Abstract
Alkaline cellobiohydrolases have the potential for application in various industries, including pulp processing and laundry where operation under high pH conditions is preferred. In this study, variants of CtCel6A cellobiohydrolase from Chaetomium thermophilum were generated by structural-based protein engineering with the rationale of increasing catalytic activity and alkaline stability. The variants included removal of the carbohydrate-binding module (CBM) and substitution of residues 173 and 200. The CBM-deleted enzyme with Y200F mutation predicted to mediate conformational change at the N-terminal loop demonstrated increased alkaline stability at 60 °C, pH 8.0 for 24 h up to 2.25-fold compared with the wild-type enzyme. Another CBM-deleted enzyme with L173E mutation predicted to induce a new hydrogen bond in the substrate-binding cleft showed enhanced hydrolysis yield of pretreated sugarcane trash up to 4.65-fold greater than that of the wild-type enzyme at the pH 8.0. The variant enzymes could thus be developed for applications on cellulose hydrolysis and plant fiber modification operated under alkaline conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03339-4.
Keywords: Cellobiohydrolase, Alkaline stability, Structure-based protein engineering, Pulp and paper
Introduction
Cellulose is a major component of the plant cell wall comprising approximately 30–50% of the dry weight. It is composed of glucose units linked by β-1,4 glycosidic bonds into linear chains. Cellulose consists of amorphous and crystalline regions, in which the latter with a highly organized structure makes cellulose recalcitrant to enzymatic degradation (Arantes and Saddler 2010). Cellulolytic microorganisms degrade cellulose via three types of cellulase enzymes that work synergistically. Endoglucanases (EG) (EC 3.2.1.4) attack internal β-1,4-glycosidic linkages among amorphous regions to create oligosaccharides and new free chain ends. Cellobiohydrolases (CBH) or exoglucanases (EC 3.2.1.91 and 3.2.1.176) act against the crystalline region of cellulose to progressively digest the cellulose chain ends to cellobiose. Finally, β-glucosidases (BGL) (EC 3.2.1.21) further cleave soluble oligosaccharides into glucose (Champreda et al. 2019; Howard et al. 2003; Lynd et al. 2002).
CBH enzymes are exo-acting glycosyl hydrolases with a preference for the crystalline regions of the cellulose chain. Based on amino acid sequence similarity and enzyme activity, CBH enzymes are classified into two subclasses, namely Cel6A (CBH-II) which hydrolyzes the cellulose chain from the non-reducing ends (EC 3.2.1.91) and Cel7A (CBH-I) which attacks the substrate at the reducing ends (EC 3.2.1.176). Both Cel6A and Cel7A enzymes act progressively by liberating cellobiose from the ends of exposed cellulose chains. (Baramee et al. 2017; Cerda-Mejía et al. 2017; Christensen et al. 2018). Cel6A belongs to the glycoside hydrolase family 6 (GH6) and Cel6A enzymes have been identified from diverse microbial origins, for example, Paenibacillus curdlanolyticus, Trichoderma reesei, Aspergillus fumigatus, Penicillium decumbens, Magnaporthe grisea and Chaetomium thermophilum (Gao et al. 2011; Takahashi et al. 2010; Thompson et al. 2012; Zhou et al. 2017). The Cel6A structure includes a characteristic (β/α)7 barrel fold comprising a buried tunnel-shaped active site that allows the cellulose chain to be threaded through the enzyme and sequentially cleaved at glycosidic bonds (Thompson et al. 2012). Interestingly, some of these enzymes have endo- and exo-action, for example, the Cel6A from Humicola insolens that contains two active-site conformations, i.e., closed tunnel-shape and open cleft-shape (Varrot et al. 2003). Many of these enzymes display a modular structure consisting of a GH6 catalytic module linked with one or more non-catalytic carbohydrate-binding modules (CBMs). The CBM can significantly enhance the enzyme activity against crystalline cellulose by allowing the enzyme’s active site to attach to the cellulose chain and enable its progressive hydrolysis (Gilbert et al. 2013; Hervé et al. 2010).
Cellulases working optimally under alkaline conditions have great potential for application in several industries including pulp and paper, laundry and detergents, and food (Ben Hmad and Gargouri 2017; Jayasekara and Ratnayake 2019). However, most CBHs work optimally under acidic conditions (Díaz-Rincón et al. 2017; Gu et al. 2019; Lantz et al. 2010; Ogunmolu et al. 2017) and there are only a few reports of native alkaline CBHs, including H. insolens CBH-II which works optimally at pH 9.0 (Schülein 1997), M. oryzae GH-6 CBH which is active from pH 5.5–9 (Takahashi et al. 2010), and H. grisea CBH which has an optimal pH of 8.0 (Oliveira et al. 2013).
Enzyme engineering has been applied for enhancing the performance and kinetic properties of CBHs, with an emphasis on increasing activity and thermostability. A mutant of CBH-II from C. thermophilum demonstrated increased catalytic activity by 1.82-, 1.65- and 1.43-fold against β-d-glucan, phosphoric acid swollen cellulose (PASC) and carboxymethyl cellulose-sodium salt (CMC-Na), respectively, under the optimal conditions at 70 °C, pH 5.0 (Han et al. 2018). A mutant of CBH-I from Penicillium verruculosum showed 2.48-fold higher thermostability after 1 h incubation at 65 °C compared with the wild type (Pramanik et al. 2021). Site-directed mutagenesis of Cel5A from Thermotoga maritima revealed a shift in optimal pH from 5.0 to 5.4 and 14 to 18-fold higher hydrolytic activity against Avicel at 70 °C, pH 5.0 (Mahadevan et al. 2008). To our knowledge, there are only two reports of CBH engineering for improving activity and stability under alkaline conditions. A mutant of T. reesei Cel7A exhibited increased pK1 and pK2 compared with that of the wild-type (Becker et al. 2001). In another study, the T. reesei Cel6A E107Q/D170N/D366N mutant showed enhanced alkaline stability with a four-fold increased half-life at pH 8.0 compared with that of wild-type (Wohlfahrt et al. 2003). Apart from mutagenesis, modification of CBM has been reported for improving of cellulolytic activity and specificity (Cuskin et al. 2012). CBM enhanced the accessibility of enzyme targeted to the substrate (Bernardes et al. 2019) and allow the catalytic module to destroy the crystallinity of the insoluble polysaccharides (Thongekkaew et al. 2013). The deletion of CBM can affect on decrease the enzymatic activity and stability (Courtade et al. 2018). However, it has been reported that the removal of CBM enhanced the hydrolysis activity (Park et al. 2002). CBM-truncated endo-β-1,4-xylanase (X11) resulted in 3.4-fold greater specific enzyme activity compared to the wild type (Boonyapakron et al. 2021). GH11 XynATM1 lacking CBM exhibited a considerable improvement in specific activity for 2.3-fold (Meng et al. 2015). The catalytic efficiency of CBD truncated mutant Egl330, an endo-β-1,4-glucanase from Bacillus subtilis JA18 increased for the CMC substrate with a great improvement in thermal stability (Wang et al. 2009). The Chaetomium thermophilum Cel6A enzyme (CtCel6A) is an exocellulase consisting of a GH6 catalytic domain and a family 1 CBM1. The crystal structure of the catalytic domain of CtCel6A was solved in complex with cellotetraose (PDB: 4A05), which revealed that the catalytic domain of CtCel6A adopts a distorted α/β-barrel fold with a buried tunnel-like active site (Thompson et al. 2012). In this study, the enzymatic properties of CtCel6A and variants created by site-directed mutagenesis were investigated. We expressed CtCel6A recombinant proteins in Pichia pastoris and obtained high yields of secreted enzymes. We show that wild-type CtCel6A works optimally at 60 °C and pH 5.0. Moreover, the wild-type enzyme is thermostable showing ~ 70% activity remaining after incubation at 60 °C for 48 h. Structure-based site-directed mutagenesis of the full-length enzyme and the catalytic domain of CtCel6A was applied with the aim of increasing the activity and stability under alkaline conditions as needed for the pulp and paper industry.
Materials and methods
Strains and plasmids
The Cel6A gene from C. thermophilum (accession number XP_006695418, CtCel6A) was synthesized by GenScript (New Jersey, USA). Expression vector pPICZαA, Escherichia coli DH5α and Pichia pastoris KM71 were purchased from Invitrogen (Carlsbad, CA, USA). Analytical or molecular biology grade chemicals and reagents were procured from major chemical suppliers (Sigma-Aldrich, Fluka, and Merck).
Molecular docking
The crystal structure of C. thermophilum cellobiohydrolase Cel6A (PDB: 4A05) was used as a template for docking of a cellotetraose molecule at subsites − 1 to − 4 in AutoDockTools (https://vina.scripps.edu/). Cellobiose and cellotetraose at the active cleft of a distorted α/β barrel architecture CtCel6A were deleted from the subsites − 2 and − 3 and + 1 to + 4, respectively (Thompson et al, 2012). Then, a cellotetraose molecule was docked into the subsites − 1 to − 4 to link to the original cellotetraose molecule at the subsite + 1 to + 4 to create cellooctaose at the active site of CtCel6A. The cellooctaose-bound structure was analyzed to determine candidate residues in the substrate-binding region for mutagenesis. Figures were prepared by PyMol molecular graphics system, version 2.1 (http://www.pymol.org).
Mutagenesis of CtCel6A
The CBM deletion (residues 19–115) of full-length CtCel6A was performed by polymerase chain reaction (PCR) using CtCel6A_CD_F_EcoRI and CtCel6A_CD_R_XbaI primer pairs (Table S1). Variants of both full-length CtCel6A (FL) and the CBM-deleted (catalytic domain; CD) of CtCel6A were generated by site-directed mutagenesis as shown in Fig. 1. Briefly, a 50 µl reaction of 10 ng pPICZαA plasmid DNA of either the full-length or CD of CtCel6A, 0.2 µM of each corresponding forward and reverse primer pairs (Table S1), 0.2 mM of each dNTP, and 0.5 U of Phusion High-Fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) was run using the following PCR condition: one cycle of 5 min at 94 °C, 25 cycles of 30 s at 94 °C, 30 s at 55 °C and 5 min at 72 °C, then 10 min at 72 °C. The amplified products were digested with 10 U of DpnI at 37 °C for 24 h and transformed into E. coli DH5α. All the constructs were confirmed by nucleotide sequence analysis (1st Base, Singapore).
Fig. 1.
Schematic diagram of CtCel6A mutants construction. The CBM1 was removed from wild-type CtCel6A (FL) resulting in the CBM-deleted mutant (CD) containing only the catalytic domain. The mutant with single (L173E, Y200F) and double mutation (L173E/Y200F) were generated by PCR-based mutagenesis. The wild-type and all mutants were constructed and expressed in yeast P. pastoris
Transformation and expression of P. pastoris KM71
The recombinant pPICZαA plasmids for CtCel6A full-length and CD expression were linearized and integrated into the P. pastoris KM71 genome by electroporation. Integrants were selected on YPD plates supplemented with 100 µg/ml of Zeocin. CtCel6A gene integrants were confirmed by colony PCR. A 25 µl of PCR reaction containing a single colony of integrant in 1 × Gotaq® Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), 0.4 µM 5’-AOX1 primer and 3’-AOX1 primer was run with the following PCR condition: one cycle of 5 min at 94 °C, 25 cycles of 30 s at 94 °C, 30 s at 50 °C and 1 min 30 s at 72 °C, then 10 min at 72 °C. For recombinant protein expression, the integrants were pre-cultured in YPD medium at 30 °C with shaking at 250 rpm for 72 h. The pre-culture with OD600 = 0.2 was transferred into BMGY (Buffered Glycerol-complex medium, Invitrogen) and incubated at 30 °C with shaking at 250 rpm for 16 h to reach an OD600 of 5–6. After harvesting, the cell pastes with total OD600 of 30 were resuspended in BMMY (Buffered Methanol-complex medium, Invitrogen) with 3% (v/v) methanol added as an inducer for the production of recombinant CtCel6A protein. The cells were then cultured at 30 °C for 48 h with continuous shaking at 250 rpm, in which methanol was added to 3% (v/v) after 24 h. To remove the medium components and concentrate the enzyme, buffer exchange with 50 mM sodium acetate buffer pH 5.0 was performed using Amicon Ultra-15 Centrifugal Filter Units with a 10-kDa cut-off (Millipore, Darmstadt, Germany). The protein concentration was determined by the Bradford protein assay (Bio-Rad, Hercules, CA, USA) with Bovine Serum Albumin (BSA) standards. Protein purity was assessed by SDS-PAGE.
Cellulase activity assay
Cellulase activity of CtCel6A variants was measured by the dinitrosalicylic acid (DNS) method (Miller 1959) at 60 °C and pH 5 (the optimal conditions for CtCel6A wild-type) for 30 min. The reaction was performed using 18 µM of enzyme and 90 µg phosphoric acid swollen cellulose (PASC) (Zhang et al. 2006) in 50 mM sodium acetate buffer pH 5.0 at 60 °C for 30 min. Next, 200 µl of the DNS reagent was added into the reaction. The reaction was terminated by boiling for 10 min. The amount of reducing sugars was determined from the absorbance at 540 nm wavelength using glucose as a standard. The relative activity refers to the percent of reducing sugar released by the enzyme in comparison with that of the maximum value observed for each enzyme.
Temperature and pH profiles of CtCel6A variants were determined from cellulase activity as described above. For the temperature profile, the activity was measured at pH 5.0 for 30 min from 40 to 90 °C. For the pH profile, the activity was measured at pH 4.0–10.0 at 60 °C for 30 min. Buffers used to maintain different pH included 50 mM sodium acetate buffer for pH 4.0–6.0, 50 mM sodium phosphate buffer for pH 6.0–8.0 and 50 mM Tris–HCl buffer for pH 8.0–10.0. All the experiments were performed in triplicate. The differences between groups were analyzed by one-way ANOVA using Tukey’s Post Hoc test. The corrected p value < 0.05 was considered as statistically significant.
Stability of recombinant CtCel6A
The enzyme stability was determined by incubating 180 µM of enzyme at 60 °C in 50 mM sodium acetate buffer pH 5.0 or 100 mM Tris–HCl buffer pH 8.0 for 0, 1, 2, 3, 6, 9, 18, 24 and 48 h. After pre-incubation, enzyme activity was determined by DNS method. Briefly, a reaction of 18 µM of enzyme and 90 µg of PASC was incubated at 60 °C for 30 min, follow by adding DNS reagent to terminate the reaction. The reducing sugar was determined by absorbance at 540 nm (A540). The relative activity was defined as a percentage of the released reducing sugar compared with the maximum of 100% with no pre-incubation (t0).
Substrate specificity of recombinant CtCel6A
Substrate specificity of the enzyme was determined based on reducing sugar liberated from various substrates including β-d-glucan, carboxymethyl cellulose (CMC-Na) and PASC. The reaction contained 90 µl of the substrate (0.2% β-d-glucan, 1% CMC-Na or 1 mg/ml PASC) and 18 µM of enzyme. The reaction was incubated at 60 °C for 10 min when β-d-glucan and CMC-Na were used as substrates and for 30 min when PASC was utilized. After incubation, the reaction was terminated by adding 200 µl of the DNS reagent and boiled for 10 min before measuring A540. All experiments were performed in triplicate.
Substrate hydrolysis by recombinant CtCel6A and mutants
Enzymatic hydrolysis of cellulosic substrates by CtCel6A proteins was performed with non-complex and complex substrates. Non-complex substrates, including 0.2% of β-d-glucan, 1% of CMC-Na, 1 mg/ml PASC and 1 mg/ml Avicel, were dissolved individually in 50 mM sodium acetate buffer pH 5.0. Pre-treated sugarcane trash (SCT) was prepared by liquid hot water pretreatment at 180 °C for 30 min (Imman et al. 2013) and was used as a complex substrate. SCT was composed of cellulose (51.52 ± 1.80%), hemicellulose (6.21 ± 0.97%), lignin (25.96 ± 0.54%) and ash (1.73 ± 0.47%). Enzymatic hydrolysis reactions were carried out using 900 µl of non-complex substrate solution and 4.6 µM of enzyme in a 1 ml reaction. For hydrolysis of complex substrate, 10 and 50 mg (dry weight) of pretreated SCT were used for low and high substrate consistencies, respectively. The assay was performed at 60 °C with shaking at 200 rpm for 72 h. After incubation, the reaction was terminated by boiling for 10 min and centrifuged at 12,396 × g for 5 min. The products from hydrolysates were determined by Dionex3000 high-performance liquid chromatography (Thermo Fisher Scientific, Waltham, MA) equipped with an Aminex HPX-87H column and refractive index detector (Shodex, Kyoto, Japan) using 5 mM sulfuric acid as the mobile phase at 65 °C with a 0.5 ml/min flow rate. The product profile and yield were determined by calibrating with the standards of glucose (C1), cellobiose (C2), cellotriose (C3), cellotetraose (C4), cellopentaose (C5) and cellohexaose (C6). All experiments were performed in triplicate. The differences between groups were analyzed by one-way ANOVA using Tukey’s post hoc test. The corrected p value < 0.05 was considered as statistically significant.
Characterization of kinetic parameters
The enzyme kinetic parameters of CtCel6A proteins were determined with various substrates, including β-d-glucan (1–5 mg/ml), CMC-Na (2–10 mg/ml), PASC (1–5 mg/ml) dissolved individually in 50 mM sodium acetate buffer pH 5.0. The reactions were carried out in 100 μl reaction containing 90 μl of substrates at different concentrations and 5 µM of enzyme. The assay reaction was incubated at 60 °C for 0–300 min with shaking at 1000 rpm in a ThermoMixer (Eppendorf, Hamburg, Germany). After incubation, the reaction was terminated by adding 200 μl of the DNS reagent. The reducing sugar was developed by boiling for 10 min and measuring A540. All of experiments were performed in triplicate. Kinetic parameters were calculated according to the Michaelis–Menten equation using SigmaPlot 14.0 program.
Differential scanning fluorimetry (DSF)
Enzyme stability was conducted by differential scanning fluorimetry with Sypro Orange dye (Sigma-Aldrich, USA) following the standard protocol (Niesen et al. 2007). The mixture contained 6.25 µM of enzyme, 12.5 × Sypro Orange in 50 mM sodium acetate buffer pH 5.0 or 50 mM Tris–HCl buffer pH 8.0. Reactions were heated from 20 to 100 °C at a constant heating rate of 1 °C/1 min. The fluorescence intensities for the thermal unfolding profile were quantified using the FRET filter (excitation 450–290 nm, emission 560–580 nm) with the CFX real-time PCR machine (BioRad, Hercules, CA).
Molecular dynamics simulations
The 3D structures of the CD-CtCel6A (PDB: 4A05) and the FL-CtCel6A from AlphaFold protein structure prediction (Jumper et al. 2021) were subjected to molecular dynamic simulations (MD simulations) (Case et al. 2022). Discovery studio (BIOVIA Dassault Systèmes 2021) was used to prepare and visualized the structures. Molecular dynamics simulations were carried out using Amber20 (Case et al. 2022). Leap module was used to add hydrogen and missing atoms. System was solved in TIP3P water model with 9 Å box and Cl ions were added to keep the system's charge neutral. A force field of the protein was FF99SB. Before the production run, the water system was minimized for 1000 of steepest descent and 3000 of conjugated gradient, while all non-water atoms were fixed. After that all atoms were minimized for 1000 of steepest descent and 2000 of conjugated gradient. The system temperature was gradually increased until the temperature of 300 K or 338 K and 343 K depending on the system. After minimization, each of the simulation systems was heated gradually from 0 K to targeted temperature by Langevin dynamics followed by 50 ns of production simulation.
Far-UV circular dichroism
Secondary structure of the CD-CtCel6A and its mutant was measured by circular dichroism spectroscopy using a J-815 CD spectrometer (Jasco Corp., Tokyo, Japan). Far-UV CD spectra were collected at 190–260 nm using 1 nm bandwidth and 5 acquisition times. Each enzyme at 5.0 µM was incubated in 50 mM sodium phosphate buffer pH 8.0 at 60 °C for 24 h and diluted to 0.5 µM for far-UV measurement.
Results
Design of CtCel6A mutants
To enhance the activity and stability of CtCel6A, we investigated and used the crystal structure of CtCel6A catalytic domain (CD-CtCel6A; PDB: 4A05) with bound ligands cellobiose and cellotetraose as a source for this study (Thompson et al, 2012). Molecular docking of a cellotetraose molecule at the subsites − 1 to − 4 of an apo-CD-CtCel6A was carried out to create an oligosaccharide (cellooctaose) substrate occupying the whole active cleft. We hypothesized that catalytic activity and stability of CtCel6A could be affected by the interactions and hindrance of the active site residues involving in the substrate binding. For Cel6A, it is thought that the binding of substrate proceeds from subsites + 4 to − 4, in which the glycosidic bond at the catalytic subsites + 1 and − 1 will be cleaved (Bu et al. 2012; Mayes et al. 2016). In this study, the rationale for design of mutants involved establishing more favorable interactions of residues at the substrate-binding cleft with the cellooctaose substrate model.
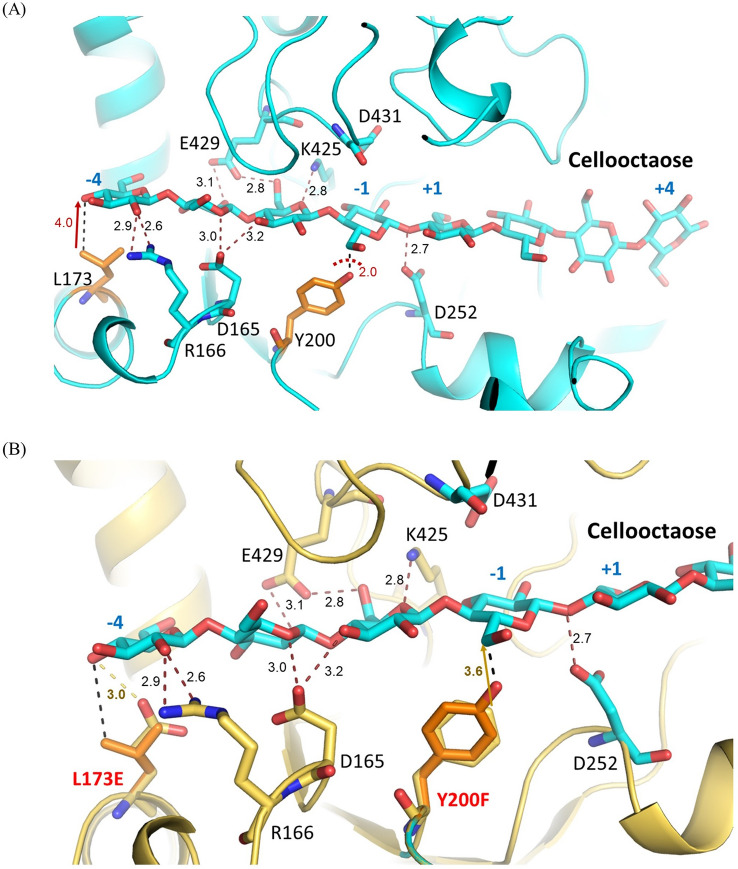
Analysis of the cellooctaose-enzyme structure revealed that along the subsites − 1 to − 4, Asp165, Arg166, Lys425 and Glu429 provide potential H-bond interactions with the OH groups of the cellooctaose at subsites − 2 to − 4 (Fig. 2A). On the other hand, the hydroxy group of Tyr200 collided with the saccharide unit at the subsite-1 causing an unfavorable interaction to the docked substrate (Fig. 2A); however, it is worth to mention that Tyr200 is located on the long tunnel-forming loop hence it can also adjust itself to accommodate binding of substrate with H-bonding interaction. While Leu173 is located at the subsite − 4 which is more solvent-exposed and lacks interaction with the substrate. A catalytic residue Asp252 is 2.7 Å from the glycosidic oxygen at the subsite − 1 to + 1 while Asp431 located on the flexible loop is far from the catalytic site as depicted from the crystal structure of CtCel6A. Therefore, two residues namely Tyr200 and Leu173 were chosen for engineering to either avoiding clashes or adding binding interactions to the substrate. Tyr200, located within 2.0 Å distance from the docked substrate, was mutated to Phe to void a steric hindrance. Hence, a potential H-bond between the OH group of Tyr200 and the 5-hydroxy methyl group of the saccharide unit at the subsite − 1 will be disrupted to create a wider tunnel for substrate binding (Fig. 2B). Leu173, situated 4 Å away from the substrate, was changed to Glu to stabilize the 4-hydroxy group of the saccharide unit at the subsite − 4 of the substrate which was expected to pick up 3.0 Å hydrogen bond (Fig. 2B). This would secure the long substrate like pretreated sugarcane trash (SCT) and lead to higher catalytic efficiency.
Fig. 2.
3D structures of CtCel6A complexed with cellooctaose from a cellotetraose at subsites + 1 to + 4 (PDB: 4A05) and a docked cellotetraose at the subsites − 1 to − 4. A Wild-type CtCel6A showing H-bond interactions (Å) at the subsites − 1 to − 4, and distances (Å) between Leu173 and Tyr200 with the substrate. B Overlaid of Leu173 to Glu and Tyr200 to Phe in mutants to the wild-type CtCel6A revealing 3.0 Å hydrogen bond of Leu173Glu and the saccharide unit at subsite − 4 and a release of steric clash of Tyr200Phe to the cellooctaose substrate at subsite − 1. Figures were generated with PyMol2.1
Optimal activity and stability
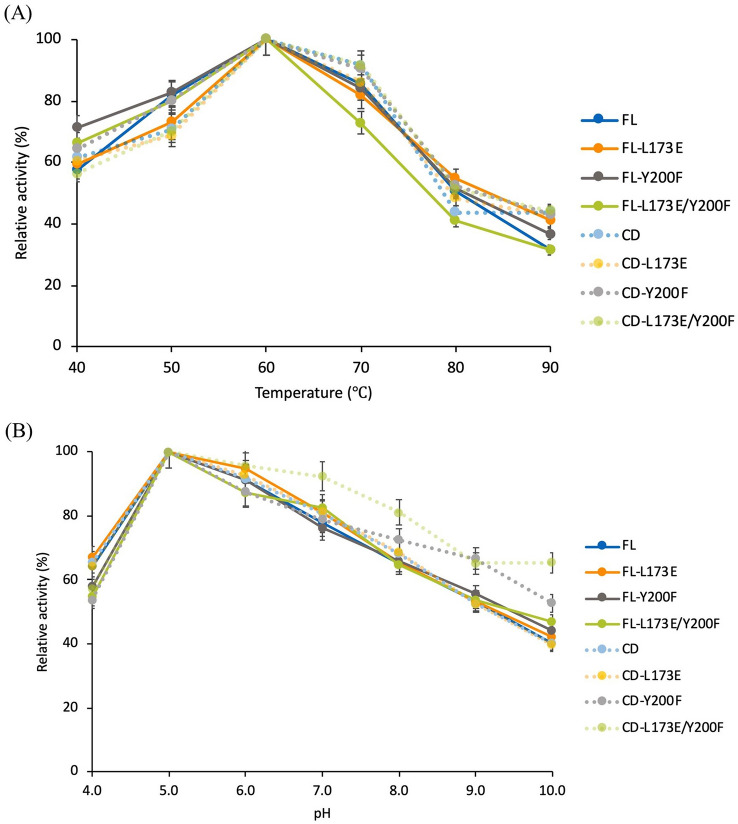
The enzymatic activities of mutant enzymes at different temperatures and pH were investigated for the full-length CtCel6A (FL) and CBM-truncated mutants. The temperature effect was studied at 40–90 °C. The FL and all mutants displayed a similar pattern in their temperature profiles with the optimal temperature at 60 °C (Fig. 3A). However, the CBM-deleted mutant substituted with Y200F (CD-Y200F, CD-L173E/Y200F), which exhibited a significantly higher enzyme activity at pH 10.0 compared with the wild-type CD (Fig. 3B). The stability profiles of the enzymes were compared under different pH conditions. No significant differences in enzyme stability were observed at the optimal pH 5.0 (Fig. 4A); however, CBM-truncated variants showed markedly higher stability compared with the corresponding FL enzyme mutants at pH 8.0 (Fig. 4B). Moreover, mutants with the Y200F substitution (CD-Y200F and CD-L173E/Y200F) showed 25–29% increased stability compared with CD under the same alkaline condition (Fig. 4B).
Fig. 3.
Temperature and pH activity profiles of wild-type full-length CtCel6A (FL) and mutants. Mutants included deletion of residues 19–115 of the CBM (CD) and substitution of residues 173 and/or 200 in FL and CD. A Cellulase activities on PASC substrate were determined at temperatures ranging from 40 to 90 °C relative to the mean maximum value observed for each enzyme (100%). B pH profile was determined in buffer ranging from pH 3.0 to 10.0. Reducing sugar production was assessed using DNS reagent. Mean values are shown (n = 3) and error bars indicate standard deviation
Fig. 4.
Stability of CtCel6A and its mutants. Reactions on PASC substrate were performed by incubating enzyme in buffer pH 5.0 and pH 8.0 at 60 °C from 0 to 48 h. A Sodium acetate buffer pH 5.0. B Tris–HCl buffer pH 8.0. The remaining activity was determined using DNS reagent. Mean values are shown (n = 3) and error bars indicate standard deviation
Thermostability was also assessed by monitoring the melting temperature (Tm) of purified CtCel6A protein with differential scanning fluorimetry (DSF). The Tm values of each protein were 5–6 °C greater at the optimal pH 5.0 compared with pH 8.0 (Table S2). The Tm values of CBM-deleted proteins were higher than that of the FL CtCel6A proteins tested under the same condition; however, no difference was observed among the mutants compared with the corresponding wild-type FL or CBM-deleted enzyme tested under the same condition (Table S2).
Substrate specificities and kinetics of CtCel6A mutants
Substrate specificities and kinetic properties of CtCel6A enzymes were determined on non-complex cellulosic substrates with different crystallinity index including β-d-glucan, CMC-Na and PASC under the optimal conditions of 60 °C and pH 5.0. The specific activity of CD on β-d-glucan and CMC-Na was significantly increased by 1.22-fold and 1.29-fold compared with FL, respectively, with corresponding differences in catalytic efficiency (kcat/Km) (Table 1). Of all the enzymes tested, the greatest specific activity on β-d-glucan and CMC-Na substrates was observed for the CD-Y200F mutant, whereas the FL-L173E mutant showed the greatest specific activity on PASC (Table 1). Furthermore, the km and Vmax values of FL enzymes for PASC substrate were markedly increased compared with the corresponding CBM-deleted enzymes (Table 1).
Table 1.
Kinetic parameters and specific activity of CtCel6A and its mutants
| Enzyme | Substrate | Kinetic parameters | Specific activity – pH5 (nmole/mg.min) | |||
|---|---|---|---|---|---|---|
| Vmax (nmole/min) | Km (mg/ml) | kcat × 10–3 (s−1) | kcat/Km (µL/s/mg) | |||
| FL | β-d-glucan | 16.8 ± 0.4 | 0.65 ± 0.08 | 0.56 | 0.86 | 246 ± 6 |
| FL-L173E | 13.3 ± 7.2 | 0.53 ± 0.02 | 0.44 | 0.83 | 202 ± 9 | |
| FL-Y200F | 9.0 ± 1.4 | 0.39 ± 0.02 | 0.30 | 0.76 | 210 ± 4 | |
| FL-L173E/Y200F | 8.3 ± 2.4 | 0.41 ± 0.05 | 0.28 | 0.67 | 180 ± 7 | |
| CD | 17.5 ± 0.5 | 0.63 ± 0.09 | 0.58 | 0.93 | 300 ± 4 | |
| CD-L173E | 17.8 ± 0.4 | 0.74 ± 0.01 | 0.59 | 0.80 | 290 ± 2 | |
| CD-Y200F | 22.7 ± 1.8 | 0.82 ± 0.04 | 0.76 | 0.92 | 361 ± 2 | |
| CD-L173E/Y200F | 17.3 ± 1.4 | 1.81 ± 0.04 | 0.58 | 0.32 | 216 ± 2 | |
| FL | CMC-Na | 21.7 ± 1.3 | 2.10 ± 0.44 | 0.36 | 0.17 | 130 ± 7 |
| FL-L173E | 17.4 ± 0.8 | 1.69 ± 0.24 | 0.29 | 0.17 | 105 ± 8 | |
| FL-Y200F | 18.0 ± 0.4 | 1.47 ± 0.13 | 0.30 | 0.20 | 111 ± 4 | |
| FL-L173E/Y200F | 23.0 ± 2.1 | 2.35 ± 0.48 | 0.38 | 0.16 | 108 ± 6 | |
| CD | 27.4 ± 1.4 | 2.22 ± 0.45 | 0.46 | 0.21 | 168 ± 5 | |
| CD-L173E | 27.8 ± 1.3 | 2.17 ± 0.18 | 0.46 | 0.21 | 154 ± 3 | |
| CD-Y200F | 20.7 ± 0.6 | 1.51 ± 0.19 | 0.35 | 0.23 | 212 ± 4 | |
| CD-L173E/Y200F | 16.4 ± 0.9 | 1.13 ± 0.33 | 0.27 | 0.24 | 155 ± 4 | |
| FL | PASC | 43.8 ± 2.0 | 1.90 ± 0.13 | 0.73 | 0.39 | 80 ± 5 |
| FL-L173E | 41.2 ± 1.4 | 1.86 ± 0.01 | 0.69 | 0.37 | 85 ± 1 | |
| FL-Y200F | 35.2 ± 0.5 | 1.92 ± 0.04 | 0.59 | 0.31 | 62 ± 4 | |
| FL-L173E/Y200F | 27.5 ± 2.3 | 2.17 ± 0.02 | 0.46 | 0.21 | 50 ± 2 | |
| CD | 22.9 ± 1.0 | 1.09 ± 0.09 | 0.38 | 0.35 | 64 ± 6 | |
| CD-L173E | 22.2 ± 1.4 | 1.03 ± 0.08 | 0.37 | 0.36 | 59 ± 7 | |
| CD-Y200F | 18.8 ± 0.8 | 0.82 ± 0.07 | 0.31 | 0.38 | 71 ± 2 | |
| CD-L173E/Y200F | 13.6 ± 0.6 | 0.54 ± 0.06 | 0.23 | 0.42 | 57 ± 2 | |
Hydrolysis of pretreated sugarcane trash
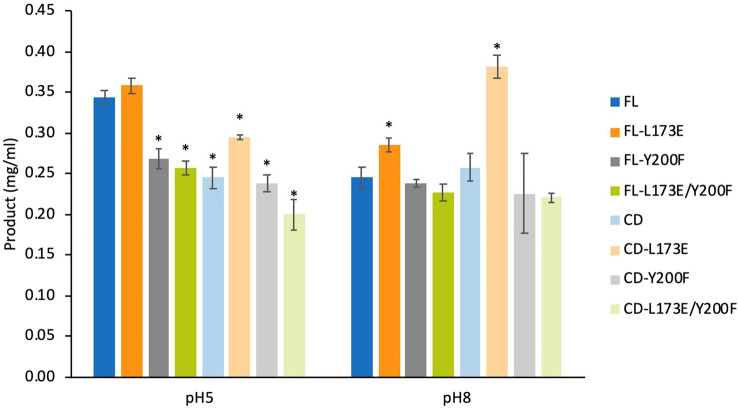
The performance of mutant enzymes on biomass hydrolysis was initially studied with 10 mg of pretreated sugarcane trash and 4.6 µM enzyme variants. Comparison of product yield at pHs 5 and 8 revealed that the cellobiose product from each variant at the optimal pH 5.0 was higher than that at the alkaline pH 8.0 (Fig. 5) except for CD-L173E. The cellobiose product from CD-L173E catalysis at pH 8.0 was increased by 29.5%, compared to at pH 5.0 (Fig. 5). The FL-L173E enzyme exhibited the highest product yield of 0.36 mg/ml at the optimal pH 5.0 (Fig. 5) and reduced to 0.30 mg/ml at pH 8.0. Interestingly, the CD-L173E mutant demonstrated 20.2% and 47.9% enhanced product yield compared to the wild-type CD under the hydrolysis conditions at pH 5.0 and pH 8.0, respectively (Fig. 5). In contrast, the mutants with Y200F substitution (FL-Y200F and CD-Y200F) demonstrated decreased hydrolysis yield compared with the corresponding wild-type enzyme (Fig. 5). To evaluate the effect of substrate loading on enzymatic hydrolysis, increased solid loading (50 mg) of the pretreated sugarcane trash was studied for the wild-type (FL and CD) and L173E mutant (FL-L173E and CD-L173E) enzymes. In the hydrolysis condition at pH 5.0, the CD and CD-L173E enzymes showed significantly greater yields than FL and FL-L173E enzymes (Fig. 6A). In the hydrolysis condition at pH 8.0, the difference in yield for the CD and CD-L173E enzymes compared with FL and FL-L173E was even more marked (Fig. 6B). In addition, the product yield of FL and FL-L173E enzymes was significantly lower at the 50 mg substrate loading than at the 10 mg substrate loading (Fig. 6B).
Fig. 5.
Enzymatic hydrolysis of CtCel6A and its mutants. Reactions were performed with 10 mg of pretreated sugarcane trash as the substrate in buffer pH 5.0 and pH 8.0 at 60 °C for 72 h. The product yield was determined by comparison of HPLC profiles with C1–C6 standards. Mean values are shown (n = 3) and error bars indicate standard deviation. Asterisks indicate significant differences (p < 0.05) compared to FT in each pH
Fig. 6.
Hydrolysis of pretreated sugarcane trash with different substrate concentration by FL, FL-L173E, CD and CD-L173E. The reactions were performed with pretreated sugarcane trash (10 and 50 mg) in buffer pH 5.0 and pH 8.0 at 60 °C for 72 h. A Sodium acetate buffer pH 5.0. B Tris–HCl buffer pH 8.0. The product yield was determined by comparison of HPLC profiles with C1–C6 standards. Mean values are shown (n = 3) and error bars indicate standard deviation. Asterisks indicate significant differences (p < 0.05) compared to FT in each condition
Discussion
In this work, the effect of CBM deletion and substrate-binding site mutations on enzymatic properties and substrate preference of the thermostable CtCel6A from C. thermophilum was elucidated. Non-catalytic CBMs have been reported to play an important role on enhancing the accessibility of enzyme to substrate, thereby promoting the enzymatic hydrolysis of cellulose (Boraston et al. 2004). Recently, the influences of CBM on the properties of GH6 exoglucanase from C. thermophilum (CtCBH) have been elucidated. The study reported that the deletion of its own CBM significantly reduced the binding ability of CtCBH to Avicel, but its catalytic activity was not obviously reduced, implying that binding ability of CBM1 is not necessary for the catalytic activity of CtCBH (Hu et al. 2021). In our study, we found that removal of the CBM showed no effect on binding affinity (Km) and catalytic efficiency (kcat/Km) to the soluble substrate β-d-glucan and CMC-Na (Table 1). However, the Km and kcat values of FL on PASC was greater compared with CD (Table 1). We think that the CBM targets the enzyme to the PASC surface and allows the catalytic module to hydrolyze the crystalline cellulose in this substrate (Várnai et al. 2013). In addition to processing of certain substrates, the CBM can also affect the optimal pH and pH stability of enzymes (Hamada et al. 2001). In the present study, the CBM-deleted mutant exhibited an increased alkaline tolerant property (Fig. 4B) and greater thermal stability (increased Tm) (Table S2) compared with the FL enzyme. Previous study has found that the enzyme increases its hydrophobic contact to compensate the absence of CBM (Prates et al. 2013). The increase in repulsion force between enzyme and polar solvent could imply a more compact structure of the enzyme which could lead to an increase in alkali and heat tolerance. Although CBM deletion could have an impact on protein folding, point mutations were not predicted to introduce major conformational changes on the enzyme’s backbone (Fig. 2), which was reflected by the similar Tm and far-UV CD spectra of the mutants compared with the wild-type (Table S2 and Fig. S4). However, the far-UV CD data of the CD variants upon incubation at 80 °C for 24 h showed a slightly higher ellipticity at 200 nm of the Y200F mutants CD-Y200F, CD-L173E/Y200F implying for higher remaining secondary structure content, compared to the wild-type CD (Fig. S4). This agrees with our preliminary molecular dynamic simulation of the CBM-deleted structure and the predicted FL structure that showed high fluctuation of the CBM region but the stable catalytic domain regardless of the temperatures (Figs. S1–S3). As expected, the root mean square deviation (RMSD) of FL structure exhibited a high variation (> 3 Å) at all temperatures (300 K (27 °C), 338 K (65 °C) and 343 K (70 °C)), whereas the RMSD of CD structure remained stable (Fig. S2). Likewise, the root mean square fluctuation (RMSF) indicated the N-terminal CBM region had high structural fluctuation (> 2 Å), whereas the catalytic domain of both FL or CD structures was rather stable with 1 Å fluctuation range. Nevertheless, the catalytic domain region of FL showed higher fluctuation than that of CD structure. Increased temperature caused higher fluctuation in FL structure but not in CD structure (Fig. S3). Together, the results suggested that N-terminal CBM region might decrease stability of the FL structure. This also agrees well with the higher Tm and thermostability of the CBM deleted variants compared with FL.
The effects of mutations L173E in subsite − 4 and Y200F at subsite − 1 in the tunnel-forming loop of CtCel6A were elucidated by the kinetics parameters. The increase in binding affinity (lower ) could be observed from CD-Y200F variants for CMC-Na and PASC substrates. The Y200F mutation may release a restraint in the saccharide unit of the substrate due to lack of H-bonding, thereby enhancing the movement of substrate, the release the product in the enzyme active tunnel and the turnover of the catalysis. CtCel6A functioned as exocellulase cleaving and releasing the product at the end of cellulose chain leaving the remaining celluloses binding to the enzyme unproductively. Unlike wild type, the remaining celluloses in Y200F variants then moved through the substrate binding channel and the productive binding between substrate and enzyme is recovered. By widening of this channel, Y200F could facilitate this process resulting in higher ratio of productive binding form of the enzyme. Considering of the possible unproductive binding form of the enzyme, modified Michaelis–Menten kinetic model was derived, and the true substrate binding capabilities of the enzyme variants could be compared based on productive binding recovery rate of the wild-type enzyme (Fig. S5). With the corrected binding constant, the decrease in substrate binding capability of Y200F variant to PASC could be observed in agreement with the initial hypothesis (Table S3). However, when β-d-glucan or CMC-Na were used as substrate, no significant change in corrected binding constant could be observed for both L173E and Y200F variants. The structure of β-d-glucan consisted of 1,3 glycosidic linkage; and therefore, might exhibit different binding conformation than the modeled substrates. In the case of CMC-Na, O6 is carboxymethylated and lose H-bond interaction with Tyr200 residue. Hence, the prediction might not be able to apply for these two substrates.
The presence of lignin in lignocellulose has been reported to be one of the major obstacles to enzymatic hydrolysis (Börjesson et al. 2007). Lignin in pretreated crop plant biomass adsorbs the enzyme unproductively and forms a physical barrier to restrict the access of cellulolytic enzymes to substrates (Palonen et al. 2004). In this study, the role of the family-1 CBM in CtCel6A was elucidated for hydrolysis of a lignin-containing substrate (SCT). Consistent with the assumption that the L173E substitution introduced an H-bond at the − 4 subsite (Fig. 2), the L173E mutant demonstrated increased SCT hydrolysis yield compared with wild type (Figs. 5, 6). Furthermore, the CD-L173E variant was found to exhibit the highest SCT hydrolysis activity under alkaline condition. On the other hand, despite the Y200F mutation was predicted to enhance enzymatic activity due to a more relaxed conformation of substrate binding site at the − 1 subsite (Fig. 2), the SCT hydrolysis enzyme variants with Y200F showed no significant improvement. A further kinetic study by the CBM truncated variants suggested that the hydrolysis of SCT took place under kcat/Km regime of the Michaelis–Menten kinetic model (Table S4). The limited solubility of SCT prevented the access to the hydrolysis reaction at high substrate concentration, and hence, both kinetic parameters could not be obtained individually. As shown by observed kinetic against PASC (Table 1), Y200F variants exhibited the reduction in catalytic constant (kcat), which could lead to adverse effect in SCT hydrolysis. Likewise, the L173E variant showed the highest kcat/Km in agreement with the hydrolysis results obtained in Fig. 5.
Interestingly, at the higher SCT loading (50 mg), the CD enzymes lacking CBM showed greater product yields than the FL enzymes (Fig. 6). Typically, the CBM enhances hydrolysis, especially under substrate limiting conditions by facilitating recognition and adsorption of enzyme on the substrate surface (Chundawat et al. 2021). Nevertheless, it has been reported that the adsorption of core enzymes to the substrate can be enhanced in the absence of a CBM by increasing the substrate concentration and reducing the amount of water in the hydrolytic system (Le Costaouec et al. 2013). Moreover, the CBM can bind unproductively with the remaining lignin in pretreated biomass (Várnai et al. 2011), which leads to decrease of free enzyme in the supernatant (Palonen et al. 2004).
Conclusions
CtCel6A cellobiohydrolase mutants were created by structure-guided protein engineering. Removal of the CBM enhanced hydrolysis efficiency and alkaline stability for all substrates except PASC. The CD-Y200F mutant with predicted disruption of H-bonds in the substrate binding region showed increased alkaline stability and specific activity with non-complex substrates. The CD-L173E mutant showed the highest product yield from pretreated sugarcane trash substrate under different conditions. These engineered cellobiohydrolases have the potential for further application where hydrolysis of cellulose under alkaline condition is preferred.
Accession numbers
CtCel6A-FL sequence (GenBank accession no: OM978548), CtCel6A-FL-L173E sequence (GenBank accession no: OM978549), CtCel6A-FL-Y200F sequence (GenBank accession no: OM978550), CtCel6A-FL-L173E-Y200F sequence (GenBank accession no: OM978551), CtCel6A-CD sequence (GenBank accession no: OM978552), CtCel6A-CD-L173E sequence (GenBank accession no: OM97853), CtCel6A-CD-Y200F sequence (GenBank accession no: OM978554), CtCel6A-CD-L173E-Y200F sequence (GenBank accession no: OM978555).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to gratefully thank Dr. Jirondon Yuvaniyama for helpful suggestion in mutation site design. We would like to thank Dr. Philip J. Shaw for manuscript proofreading and comment. We would like to thank Mr. Danoo Vitsupakorn for suggestion and comment. The project was funded by the e-Asia project, “Integrated bio-refinery of sugarcane trash,” supported by the Japan Science and Technology Agency [grant number JPMJSC18E1] under collaboration with the National Science and Technology Development Agency [grant number P-19-51656].
Author contributions
KP, KB, BB, NA, TU, PC & VC conceptualized and designed the study; KP, KB, TU performed research and analyzed data; and KP, KB, BB, NA, PC and VC wrote the paper. All authors reviewed and edited the paper.
Funding
Japan Science and Technology Agency (Grant no: JPMJSC18E1); National Science and Technology Development Agency (Grant no: P-19-51656).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Kanoknart Prabmark and Katewadee Boonyapakron have contributed equally to this work.
References
- Arantes V, Saddler JN. Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol Biofuels. 2010;3:4. doi: 10.1186/1754-6834-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baramee S, Teeravivattanakit T, Phitsuwan P, Waeonukul R, Pason P, Tachaapaikoon C, Kosugi A, Sakka K, Ratanakhanokchai K. A novel GH6 cellobiohydrolase from Paenibacillus curdlanolyticus B-6 and its synergistic action on cellulose degradation. Appl Microbiol Biotechnol. 2017;101(3):1175–1188. doi: 10.1007/s00253-016-7895-8. [DOI] [PubMed] [Google Scholar]
- Becker D, Braet C, Brumer H, Claeyssens M, Divne C, Fagerström R, Harris M, Jones T, Kleywegt G, Koivula A, Mahdi S, Piens K, Sinnott M, Ståhlberg J, Teeri T, Underwood M, Wohlfahrt G. Engineering of a glycosidase Family 7 cellobiohydrolase to more alkaline pH optimum: the pH behaviour of Trichoderma reesei CeI7A and its E223S/A224H/L225V/T226A/D262G mutant. Biochem J. 2001;356:19–30. doi: 10.1042/0264-6021:3560019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hmad I, Gargouri A. Neutral and alkaline cellulases: production, engineering, and applications. J Basic Microbiol. 2017;57(8):653–658. doi: 10.1002/jobm.201700111. [DOI] [PubMed] [Google Scholar]
- Bernardes A, Pellegrini VOA, Curtolo F, Camilo CM, Mello BL, Johns MA, Scott JL, Guimaraes FEC, Polikarpov I. Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohydr Polym. 2019;211:57–68. doi: 10.1016/j.carbpol.2019.01.108. [DOI] [PubMed] [Google Scholar]
- BIOVIA Dassault Systèmes (2021) Discovery Studio Visualizer Software, Version 4.0, Dassault Systèmes, San Diego
- Boonyapakron K, Chitnumsub P, Kanokratana P, Champreda V. Enhancement of catalytic performance of a metagenome-derived thermophilic oligosaccharide-specific xylanase by binding module removal and random mutagenesis. J Biosci Bioeng. 2021;131(1):13–19. doi: 10.1016/j.jbiosc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J. 2004;382(Pt 3):769–781. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson J, Engqvist M, Sipos B, Tjerneld F. Effect of poly(ethylene glycol) on enzymatic hydrolysis and adsorption of cellulase enzymes to pretreated lignocellulose. Enzyme Microb Technol. 2007;41(1):186–195. doi: 10.1016/j.enzmictec.2007.01.003. [DOI] [Google Scholar]
- Case DA, Aktulga HM, Belfon K, Ben-Shalom IY, Berryman JT, Brozell SR, Cerutti DS, Cheatham TE, III, Cisneros GA, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Izmailov SA, Kasavajhala K, Kaymak MC, King E, Kovalenko A, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Machado M, Man V, Manathunga M, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, O'Hearn KA, Onufriev A, Pan F, Pantano S, Qi R, Rahnamoun A, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shajan A, Shen J, Simmerling CL, Skrynnikov NR, Smith J, Swails J, Walker RC, Wang J, Wang J, Wei H, Wolf RM, Wu X, Xiong Y, Xue Y, York DM, Zhao S, Kollman PA. Amber 2022. San Francisco: University of California; 2022. [Google Scholar]
- Cerda-Mejía L, Valenzuela SV, Frías C, Diaz P, Pastor FI. A bacterial GH6 cellobiohydrolase with a novel modular structure. Appl Microbiol Biotechnol. 2017;101(7):2943–2952. doi: 10.1007/s00253-017-8129-4. [DOI] [PubMed] [Google Scholar]
- Champreda V, Mhuantong W, Lekakarn H, Bunterngsook B, Kanokratana P, Zhao XQ, Zhang F, Inoue H, Fujii T, Eurwilaichitr L. Designing cellulolytic enzyme systems for biorefinery: from nature to application. J Biosci Bioeng. 2019;128(6):637–654. doi: 10.1016/j.jbiosc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Christensen SJ, Kari J, Badino SF, Borch K, Westh P. Rate-limiting step and substrate accessibility of cellobiohydrolase Cel6A from Trichoderma reesei. FEBS J. 2018;285(23):4482–4493. doi: 10.1111/febs.14668. [DOI] [PubMed] [Google Scholar]
- Chundawat SPS, Nemmaru B, Hackl M, Brady SK, Hilton MA, Johnson MM, Chang S, Lang MJ, Huh H, Lee S-H, Yarbrough JM, López CA, Gnanakaran S. Molecular origins of reduced activity and binding commitment of processive cellulases and associated carbohydrate-binding proteins to cellulose III. J Biol Chem. 2021;296:100431–100431. doi: 10.1016/j.jbc.2021.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtade G, Forsberg Z, Heggset EB, Eijsink VGH, Aachmann FL. The carbohydrate-binding module and linker of a modular lytic polysaccharide monooxygenase promote localized cellulose oxidation. J Biol Chem. 2018;293(34):13006–13015. doi: 10.1074/jbc.RA118.004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskin F, Flint JE, Gloster TM, Morland C, Baslé A, Henrissat B, Coutinho PM, Strazzulli A, Solovyova AS, Davies GJ, Gilbert HJ. How nature can exploit nonspecific catalytic and carbohydrate binding modules to create enzymatic specificity. PNAS. 2012;109(51):20889. doi: 10.1073/pnas.1212034109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Rincón DJ, Duque I, Osorio E, Rodríguez-López A, Espejo-Mojica A, Parra-Giraldo CM, Poutou-Piñales RA, Alméciga-Díaz CJ, Quevedo-Hidalgo B. Production of recombinant Trichoderma reesei cellobiohydrolase II in a new expression system based on Wickerhamomyces anomalus. Enzyme Res. 2017;2017:6980565. doi: 10.1155/2017/6980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Wang F, Gao F, Wang L, Zhao J, Qu Y. Purification and characterization of a novel cellobiohydrolase (PdCel6A) from Penicillium decumbens JU-A10 for bioethanol production. Bioresour Technol. 2011;102(17):8339–8342. doi: 10.1016/j.biortech.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Gilbert HJ, Knox JP, Boraston AB. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol. 2013;23(5):669–677. doi: 10.1016/j.sbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Gu Y, Zheng F, Wang Y, Su X, Bai Y, Yao B, Huang H, Luo H. Characterization of two thermophilic cellulases from Talaromyces leycettanus JCM12802 and their synergistic action on cellulose hydrolysis. PLoS ONE. 2019;14(11):e0224803. doi: 10.1371/journal.pone.0224803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N, Kodaira R, Nogawa M, Shinji K, Ito R, Amano Y, Shimosaka M, Kanda T, Okazaki M. Role of cellulose-binding domain of exocellulase I from white rot basidiomycete Irpex lacteus. J Biosci Bioeng. 2001;91(4):359–362. doi: 10.1263/jbb.91.359. [DOI] [PubMed] [Google Scholar]
- Han C, Li W, Hua C, Sun F, Bi P, Wang Q. Enhancement of catalytic activity and thermostability of a thermostable cellobiohydrolase from Chaetomium thermophilum by site-directed mutagenesis. Int J Biol Macromol. 2018;116:691–697. doi: 10.1016/j.ijbiomac.2018.05.088. [DOI] [PubMed] [Google Scholar]
- Hervé C, Rogowski A, Blake AW, Marcus SE, Gilbert HJ, Knox JP. Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. PNAS. 2010;107(34):15293–15298. doi: 10.1073/pnas.1005732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RL, Abotsi E, Jansen van Rensburg EL, Howard S. Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol. 2003;2(12):602–619. doi: 10.5897/AJB2003.000-1115. [DOI] [Google Scholar]
- Hu Y, Li H, Ran Q, Liu J, Zhou S, Qiao Q, Song H, Peng F, Jiang Z. Effect of carbohydrate binding modules alterations on catalytic activity of glycoside hydrolase family 6 exoglucanase from Chaetomium thermophilum to cellulose. Int J Biol Macromol. 2021;191:222–229. doi: 10.1016/j.ijbiomac.2021.09.002. [DOI] [PubMed] [Google Scholar]
- Imman S, Arnthong J, Burapatana V, Laosiripojana N, Champreda V. Autohydrolysis of tropical agricultural residues by compressed liquid hot water pretreatment. Appl Biochem Biotechnol. 2013;170(8):1982–1995. doi: 10.1007/s12010-013-0320-1. [DOI] [PubMed] [Google Scholar]
- Jayasekara S, Ratnayake R. Microbial cellulases: an overview and applications. Cellulose, IntechOpen, London. 2019 doi: 10.5772/intechopen.84531. [DOI] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz SE, Goedegebuur F, Hommes R, Kaper T, Kelemen BR, Mitchinson C, Wallace L, Ståhlberg J, Larenas EA. Hypocrea jecorina CEL6A protein engineering. Biotechnol Biofuels. 2010;3(1):20. doi: 10.1186/1754-6834-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Costaouec T, Pakarinen A, Varnai A, Puranen T, Viikari L. The role of carbohydrate binding module (CBM) at high substrate consistency: comparison of Trichoderma reesei and Thermoascus aurantiacus Cel7A (CBHI) and Cel5A (EGII) Bioresour Technol. 2013;143:196–203. doi: 10.1016/j.biortech.2013.05.079. [DOI] [PubMed] [Google Scholar]
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66(3):506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan SA, Wi SG, Lee DS, Bae HJ. Site-directed mutagenesis and CBM engineering of Cel5A (Thermotoga maritima) FEMS Microbiol Lett. 2008;287(2):205–211. doi: 10.1111/j.1574-6968.2008.01324.x. [DOI] [PubMed] [Google Scholar]
- Mayes HB, Knott BC, Crowley MF, Broadbelt LJ, Ståhlberg J, Beckham GT. Who's on base? Revealing the catalytic mechanism of inverting family 6 glycoside hydrolases. Chem Sci. 2016;7(9):5955–5968. doi: 10.1039/C6SC00571C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D-D, Ying Y, Chen X-H, Lu M, Ning K, Wang L-S, Li F-L. Distinct roles for carbohydrate-binding modules of glycoside hydrolase 10 (GH10) and GH11 xylanases from Caldicellulosiruptor sp. strain F32 in thermostability and catalytic efficiency. Appl Environ Microbiol. 2015;81(6):2006–2014. doi: 10.1128/AEM.03677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Ogunmolu FE, Jagadeesha NBK, Kumar R, Kumar P, Gupta D, Yazdani SS. Comparative insights into the saccharification potentials of a relatively unexplored but robust Penicillium funiculosum glycoside hydrolase 7 cellobiohydrolase. Biotechnol Biofuels. 2017;10(1):71. doi: 10.1186/s13068-017-0752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira GS, Ulhoa CJ, Silveira MH, Andreaus J, Silva-Pereira I, Poças-Fonseca MJ, Faria FP. An alkaline thermostable recombinant Humicola grisea var. thermoidea cellobiohydrolase presents bifunctional (endo/exoglucanase) activity on cellulosic substrates. World J Microbiol Biotechnol. 2013;29(1):19–26. doi: 10.1007/s11274-012-1153-8. [DOI] [PubMed] [Google Scholar]
- Palonen H, Tjerneld F, Zacchi G, Tenkanen M. Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol. 2004;107(1):65–72. doi: 10.1016/j.jbiotec.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Park SR, Cho SJ, Kim MK, Ryu SK, Lim WJ, An CL, Hong SY, Kim JH, Kim H, Yun HD. Activity enhancement of Cel5Z from Pectobacterium chrysanthemi PY35 by removing C-terminal region. Biochem Biophys Res Commun. 2002;291(2):425–430. doi: 10.1006/bbrc.2002.6437. [DOI] [PubMed] [Google Scholar]
- Pramanik S, Semenova MV, Rozhkova A, Zorov IN, Korotkova O, Sinitsyn AP, Davari MD. An engineered cellobiohydrolase I for sustainable degradation of lignocellulosic biomass. Biotechnol Bioeng. 2021;118(10):4014–4027. doi: 10.1002/bit.27877. [DOI] [PubMed] [Google Scholar]
- Prates ÉT, Stankovic I, Silveira RL, Liberato MV, Henrique-Silva F, Pereira N, Jr, Polikarpov I, Skaf MS. X-ray structure and molecular dynamics simulations of endoglucanase 3 from Trichoderma harzianum: structural organization and substrate recognition by endoglucanases that lack cellulose binding module. PLoS ONE. 2013;8(3):e59069. doi: 10.1371/journal.pone.0059069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülein M. Enzymatic properties of cellulases from Humicola insolens. J Biotechnol. 1997;57(1–3):71–81. doi: 10.1016/s0168-1656(97)00090-4. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Takahashi H, Nakano Y, Konishi T, Terauchi R, Takeda T. Characterization of a cellobiohydrolase (MoCel6A) produced by Magnaporthe oryzae. Appl Environ Microbiol. 2010;76(19):6583–6590. doi: 10.1128/AEM.00618-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Heu T, Shaghasi T, Benyamino R, Jones A, Friis EP, Wilson KS, Davies GJ. Structure of the catalytic core module of the Chaetomium thermophilum family GH6 cellobiohydrolase Cel6A. Acta Cryst D. 2012;68(8):875–882. doi: 10.1107/S0907444912016496. [DOI] [PubMed] [Google Scholar]
- Thongekkaew J, Ikeda H, Masaki K, Iefuji H. Fusion of cellulose binding domain from Trichoderma reesei CBHI to Cryptococcus sp. S-2 cellulase enhances its binding affinity and its cellulolytic activity to insoluble cellulosic substrates. Enzyme Microb Technol. 2013;52(4–5):241–246. doi: 10.1016/j.enzmictec.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Várnai A, Viikari L, Marjamaa K, Siika-aho M. Adsorption of monocomponent enzymes in enzyme mixture analyzed quantitatively during hydrolysis of lignocellulose substrates. Bioresour Technol. 2011;102(2):1220–1227. doi: 10.1016/j.biortech.2010.07.120. [DOI] [PubMed] [Google Scholar]
- Várnai A, Siika-Aho M, Viikari L. Carbohydrate-binding modules (CBMs) revisited: reduced amount of water counterbalances the need for CBMs. Biotechnol Biofuels. 2013;6(1):30–30. doi: 10.1186/1754-6834-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrot A, Frandsen TP, von Ossowski I, Boyer V, Cottaz S, Driguez H, Schülein M, Davies GJ. Structural basis for ligand binding and processivity in cellobiohydrolase Cel6A from Humicola insolens. Structure. 2003;11(7):855–864. doi: 10.1016/s0969-2126(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yuan H, Wang J, Yu Z. Truncation of the cellulose binding domain improved thermal stability of endo-beta-1,4-glucanase from Bacillus subtilis JA18. Bioresour Technol. 2009;100(1):345–349. doi: 10.1016/j.biortech.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt G, Pellikka T, Boer H, Teeri TT, Koivula A. Probing pH-dependent functional elements in proteins: modification of carboxylic acid pairs in Trichoderma reesei cellobiohydrolase Cel6A. Biochem. 2003;42(34):10095–10103. doi: 10.1021/bi034954o. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Cui J, Lynd LR, Kuang LR. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromol. 2006;7(2):644–648. doi: 10.1021/bm050799c. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Jia J, Ji P, Han C. A novel application potential of GH6 cellobiohydrolase CtCel6 from thermophilic Chaetomium thermophilum for gene cloning, heterologous expression and biological characterization. Int J Agric Biol. 2017;19:355–362. doi: 10.17957/IJAB/15.0290. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.