<0.001). Overall, Westin and his colleagues’ findings suggest that US measurement of the gap between the ruptured AT ends may be one factor that can help guide providers’ clinical decision making between surgical and nonsurgical treatments, based on the indication that larger gaps, especially those larger than ten millimeters, when treated nonoperatively may increase risk of re-rupture and poor outcomes. Additional research will need to be conducted in order to validate the use of US in guiding clinical decision making and treatment approaches for ATR [53, 54].
Modes of Fixation and Author’s Preferred Surgical Technique
The standard repair for ATR involves a locked suture repair with core strands as well as an epitendinous suture (i.e., Krackow) [55]. Suture techniques, including the double loop knot stitch and Kessler stitch, have been biomechanically compared in cadaveric models displaying comparable gap-resistance in relation to elongation under cyclic loading [31]. Suture caliber and the number of core strands have also been studied to assess optimal techniques with regard to the strength, stiffness, and elongation of tendon constructs compared to the native tendon [33]. The addition of suture tape or doubling of the core-strands of a smaller caliber sutures show significant biomechanical advantages with regard to endurance limits [33]. Bone suture anchors and biotenodesis screws are commonly used treatments to augment a degenerative AT through a flexor hallucis longus tendon transfer anchored to the calcaneus. Concerns regarding these procedures involve pullout, suture integrity, and inadequate lengthening. Cadaveric models evaluating and comparing these fixations have determined both to be suitable; however, tendon stiffness is found to be greater using screws in female donors and significantly less tendon is required for the transfer when using suture anchors.
Percutaneous and open repair techniques in cadaveric models display a higher susceptibility to elongation in percutaneous repairs in early weight-bearing, although ultimate load to failure was similar between techniques [32]. Although a minimally invasive percutaneous approach provides similar ultimate, and fatigue load to failure and can potentially mitigate complications involving infections, adhesions, and nerve damage, the lack of visibility may raise the concern of insufficient tendon capture [32].
Preferred Technique
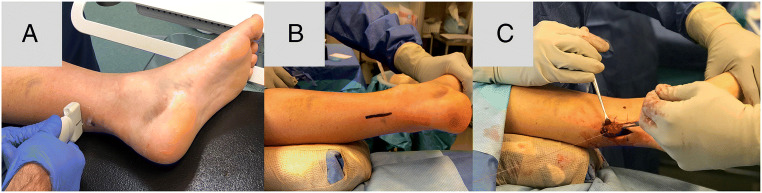
Ideally, active individuals, athletes, and active laborers should be considered for repair, whereas patients who are elderly, obese, sedentary individuals, poorly controlled diabetics, and/or smokers should attempt nonsurgical repair and management due to their increased risk of wound complications. For those receiving repair, the senior author (MA) prefers the mini-open technique with pre-operative localization of ruptured ends with ultrasound to minimize incision length. Supine positioning and a medial-based incision if favored as it minimizes anesthesia complications, operative time, and decreases wound complications (Fig. 3). The tendon is repaired and overtightened in plantarflexion that barely exceeds the contralateral side to avoid elongation during healing. The contralateral side tone is measured preoperatively or may be prepped in the surgical field to compare. Suture repair is performed with a Krackow suture repair as well as an epitendinous running stitch. The suture type is left to the discretion of the surgeon, but our institution utilizes suture tape for repair. Postoperatively, patients remain in a splint for 10 days. After 10 days, patients’ transition to a boot with wedges, weight bearing as tolerated (WBAT), early plantarflexion strengthening, and no dorsiflexion past neutral until the eighth week. During this transition, at 6 weeks, one wedge a week will be removed from the boot and patients can start to bike. Between weeks 8 and 10, patients can begin to wean off the boot and then progress towards walking and jogging during weeks 10–12. Lastly, if muscle strength returns, patients can begin to cut, jump, etc., at weeks 16s–20.
Fig. 3.
Example of preferred mini-open technique repair involving A ultrasound imaging, B incision length marking, and C using end to end Krakow repair
Return to Play
RTP is a major concern for athletes following an ATR. A recent meta-analysis by John et al. based on 15 studies demonstrated that the RTP rate was 76% among professional athletes, where the mean time to RTP was 11 months [56]. This is consistent with the RTP rate (80%) demonstrated in Zellers et al.’s systematic review and meta-analysis, which included 85 studies [57]. In comparison to other common lower extremity orthopedic procedures, including knee microfracture surgery, ACL reconstruction, patellar tendon repair, ankle, or tibial shaft fracture fixation, AT repair is associated with significantly lower RTP rates and significantly longer mean time to RTP [56]. Table 1 shows RTP rates for different sports observed across studies. Calf strength asymmetry, observed in many patients after ATR, likely contributes to the challenges athletes face to RTP [58, 59]. This has been found to be most prevalent in athletic competitions, including basketball and football, where the nature of the games demands a stop/start playing style, repetitive jumping, and short sprints [3, 60–62]. Muscle weakness, decreased endurance, and other functional deficits observed after ATR, which ultimately limit athletes’ abilities to execute the physical demands of sport, in some cases persist for 10 years following injury [57]. In these cases, it is valuable to note prolonged functional deficits experienced by patients after a surgically repaired AT, as this could be associated with the AT healing in an elongated position. This may be misdiagnosed as muscle weakness; however, most muscle will be regained within the first two of years of recovery [29, 34]. In instances when a muscle tendon unit length-tension relationship is compromised, the only treatment to restore the tension is through revision surgery; in some instances, a deep tendon transfer, such as a flexor hallucis longus transfer, is necessary to augment the repair.
Table 1.
Return to play rate observed across eight studies from 2016 to 2020 across five different sports
| Author | Year | Sample (n) | Sport | Return to play rate |
|---|---|---|---|---|
| Wise et al. [38] | 2020 | 57 | Football (NCAA Division I FBS) | 92.5% |
| Trofa et al. [11] | 2018 | 62 (25 NBA, 32 NFL, and 5 MLB, 0 NHL) | Basketball (NBA), Football (NFL), Baseball (MLB), Hockey (NHL) | 69.4% (68.0% NBA, 65.6% NFL, 100%, MLB) |
| Jack et al. [60] | 2017 | 98 | Football (NFL) | 72.4% |
| Mai et al. [64] | 2016 | 80 | Football (NFL) | 72.5% |
| Yang et al. [65] | 2019 | 80 | Football (NFL) | 61.3% |
| Lemme et al. [61] | 2019 | 44 | Basketball (NBA) | 79.5% |
| Grassi et al. [63] | 2020 | 118 | Soccer (European) | 96.0% |
| Minhas et al. [62] | 2016 | 24 | Basketball (NBA) | 70.8% |
Despite these challenges, a majority of athletes are able to RTP; however, decreased performance levels affect many athletes’ abilities to play at their baseline levels [38••, 63]. For NFL players, in their first year returning from ATR, studies demonstrate that athletes play in significantly fewer games, with reduced play time, and worse performance compared to age-matched controls and their preinjury baseline [11, 40, 64]. At 2 to 3 years after surgery, athletes recover to their baseline performance levels and numbers of games played; however, the duration of their NFL career is significantly shorter than controls (3.6 ± 2.1 years, p<0.05) [60, 64].
Among NCAA Football Bowl Subdivision (FBS), defensive players with ATRs do not suffer the same impacts in regards to their RTP rates (92.5%) and performance scores when compared to NFL players with ATR [38••]. Across all FBS defensive positions, players did not experience a drop-off in performance from their preinjury baseline after ATR. With the exception of defensive backs demonstrating decreased improvements with regard to tackling against matched controls, defensive lineman, linebackers, and defensive backs overall show no significant changes with regard to their abilities to rush the passer, stop running plays at the line of scrimmage, and provide pass coverage when compared against controls [38••].
While there are many factors that may contribute to the higher RTP rate and the overall improved performance observed among collegiate athletes in contrast to professional athletes, it is likely that the player’s age at time of injury is one notable difference [38••]. For example, among professional soccer players with ATR, age was identified as an important risk factor for worse outcomes [63]. Age greater than 30 years is associated with an increased risk of not returning to the same level of play (OR=4.46, p=0.030), increased risk of re-rupture within the first two seasons after RTP (OR=6.36, p= 0.05), as well as increased risk of postoperative complications, such as surgical site infection, nerve injury, and wound healing complications. Carmont et al.’s study, which focused on identifying factors that influence functional outcomes at 1 year from postoperative intervention (percutaneous and minimally invasive repair), indicates age to be the strongest predictor of outcome after ATR through multiple regression analyses of symmetrical heel-rise to height index (HRHI) [66•]. In this study, HRHI was selected as the primary variable of interest due to its association with reduced tendon elongation, which contributes to decreased plantar flexion and overall worse ATR outcomes in the long term [66•]. When considering age and its relationship to HRHI, these findings suggest that younger patients with ATRs experience reduced tendon elongation, thus increasing their likelihood of regaining ankle plantar flexion and achieving more successful AT recovery based on long-term outcomes.
Beyond age, other differences between the NFL and NCAA athletes that may provide insight to potential factors impacting ATR outcomes, such as RTP, include degeneration and level of play [38••]. Tendinosis is a naturally occurring process that accompanies aging as the tensile strength of collagen decreases with the decreasing blood flow to regions [7]. With these changes, the tendon stiffness increases, decreasing its ability to withstand repetitive stress. Failed healing responses observed in the setting of tendinosis are often impacted by continued exposure to ongoing mechanical forces, poor blood supply, or a combination of both factors [67]. More specifically, ongoing exposure to mechanical forces play a major role in many foot and ankle tendinopathies, such that the term tendinopathy is used to describe overuse tendon injuries in the absence of a pathologic diagnosis [68, 69]. Tendinopathies of the AT, posterior tibial tendon, and peroneal tendon, as well as other foot and ankle tendinopathies, often occur when patients’ physical activity or athletic training changes with respect to mode, intensity, or duration. While overuse injuries affecting the peroneal tendon most often result in chronic tendonitis and interstitial tears, the same overuse injuries affecting the AT are more prone to rupture due to the added insult of poor blood supply that often occurs in the vascular watershed region of the AT [70]. In turn, it is this combined progression of decreasing blood supply and decreasing collagen strength that occurs with aging and ongoing overuse, that increases the likelihood of rupture of the AT, unlike other foot and ankle tendinopathies. Furthermore, the same poor vascular supply that contributes to the weakening of the tendon that increases the risk of rupture also creates an environment that is less conducive to healing and recovery when an ATR takes place [7].
Understanding that NFL players are generally older than their NCAA counterparts, it is likely that there is a higher incidence of tendinosis and tendinopathy. The degeneration older athletes’ experience and the corresponding challenges these degenerative changes produce during the recovery phase following ATR are only exacerbated by the increased stress and physical demands professional athletes endure when trying to return and compete at an elite level. In turn, considering an athlete’s age, their history of degeneration, and the level of competition are all factors the clinical team and patient should appraise when selecting a treatment plan and discussing the athlete’s likelihood of returning to play.
Conclusion
In conclusion, the Achilles tendon plays an important role in sports performance due to its vital role in ankle stability and allowing athletes to perform explosive activities. While nonoperative management may be sufficient in some non-athletes, multiple studies support operative intervention in elite athletes. Recent studies have shown that operative intervention improves postoperative strength and functional outcomes as well as lowers re-rupture rates compared to nonoperative management with rehabilitation. The current literature supports operative intervention in elite athletes to improve performance and shorten their RTP. However, we acknowledge that surgical intervention does have inherent risks. Ultimately, most if not all athletes with an ATR benefit from surgical repair, but it is crucial to take a critical stepwise algorithmic approach and consider other factors, which may lead towards nonoperative intervention. These factors include age, chronicity of injury, gap of ATR, social factors, medical history, and other factors which are discussed in this review. The RTP rate for athletes that experience an ATR still remains to be 80%, with difficulty in achieving baseline performance until 2 to 3 years after surgery, impacting the overall career of an athlete. This is an area where post-operative rehabilitation, with an emphasis on early weight-bearing, may prove to be beneficial in reducing the time back to peak performance.
Declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work. Dr. Michael Aynardi is a consultant for Arthrex Inc., Zimmer Biomet, and Stryker. The other authors have no relevant financial or non-financial interests to disclose.
Footnotes
This article is part of the Topical Collection on Sports Injuries and Rehabilitation: Getting Athletes Back to Play
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirsten Mansfield, Email: kmansfield@pennstatehealth.psu.edu.
Kelly Dopke, Email: kdopke@pennstatehealth.psu.edu.
Zachary Koroneos, Email: zkoroneos@pennstatehealth.psu.edu.
Vincenzo Bonaddio, Email: vbonaddio@pennstatehealth.psu.edu.
Adeshina Adeyemo, Email: aadeyemo@pennstatehealth.psu.edu.
Michael Aynardi, Email: maynardi@pennstatehealth.psu.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Lantto I, Heikkinen J, Flinkkilä T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand J Med Sci Sports. 2015;25(1):e133–e138. doi: 10.1111/sms.12253. [DOI] [PubMed] [Google Scholar]
- 2.Okewunmi J, Guzman J, Vulcano E. Achilles tendinosis injuries-tendinosis to rupture (getting the athlete back to play) Clin Sports Med. 2020;39(4):877–891. doi: 10.1016/j.csm.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Winnicki K, Ochała-Kłos A, Rutowicz B, Pękala PA, Tomaszewski KA. Functional anatomy, histology and biomechanics of the human Achilles tendon - a comprehensive review. Ann Anat. 2020;229:151461. doi: 10.1016/j.aanat.2020.151461. [DOI] [PubMed] [Google Scholar]
- 4.Westermann RW, Kerr ZY, Wehr P, Amendola A. Increasing lower extremity injury rates across the 2009-2010 to 2014-2015 seasons of National Collegiate Athletic Association Football: an unintended consequence of the “targeting” rule used to prevent concussions? Am J Sports Med. 2016;44(12):3230–3236. doi: 10.1177/0363546516659290. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari M, Guyatt GH, Siddiqui F, Morrow F, Busse J, Leighton RK, Sprague S, Schemitsch EH. Treatment of acute Achilles tendon ruptures: a systematic overview and metaanalysis. Clin Orthop Relat Res. 2002;400:190–200. doi: 10.1097/00003086-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005;10(2):225–238. doi: 10.1016/j.fcl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- 8.• Yin NH, Fromme P, Mc Carthy I, Birch HL. Individual variation in Achilles tendon morphology and geometry changes susceptibility to injury. Elife. 2021;10:e63204. Published 2021 Feb 16. This biomechanical study demonstrates the variability among Achilles tendons in individuals which impacts the physiological behavior of the Achilles tendon when subjected to stress. This is significant to note when identifying possible athletes who may be at risk of rupture and how to personalize follow up care and management [DOI] [PMC free article] [PubMed]
- 9.Dederer KM, Tennant JN. Anatomical and functional considerations in Achilles tendon lesions. Foot Ankle Clin. 2019;24(3):371–385. doi: 10.1016/j.fcl.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen TM, Rozen WM, Pan WR, Ashton MW, Richardson MD, Taylor GI. The arterial anatomy of the Achilles tendon: anatomical study and clinical implications. Clin Anat. 2009;22(3):377–385. doi: 10.1002/ca.20758. [DOI] [PubMed] [Google Scholar]
- 11.Trofa DP, Miller JC, Jang ES, Woode DR, Greisberg JK, Vosseller JT. Professional athletes’ return to play and performance after operative repair of an Achilles tendon rupture. Am. J. Sports Med. 2017;45:2864–2871. doi: 10.1177/0363546517713001. [DOI] [PubMed] [Google Scholar]
- 12.Tarantino D, Palermi S, Sirico F, Corrado B. Achilles tendon rupture: mechanisms of injury, principles of rehabilitation and return to play. J Funct Morphol Kinesiol. 2020;5(4):95. doi: 10.3390/jfmk5040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aicale R, Tarantino D, Maulli N. Basic science of tendons. In Bio-Orthopaedics: a new approach; Gobbi, A., Espregueira-Mendes, J., Lane, J.G., Karahan, M., Eds. Berlin: Springer; 2017. pp. 249–273. [Google Scholar]
- 14.Egger AC, Berkowitz MJ. Achilles tendon injuries. Curr Rev Musculoskelet Med. 2017;10(1):72–80. doi: 10.1007/s12178-017-9386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barfred T. Achilles tendon rupture: aetiology and pathogenesis of subcutaneous rupture assessed on the basis of the literature and rupture experiments on rats. Acta Orthop Scand. 1973;44(sup152):1–126. doi: 10.3109/ort.1973.44.suppl-152.01. [DOI] [PubMed] [Google Scholar]
- 16.Riggin CN, Morris TR, Soslowsky LJ. In: Tendinopathy II: etiology, pathology, and healing of tendon injury and disease. In Tendon Degeneration: Understanding Tissue Physiology and Development to Engineer Functional Substitutes. Gomes ME, Reis RL, Rodrigues MT, editors. Amsterdam: Elsevier; 2015. pp. 149–183. [Google Scholar]
- 17.Alsousou J, Keene DJ, Harrison P, Hulley P, Wagland S, Thompson JY, Parsons SR, Byrne C, Schussel MM, O’Connor HM, et al. Platelet-rich plasma injection for adults with acute Achilles tendon rupture: the PATH-2 RCT. Ec. Mech. Eval. 2019;6:1–98. [PubMed] [Google Scholar]
- 18.Cheung JT, Zhang M, An KN. Effect of Achilles tendon loading on plantar fascia tension in the standing foot. Clin Biomech (Bristol, Avon). 2006;21(2):194–203. doi: 10.1016/j.clinbiomech.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Fukashiro S, Komi PV, Järvinen M, Miyashita M. In vivo Achilles tendon loading during jumping in humans. Eur J Appl Physiol Occup Physiol. 1995;71(5):453–458. doi: 10.1007/BF00635880. [DOI] [PubMed] [Google Scholar]
- 20.Komi PV, Fukashiro S, Järvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11(3):521–531. doi: 10.1016/S0278-5919(20)30506-8. [DOI] [PubMed] [Google Scholar]
- 21.Oda H, Sano K, Kunimasa Y, Komi PV, Ishikawa M. Neuromechanical modulation of the Achilles tendon during bilateral hopping in patients with unilateral Achilles tendon rupture, over 1 year after surgical repair. Sports Med. 2017;47(6):1221–1230. doi: 10.1007/s40279-016-0629-3. [DOI] [PubMed] [Google Scholar]
- 22.Wearing SC, Davis IS, Brauner T, Hooper SL, Horstmann T. Do habitual foot-strike patterns in running influence functional Achilles tendon properties during gait? J Sports Sci. 2019;37(23):2735–2743. doi: 10.1080/02640414.2019.1663656. [DOI] [PubMed] [Google Scholar]
- 23.Wade FE, Lewis GS, Piazza SJ. Estimates of Achilles tendon moment arm differ when axis of ankle rotation is derived from ankle motion. J Biomech. 2019;90:71–77. doi: 10.1016/j.jbiomech.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Rasske K, Thelen DG, Franz JR. Variation in the human Achilles tendon moment arm during walking. Comput Methods Biomech Biomed Engin. 2017;20(2):201–205. doi: 10.1080/10255842.2016.1213818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi KZ, Gross MT, van Werkhoven H, Piazza SJ, Sawicki GS. Adding stiffness to the foot modulates soleus force-velocity behaviour during human walking. Sci Rep. 2016;6:29870. doi: 10.1038/srep29870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxter JR, Piazza SJ. Plantar flexor moment arm and muscle volume predict torque-generating capacity in young men. J Appl Physiol (1985). 2014;116(5):538–544. doi: 10.1152/japplphysiol.01140.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olszewski K, Dick TJ, Wakeling JM. Achilles tendon moment arms: the importance of measuring at constant tendon load when using the tendon excursion method. J Biomech. 2015;48(6):1206–1209. doi: 10.1016/j.jbiomech.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59–64. doi: 10.1177/0363546506293255. [DOI] [PubMed] [Google Scholar]
- 29.Silbernagel KG, Steele R, Manal K. Deficits in heel-rise height and Achilles tendon elongation occur in patients recovering from an Achilles tendon rupture. Am J Sports Med. 2012;40(7):1564–1571. doi: 10.1177/0363546512447926. [DOI] [PubMed] [Google Scholar]
- 30.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Frosch S, Buchhorn G, Hawellek T, Walde TA, Lehmann W, Hubert J. Comparison of the double loop knot stitch and Kessler stitch for Achilles tendon repair: a biomechanical cadaver study. PLoS One. 2020;15(12):e0243306. doi: 10.1371/journal.pone.0243306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clanton TO. Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957–1964. doi: 10.1177/0363546515587082. [DOI] [PubMed] [Google Scholar]
- 33.Backus JD, Marchetti DC, Slette EL, Dahl KD, Turnbull TL, Clanton TO Effect of suture caliber and number of core strands on repair of acute Achilles ruptures: a biomechanical study. Foot Ankle Int. 2017;38(5):564–570. doi: 10.1177/1071100716687368. [DOI] [PubMed] [Google Scholar]
- 34.Brorsson A, Willy RW, Tranberg R, Grävare SK. Heel-rise height deficit 1 year after Achilles tendon rupture relates to changes in ankle biomechanics 6 years after injury. Am J Sports Med. 2017;45(13):3060–3068. doi: 10.1177/0363546517717698. [DOI] [PubMed] [Google Scholar]
- 35.Agres AN, Duda GN, Gehlen TJ, Arampatzis A, Taylor WR, Manegold S. Increased unilateral tendon stiffness and its effect on gait 2-6 years after Achilles tendon rupture. Scand J Med Sci Sports. 2015;25(6):860–867. doi: 10.1111/sms.12456. [DOI] [PubMed] [Google Scholar]
- 36.Eliasson P, Agergaard AS, Couppé C, Svensson R, Hoeffner R, Warming S, Warming N, Holm C, Jensen MH, Krogsgaard M, Kjaer M, Magnusson SP. The ruptured Achilles tendon elongates for 6 months after surgical repair regardless of early or late weightbearing in combination with ankle mobilization: a randomized clinical trial. Am J Sports Med. 2018 Aug;46(10):2492–502. 10.1177/0363546518781826. Epub 2018 Jul 2 [DOI] [PubMed]
- 37.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 38.•• Wise PM, King JL, Stauch CM, Walley KC, Aynardi MC, Gallo RA. Outcomes of NCAA defensive football players following Achilles tendon repair. Foot Ankle Int. 2020;41(4):398–402. This retrospective comparative study details return to play rates for Division I college football players after an Achilles rupture followed by repair and found that players returned at a high rate without a decline in their performance but at the expense of pursuing a professional career. This demonstrates the significance of how an Achilles tendon rupture impacts athletes and the need for further research on how to approach an elite athlete after Achilles tendon rupture [DOI] [PubMed]
- 39.Ardern CL, Glasgow P. Schneiders A, et al2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy. BernBritish Journal of Sports Medicine. 2016;50:853–864. doi: 10.1136/bjsports-2016-096278. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell JE, Vosseller JT. Maximizing return to sports after Achilles tendon rupture in athletes. Foot Ankle Clin. 2019;24(3):439–445. doi: 10.1016/j.fcl.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Holm C, Kjaer M, Eliasson P. Achilles tendon rupture--treatment and complications: a systematic review. Scand J Med Sci Sports. 2015;25(1):e1–e10. doi: 10.1111/sms.12209. [DOI] [PubMed] [Google Scholar]
- 42.• Manent A, López L, Corominas H, Santamaría A, Domínguez A, Llorens N, Sales M, Videla S. Acute Achilles tendon ruptures: efficacy of conservative and surgical (percutaneous, open) treatment-a randomized, controlled, clinical trial. J Foot Ankle Surg. 2019;58(6):1229–34. With controversy over the optimal treatment option for an Achilles tendon repair, this clinical trial details the importance of early weight-bearing as part of the rehabilitation program which reduces the likelihood of rerupture in the future. This may prove to be very helpful for athletes seeking early return to play without any deficits in performance [DOI] [PubMed]
- 43.Suchak, Amar A. Bostick, Geoff P. Beaupré, Lauren A. Durand D’AC. Jomha, Nadr M. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon, The Journal of Bone & Joint Surgery: September 01, 2008 - Volume 90 - Issue 9 - p 1876-1883 10.2106/JBJS.G.01242 [DOI] [PubMed]
- 44.Zhou K, Song L, Zhang P, Wang C, Wang W. Surgical versus non-surgical methods for acute Achilles tendon rupture: a meta-analysis of randomized controlled trials. J Foot Ankle Surg. 2018;57(6):1191–1199. doi: 10.1053/j.jfas.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Hussien DG, Villarreal JV, Panchbhavi V, Jupiter DC. Predisposing factors for 30-day complications following Achilles tendon repair. J Foot Ankle Surg. 2021;60(2):288–291. doi: 10.1053/j.jfas.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 46.Lantto I, Heikkinen J, Flinkkila T, Ohtonen P, Siira P, Laine V, Leppilahti J. A prospective randomized trial comparing surgical and nonsurgical treatments of acute Achilles tendon ruptures. Am J Sports Med. 2016;44(9):2406–2414. doi: 10.1177/0363546516651060. [DOI] [PubMed] [Google Scholar]
- 47.Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. doi: 10.1136/bmj.k5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel KA, O'Malley MJ. Management of Achilles tendon injuries in the elite athlete. Orthop Clin North Am. 2020;51(4):533–539. doi: 10.1016/j.ocl.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Lerch TD, Schwinghammer A, Schmaranzer F, Anwander H, Ecker TM, Schmid T, Weber M, Krause F. Return to sport and patient satisfaction at 5-year follow-up after nonoperative treatment for acute Achilles tendon rupture. Foot Ankle Int. 2020;41(7):784–792. doi: 10.1177/1071100720919029. [DOI] [PubMed] [Google Scholar]
- 50.Westin O, Nilsson Helander K, Grävare Silbernagel K, Möller M, Kälebo P, Karlsson J. Acute ultrasonography investigation to predict reruptures and outcomes in patients with an Achilles tendon rupture. Orthop J Sports Med. 2016;4(10):2325967116667920. doi: 10.1177/2325967116667920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thermann H, Zwipp H. Achillessehnenruptur [Achilles tendon rupture] Orthopade. 1989;18(4):321–335. [PubMed] [Google Scholar]
- 52.Kotnis R, David S, Handley R, Willett K, Ostlere S. Dynamic ultrasound as a selection tool for reducing Achilles tendon reruptures. Am J Sports Med. 2006;34(9):1395–1400. doi: 10.1177/0363546506288678. [DOI] [PubMed] [Google Scholar]
- 53.Amlang MH, Zwipp H, Friedrich A, Peaden A, Bunk A, Rammelt S. Ultrasonographic classification of Achilles tendon ruptures as a rationale for individual treatment selection. ISRN Orthop. 2011;2011:869703. doi: 10.5402/2011/869703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen MS, Vestermark MT, Hölmich P, Kristensen MT, Barfod KW. Individualized treatment for acute Achilles tendon rupture based on the Copenhagen Achilles Rupture Treatment Algorithm (CARTA): a study protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):399. doi: 10.1186/s13063-020-04332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drakos MC, Gott M, Karnovsky SC, Murphy CI, DeSandis BA, Chinitz N, Grande D, Chahine N. Biomechanical analysis of suture anchor vs tenodesis screw for FHL transfer. Foot Ankle Int. 2017;38(7):797–801. doi: 10.1177/1071100717702848. [DOI] [PubMed] [Google Scholar]
- 56.Johns W, Walley KC, Seedat R, Thordarson DB, Jackson B, Gonzalez T. Career outlook and performance of professional athletes after Achilles tendon rupture: a systematic review. Foot Ankle Int. 2021;42(4):495–509. doi: 10.1177/1071100720969633. [DOI] [PubMed] [Google Scholar]
- 57.Zellers JA, Carmont MR, Grävare SK. Return to play post-Achilles tendon rupture: a systematic review and meta-analysis of rate and measures of return to play. Br J Sports Med. 2016;50(21):1325–1332. doi: 10.1136/bjsports-2016-096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyer CF, Berlet G, Philbin T, et al. Does functional neuromuscular electrical stimulation (NMES) influence calf atrophy following Achilles tendon surgery? Prospective Double-Blind Randomized Controlled Trial on the Use of Immediate Postoperative Electrical Muscle Stimulation to Preserve Muscle Function and Volume [published online ahead of print, 2021 Jan 8] J Foot Ankle Surg. 2021;S1067-2516(21):00001–00006. doi: 10.1053/j.jfas.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Mavrodontidis A, Lykissas M, Koulouvaris P, Pafilas D, Kontogeorgakos V, Zalavras C. Percutaneous repair of acute Achilles tendon rupture: a functional evaluation study with a minimum 10-year follow-up. Acta Orthop Traumatol Turc. 2015;49(6):661–667. doi: 10.3944/AOTT.2015.14.0196. [DOI] [PubMed] [Google Scholar]
- 60.Jack RA, 2nd, Sochacki KR, Gardner SS, McCulloch PC, Lintner DM, Cosculluela PE, Varner KE, Harris JD. Performance and return to sport after Achilles tendon repair in National Football League players. Foot Ankle Int. 2017;38(10):1092–1099. doi: 10.1177/1071100717718131. [DOI] [PubMed] [Google Scholar]
- 61.Lemme NJ, Li NY, Kleiner JE, Tan S, DeFroda SF, Owens BD. Epidemiology and video analysis of Achilles tendon ruptures in the National Basketball Association. Am J Sports Med. 2019;47(10):2360–2366. doi: 10.1177/0363546519858609. [DOI] [PubMed] [Google Scholar]
- 62.Minhas SV, Kester BS, Larkin KE, Hsu WK. The effect of an orthopaedic surgical procedure in the National Basketball Association. Am J Sports Med. 2016;44(4):1056–1061. doi: 10.1177/0363546515623028. [DOI] [PubMed] [Google Scholar]
- 63.Grassi A, Rossi G, D'Hooghe P, Aujla R, Mosca M, Samuelsson K, Zaffagnini S. Eighty-two percent of male professional football (soccer) players return to play at the previous level two seasons after Achilles tendon rupture treated with surgical repair. Br J Sports Med. 2020;54(8):480–486. doi: 10.1136/bjsports-2019-100556. [DOI] [PubMed] [Google Scholar]
- 64.Mai HT, Alvarez AP, Freshman RD, Chun DS, Minhas SV, Patel AA, Nuber GW, Hsu WK. The NFL Orthopaedic Surgery Outcomes Database (NO-SOD): the effect of common orthopaedic procedures on football careers. Am J Sports Med. 2016;44(9):2255–2262. doi: 10.1177/0363546516651426. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Hodax JD, Machan JT, Krill MK, Lemme NJ, Durand WM, Hoffman JT, Hewett TE, Owens BD. Factors affecting return to play after primary Achilles tendon tear: a cohort of NFL players. Orthop J Sport Med. 2019;7(3):1–8. doi: 10.1177/2325967119830139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.• Carmont MR, Zellers JA, Brorsson A, Nilsson-Helander K, Karlsson J, Grävare Silbernagel K. Age and tightness of repair are predictors of heel-rise height after Achilles tendon rupture. Orthop J Sports Med. 2020;8(3):2325967120909556. This prospective cohort study of patients sustaining Achilles tendon rupture receiving operative repair demonstrates age as the strongest predictor of functional outcomes at one year from postoperative intervention. Among modifiable risk factors, tightness of repair was the most important risk factor identified to optimize heel-rise height, as well as other functional outcomes, at 1 year after operative repair. While repairing in greater tightness to the contralateral side is commonly practiced, as tendon elongation during healing and rehabilitation contributes to poor functional outcomes, this study provides novel data to support the influence of tightness of repair on functional (heel-rise) performance [DOI] [PMC free article] [PubMed]
- 67.Simpson MR, Howard TM. Tendinopathies of the foot and ankle. Am Fam Physician. 2009;80(10):1107–1114. [PubMed] [Google Scholar]
- 68.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. doi: 10.1016/S0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 69.Wilder RP, Sethi S. Overuse injuries: tendinopathies, stress fractures, compartment syndrome, and shin splints. Clin Sports Med. 2004;23(1):55–81. doi: 10.1016/S0278-5919(03)00085-1. [DOI] [PubMed] [Google Scholar]
- 70.Jones DC. Tendon disorders of the foot and ankle. J Am Acad Orthop Surg. 1993;1(2):87–94. doi: 10.5435/00124635-199311000-00003. [DOI] [PubMed] [Google Scholar]