Abstract
Background
Wearable cardioverter-defibrillators (WCDs) are currently used in patients at temporarily heightened risk for sudden cardiac death (SCD) who are temporarily unable to receive an implantable cardioverter-defibrillator (ICD). WCD can safely record and terminate life-threatening arrhythmias through a non-invasive electrode-based system. The current clinical indications for WCD use are varied and keep evolving as experience with this technology increases.
Methods
We reviewed and explored the data behind indications for WCD use and discuss its usefulness in congenital heart disease (CHD) patients.
Results
We considered 8 consecutive patients (mean age 35.25 years, range 18–51 years, average duration of WCD use 4 months, range 3–6 months) with complex CHD, in which a WCD was used between June 2018 and January 2022. No sustained ventricular arrhythmias requiring shocks were recorded in the observation period. No inappropriate shocks were recorded. All the patients showed a good compliance and a very high mean wear time per day (21.2 ± 1 h a day). Four patients implanted a permanent device (3 CRT-D, 1 ICD), three underwent cardiac surgery at the end of the WCD period and one is still on the waiting list for the operation.
Conclusions
Larger trial could confirm the possible conceivable benefit from an extended use of the WCD in certain populations with complex CHD as in our case series, especially in patients with life-treating ventricular arrhythmias waiting for surgery for residual cardiac defects or in the early phases following the surgical/hemodynamic interventions, patients with tachycardiomyopathy expected to improve after the arrhythmias are removed and patients awaiting implantation of an ICD at high risk due to active infection.
Keywords: Wearable cardioverter defibrillator, Congenital heart disease, Quality of life, Ventricular arrhythmias, Sudden death
Abbreviations
- WCD =
Wearable cardioverter-defibrillator
- SCD =
Sudden cardiac death
- ICD =
Implantable cardioverter defibrillator
- CHD =
Congenital heart disease
- CRTD =
Cardiac resynchronization therapy defibrillator
- VT =
Ventricular tachycardia
- VF =
Ventricular fibrillation
- AS =
Appropriate Shock
- IAS =
Inappropriate Shock
1. Introduction
The implantable cardioverter/defibrillator (ICD) has been used for CHD population more than 35 years and it is central in prevention of SCD in populations at high risk for life-threatening sustained ventricular tachycardia (VT) and ventricular fibrillation (VF). However, a significant number of patients have an increased risk but do not qualify for implantation of ICD because of multiple reasons such as, but not limited to, unestablished chronicity of severe impairment of ventricular function or active infection and the ICD implantation may be inappropriate or delayed [1]. For instance, ICD implantation may be initially deferred and become unnecessary if the arrhythmic substrate is temporary, or if the risk for ICD implantation is high or without evidence for survival benefit [2]. Wearable cardioverter-defibrillators (WCDs) have been presented as the solution to bridge this vulnerable population to the implantable device during the waiting period.
The purpose of this case series was to analyse our experience in terms of appropriateness and usefulness of WCD in complex high risk CHD patients.
2. Methods
This is an observational, retrospective study on WCD indications and follow-up in complex adult CHD patients. Data were collected prospectively in the WCD “Monaldi Adult Congenital Heart Disease Registry” and analysed retrospectively. The study was approved by Institutional Review Board. We prospectively entered data from all patients who underwent WCD implantation in our Unit. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. Informed consent for data storage and analysis was obtained from the patients or their guardians, respectively.
2.1. WCD description
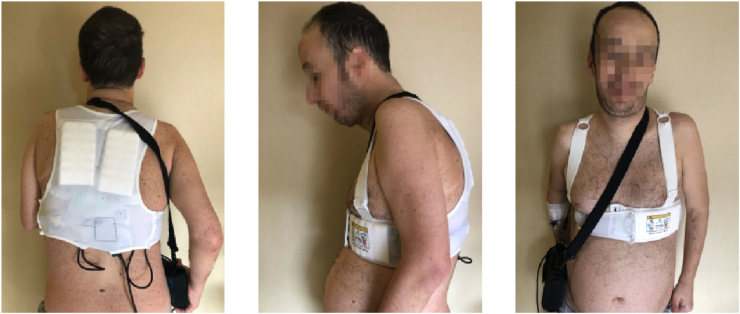
The WCD is an alternative device that combines the advantages of continuous cardiac monitoring with a non-invasive defibrillation system. The WCD is a vest-like device intended to be worn underneath clothing (Fig. 1– Patient A.M. Case n°2). It is composed of four monitoring electrodes and three defibrillation electrodes incorporated into a chest strap assembly. A defibrillation unit is carried on a waist belt. Collected ECG signals from two leads are continuously analysed for the presence of VT and VF using a digital signal processor. Real time arrhythmia monitoring is an added benefit of this device. If an arrhythmia is detected, ringing alarms demand the patient's or bystanders' attention. A conscious patient can respond to the alarms by holding the ‘response buttons’ in order to prevent the shock. In the absence of a patient response, the WCD charges and delivers a programmed energy level shock [[3], [4], [5]]. The device also works as a loop recorder and continuously records and transmits data on tachyarrhythmias and brady-arrhythmias. The device may be programmed to different VT or VF zones with different response times (time from detection to defibrillation sequence activation) and shock energy (between 75 and 150 J, biphasic).
Fig. 1.
Wearable Cardioverter Defibrillator (WCD) and Monitor Unit. Patient A.M. Case n°2.
The only WCD currently commercially available is the Zoll Life Vest (ZOLL Lifecor Corp., Pittsburgh, PA, USA).
2.2. Selection of the patients
All the patients aged more than 16 years, affected by complex CHD, in which a WCD was used between June 2018 and January 2022 were included in the study. A complex CHD was defined according to the latest ACC/AHA [6].
2.2.1. Inclusion criteria
The indication for WCD was considered for patients with complex CHD who presented the following conditions:
-
-
patients with a temporarily elevated risk of life-threatening arrhythmias in the absence of a proven ICD indication
-
-
short-term use in patients with multiple conditions associated with possible high risk of VT/VF; including myocarditis, newly diagnosed or prior severe impairment of ventricular function, recent surgery complicated by a low LVEF
-
-
patients who qualify for an ICD but who have contraindications for implantation, or as a bridge to more definitive therapy, and/or while potentially reversible underlying risks for SCD are being treated
-
-
patients who have a conventional ICD indication but are not candidates for immediate surgical implantation.
2.2.2. Exclusion criteria
-
-
All the patients with a simple heart defect (isolated defect, defects repaired or unrepaired without any haemodynamic impairment)
-
-
Those with an inherited arrhythmia (long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia), cardiomyopathies (dilated, hypertrophic, restrictive, non-compaction, arrhythmogenic right ventricular), myocarditis were excluded.
2.3. Data collection
Following data were collected:
-
-
patient demographics,
-
-
pre WCD use: clinical characteristics (congenital diagnosis and details regarding surgical repair/palliation; results of the most recent catheterization and non-invasive imaging; existing cardiac implantable electronic device details; drug therapy; WCD indication and motivation for its use).
Patients were regularly followed between June 2018 and January 2022 at Adult CHD Unit in accordance with the following protocol: patients underwent clinical evaluation, ECG, trans-thoracic echocardiography 2 weeks after the implant and every month thereafter. Daily remote-monitoring was carried-on.
The outcomes analysed included patients’ characteristics, long-term complications, all appropriate and inappropriate shocks during follow-up. Therapies were classified as appropriate (AS = Appropriate Shock) if delivered for ventricular tachycardia/ventricular fibrillation (VT/VF); otherwise, they were considered inappropriate (IAS = Inappropriate Shock).
Time for the follow-up and WCD use was decided on the clinical scenario and the indication for WCD.
2.4. Data analysis
Data are presented as mean ± standard deviation or median (interquartile range) for continuous variables as appropriate and as frequencies and percentages for dichotomous variables. The study is descriptive, with no inferential statistics performed.
3. Results
All the patient's data are reported in Table 1.
Table 1.
Baseline clinical characteristics of the study population.
| Pt. | Sex/Age | CHD | Indications | Drugs at discharge | AS/IAS | Wear time (hours) | F–U (months) | Outcome |
|---|---|---|---|---|---|---|---|---|
| #1 | M/51 | TOF s/p Repair | Persistent SVT. LVEF:25% Possible tachycardiomyopathy |
Furosemide, Ramipril, Apixaban, Bisoprolol, Amiodarone |
No/No | 20 | 5 | -RFCA -LVEF:50% -No CIED |
| #2 | M/45 | Shone Syndrome s/p decoartaction, VSD closure, Bentall procedure | Infective endocarditis. LVEF:17% |
Furosemide, Ramipril Bisoprolol, Antibiotics |
No/No | 21 | 6 | -LVEF:45%. -No Endocarditis -No CIED |
| #3 | M/27 | TOF s/p Repair | Severe pulm. regurg (PHT:73 ms). RV dilatation. TAPSE:14 mm nsVT runs. WCD during workup prior to and in the early phases following surgery |
Bisoprolol, Spironolactone Furosemide, Amiodarone |
No/No | 23 | 3 | -Pulm. valve replaced -LVEF:50% -No CIED |
| #4 | M/37 | DORV s/p Repair | Severe LV dilatation. LVEF:20% Severe RV dilatation. TAPSE:16 mm Severe pulm. regurg (PHT:43 ms) WCD during workup prior to and in the early phases following surgery |
Bisoprolol, Spironolactone Sacubitril/Valsartan Furosemide, Amiodarone, Apixaban |
No/No | 20.5 | 3 | -Pulm. valve replaced -LVEF:35% -CRT-D |
| #5 | M/18 | ccTGA s/p VVI PMK |
Severe systemic AV valve regurgitation Severe dysfunction of morphological right systemic ventricle (TAPSE:13 mm) WCD during workup prior to and in the early phases following surgery |
Bisoprolol, Sacubitril/Valsartan Amiodarone, Warfarin |
No/No | 22 | 5 | -Systemic AV valve replaced -LVEF:35% -CRT-D |
| #6 | M/41 | VSD + LVOTO s/p Repair | Severe LV dilatation. LVEF:25% |
Sacubitril/Valsartan, Bisoprolol, Amiodarone, Furosemide, Rivaroxaban, Spironolactone | No/No | 21 | 4 | -LVEF:35% -CRT-D |
| #7 | M/34 | TGA s/p Mustard | Endocardial ICD lead extraction Severe dysfunction of morphological right systemic ventricle (TAPSE:5 mm) |
Furosemide, Rivaroxaban, Bisoprolol, Sacubitril/Valsartan Spironolactone, Amiodarone | No/No | 20.5 | 3 | -ICD |
| #8 | M/29 | TOF s/p Repair | Persistent SVT. Severe pulm. regurg (PHT:78 ms) LVEF:25% Possible tachycardiomyopathy |
Furosemide, Rivaroxaban, Bisoprolol, Sacubitril/Valsartan Spironolactone, Amiodarone | No/No | 21 | 3 | -RFCA -Waiting-List for pulm valve replacement -No CIED |
CHD= Congenital heart disease; TOF = Tetralogy of Fallot; VSD= Ventricular septal defect; DORV = Double outlet right ventricle; ccTGA = Congenital corrected transposition of great arteries; TGA = Transposition of great arteries; PMK = pacemaker; LVOTO = Left ventricle outflow tract obstruction; SVT= Supraventricular tachycardia; LVEF = Left ventricle ejection fraction, PHT= Pressure half-time; RV = right ventricle, TAPSE = Tricuspid annular plane systolic excursion; AS = Appropriate shock; IAS= Inappropriate shock; CIED: cardiovascular implantable electronic device.
All the patients showed a good compliance and a very high mean wear time per day (21.25 ± 1 h a day). Two patients (#1 and #3) showed, during WCD monitoring, supraventricular un-sustained arrhythmias (intra-atrial reentry tachycardia as confirmed through electrophysiological study). No asystole was detected.
No sustained ventricular arrhythmias requiring shocks were recorded in the observation period (mean time 4 months - range 3–6 months). No inappropriate shocks were recorded. Four patients implanted a permanent device (CRT-D patients #4, #5 and #6, and ICD #7) at the end of the WCD period.
4. Discussion
4.1. Clinical use of WCD therapy
WCD is ideally indicated as temporary therapy (three to six months) for patients at a high risk for SCD [[7], [8], [9], [10]]. Current ICD implantation guidelines stress the importance of avoiding ICD therapy in patients with reversible arrhythmic disorders or risk factors [7,11]. Given these issues, WCD therapy is best suited for clinical scenarios in which the risk of SCD is temporary, or when the wearable device can be used to bridge the patient to a more definitive treatment (ICD implant or surgery).
4.2. Possible indications and advantages of WCD in complex CHD
The most significant long-term sequelae of repaired complex CHD and the most important reasons for hospitalisation in this population of patients are arrhythmias [[12], [13], [14], [15]]. Postoperative tachycardias occurring in patients who have undergone palliation or repair of CHD remain one of the most challenging problems facing the field of modern electrophysiology. The aetiology of arrhythmias in adult patients with congenital heart disease (ACHD) is multifactorial, including electrical disturbances that are inherent components of certain malformations or that develop as a consequence of previous operations or are the result of haemodynamic abnormalities during follow-up [16]. The most detailed data for primary prevention of SCD in CHD relates to those with repaired tetralogy of Fallot (TOF) and a wide number of risk factors have been identified that delineate increased risk and therefore the ones that will derive greatest benefit from ICD implantation [[17], [18], [19]]. WCD could be prescribed in these high-risk patients during the hemodynamic and electrophysiologic workup and in the early phases following the surgical/hemodynamic interventions, when the arrhythmic risk is even increased due to the scars or mechano-electrical adaptation to the intervention, allowing the patient to recover avoiding a premature ICD implant. In patients with other complex CHD conditions, such as systemic right ventricle encountered in transposition of the great arteries treated by an atrial switch procedure (Mustard or Senning), or in congenital corrected transposition of great arteries, the risk of SCD is approximately 5% per decade of life [20]. Pharmacological strategies addressed to improve systemic ventricle function should be tested before the indication to ICD implant, whose lead placement can be technically difficult, other than, sometimes, contraindicated due to the possibility of atrial buffle obstruction (transposition of the great arteries after Mustard or Senning procedure). In these conditions, WCD could be indicated for a prolonged period, even beyond the classical 3-months waiting time, to prevent SCD while allowing systemic right ventricle function to improve following intensified heart failure therapy optimization [21]. Similarly, in patients with univentricular circulation following Fontan or modified Fontan operation with a direct atrial-pulmonary or bi-caval-pulmonary artery connection a WCD use could be the option, in presence of life-threatening arrhythmias due to decreased systemic function or haemodynamic causes before any possible therapeutical interventions.
Our study investigated eight patients with complex CHD at high risk for life-threatening arrhythmias in which WCD was decided to be used to prevent SCD. This study includes the largest single center population of patients with complex CHD and WCD analysed so far.
Although the present cohort study is limited by the small number of patients and, subsequently, the absence of malignant arrhythmic events, interesting observations can be drawn.
Our patient population did not experience any inappropriate shock. Patients were prescribed WCD for primary prevention and were 7/8 on Amiodarone, which may have prevented ventricular arrhythmias.
In our study the mean wear time per day was quite high, in this unique previously unreported subset of patients, when compared with the literature; for instance, the PROLONG study [21] and Olgin et al. [22] reported a mean wear time per day of 21.7 ± 4 and 14.0 ± 9 h, respectively. However, as no arrhythmic events occurred in our patient population, any interference about these aspects and malignant arrhythmic episode or mortality could not be done. The ease of discontinuation of use heightens the importance of maximizing patient compliance to ensure effective treatment. It has been postulated that the compliance and the mean wear time might probably be crucial for the decrease of arrhythmic and overall mortality for all the WCD users. Usually, the rate of patients who did not implant ICD at the end of the WCD period is very high, almost 70% in some series [22]. In our series only four patients (50% of patients), at the end of the observational period, implanted an ICD or CRT, according to the latest ACC/AHA ESC guidelines. In some studies, the prolongation of WCD period >3 months because of initial increase of LVEF resulted in a higher proportion of patients with a recovery of LVEF >35%. Finally, the WCD might also be a helpful tool for the detection of supraventricular arrhythmias during the follow up of these patients, as showed in our case series (patients #1 and #3).
Overall, the WCD should not be considered as an alternative to the ICD, but as a device that may contribute to better selection of patients for ICD therapy, even in patients with complex CHD.
The WCD provides a mode of arrhythmia detection, recording and defibrillation in patients at risk of SCD. Its benefit as temporary therapy for patients at high risk of SCD has been reported in a few large but non-randomised observational studies, and a growing number of single centre experiences.
Undoubtedly, there are limitations to its use, including user dependency, inappropriate shocks, lack of pacemaker functionality, adherence issues and improper wear.
5. Conclusions
In our case series, no sustained ventricular arrhythmias requiring shocks were recorded in the observation period. No inappropriate shocks were recorded. All the patients showed a good compliance and a very high mean wear time per day.
Larger trial could confirm the possible conceivable benefit from use of the WCD in certain populations with CHD as in our case series, especially in patients with life-treating ventricular arrhythmias waiting for surgery for residual defects (Fig. 2) or in the early phases following the surgical/hemodynamic interventions, patients with tachycardiomyopathy expected to improve after the arrhythmias are removed (Fig. 3) and patients awaiting implantation of an ICD at high risk due to active infection (Fig. 4).
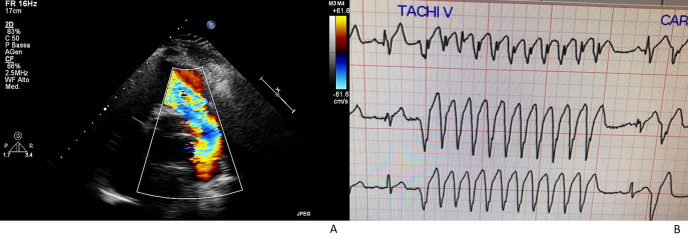
Fig. 2.
Clinical case n 3. Patient D.Z.
Fig. 2A: Echocardiographic evidence of severe pulmonary regurgitation
Fig. 2B: Telemetric electrocardiography showing non-sustained VT run.
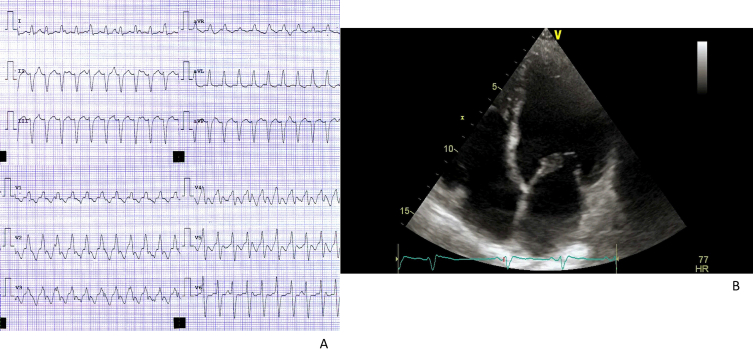
Fig. 3.
Clinical case n.1. Patient C.D.G.
Fig. 3A: High-rate supraventricular tachycardia (intra-atrial reentry tachycardia as confirmed through electrophysiological study)
Fig. 3B: Echocardiographic evidence of dilated left ventricle.
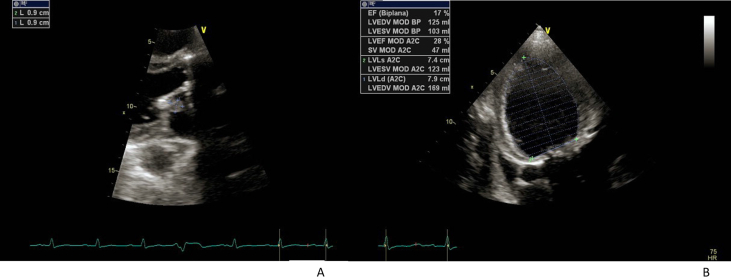
Fig. 4.
Clinical case n 2. Patient A.M.
Fig. 4A Echocardiographic evidence of a vegetation of 0.9 × 0.9 mm attached to the atrial side of the stenotic mitral valve.
Fig. 4B: severe biventricular function depression (LVEF 17%).
Declaration of competing interest
No funding sources have been given for this study.
All authors report no relationships that could be construed as a conflict of interest.
Acknowledgement
Special thanks to the Adult Congenital Heart Disease Unit nursing staff and specially to the head nurse Mrs. Assunta Carandente for their essential contribution and support in maintaining high-quality standard of care for our complex patients. We thank furthermore Dr. Gabriella Piccolo and Dr. Nadia Puzone, data manager, for data collecting and analysis and Dr. Cecilia Spinelli Barrile for her professional support in reviewing the English language and style of the manuscript and reviewing the data.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Kalarus Z., Svendsen J.H., Capodanno D., et al. Cardiac arrhythmias in the emergency settings of acute coronary syndrome and revascularization: an European heart rhythm association (EHRA) consensus document, endorsed by the European association of percutaneous cardiovascular interventions (EAPCI), and European acute cardiovascular care association (ACCA) Europace. 2019;21:1603–1604. doi: 10.1093/europace/euz163. [DOI] [PubMed] [Google Scholar]
- 2.Klein H.U., Meltendorf U., Reek S., et al. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defibrillator (WCD) Pacing Clin Electrophysiol. 2010 Mar;33(3):353–367. doi: 10.1111/j.1540-8159.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 3.Reek S., Geller J.C., Meltendorf U., et al. Clinical efficacy of a wearable defibrillator in acutely terminating epi- sodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol. 2003;26(10):2016–2022. doi: 10.1046/j.1460-9592.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Said A., Suleman I., Fayez S., et al.: Keeping up to date: a current review of wearable cardioverter defibrillator use, Acta Cardiol, DOI: 10.1080/00015385.2019. [DOI] [PubMed]
- 5.Reek S., Burri H., Roberts P.R., et al. The wearable cardioverter-defibrillator: current technology and evolving indications. Europace. 2017 Mar 1;19(3):335–345. doi: 10.1093/europace/euw180. [DOI] [PubMed] [Google Scholar]
- 6.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019 Apr 2;73(12):1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm. 2018 Oct;15(10):e73–e189. doi: 10.1016/j.hrthm.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP Sr, Allen L.A., Kudenchuk P.J., Page R.L., Patel M.R., Turakhia M.P. American heart association electrocardiography and arrhythmias committee of the council on clinical cardiology and council on cardiovascular and stroke nursing. Wearable cardioverter- defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American heart association. Circulation. 2016;133(17):1715–1727. doi: 10.1161/CIR.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 9.Priori S.G., Blomström-Lundqvist C., Mazzanti A., et al. Esc Scientific Document Group 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: association for European paediatric and congenital cardiology (AEPC) Eur Heart J. 2015 Nov 1;36(41) doi: 10.1093/eurheartj/ehv445. 2793-286. [DOI] [PubMed] [Google Scholar]
- 10.Botto G.L., Mantovani L.G., Cortesi P.A., et al. On behalf of the Italian Association of Arrhythmology and Cardiac Pacing (AIAC) The value of wearable cardioverter defibrillator in adult patients with recent myocardial infarction: economic and clinical implications from a health technology assessment perspective. Int J Cardiol. 2022;356:12–18. doi: 10.1016/j.ijcard.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu U., Rajyaguru C., Cheung C.C., Morin D.P., Lee B.K. The wearable cardioverter-defibrillator vest: indications and ongoing questions. Prog Cardiovasc Dis. 2019;62:256–264. doi: 10.1016/j.pcad.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019 Apr 2;73(12):1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 13.Limongelli G., Sarubbi B. Atrial arrhythmias in adults with congenital heart disease. Listening to your heart sound can save your life. Int J Cardiol. 2017;248:159–160. doi: 10.1016/j.ijcard.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Bouchardy J., Therrien J., Pilote L., et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 15.Khairy P., Aboulhosn J., Gurvitz M.Z., et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–875. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 16.Sarubbi B., Pacileo G., Ducceschi V., et al. Arrhythmogenic substrate in young patients with repaired tetralogy of Fallot. Role of an abnormal ventricular repolarization. Int J Cardiol. 1999;72(1):73–82. doi: 10.1016/s0167-5273(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 17.Khairy P., Harris L., Landzberg M.J., et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117(3):363–370. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H., Bonhoeffer P., De Groot N.M.S., et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner H., De Backer J., Babu-Narayan S.V., et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2020 Aug;29 ehaa554. [Google Scholar]
- 20.Kammeraad J.A., Van Deurzen C.H., Sreeram N., et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44(5):1095–1102. doi: 10.1016/j.jacc.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 21.Duncker D., Keonig T., Hohmann S., Bauersachs J., Veltmann C. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator—the PROLONG study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olgin J.E., Pletcher M.J., Vittinghoff E., et al. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med. 2018;379:1205–1215. doi: 10.1056/NEJMoa1800781. [DOI] [PMC free article] [PubMed] [Google Scholar]