Abstract
Berberine (BBR), an isoquinoline alkaloid, has been found in many plants, such as Coptis chinensis Franch and Phellodendron chinense Schneid. Although BBR has a wide spectrum of pharmacological effects, its oral bioavailability is extremely low. In recent years, gut microbiota has emerged as a cynosure to understand the mechanisms of action of herbal compounds. Numerous studies have demonstrated that due to its low bioavailability, BBR can interact with the gut microbiota, thereby exhibiting altered pharmacological effects. However, no systematic and comprehensive review has summarized these interactions and their corresponding influences on pharmacological effects. Here, we describe the direct interactive relationships between BBR and gut microbiota, including regulation of gut microbiota composition and metabolism by BBR and metabolization of BBR by gut microbiota. In addition, the complex interactions between gut microbiota and BBR as well as the side effects and personalized use of BBR are discussed. Furthermore, we provide our viewpoint on future research directions regarding BBR and gut microbiota. This review not only helps to explain the mechanisms underlying BBR activity but also provides support for the rational use of BBR in clinical practice.
Keywords: Berberine, Gut microbiota, Oral bioavailability, Traditional Chinese medicines, Short chain fatty acids
Graphical abstract
Highlights
-
•
Low bioavailability enables interactions between berberine and the gut microbiota.
-
•
Berberine can shape the composition and metabolism of the gut microbiota.
-
•
Gut microbiota can metabolize and transform berberine.
-
•
Personalized use of berberine can reduce the occurrence of side effects.
1. Introduction
Berberine (BBR) is alkaloid that belongs to isoquinoline group and has a molecular formula of C20H18NO4. BBR can be extracted from various plant families, including Annonaceae, Berberidaceae, Menispermaceae, and Papaveraceae [1]. Over the past decades, BBR has emerged as an important compound in traditional Chinese medicine because of its wide range of applications [2,3]. Numerous studies have demonstrated that BBR exhibits positive pharmacological effects in many diseases such as cancer [4,5], inflammation [6], bacterial infection [7], reperfusion injury [8], and non-alcoholic fatty liver (NAFL) disease [9]. Therefore, it has a great clinical value. In contrast to its strong pharmacological effects, the oral bioavailability of BBR is extremely low [10]. The absolute bioavailability and peak concentration (cmax) of orally administered 100 mg/kg BBR in rats were 0.68% and 9.48 ± 3.40 ng/mL, respectively [11]. The cmax value of 50 mg/kg BBR in dogs was 36.88 ± 23.45 ng/mL [12]. The contradiction between the low oral bioavailability of BBR and strong pharmacological actions of BBR has perplexed pharmacologists for a long time [13].
A large number of microorganisms are distributed in almost all parts of the body, such as the mouth, gastrointestinal tract, and vagina [14]. Gut microbiota is a group of microbes that mainly inhabit the intestine and account for over 70% of human microorganisms. Most of the gut microbiota are bacteria, mainly belonging to four phyla: Bacteroidetes, Actinobacteria, Firmicutes, and Proteobacteria [15]. Normal composition and metabolism of gut microbiota is vital for the maintenance of health, and disturbances in the gut microbiota can lead to the occurrence of various diseases, such as diabetes [16], diarrhea [17], and colitis [18]. In contrast, restoration of gut microbiota by various methods such as administration of antibiotics and probiotics and fecal microbiota transplantation can ameliorate diseases [19,20]. Besides the direct roles of gut microbiota in disease development and treatment, many researches have demonstrated that gut microbiota can interact with drugs in the past two decades [21]. On the one hand, gut microbiota can metabolize drugs into products with different potencies; on the other hand, drugs can influence gut microbiota composition and its metabolism [22,23]. Because these interactions can significantly influence the efficacy and toxicity of drugs, it has become essential to study the mechanisms of drugs with respect to the gut microbiota [24].
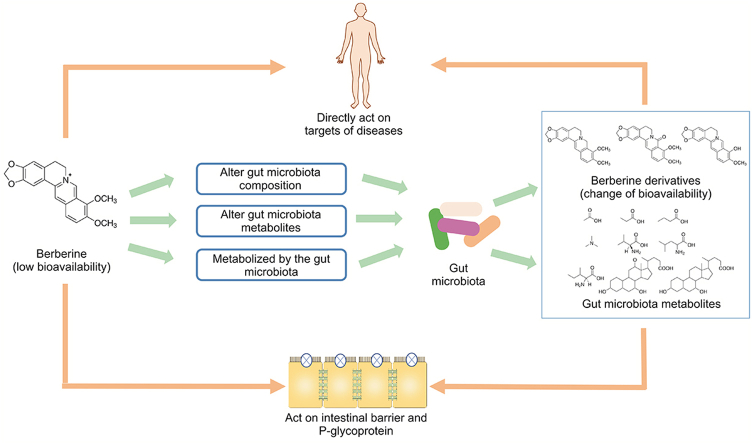
Due to the extremely low bioavailability of BBR, BBR can easily reach the intestines and interact with the gut microbiota. These direct interactions include modulation of composition and metabolism of gut microbiota by BBR and transformation of BBR by gut microbiota [25,26]. Gut microbiota has the capability to synthesize an array of metabolites essential for maintaining normal body functions, such as trimethylamine (TMA), short-chain fatty acids (SCFAs), bile acids (BAs), and branched chain amino acids (BCAAs) [27,28]. BBR can regulate these metabolites by regulating the amounts of gut bacteria that produce these metabolites and the expression or activity of enzymes responsible for the production of these metabolites [29,30]. In addition, BBR can be metabolized in the intestinal tract by gut microbiota through processes including oxidation, reduction, and demethylation, thereby forming products with different properties [31]. The pharmacological activity of BBR can be significantly influenced by these interactions. Although one review has discussed the influence of BBR on gut microbiota composition [25], no systemic summary or discussion about the interactive relationship between gut microbiota and BBR has been reported. In this review, we summarize the interactions between gut microbiota and BBR and the influences of these interactions on pharmacological effects, hoping to provide an explanation for the contradiction between low bioavailability and strong pharmacological effects of BBR, and to rationally apply BBR in clinical practice.
2. BBR directly regulates gut microbiota
In the gastrointestinal tract, the normal composition of gut microbiota can restrain the invasion of pathogens by inducing host release of antimicrobial materials, competitive consumption of nutrient sources, and occupation of attachment sites [32]. In addition, gut microbiota can generate and release a battery of metabolites such as BCAAs and trimethylamine N-oxide (TMAO), which are favorable or unfavorable to hosts depending on context. Therefore, regulating gut microbiota composition and metabolism is regarded as an important approach to treating diseases [33]. Because of its low bioavailability, BBR can influence the gut microbiota composition and metabolism by directly interacting with gut microbiota, thus assisting in the amelioration of diseases.
2.1. BBR regulates the composition of gut microbiota
BBR is a natural compound with direct antibacterial effects [34]. BBR (78 μg/mL) has a direct antibacterial effect on Streptococcus agalactiae, and the antibacterial effect increases as BBR concentration and exposure time increase [35]. BBR (32 μg/mL) can inhibit the growth of Candida albicans by inhibiting biofilm formation [36], and 128 μg/mL BBR can inhibit methicillin-resistant Staphylococcus aureus (S. aureus) [37]. When Escherichia coli (E. coli) is exposed to BBR (750 μg/mL), the cellular lifespan of E. coli decreases with prolonged exposure time [38]. In addition, BBR has direct inhibitory effects on bacteria such as Clostridium difficile, Actinobacillus, and Bacillus subtilis [[39], [40], [41]]. These studies indicate the direct antibacterial activity of BBR.
Due to its low oral availability and direct antibacterial effect, BBR can easily reach the colon and change the gut microbiota composition. Some researchers have revealed that BBR can regulate gut microbiota composition in normal animals (Table 1) [[29], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]]. Pan et al. [42] studied the influence of BBR on the gut microbiota composition of juvenile grass carp and observed the increased abundances of Bacteroidetes, Proteobacteria, and Firmicutes and the significantly decreased abundance of Fusobacteria. In normal mice, BBR can decrease the levels of Clostridium cluster (C. cluster) XIVa and C. cluster IV in the intestines [29]. In another study, a decrease in Ruminococcus gnavus and Ruminococcus schinkii and an increase in Bacteroides were observed in normal mice after treatment with BBR [43].
Table 1.
The changes in gut microbiota composition caused by BBR.
| Animal Models | Dosage of BBR | Key findings | Refs. |
|---|---|---|---|
| Normal C57BL/6 wild type mice | 100 mg/kg daily for one week | Clostridiumcluster XIVa and Clostridiumcluster IV were decreased; the proportion of Firmicutes/Bacteriodetes was decreased. | [29] |
| Normal juvenile grass carp | 30 mg/kg daily for 7 days | The relative abundances of Bacteroides and Proteus were increased; Fusobacteria was decreased. | [42] |
| Seven-week-old male C57BL/6 mice | 100 mg/kg daily for two weeks | Bacteroides was increased; Lactobacillus acidophilus, Lactobacillus murinus, Lactococcus lactis, Ruminococcusgnavus, and Ruminococcusschinkii were decreased. | [43] |
| HFD-induced insulin resistance rats | 200 mg/kg daily for eight weeks | Bifidobacterium and Lactobacillus were enriched; Escherichiacoli was inhibited. | [44] |
| HFD-induced atherosclerosis male ApoE-/- mice | 0.5 g/L in drinking water for 14 weeks. | The diversity of intestinal microbial community was decreased; Verrucomicrobia, Akkermansia, and Bacteroides were increased. | [45] |
| HFD-induced atherosclerosis male ApoE-/- mice | 50 mg/kg twice weekly for 12 weeks | Firmicutes and Verrucomicrobia were enriched; Bacteroidetes and Proteobacteria were decreased. | [46] |
| HFD-induced atherosclerosis in mice | 100 mg/kg daily for 13 weeks | Proteobacteria was reduced; Actinobacteria, Blautia, Roseburia, Blautia, Allobaculum, Alistipes, and Turicibacter were enriched. | [47] |
| HFD-induced NAFL in rats | 150 mg/kg daily for 4 weeks | Bacteroidetes and Proteobacteria were significantly increased; Firmicutes and Cyanobacteria were significantly decreased. | [48] |
| HFD-induced NAFL disease in rats | 150 mg/kg daily for 4 weeks | Faecalibacterium prausnitzii was reduced; Bacteroides was elevated. | [49] |
| DSS-induced ulcerative colitis in rats | 100 mg/kg daily for 6 days | Mucispirillum schaedleri and Bacteroides uniformis were decreased; Lachnospiraceae bacterium, Faecalibaculum rodentium, Corynebacterium glutamicum, Akkermansia muciniphila, Ruminococcus flavefaciens, and Bifidobacterium pseudolongum were increased. | [50] |
| DSS-induced ulcerative colitis in rats | 40 mg/kg daily for 10 days | Desulfovibrio was decreased; Eubacterium and Bacteroides were increased. | [51] |
| Colorectal cancer mice induced by azoxymethane/DSS | 100 mg/kg daily for 10 weeks | Actinobacteria, Verrucomicrobia, Bifidobacterium, Barnesiella, and Odoribacter were decreased; Alloprevotella, Flavonifractor, Oscillibacter, and Parabacteroides were increased. | [52] |
| ob/ob mice | 100 mg/kg daily for 10 days | Enterobacter, Escherichia-Shigella, Incertae sedis, Akkermansia, and Bacteroides were increased. | [53] |
| db/db mice with T2D | 100 mg/kg daily for 55 days | Saccharibacteria, Deferribacteres, Actinobacteria, and Firmicutes were reduced; Verrucomicrobia was increased. | [54] |
| db/db mice with T2D | 136.5 mg/kg daily for 11 weeks | Butyricimonas, Lactobacillus, Coprococcus, Ruminococcus, and Akkermansia were increased; Prevotella and Proteuswere were reduced. | [55] |
| Collagen induced arthritis in rats | 200 mg/kg daily for 14 days | Blautia, Butyricicoccus, and Parabacteroides were increased; Prevotella, Paraprevotella, and Coprococcus were decreased. | [56] |
| Ovariectomized rat with periodontitis | 120 mg/kg daily for 7 weeks | Blautia, Allobaculum, norank_f_Bacteroidales_S24-7_group, and Roseburia were increased; Lactobacillus was decreased. | [57] |
| HFD-induced obese rats | 100 mg/kg orally once a day for 18 weeks | 174 key OTUs were decreased, and 94 OTUs were enriched; Allobaculum, Blautia, Bacteroides, Butyricimonas, Phascolarctobacterium, Prevotella, unclassified Porphyromonadaceae, and unclassified Ruminococcaceae were enriched. | [58] |
| Experimental autoimmune uveitis mice induced by interphotoreceptor retinoid binding protein peptide 161–180 | 100 mg/kg daily for 14 days | Five genera were reduced including Lactobacillus; thirteen genera were increased including Akkermansia and Oscillibacter. | [59] |
| 5% ethanol-induced alcoholic liver disease in C57BL/6J male mice | 10, 50, and 100 mg/kg daily for 33 days | Proteobacteria, Terrisporobacter, and Helicobacter were increased; Pseudoflavonifractor, Mucisirillum, Alistipes, Ruminiclostridium, and Lachnoclostridium were decreased. | [60] |
| T2D rats induced by HFD | 200 mg/kg/day for 6 weeks | Bacteroidetes, Spirochaetaceae, Lactobacillaceae, and Peptostreptococcaceae were increased; Proteobacteria, Verrucomicrobi, and Enterobacteriaceae were decreased. | [61] |
| Hepatitis rats induced by transplanting the stool of patients with diarrhea-predominant irritable bowel syndrome | 200 mg/kg daily for 2 weeks | Faecalibacterium, Ruminococcus, Clostridium IV, Gemmiger, Roseburia, and Clostridium XI were reduced; Clostridium XIVa and Bacteroides were increased. | [62] |
| HFD-induced obese rats | 150 mg/kg daily for 4 months | The species diversity and richness of gut microbiota were declined; Bacteroidetes/Firmicutes ratio was increased; Bacteroidaceae, Rikenellaceae, and Bacteriodes were enriched; Coriobacteriia, Erysipelotrichi, Gammaproteobacteria, Christensenellaceae, Dehalobacteriaceae, Peptococcaceae, Dorea, Roseburia, and Blautia were decreased. | [63] |
| HFD-induced obese rats | Oral 150 mg/kg daily for 6 weeks | The diversity and richness of gut microbiota was decreased. Fusobacteria, Proteobacteria, Erysipelotrichaceae_incertae_sedis, Peptostreptococcaceae_incertae_sedis, Bacteroides, Escherichia-Shigella, Anaerostipes, Fusobacterium, and Phascolarctobacterium were enciched; Roseburia, Prevotella, Allobaculum, Faecalibacterium, Oscillibacter, and Desulfovibrio were reduced. |
[64] |
| HFD-induced obese rats | 100 mg/kg daily for 8 weeks | The species diversity and richness of gut microbiota was declined; Clostridium XlVa, Flavonifractor, Lachnospiracea_incertae_sedis, Roseburia, and Clostridium XI were inhibited. Butyricicoccus, Fusobacteria, Allobaculum, Parasutterella, Bacteroides, Blautia, Lactobacillus, Phascolarctobacterium, and Klebsiella were enriched; | [65] |
| HFD-induced hyperlipidemia in male wistar rats | Oral 150 mg/kg daily for 4 weeks | Prevotella, Clostridium, Sutterella, and Escherichia were decreased; Bacteroides, Blautia, and Parabacteroides were increased. | [66] |
| 5-fluorouracil-induced intestinal mucositis in rats | 100 mg/kg daily for 8 days | Proteobacteria and Verrucomicrobia were decreased; unclassified_f_Porphyromonadaceae, Firmicutes, Tenericutes, unclassified_f_Lachnospiraceae, Lactobacillus, Prevotella, unclassified_o_Clostridiales, Ruminococcus, and Clostridium IV were enriched. | [67] |
| T2D mice induced by streptozotocin and HFD | 100 mg/kg daily for 6 weeks | Enterobacter and Enterococcus were decreased; Lactobacillus, Bifidobacterium, and Bacteroidetes were increased. | [68] |
| T2D rats induced by HFD and high sucrose | 500 mg/kg daily for 4 weeks | Clostridia, Bacteroidetes, Prevotellaceae, Alloprevotella, and Lactobacillales, were increased; Lachnospiraceae, Bacteroidales, Desulfovibrio, and Rikenellaceae were reduced | [69] |
| Apcmin/+ mice feeding HFD | Blended into HFD at 500 mg/kg for 12 weeks | Verrucomicrobia, Bacteroidetes, Akkermansia, Bacteroides, and Prevotellaceae_uncultured were decreased; Firmicutes, Lachnospiraceae_incertae_sedis, Bacteroidaceae, Bacteroides, and Bilophila were enriched | [70] |
BBR: berberine; DSS: dextran sodium sulfate; HFD: high-fat diet; NAFL: non-alcoholic fatty liver; T2D: type 2 diabetes.
In recent years, plenty of research using animal models of diseases has disclosed that BBR can ameliorate diseases by influencing the gut microbiota composition (Table 1). Among these studies, a high-fat diet (HFD)-induced disease model, dextran sodium sulfate (DSS)-induced disease model, and type 2 diabetes (T2D) model are the three most commonly used.
The disease model induced by HFD is commonly used to investigate the effect of BBR on the gut microbiota. In HFD feeding-induced insulin resistance, atherosclerosis, NAFL, and other diseases, the alleviation of disease and changes in the composition of gut microbiota were detected after BBR treatment. Liu et al. [44] studied the action of BBR on the gut microbiota of rats with HFD-mediated insulin resistance. The increase in fasting plasma glucose, fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) caused by HFD feeding decreased after BBR treatment; in addition, Bifidobacterium and Lactobacillus were enriched and E. coli was inhibited after BBR treatment. In HFD-induced atherosclerotic mice, the symptoms and the development of atherosclerosis in mice were improved after BBR treatment, and it was also observed that the diversity and richness of gut microbiota of mice decreased and the abundances of Akkermansia and Bacteroides increased [45]. In another study, BBR treatment could enrich Firmicutes and Verrucomicrobia and decrease Bacteroidetes and Proteobacteria in HFD-induced atherosclerotic mice [46]. In addition, in HFD-induced atherosclerotic mice, Actinobacteria, Roseburia, Blautia, Allobaculum, Alistipes, and Turicibacter were increased and Proteobacteria was reduced after treatment with BBR [47]. In HFD-induced NAFL rats, BBR reduced the weight of rats, total cholesterol, triglycerides, and low-density lipoprotein-cholesterol contents, and the improved phenotype was associated by an increase in Bacteroidetes and Proteobacteria and a decrease in Firmicutes and Cyanobacteria [48]. In another study, F. prausnitzii was reduced and the level of Bacteroides was elevated in HFD-induced NAFL rats after treatment with BBR [49].
DSS is a chemical agent widely used to induce colon disease. In these models, the gut microbiota composition plays a vital role in disease progression. However, BBR can reshape gut microbiota disorders and alleviate DSS-induced intestinal diseases. After treating DSS-induced ulcerative colitis with BBR, inflammation and intestinal barrier function were improved [50]. In addition, compared with the model group, the relative abundances of Mucispirillum, Proteus, Oscillospira, and Allobaculum decreased and the relative abundances of Lactobacillus, Desulfovibrio, Ruminococcus, Parabacteroides, Sutterella, and Akkermansia increased [50]. In another DSS-induced colitis model, the relative abundances of Desulfovibrio, Proteobacteria, Streptococcaceae, Enterococcaceae, and Erysipelotrichale decreased, while the relative abundances of Eubacterium and Bacteroides increased after BBR treatment [51]. In a colon cancer model induced by azoxymethane/DSS, BBR treatment improved the hypoplasia of the crypt and hyperplasia of adenoma in the mucosa and reduced the occurrence of colon cancer [52]. In addition, the relative abundances of Actinobacteria, Verrucomicrobia, Bifidobacterium, Barnesiella, and Odoribacter decreased and the relative abundances of Alloprevotella, Flavonifractor, Oscillibacter, and Parabacteroides increased after BBR treatment [52].
Several studies have used T2D animal models to investigate the relationships among BBR, diabetes, and gut microbiota composition, and the results show that BBR can relieve the symptoms of diabetes by regulating the gut microbiota composition. Using ob/ob mice as an experimental model, BBR treatment decreased blood glucose and lipids, and increased some intestinal bacteria such as Nterobacter, Escherichia-Shigella, I. sedis, Akkermansia, and Bacteroides [53]. In db/db mice with T2D, BBR could effectively regulate glucose metabolism and restore glucose homeostasis, and the abundance of Saccharibacteria, Deferribacteres, Actinobacteria, and Firmicutes was reduced [54]. In another study, BBR treatment reduced Prevotella and Proteus numbers and increased Lactobacillus, Butyricimonas, Ruminococcus, Akkermansia, and Coprococcus numbers [55].
In summary, BBR can not only change the composition of the gut microbiota in normal animals, but also alleviate HFD-induced insulin resistance, DSS-induced colitis, T2D, and other diseases by regulating the composition of the gut microbiota.
2.2. BBR regulates the metabolism of gut microbiota
Gut microbiota can generate and release a range of metabolites that include, but are not limited to, SCFAs, BCAAs, BAs, and TMA [[27], [28], [29]]. These metabolites have a great significance in cardiovascular [71,72], NAFL [73,74], Alzheimer's [75], and other diseases [76,77]. Therefore, regulating the metabolism of these metabolites has become necessary in treating diseases and maintaining normal physiological functions of the body [77]. Many studies have demonstrated that the efficacy of BBR on diseases is related to regulating gut microbiota metabolites by BBR (Fig. 1, Fig. 2).
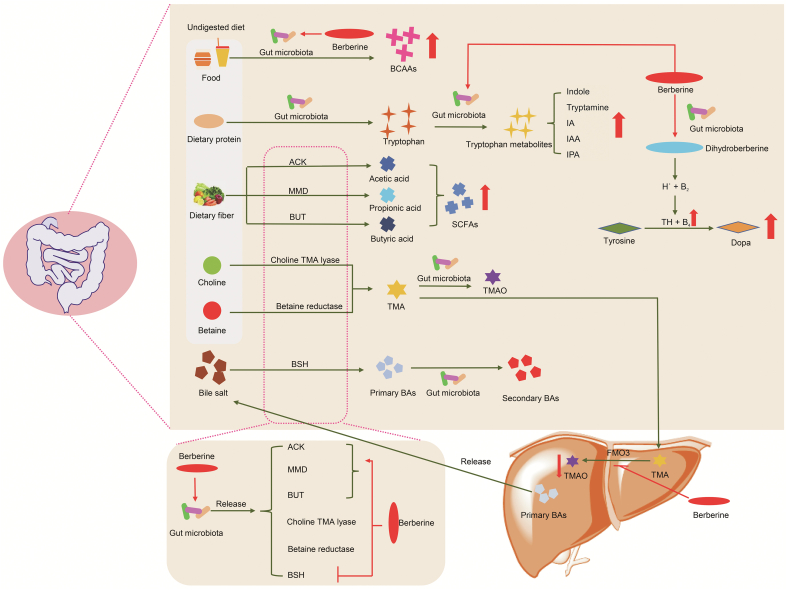
Fig. 1.
Typical gut microbiota metabolites and the influences of berberine (BBR) on production of these metabolites. ACK: acetate kinase; BAs: bile acids; BCAAs: branched-chain amino acids; BUT: butyryl-CoA:acetate-CoA transferase; BSH: bile salt hydrolase; B2: dihydrobiopterin; B4: tetrahydrobiopterin; FMO3: flavin-containing monooxygenase 3; IAA: indole-3-acetic acid; IA: indoleacrylic acid; IPA: indole-3-propionic acid; MMD: methylmalonyl-CoA decarboxylase; SCFAs: short-chain fatty acids; TMAO: trimethylamine N-oxide; TMA: trimethylamine.
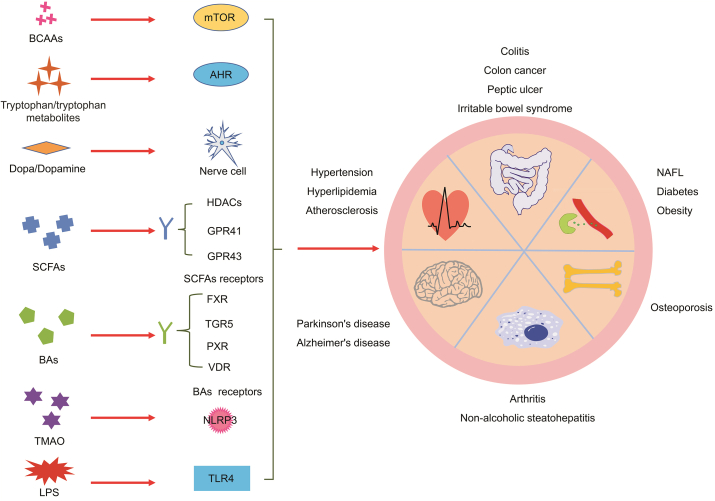
Fig. 2.
Targets of gut microbiota metabolites and the diseases related to these metabolites. AHR: aryl hydrocarbon receptor; FXR: farnesoid X receptor; GPR: G-protein-coupled receptor; HDACs: histone deacetylases; LPS: lipopolysaccharides; mTOR: mammalian target of rapamycin; NLRP3: nod-like receptor family pyrin domain containing 3; NAFL: non-alcoholic fatty liver; PXR: pregnane X receptor; TGR5: Takeda G protein-coupled receptor 5; TLR4: Toll-like receptor 4; VDR: vitamin D receptor.
2.2.1. SCFAs
SCFAs are carboxylic acids with two to six carbon atoms [78]. The gut microbiota primarily synthesizes the SCFAs, mainly including butyric acid, acetic acid, and propionic acid, through the fermentation of undigested dietary fibers [79,80]. The SCFAs produced in the intestine can directly act on receptors on the intestinal wall, or be absorbed and then act on receptors in other parts of the body to produce pharmacological effects. There are mainly two types of SCFAs receptors: histone deacetylases (HDACs) and G-protein-coupled receptors (GPR41 and GPR43) [[81], [82], [83]]. HDACs play important roles in chromosome structure modification and gene expression regulation [84]. GPR41 and GPR43 have a regulatory effect on various systems of the body, such as the central nervous system, metabolic system, and cardiovascular system [[85], [86], [87]]. SCFAs can act on these receptors to regulate multiple sclerosis [88], atherosclerosis [89], cancer [90], colitis [91], diabetes [92], and other diseases (Fig. 2). Butyryl-CoA:acetate-CoA transferase (BUT), acetate kinase (ACK), and methylmalonyl-CoA decarboxylase (MMD) are the key enzymes for the synthesis of butyric acid, acetic acid, and propionic acid, respectively [93,94] (Fig. 1). Bacteria such as Bacteroidetes, Firmicutes, Coprococcus, Fusobacteria, Actinobacteria, Roseburia, Allobaculum, Blautia, Butyricoccus, and Phascolarctobacterium are related to the formation of SCFAs [95,96]. Therefore, regulating the activity and expression of the above enzymes and the composition of gut microbiota will change the content of SCFAs, subsequently regulating the physiological functions of the body.
BBR can regulate the content of SCFAs through two main mechanisms (Fig. 1). One is by modulating the expression and activity of the enzymes responsible for SCFA biosynthesis. Wang et al. [97] used BBR to cultivate the gut microbiota isolated from rats and found that acetic acid, butyric acid, and propionic acid contents increased after treatment with BBR. Additionally, the expression and activity of key enzymes involved in SCFA formation, such as ACK, MMD, and BUT, increased. BBR can improve intestinal hypoxia, restore the balance of intestinal energy supply, reduce the infiltration of inflammatory cells, reduce synovial hyperplasia, and downregulate the arthritis index and foot swelling degree. In addition, BBR promotes the production of butyric acid by increasing the activity of BUT [56]. BBR (10 and 20 μg/mL) can increase the expressions of BUT and two butyrate synthesis precursor substances (crotonyl-CoA and butyryl-CoA), subsequently lowering blood lipid and glucose levels [53]. These studies showed that BBR can regulate the production of SCFAs by improving the expressions and activity of the enzymes, thereby exerting downstream pharmacological effects.
Another mechanism by which BBR regulates the content of SCFAs is via regulating the abundance of SCFA-producing bacteria. BBR can effectively improve alveolar bone loss by inhibiting osteoclast activity, increasing osteoblasts, and improving bone metabolism in OVX rats with periodontitis; further, increased butyric acid concentration in feces was detected in BBR-administered rats. Furthermore, the relative abundances of Blautia, Bacteroidales, and Roseburia in the gut microbiota increased after BBR treatment [57]. In HFD-induced obese rats, BBR decreased the adiposity index and body weight. In addition, the fecal concentration of acetic acid and propionic acid, and the number of SCFA-producing bacteria such as Allobaculum and Blautia increased after BBR treatment [58].
2.2.2. BAs
BAs are a class of molecules with a steroid structure that are generated from cholesterol in the liver [98]. After synthesis in the liver, primary BAs, mainly cholic acid (CA) and chenodeoxycholic acid (CDCA), combine with glycine or taurine to form bile salts and are secreted into the upper intestine along with cholesterol and coagulum. Some bile salts that reach the intestines are partially converted into secondary BAs, predominantly deoxycholic acid (DCA) and lithocholic acid (LCA) [99]. This conversion process involves the following two steps (Fig. 1). In the first step, bile salt hydrolases (BSH) specifically cut the amide bond of bile salts to dissociate primary BAs. In the second step, primary BAs are converted into secondary BAs under the action of the gut microbiota. For example, uncoupled CA and CDCA are catalyzed by microorganism 7α-dehydroxylase into LCA and DCA, respectively [[99], [100], [101]]. The expression of BSH has been reported in many gut bacteria such as Lactobacillus, Bifidobacterium, Enterococcus, and Clostridium spp. [99]. In addition, it was reported that the genera Clostridium and Eubacterium are the predominant intestinal species exhibiting BA 7α-dehydroxylating activity [102].
The primary BAs produced in the liver and secondary BAs produced in the intestines act on the BA receptors, which are highly expressed in the liver, intestine, brown adipose tissues, immune cells, and other tissues or cells, thereby regulating NAFL [103,104], liver cancer [105], diabetes [106], and other diseases. BA receptors predominantly comprise the Takeda G protein-coupled receptor 5 (TGR5) and the nuclear receptors, including vitamin D receptor, pregnane X receptor, and farnesoid X receptor (FXR) [[107], [108], [109]] (Fig. 2). Different BAs have different affinities with different BA receptors. For instance, DCA and LCA are the most efficient agonists for TGR5; however, CDCA has the highest affinity for FXR, followed by LCA, DCA, and CA. Therefore, the change in the BA pool regulates BA receptors and regulates the normal functioning of the body [107,108].
BBR exerts pharmacological effects by regulating the BA profile (Figs. 1 and 2). The serum free BA, total BA, and primary BA content increase after BBR treatment in mice, whereas the secondary BA content decreases; the effect is enhanced with an increase in BBR concentration [43]. In normal mice, BBR can inhibit the activity of BSH and reduce BSH-expressing bacteria, such as Clostridium spp. [29]. Meanwhile, taurocholic acid, tauroursodeoxycholic acid (TUDCA), and taurochenodeoxycholic acid (TCDCA) levels in the feces and liver and serum BA content are increased, and the cholesterol and free fatty acids are reduced [29]. Sun et al. [110] studied the influence of BBR on hepatic lipid metabolism of male mice. After treatment with BBR, the activity of BSH was inhibited, and the levels of some BAs such as CDCA, hyodeoxycholic acid, and DCA were reduced, while the levels of taurodeoxycholic acid, TCDCA, and TUDCA increased. Additionally, BAs can activate FXR to inhibit the expression of Cd36, which is a crucial protein allowing long-chain fatty acids to enter the liver and prevent obesity and triglyceride accumulation.
2.2.3. TMAO
TMAO is produced by oxidation of TMA. Undigested choline, carnitine, and betaine in food (such as fish, eggs, offal, and beans) are converted into TMA under the action of enzymes produced by the gut microbiota [111] (Fig. 1). Bacteria related to TMA production include Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Acinetobacter, Citrobacter, E. coli, Klebsiella, Proteus, Pseudomonas, Clostridium, Eubacterium, Sporomusa, Alcaligenes, and Escherichia [112]. The microbial enzymes acting in the TMA synthesis process mainly include choline TMA lyase, betaine reductase, and carnitine oxidase/reductase [111,113]. Some of the TMA produced in the intestine is directly converted into TMAO in the intestines by oxidation [114], while some of the TMA is transferred to the liver and converted into TMAO under the action of flavin-containing monooxygenase 3 (FMO3) [114,115]. TMAO affects atherosclerosis [116,117], insulin resistance [118], cancer [119,120], and myocardial fibrosis [121,122]. The mechanism of action of TMAO is related to the activation of the nod-like receptor family pyrin domain containing 3 inflammasome [[123], [124], [125]] (Fig. 2).
Studies have revealed that BBR can influence TMAO, thereby exerting ameliorative effects on diseases (Fig. 1). After BBR treatment, the development of atherosclerosis is relieved in HFD-induced atherosclerosis mice. Further, the expression levels of matrix metalloproteinase-2, interleukin (IL)-6, and intercellular adhesion molecule 1 in aortic arch sections and the expression levels of tumor necrosis factor (TNF)-α, monocyte chemoattractant protein-1, IL-1β, and vascular cellular adhesion molecule-1 in the carotid artery are reduced. In addition, FMO3 and serum TMAO expressions decrease. These results indicate that BBR can relieve atherosclerosis, and this effect is correlated to the inhibition of the FMO3-TMAO pathway [46]. In another study, treatment with BBR alleviated atherosclerosis, reduced lipid levels, and decreased the concentration of inflammatory factors, including TNF-α, IL-1β, and IL-6. In addition, the abundance of enzymes (such as choline TMA lyase and betaine reductase) related to TMA production in mice was decreased. Therefore, BBR may regulate the enzymes involved in TMA synthesis to alleviate atherosclerosis [47].
2.2.4. BCAAs
BCAAs (leucine, isoleucine, and valine) are three of nine essential amino acids that are not generated by our body; therefore, they must be obtained from the diet. The undigested part of foods such as dairy products, meat, fish, eggs, beans, nuts, and whole-grain products can be metabolized into BCAAs under the action of the gut microbiota [126,127] (Fig. 1). Many bacteria are involved in the biosynthesis of BCAAs, such as Prevotella copri, Bacteroides vulgatus, C. clusters, the Bacillus-Lactobacillus-Streptococcus group, and Proteobacteria (including E. coli and Klebsiella spp.) [[128], [129], [130]]. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that participates in regulating cell growth, proliferation, and development [131]. Abnormal activation of mTOR is correlated with various diseases, such as ischemic diseases and cancer [131,132]. BCAAs, especially leucine, are activators of the mTOR pathway and can regulate the physiological state of the body by activating mTOR [133]. For example, high levels of BCAAs contribute to activation of mTOR complex 1, which leads to insulin resistance through phosphorylation of insulin receptor substrate 1 [134]. In addition, free BCAAs are involved in protein synthesis, energy metabolism, and formation of the neurotransmitter glutamate [134,135].
BBR can regulate the biosynthesis of BCAAs to restore the function of the body, and the mechanism of regulation of BCAAs is related to inhibiting the microbial synthesis of BCAAs (Fig. 1). In obese mice, BBR can reduce body weight and the levels of serum total cholesterol, high-density lipoprotein, triglyceride, aspartate aminotransferase, and alanine aminotransferase. Furthermore, fasting serum glucose, insulin, and HOMA-IR index in insulin-resistant mice decreased after BBR treatment. Simultaneously, after BBR treatment, the relative abundances of BCAA-producing bacteria such as Clostridium, Streptococcus, Clostridium, Proflagellate, Streptococcus, and Brevibacterium were decreased, and the content of circulating BCAAs was reduced [30].
2.2.5. Dopamine
Dopamine is a major neurotransmitter in the brain, and its levels in the brain are closely related to brain function [136]. The phenylalanine-tyrosine-dopa-dopamine pathway is the main dopamine synthesis pathway. The conversion of tyrosine into dopamine by tyrosine hydroxylase (TH) is the rate-limiting process of dopamine synthesis, and tetrahydrobiopterin acts as a coenzyme in this process [137]. Dopamine is mainly synthesized in brain neurons, but the metabolic pathway of phenylalanine-tyrosine-dopa-dopamine in microorganisms has also been confirmed [138]. Recent studies have pointed out that BBR can regulate the phenylalanine-tyrosine-dopa-dopamine metabolic process of the gut microbiota to promote the production of intestinal dopa/dopamine and transfer to the circulatory system, from which it can ultimately reach the brain and improve Parkinson's symptoms. Specifically, oral BBR supplies H⋅ through dihydroberberine (DhB) (reduced product of BBR by gut bacterial nitroreductase) and promotes the generation of tetrahydrobiopterin from dihydrobiopterin, which in turn enhances the activity of TH, thus increasing the generation of l-dopa by the gut microbiota [139] (Fig. 1).
2.2.6. Tryptophan, indole, and indole derivatives
As an indispensable amino acid for the human body, tryptophan is primarily supplemented by the bio-transformation of dietary proteins. In the gastrointestinal tract, tryptophan metabolism follows three pathways: 1) the kynurenine pathway in both immune and epithelial cells, 2) the serotonin pathway in enterochromaffin cells, and 3) direct conversion by gut microbiota into metabolites including indole and indole derivatives such as indole-3-aldehyde, indole-3-acetic acid, indole-3-propionic acid, and indole-3-acetaldehyde [140]. Tryptophan and tryptophan microbial products, indole and indole derivatives, can activate the aryl hydrocarbon receptor (AHR) to regulate intestinal inflammation, gut hormone secretion, and gastrointestinal motility [141]. An article showed that BBR can significantly improve colitis in a DSS-induced colitis model [142]. This phenomenon occurs in a gut microbiota-dependent manner and is accompanied by an increase in fecal and serum tryptophan and indole derivatives, such as indole-3-propionic acid and indole-3-acetic acid. Furthermore, activation of AHR was observed in the colon tissue of the BBR group, and co-incubation of the Caco-2 cell monolayers and the culture supernatants of gut microbiota confirmed this result. This study demonstrated that BBR can improve colitis by promoting the microbial metabolism of tryptophan [142].
2.2.7. Lipopolysaccharides
Lipopolysaccharide (LPS) is the main ingredient of the cell wall of gram-negative bacteria. Upon entering the circulatory system, LPS can stimulate Toll-like receptor 4 (TLR4) and lead to recruitment and activation of MyD88 adaptor and NF-κB transcription factor, resulting in the expressions of type I interferons, interferon (IFN)-inducible chemokines, and proinflammatory cytokines such as TNF and IL-1 [143]. Increased circulating LPS can promote inflammatory responses, insulin resistance, obesity, T2D mellitus, NAFL, chronic hepatitis, and other diseases [144,145]. Therefore, regulating the amount of plasma LPS is an important therapeutic approach to treating diseases such as NAFL. Recent studies have shown that BBR can regulate the concentration of plasma LPS by modulating gut microbiota. In HFD-induced obese rats, BBR treatment reduced the concentrations of plasma LPS and decreased the gut levels of gram-negative bacteria such as E. coli. In addition, the innate immune receptor TLR4 for LPS and the subsequent immune pathway (NF-κB pathway) were inhibited [44]. In addition, in db/db mice, treatment with BBR can reduce the levels of plasma LPS and gram-negative bacteria, including Prevotella and Proteus, and increase the levels of gram-positive bacteria (Lactobacillus). Meanwhile, the ameliorative effect of BBR on intestinal barrier inflammation is correlated with the downregulation of TLR/NF-κB signaling pathways [55]. The results demonstrate that BBR can regulate gut microbiota composition and plasma LPS concentration to improve diseases.
3. Direct metabolism of BBR by gut microbiota
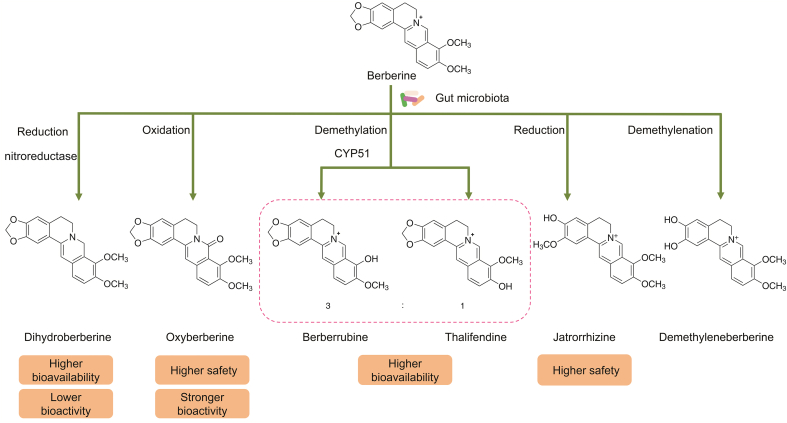
With the rapid progress of metagenomics, studies have found that gut microbiota contains rich and diverse genes, in total representing several orders of magnitude more genes than the human genome [[146], [147], [148]]. The large number, different species, and complex and diverse genes endow the gut microbiota with powerful metabolic capabilities. Diets, drugs, antibiotics, and substances secreted by human cells can be bio-transformed by the gut microbiota into various secondary metabolites. The reactions provided by the gut microbiota include reduction, oxidation, demethylation, and isomerization [31]. The application of modern technologies shows that BBR can be transformed by the gut microbiota into DhB, berberrubine, demethyleneberberine (DMB), jatrorrhizine, and oxyberberine (OBB), as shown in Fig. 3. These metabolites have similar pharmacological effects to BBR, but compared with BBR, each has its own characteristics and advantages.
Fig. 3.
Chemical transformation of BBR under the action of gut microbiota.
3.1. DhB
DhB, a reduction product of BBR, is produced under the catalytic activity of the gut microbiota [31]. Feng et al. [26] treated cultivated intestinal bacteria (S. aureus, Enterococcus faecium, Enterococcus faecalis, Enterobacter cloacae, E. coli, Staphylococcus, Pseudomonas aeruginosa, etc.) with BBR, and a decrease in BBR and DhB production was detected. Reports have pointed out that the intestinal microbial metabolite DhB transformed from BBR may be the key to explaining the intestinal absorption of BBR. Compared with BBR, DhB has a higher intestinal absorption rate (approximately five times that of BBR) and lower bioactivity [26,149]. The absorption of BBR in the form of DhB from the intestine into the circulatory system involves two processes: the conversion of BBR into DhB under the catalysis of gut microbiota enzymes and the conversion of DhB absorbed by intestinal epithelial cells into BBR through non-enzymatic oxidation reactions [26]. The conversion of BBR into DhB is carried out under the catalysis of gut microbial nitroreductase [150]. The intestinal microorganisms that produce nitroreductase include Enterobacter, Escherichia, Shigella, and Bacteroides [26,151]. The converted DhB can be absorbed by the intestinal epithelial tissue and then oxidized to BBR. The conversion rate of DhB into BBR in the intestinal epithelial tissue is approximately 80%; therefore, it is difficult to detect DhB in plasma [26]. In addition, studies have shown that the oxidation of DhB to BBR is a non-enzymatic reaction that is unrelated to cytochrome P450 (CYP450) and monoamine oxidase; however, some superoxide anions and metal ions seem to play an indispensable role in this process. Therefore, the activity and content of nitroreductase have become decisive factors that determine the speed of the BBR-DhB-BBR conversion process.
3.2. Berberrubine, thalifendine, and DMB
Demethylation is the main mechanism of microbial BBR metabolism. There are three main demethylation products of BBR: berberrubine, thalifendine, and DMB [31]. Berberrubine and thalifendine are products of the demethylation of the methoxy groups at the C-9 and C-10 positions of BBR, respectively, but DMB is a product of demethylation of the five-membered rings of the BBR dimethoxy subunit. Sterol 14α-demethylase (CYP51) belongs to the family of CYP450 enzymes and is present in many bacteria, lower eukaryotes, and mammals [152]. Berberrubine and thalifendine are both products of CYP51 (secreted by the gut microbiota) that catalyze BBR demethylation [153]. The bacteria in the gut microbiota, including Enterococcus faecalis, Staphylococcus epidermidis, Enterobacter cloacae, and Enterococcus faecium, contribute to the high yield of berberrubine [153]. In addition, cultivation of different concentrations of BBR with the same intestinal contents in vitro found that although the content of BBR increased, the percentage of BBR metabolism decreased, suggesting that the concentrations of gut bacteria and activities of CYP51 in each cultivation system were constant. Therefore, changing the number of gut bacteria and the content and activity of CYP51 may regulate the conversion of BBR into berberrubine or thalifendine, thereby affecting the pharmacological activity of oral BBR.
Research has shown that these three metabolites of BBR have similar biological activities as BBR; for example, BBR and berberrubine can alleviate intestinal inflammation [154,155], and BBR and DMB can alleviate liver diseases [156,157]. However, the content of the three BBR demethylation products was different after oral administration of BBR. Some scholars have pointed out that the primary metabolites of BBR in the small intestines are DMB glucuronide and berberrubine, and the levels of DMB and berberrubine with much higher levels than that of BBR are detected in plasma [158]. In addition, berberrubine shows better fat solubility and higher absolute bioavailability (31.6%) than BBR (<1%) [10,11,158,159]. In summary, it can be seen that the demethylated metabolites of BBR have more advantages than BBR and may be the basis for the pharmacological activity of BBR.
3.3. Jatrorrhizine
The five-membered dioxymethylene ring in BBR can be cleaved under the action of the gut microbiota to produce jatrorrhizine [31], but it is unclear what microorganisms drive this conversion process. Jatrorrhizine has a higher safety than BBR as a microbial reduction product of BBR. The LD50 of jatrorrhizine in mice is approximately 5500 mg/kg, while the LD50 of BBR is 763 mg/kg [160]. Studies have shown that both jatrorrhizine and BBR have pharmacodynamic action against hypercholesterolemia and rectal cancer [[160], [161], [162], [163]]. In addition, jatrorrhizine has been recommended as a gastric motility agent because of its effect in promoting gastric motility [164].
3.4. OBB
As a compound that naturally occurs in a variety of plants such as Coptis chinensis Franch [165], OBB is also an oxidation product of BBR after transformation by gut microbiota. Structurally, OBB is the product of oxidation of the C-8 position of BBR to hydroxyl [31]. Functionally, OBB and BBR have similar bioactivities, such as anti-inflammatory, anti-diabetic, and anti-colitis effects. However, studies have demonstrated that at the same dose, OBB is more effective in relieving inflammation and diabetes than BBR [166,167]. In addition, OBB is safer than BBR in mice; the LD50 of OBB (5243.6 mg/kg) is far greater than that of BBR (approximately 713.57 mg/kg) [168]. Therefore, OBB may be a material base for the pharmacological activity of BBR in vivo. The conversion of BBR into OBB is driven by gut microbiota, and the conversion rate can reach 12.42% in normal SD rats. It has been found that six bacteria in the gut microbiota, namely, Bifidobacterium longum, Lactobacillus acidophilus, Streptococcus faecalis, E. coli, Pseudomonas aeruginosa, and S. aureus, can convert BBR into OBB, and Streptococcus faecalis is the most productive [166].
4. Other complex interactions
In addition to the direct effects that can produce metabolites acting directly on targets of diseases, there are complex interactions between BBR and gut microbiota. These complex interactions involve tissues and proteins associated with disease development and drug transportation. Typical tissues and proteins include the intestinal barrier and P-glycoprotein.
4.1. Intestinal barrier
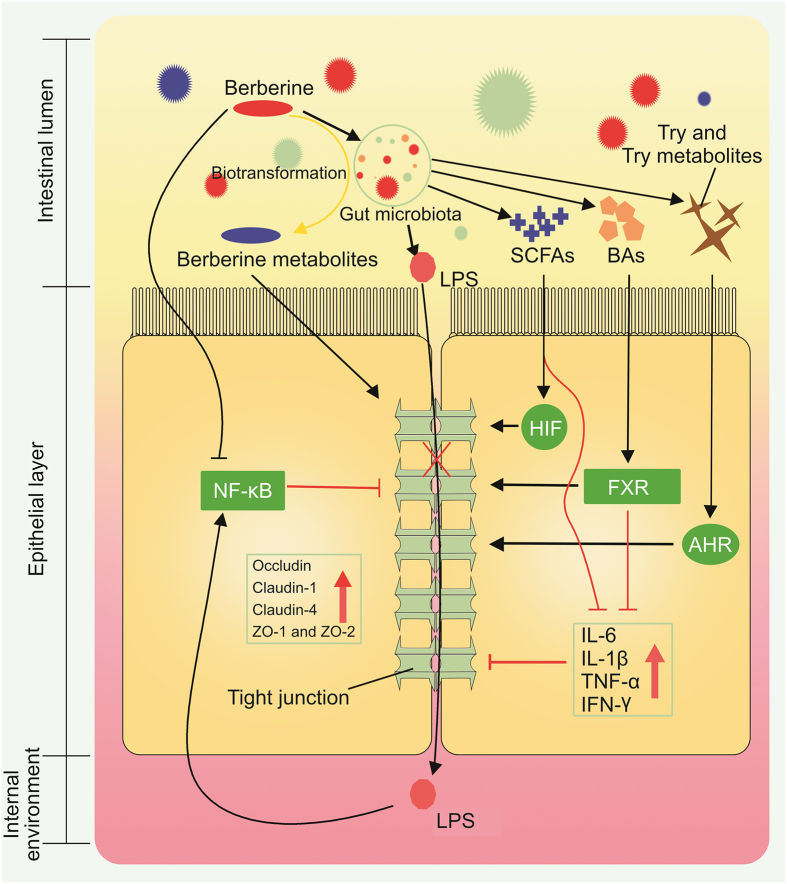
The intestinal barrier is a layer of physical and functional barriers between the gut lumen and immunocompetent submucosa [169]. The intestinal barrier comprises four distinct functional layers: 1) epithelial cells, which serve as a ‘functional barrier’ by releasing alkaline phosphatase to detoxify bacterial endotoxins (LPS), and pathogen-associated molecular patterns; 2) the mucus layer, which acts as a ‘physical barrier’ to prevent direct contact between gut microbiota and intestinal epithelia; 3) tight junctions between epithelial cells, which prevent the transport of LPS and other compounds derived from gut microbiota; and 4) specialized functional cells, such as Paneth cells, which secrete antimicrobial compounds [[169], [170], [171], [172]]. The functions of the intestinal barrier are directly correlated with a number of diseases, including metabolic diseases [173], schizophrenia [174], autism [174], irritable bowel syndrome [175], inflammatory bowel disease [176], coronary heart disease [177], chronic kidney disease [178], chronic pancreatitis [179], and various cancers [180]. In view of the significance of the intestinal barrier in the homeostasis of our body function, some scholars have conducted research on how to alleviate damage to the intestinal barrier. Among these studies, BBR has been revealed as a representative of phytotherapy (Fig. 4).
Fig. 4.
BBR regulates the intestinal barrier through the gut microbiota. IL: interleukin; HIF: hypoxia inducible factor; TNF-α: tumor necrosis factor-α; Try: tryptophan; ZO: zonula occludens.
4.1.1. BBR regulates gut microbiota to improve intestinal barrier damage
The gut microbiota and intestinal barrier are physically and functionally related as a pair of systems that live next to each other. Studies have revealed that gut microbiota has the capacity to regulate the function of intestinal barrier by regulating intestinal epithelial cell renewal, influencing intestinal permeability, and modulating antimicrobial protein expression and the mucus layer [181]. Loss of function in the intestinal barrier can contribute to a series of diseases, such as irritable bowel syndrome and inflammatory bowel disease. Improvement of the intestinal barrier function by restoring the composition of gut microbiota is now considered as an important approach to treating diseases [176,177]. In rats with NAFL induced by HFD, administering 150 mg/kg BBR relieved the symptoms of fatty liver; additionally, the intestinal mucosal villi were plump and arranged tightly and neatly compared to the model group. In addition, the richness and diversity of gut microbiota in the BBR group decreased and abundances of Bacteroidetes and Proteobacteria increased significantly, and those of Firmicutes and Cyanobacteria decreased significantly compared with those in the control group [48]. The results indicated that restoration of the intestinal barrier and gut microbiota is associated with efficacy of BBR.
Among a large number of gut bacteria species, Akkermansia has attracted the interest of more and more scholars due to its close relationship with health in animals and humans. Akkermansia, a type of bacteria, settles on the mucus layer and uses mucin as a source of nutrition [45]. Researches have disclosed that the relative abundance of Akkermansia is negatively correlated with intestinal inflammatory and metabolic diseases. In addition, Akkermansia supplementation can increase intestinal epithelial tight junction protein levels and improve the intestinal barrier [182]. Therefore, modulation of Akkermansia has attracted considerable attention for the treatment of diseases such as ulcerative colitis [183]. In HFD-induced atherosclerosis in ApoE-/- mice, BBR significantly reduced atherosclerosis in mice and increased the level of Akkermansia [45]. In addition, BBR significantly decreased HFD-induced metabolic LPS, lowered arterial and intestinal inflammatory cytokines and chemokines, increased intestinal tight junction proteins (zona occludens 1 (ZO-1) and occludin), and increased the thickness of the colonic mucus layer [45]. These results suggest that modulation of Akkermansia ameliorates atherosclerosis in mice. However, additional studies, such as fecal transplantation of Akkermansia, are required to confirm these results.
4.1.2. BBR regulates gut microbiota metabolites to improve intestinal barrier damage
SCFAs, especially butyric acid, can not only act as the main nutritional source of colon cells, but also stabilize hypoxia inducible factor (HIF) levels and promote tight junctions in intestinal epithelial cells to maintain the stability of the intestinal barrier [[184], [185], [186]]. The destruction of tight junctions between intestinal epithelial cells will help intestinal hazardous materials, such as LPS, enter the systemic circulation, and then lead to activation of the TLR/NF-κB signaling pathway and promote the release of pro-inflammatory cytokines [169]. In db/db mice, intragastric administration of BBR 136.5 mg/kg daily for 11 weeks increased the expressions of tight junction proteins (occludin and ZO-1) and alleviated the gap between intestinal epithelial cells. In addition, the content of serum LPS was reduced, the SCFA content in the feces increased, and the number of SCFA-producing bacteria (including Butyricimonas, Coprococcus, and Ruminococcus) increased [55]. The study indicated that BBR can regulate the content of SCFAs and LPS to ameliorate intestinal barrier damage. In another study, treatment with BBR can relieve collagen-induced arthritis, downregulate the diversity and richness of gut microbiota, and elevate the level of butyrate-producing bacteria. In addition, the level of the intestinal butyrate, the activity of BUT, and the intestinal expression of HIF-1α increased after BBR treatment. Furthermore, co-administration of a BUT inhibitor diminished the anti-arthritic effect of BBR [56]. These results indicate that BBR can regulate the intestinal content of SCFAs to improve the intestinal barrier and ameliorate diseases.
Other types of gut microbiota metabolites associated with the regulatory actions of BBR on the intestinal barrier are microbial BAs and tryptophan catabolites. BAs play important roles in protecting the function of the intestinal barrier by modulating the survival and death of epithelial cells, permeability of tight junctions, secretion of mucus layer, and epithelial secretion of cytokines [[109], [187]]. BA-induced activation of FXR and TGR5 can restrain the production of inflammatory cytokines such as TNF-α and IFN-γ, which is helpful for reducing inflammation and epithelial cell penetration. After oral administration, BBR can modulate lipid metabolism by regulating microbial BA metabolism and the activation of intestinal FXR [110]. Microbial tryptophan catabolites can control intestinal epithelial permeability by acting as AHR ligands that inhibit the activation of actin regulatory proteins [188]. After oral administration, BBR can improve intestinal barrier function by triggering AHR activation by microbial tryptophan catabolites [143].
4.1.3. Modulatory effects of metabolites of BBR on the intestinal barrier
In addition to gut microbiota and gut microbiota metabolites, the microbial metabolites of BBR, including berberrubine, DMB, and OBB, can also improve intestinal barrier function. OBB can improve intestinal barrier function, as evidenced by the increased length of the mouse colon, reduced inflammatory cell infiltration and intestinal tissue-related inflammatory factors (IL-6, IL-10, IL-1β, IL-17, TNF-α, and IFN-γ), and increased level of tight junction proteins (ZO-1, ZO-2, occludin, and claudin-1). In addition, the pharmacological effect of OBB was stronger than that of BBR [166]. Berberrubine can increase the level of tight junction proteins (ZO-1, ZO-2, claudin-1, and occludin) and mucins (mucin-1 and mucin-2), alleviate inflammatory cell infiltration, and reduce intestinal tissue inflammatory factors (TNF-α, IL-1β, IL-10, IL-6, IL-4, and IFN-γ) [154]. DMB can activate the NF-κB pathway to improve intestinal barrier function, and its effect shows dose-dependent tolerance [189].
4.2. P-glycoprotein
P-glycoprotein is an ATP-driven efflux pump, which is widely distributed in the large intestines, small intestines, hepatobiliary duct, and epithelial cells of the proximal tubule of the kidney. It plays an indispensable role in preventing the absorption of foreign materials into the intestine [[190], [191], [192]]. In the intestine, P-glycoprotein-mediated drug efflux is an important reason for the low bioavailability of BBR. Studies have shown that SCFAs can inhibit the activity of P-glycoprotein, and butyric acid has the strongest inhibitory effect, followed by propionic acid and acetic acid [193,194]. BAs downregulate the gene encoding P-glycoprotein and decrease the expression of P-glycoprotein [195]. Therefore, gut microbiota may regulate the absorption of BBR via the regulation of P-glycoprotein.
Previous studies have shown that P-glycoprotein inhibitors can improve the absorption of BBR and enhance its pharmacological effects. Human immunodeficiency virus protease inhibitors can inhibit intestinal P-glycoprotein activity and compete with BBR for P-glycoprotein, thus improving the intestinal absorption rate of BBR [196]. In addition, studies have shown that verapamil can increase the neuroprotective effect of BBR in rats with sporadic dementia and Alzheimer's disease induced by streptozotocin, and the mechanism is related to the inhibition of P-glycoprotein activity [197]. These results suggest that increased production of SCFAs and changes in BA profiles induced by BBR can further regulate the expression and activity of P-glycoprotein, and the intestinal absorption and pharmacological effects of BBR can eventually be altered.
5. Side effects of BBR and personalized use of BBR
The normal gut microbiota composition and metabolism are important for the maintenance of health [198]. When the ecological balance of the gut microbiota is disturbed, a series of diseases may be induced [199]. Although gut microbiota regulators can ameliorate disease by adjusting the composition and metabolism of the gut microbiota, unreasonable use of these regulators can also cause gut microbiota disorders and undesirable side effects [200]. BBR is a classic example of this sort of gut microbiota regulator. In the clinic, the main side effect of unreasonable application of BBR is diarrhea, which is similar to the side effects of antibiotics [201]. In patients with T2D, 500 mg of BBR three times a day for 13 weeks could cause multiple adverse abdominal reactions, including diarrhea, constipation, and abdominal pain [202]. In another study, BBR (200 mg/kg) administration for three weeks caused diarrhea in 62.5% of rats. In addition, changes in the metabolism and composition of gut microbiota in diarrheal rats were also detected. For example, the relative abundances of Parabacteroides, Prevotellaceae_UCG-001, and Prevotellaceae_NK3B31_group increased, the relative abundance of Christensenellaceae_R-7_group decreased, and cecal SCFA levels (acetic acid and propionic acid) were reduced. These results suggest that BBR may cause disorders in the gut microbiota and diarrhea [203]. In addition, disorders of BA metabolism can also cause diarrhea. For example, excessive BA synthesis and secretion or decreased absorption in the ileum can cause diarrhea [204]. It has been reported that BBR can cause an increase in serum BA, total BA, and primary BA content [43]. Disorders of BA metabolism may also be the mechanism by which BBR causes diarrhea; however, this mechanism needs to be further confirmed.
The composition of gut microbiota varies greatly among different persons, and this variation can lead to variations in drug toxicity and efficacy [205]. Therefore, personalized use of gut microbiota has emerged as an important way to enhance drug efficacy and reduce side effects [206,207]. Because of the common side effects, such as diarrhea and abdominal discomfort, it is necessary to use BBR personally in the clinic. Wang et al. [208] screened biomarkers for BBR use by exploring the differences in bioavailability of BBR between normal hamsters and hyperlipidemia hamsters (induced by HFD). Bioavailability of BBR in the HFD-fed hamsters, which showed higher intestinal nitroreductase activity, was higher than that in hamsters fed with normal diet. In addition, correlation analysis of clinical samples revealed a positive relationship between the activity of fecal nitroreductase and level of blood BBR. Therefore, fecal nitroreductase activity may serve as an important physiological indicator for personalized treatment of hyperlipidemia when using BBR.
6. Perspectives
6.1. The need for detection of the key gut bacteria and enzymes
Even though great achievements have been made regarding the interactions between gut microbiota and BBR, in most studies, only the associative relationships between gut microbiota species and metabolites of gut microbiota and BBR have been investigated. Specifically, the number of gut bacteria may change after BBR administration; however, not all of these bacteria might be responsible for the transformation of BBR and the production of gut microbiota metabolites such as SCFAs. To establish a causal relationship between gut microbiota and metabolites, animal models such as gnotobiotic and germ-free animals, and a variety of other methods, including in vitro and ex vivo incubation, antibiotic supplementation, and fecal microbiota transplantation should be employed. In addition to gut bacteria, the enzymes in charge of the transformation of BBR and synthesis of gut microbiota metabolites, such as BCAAs, need to be identified. In this aspect, Wang et al. [139] offered excellent examples, in which BBR ameliorates Parkinson's disease, as we have discussed in Section 2.2.5. However, the success of examples offered by Wang et al. [139] only explains a small proportion of the therapeutic effects of BBR, considering the versatility of BBR in treating diseases, and an increasing number of studies are needed to delve into the key gut bacteria and enzymes regulated by BBR when BBR is used for the treatment of other diseases such as colitis.
6.2. Separating and integrating the contributions of BBR, BBR transformed metabolites, and gut microbiota metabolites to therapeutic effects
After oral administration, three types of compounds may be absorbed: gut microbiota metabolites (such as SCFAs), BBR transformed metabolites, and BBR. All of these compounds can act on target organs, cells, and proteins to alleviate diseases such as obesity [209,210]. To predict the outcome of BBR, it is necessary to separate and integrate the contribution of each type of compound to the therapeutic effects of orally administered BBR. Nevertheless, gut microbiota and hosts are materially interlaced, and separating the contribution of each type of compound to the final therapeutic outcomes is rather challenging, considering that the gut microbiota and liver can transform BBR to the same metabolites. For example, BBR can be transformed into berberrubine, thalifendine, DMB, and jatrorrhizine in the liver [31], which are also the products of BBR when transformed by gut microbiota. To separate the contributions of the gut microbiota and liver to the production of BBR metabolites, development of new algorithms and use of gnotobiotic and germ-free animal models are necessary. Recently, Zimmermann et al. [211] offered an excellent example by separating the contribution of gut microbiota and host to the metabolism of brivudine in mice. Once the contribution of gut microbiota and hosts to the metabolism of BBR is separated, the establishment of a method to predict the therapeutic outcome of oral BBR by integrating each type of BBR metabolite to the therapeutic effects is another aim that needs to be achieved. Although no study has currently achieved this aim, we anticipate that this goal may be attained in the future with the establishment of new algorithms, animal models, organ models, and organoid models.
7. Conclusion
In recent years, gut microbiota has become a hotspot because of its role in disease development. The gut microbiota can interact with drugs, including BBR, thereby modulating their efficacy. There are two major methods by which gut microbiota and BBR directly interact with each other: BBR regulates the gut microbiota, and gut microbiota transforms BBR. Gut microbiota metabolites, such as tryptophan, and transformed BBR products, such as OBB, can directly act as targets to improve diseases. In addition to these two direct interactions, BBR may modulate the function of the intestinal barrier and activity of P-glycoprotein, two targets that can indirectly influence the development of diseases and directly influence the transportation of BBR through the body. Understanding the interactions between BBR and gut microbiota can not only explain the contradiction between the low bioavailability and conspicuous pharmacological activity of BBR, but also pave the way for further research on BBR based on gut microbiota and provide reference for clinical rational use of BBR in the treatment of diseases.
CRediT author statement
Hao Cheng: Writing - Original draft preparation; Juan Liu and Yuzhu Tan: Writing - Reviewing and Editing; Wuwen Feng: Supervision, Writing - Reviewing and Editing; Cheng Peng: Conceptualization.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 81891012, 82104409, 81891010, 81630101, and U19A2010), Science and Technology Ministry of China (Grant No.: 2108ZX09721001-008), China Postdoctoral Science Foundation (Grant No.: 2021M690490), Sichuan Science and Technology Program (Grant No.: 2021YJ0466), Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China (Grant No.: 2020BSH003), and "Xinglin Scholar" Plan of Chengdu University of Traditional Chinese Medicine (Grant No.: BSH2020017).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Wuwen Feng, Email: jiaoxiake-1@foxmail.com.
Cheng Peng, Email: pengchengcxy@126.com.
References
- 1.Neag M.A., Mocan A., Echeverría J., et al. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang Z.L., Tang Y.Q., Ying J.M., et al. Traditional Chinese medicine for anti-Alzheimer’s disease: Berberine and evodiamine from Evodia rutaecarpa. Chin. Med. 2020;15 doi: 10.1186/s13020-020-00359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., Wang F., Song Y., et al. The status of and trends in the pharmacology of berberine: A bibliometric review [1985−2018] Chin. Med. 2020;15 doi: 10.1186/s13020-020-0288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song L., Luo Y., Wang X.Y., et al. Exploring the active mechanism of berberine against HCC by systematic pharmacology and experimental validation. Mol. Med. Rep. 2019;20:4654–4664. doi: 10.3892/mmr.2019.10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M., Zhang M., Zhang Z.L., et al. Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncol. Res. 2017;25:233–239. doi: 10.3727/096504016X14742891049073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y.H., Sun W., Zhou B.J., et al. iTRAQ-based pharmacoproteomics reveals potential targets of berberine, a promising therapy for ulcerative colitis. Eur. J. Pharmacol. 2019;850:167–179. doi: 10.1016/j.ejphar.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Tan J.Q., Wang J., Yang C., et al. Antimicrobial characteristics of berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Compl. Alternative Med. 2019;19 doi: 10.1186/s12906-019-2558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao W.D., Wang X., Xiao K. Protective effect of berberine against cardiac ischemia/reperfusion injury by inhibiting apoptosis through the activation of Smad7. Mol. Cell. Probes. 2018;38:38–44. doi: 10.1016/j.mcp.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L., Cang Z., Sun H., et al. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr. Disord. 2017;17 doi: 10.1186/s12902-017-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C.-S., Zheng Y.-R., Zhang Y.-F., et al. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. doi: 10.1016/j.fitote.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Miao Y.-Q., Fan D.-J., et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12:705–711. doi: 10.1208/s12249-011-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng R., Zhao Z.-X., Ma S.-R., et al. Gut microbiota-regulated pharmacokinetics of berberine and active metabolites in beagle dogs after oral administration. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F., Wen Q., Jiang J., et al. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016;179:253–264. doi: 10.1016/j.jep.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Pascale A., Marchesi N., Marelli C., et al. Microbiota and metabolic diseases. Endocrine. 2018;61:357–371. doi: 10.1007/s12020-018-1605-5. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt B., Mulder I.E., Musk C.C., et al. Establishment of normal gut microbiota is compromised under excessive hygiene conditions. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson E., Ryan P.M., Cryan J.F., et al. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016;92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 17.Eliakim-Raz N., Bishara J. Prevention and treatment of Clostridium difficile associated diarrhea by reconstitution of the microbiota. Hum. Vaccines Immunother. 2019;15:1453–1456. doi: 10.1080/21645515.2018.1472184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X.Y., Liu X.J., Hao J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020;21:147–159. doi: 10.1111/1751-2980.12849. [DOI] [PubMed] [Google Scholar]
- 19.Weingarden A.R., Vaughn B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microb. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelucci F., Cechova K., Amlerova J., et al. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation. 2019;16 doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.H., Zhang J.M., Wang R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018;50:357–368. doi: 10.1080/03602532.2018.1497647. [DOI] [PubMed] [Google Scholar]
- 22.Swanson H.I. Drug metabolism by the host and gut microbiota: A partnership or rivalry? Drug Metab. Dispos. 2015;43:1499–1504. doi: 10.1124/dmd.115.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh K., Kang Y.R., Nepal M.R., et al. Impact of gut microbiota on drug metabolism: An update for safe and effective use of drugs. Arch. Pharm. Res. 2017;40:1345–1355. doi: 10.1007/s12272-017-0986-y. [DOI] [PubMed] [Google Scholar]
- 24.Feng W., Ao H., Peng C., et al. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharm. Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Habtemariam S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104722. [DOI] [PubMed] [Google Scholar]
- 26.Feng R., Shou J.-W., Zhao Z.-X., et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015;5 doi: 10.1038/srep12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Y., Yin Y., Li Z.R., et al. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD) Nutrients. 2019;11 doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brial F., Lay A.L., Dumas M., et al. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018;75:3977–3990. doi: 10.1007/s00018-018-2901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian Y., Cai J.W., Gui W., et al. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019;47:86–93. doi: 10.1124/dmd.118.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue S.-J., Liu J., Wang A.-T., et al. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol. Endocrinol. Metab. 2019;316:E73–E85. doi: 10.1152/ajpendo.00256.2018. [DOI] [PubMed] [Google Scholar]
- 31.Wang K., Feng X.C., Chai L.W., et al. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017;49:139–157. doi: 10.1080/03602532.2017.1306544. [DOI] [PubMed] [Google Scholar]
- 32.Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019;147 doi: 10.1016/j.phrs.2019.104367. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Yin Y.M., Wang X.Y., et al. Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 2018;20:148–162. doi: 10.1080/10286020.2017.1329300. [DOI] [PubMed] [Google Scholar]
- 35.Peng L.C., Kang S., Yin Z.Q., et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015;8:5217–5223. [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X.X., Zheng M.Y., Yi Y.L., et al. Inhibition of berberine hydrochloride on Candida albicans biofilm formation. Biotechnol. Lett. 2020;42:2263–2269. doi: 10.1007/s10529-020-02938-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X.J., Sun X.Y., Wu J.X., et al. Berberine damages the cell surface of methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budeyri Gokgoz N., Avci F.G., Yoneten K.K., et al. Response of Escherichia coli to prolonged berberine exposure. Microb. Drug Resist. 2017;23:531–544. doi: 10.1089/mdr.2016.0063. [DOI] [PubMed] [Google Scholar]
- 39.Wultańska D., Piotrowski M., Pituch H. The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1391–1399. doi: 10.1007/s10096-020-03857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S., Li Z.W., Yin Z.Q., et al. The antibacterial mechanism of berberine against Actinobacillus pleuropneumoniae. Nat. Prod. Res. 2015;29:2203–2206. doi: 10.1080/14786419.2014.1001388. [DOI] [PubMed] [Google Scholar]
- 41.Kong W.J., Xing X.Y., Xiao X.H., et al. Effect of berberine on Escherichia coli, Bacillus subtilis, and their mixtures as determined by isothermal microcalorimetry. Appl. Microbiol. Biotechnol. 2012;96:503–510. doi: 10.1007/s00253-012-4302-y. [DOI] [PubMed] [Google Scholar]
- 42.Pan H.J., Li Z.F., Xie J., et al. Berberine influences blood glucose via modulating the gut microbiome in grass carp. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y., Zhang Y.C., Huang W.H., et al. Dose-response effect of berberine on bile acid profile and gut microbiota in mice. BMC Compl. Alternative Med. 2016;16 doi: 10.1186/s12906-016-1367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D., Zhang Y.Y., Liu Y.H., et al. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes. 2018;126:513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- 45.Zhu L., Zhang D.Y., Zhu H., et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in ApoE-/- mice. Atherosclerosis. 2018;268:117–126. doi: 10.1016/j.atherosclerosis.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y.F., Hu J.X., Geng J., et al. Berberine treatment reduces atherosclerosis by mediating gut microbiota in ApoE-/- mice. Biomed. Pharmacother. 2018;107:1556–1563. doi: 10.1016/j.biopha.2018.08.148. [DOI] [PubMed] [Google Scholar]
- 47.Wu M., Yang S.J., Wang S.Z., et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y.Z., Zheng J.M., Hou H.T., et al. Effects of berberine on intestinal flora of non-alcoholic fatty liver induced by high-fat diet through 16S rRNA gene segmentation. J. King Saud Univ. Sci. 2020;32:2603–2609. [Google Scholar]
- 49.Li D.H., Zheng J.M., Hu Y.T., et al. Amelioration of intestinal barrier dysfunction by berberine in the treatment of nonalcoholic fatty liver disease in rats. Pharmacogn. Mag. 2017;13:677–682. doi: 10.4103/pm.pm_584_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao Z.Q., Xie Y.Z., Zhou B.J., et al. Berberine ameliorates colonic damage accompanied with the modulation of dysfunctional bacteria and functions in ulcerative colitis rats. Appl. Microbiol. Biotechnol. 2020;104:1737–1749. doi: 10.1007/s00253-019-10307-1. [DOI] [PubMed] [Google Scholar]
- 51.Cui H.T., Cai Y.Z., Wang L., et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H.T., Zhang F., Zhang J., et al. A holistic view of berberine inhibiting intestinal carcinogenesis in conventional mice based on microbiome-metabolomics analysis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.588079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., Shou J.W., Li X.Y., et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism. 2017;70:72–84. doi: 10.1016/j.metabol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Li C.N., Wang X., Lei L., et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020;34:1166–1174. doi: 10.1002/ptr.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W., Xu J.-H., Yu T., et al. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109131. [DOI] [PubMed] [Google Scholar]
- 56.Yue M.F., Tao Y., Fang Y.L., et al. The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. Faseb. J. 2019;33:12311–12323. doi: 10.1096/fj.201900425RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia X., Jia L., Mo L., et al. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J. Dent. Res. 2019;98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X., Zhao Y.F., Zhang M.H., et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du Z., Wang Q., Huang X., et al. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int. Immunopharm. 2020;81 doi: 10.1016/j.intimp.2020.106270. [DOI] [PubMed] [Google Scholar]
- 60.Li S., Wang N., Tan H.-Y., et al. Modulation of gut microbiota mediates berberine-induced expansion of immuno-suppressive cells to against alcoholic liver disease. Clin. Transl. Med. 2020;10 doi: 10.1002/ctm2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Y., Chen H., Yan L., et al. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110669. [DOI] [PubMed] [Google Scholar]
- 62.Jia Q., Zhang L., Zhang J., et al. Fecal microbiota of diarrhea-predominant irritable bowel syndrome patients causes hepatic inflammation of germ-free rats and berberine reverses it partially. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4530203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun H.L., Wang N.J., Cang Z., et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes. Facts. 2016;9:365–378. doi: 10.1159/000449507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J.H., Liu X.Z., Pan W., et al. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017;15:2765–2787. doi: 10.3892/mmr.2017.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X., Zhao Y.F., Xu J., et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015;5 doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li M., Shu X., Xu H., et al. Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 2016;14 doi: 10.1186/s12967-016-0987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H., Zhang F., Li R., et al. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109829. [DOI] [PubMed] [Google Scholar]
- 68.Cao W., Hu L., Chen H., et al. Berberine alleviates chronic inflammation of mouse model of type 2 diabetes by adjusting intestinal microbes and inhibiting TLR4 signaling pathway. Int. J. Clin. Exp. Med. 2017;10:10267–10276. [Google Scholar]
- 69.Cui H.-X., Hu Y.-N., Li J.-W., et al. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/8930374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H., Guan L., Li J., et al. The effects of berberine on the gut microbiota in Apc min/+ mice fed with a high fat diet. Molecules. 2018;23 doi: 10.3390/molecules23092298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng Q., Chen W.-D., Wang Y.-D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers E.S., Preston T., Frost G., et al. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canfora E.E., Meex R.C.R., Venema K., et al. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Z.H., Lai J.K.L., Qiao L., et al. Role of gut microbial metabolites in nonalcoholic fatty liver disease. J. Dig. Dis. 2019;20:181–188. doi: 10.1111/1751-2980.12709. [DOI] [PubMed] [Google Scholar]
- 75.Doifode T., Giridharan V.V., Generoso J.S., et al. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2020;24 doi: 10.1016/j.phrs.2020.105314. [DOI] [PubMed] [Google Scholar]
- 76.Hernández M.A.G., Canfora E.E., Jocken J.W.E., et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11 doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sittipo P., Shim J.-W., Lee Y.K. Microbial metabolites determine host health and the status of some diseases. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20215296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atherosclerosis Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu J.M., Lin S.L., Zheng B.D., et al. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018;58:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 81.Tan J., McKenzie C., Potamitis M., et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 82.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. 2012;3 doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng W., Ao H., Peng C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falkenberg K.J., Johnstone R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 85.Alavi M.S., Shamsizadeh A., Azhdari-Zarmehri H., et al. Orphan G protein-coupled receptors: The role in CNS disorders. Biomed. Pharmacother. 2018;98:222–232. doi: 10.1016/j.biopha.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 86.Recio C., Lucy D., Iveson P., et al. The role of metabolite-sensing G protein-coupled receptors in inflammation and metabolic disease. Antioxidants Redox Signal. 2018;29:237–256. doi: 10.1089/ars.2017.7168. [DOI] [PubMed] [Google Scholar]
- 87.Wang J., Gareri C., Rockman A.H. G-protein-coupled receptors in heart disease. Circ. Res. 2018;123:716–735. doi: 10.1161/CIRCRESAHA.118.311403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melbye P., Olsson A., Hansen T.H., et al. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2019;139:208–219. doi: 10.1111/ane.13045. [DOI] [PubMed] [Google Scholar]
- 89.Verhaar B.J.H., Prodan A., Nieuwdorp M., et al. Gut microbiota in hypertension and atherosclerosis: A review. Nutrients. 2020;12 doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang G., Yu Y., Wang Y.Z., et al. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J. Cell. Physiol. 2019;234:17023–17049. doi: 10.1002/jcp.28436. [DOI] [PubMed] [Google Scholar]
- 91.Venegas D.P., De la Fuente M.K., Landskron G., et al. Corrigendum: Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mandaliya D.K., Seshadri S. Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology. 2019;19:280–284. doi: 10.1016/j.pan.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 93.Xiao S., Zhang Z., Chen M., et al. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J. Ethnopharmacol. 2019;241 doi: 10.1016/j.jep.2019.112032. [DOI] [PubMed] [Google Scholar]
- 94.Khan I., Pathan S., Li X.A., et al. Far infrared radiation induces changes in gut microbiota and activates GPCRs in mice. J. Adv. Res. 2019;22:145–152. doi: 10.1016/j.jare.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vital M., Howe A.C., Tiedje J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5 doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun M., Wu W., Liu Z., et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L.L., Guo H.H., Huang S., et al. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J. Chromatogr. B. 2017;1057:70–80. doi: 10.1016/j.jchromb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 98.Marin J.J.G., Macias R.I., Briz O., et al. Bile acids in physiology, pathology and pharmacology. Curr. Drug Metabol. 2015;17:4–29. doi: 10.2174/1389200216666151103115454. [DOI] [PubMed] [Google Scholar]
- 99.Long S.L., Gahan C.G.M., Joyce S.A. Interactions between gut bacteria and bile in health and disease. Mol. Aspect. Med. 2017;56:54–65. doi: 10.1016/j.mam.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 100.Enright E.F., Griffin B.T., Gahan C.G.M., et al. Microbiome-mediated bile acid modification: Role in intestinal drug absorption and metabolism. Pharmacol. Res. 2018;133:170–186. doi: 10.1016/j.phrs.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Ridlon J.M., Kang D.J., Hylemon P.B., et al. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doerner K.C., Takamine F., LaVoie C.P., et al. Assessment of fecal bacteria with bile acid 7 alpha-dehydroxylating activity for the presence of Bai-like genes. Appl. Environ. Microbiol. 1997;63:1185–1188. doi: 10.1128/aem.63.3.1185-1188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chow M.D., Lee Y.H., Guo G.L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Aspect. Med. 2017;56:34–44. doi: 10.1016/j.mam.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang C., Zhu C., Shao L., et al. Role of bile acids in dysbiosis and treatment of nonalcoholic fatty liver disease. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/7659509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y., Tang R.Q., Leung P.S.C., et al. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017;16:885–896. doi: 10.1016/j.autrev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 106.González-Regueiro J.A., Moreno-Castañeda L., Uribe M., et al. The role of bile acids in glucose metabolism and their relation with diabetes. Ann. Hepatol. 2017;16:16–21. doi: 10.5604/01.3001.0010.5672. [DOI] [PubMed] [Google Scholar]
- 107.Martinot E., Sèdes L., Baptissart M., et al. Bile acids and their receptors. Mol. Aspect. Med. 2017;56:2–9. doi: 10.1016/j.mam.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Chiang J.Y. Bile acid metabolism and signaling. Comp. Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fiorucci S., Biagioli M., Zampella A., et al. Bile acids activated receptors regulate innate immunity. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun R., Yang N., Kong B., et al. Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Mol. Pharmacol. 2017;91:110–122. doi: 10.1124/mol.116.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Velasquez M.T., Ramezani A., Manal A., et al. Trimethylamine N-oxide: The good, the bad and the unknown. Toxins. 2016;8 doi: 10.3390/toxins8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rath S., Heidrich B., Pieper D.H., et al. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5 doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chhibber-Goel J., Gaur A., Singhal V., et al. The complex metabolism of trimethylamine in humans: Endogenous and exogenous sources. Expert Rev. Mol. Med. 2016;18 doi: 10.1017/erm.2016.6. [DOI] [PubMed] [Google Scholar]
- 114.Fennema D., Phillips I.R., Shephard E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016;44:1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Subramaniam S., Fletcher C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018;175:1344–1353. doi: 10.1111/bph.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilson A., McLean C., Kim R.B. Trimethylamine-N-oxide: A link between the gut microbiome, bile acid metabolism, and atherosclerosis. Curr. Opin. Lipidol. 2016;27:148–154. doi: 10.1097/MOL.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 117.Din A.U., Hassan A., Zhu Y., et al. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl. Microbiol. Biotechnol. 2019;103:9217–9228. doi: 10.1007/s00253-019-10142-4. [DOI] [PubMed] [Google Scholar]
- 118.DiNicolantonio J.J., McCarty M., OKeefe J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart. 2019;6 doi: 10.1136/openhrt-2018-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan C.W.H., Law B.M.H., Waye M.M.Y., et al. Trimethylamine-N-oxide as one hypothetical link for the relationship between intestinal microbiota and cancer - where we are and where shall we go? J. Cancer. 2019;10:5874–5882. doi: 10.7150/jca.31737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oellgaard J., Winther S.A., Hansen T.S., et al. Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr. Pharm. Des. 2017;23:3699–3712. doi: 10.2174/1381612823666170622095324. [DOI] [PubMed] [Google Scholar]
- 121.Li Z., Wu Z., Yan J., et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Invest. 2019;99:346–357. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 122.Li X., Geng J., Zhao J., et al. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X., Jiao X., Ma Y., et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 124.Chen M.-L., Zhu X.-H., Ran L., et al. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart. Assoc. 2017;6 doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boini K.M., Hussain T., Li P.-L., et al. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 2017;44:152–162. doi: 10.1159/000484623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Z., Huang S., Zou D.Y., et al. Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice. Amino Acids. 2016;48:2731–2745. doi: 10.1007/s00726-016-2308-y. [DOI] [PubMed] [Google Scholar]
- 127.Franco T.M.A., Blanchard J.S. Bacterial branched-chain amino acid biosynthesis: Structures, mechanisms, and drugability. Biochemistry. 2017;56:5849–5865. doi: 10.1021/acs.biochem.7b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 129.Neis E.P., Dejong C.H., Rensen S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7:2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai Z.-L., Wu G.Y., Zhu W.-Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. (Landmark Ed). 2011;16:1768–1786. doi: 10.2741/3820. [DOI] [PubMed] [Google Scholar]
- 131.Hwang S.-K., Kim H.-H. The functions of mTOR in ischemic diseases. BMB Rep. 2011;44:506–511. doi: 10.5483/bmbrep.2011.44.8.506. [DOI] [PubMed] [Google Scholar]
- 132.Hua H., Kong Q., Zhang H., et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019;12 doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoon M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8 doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blomstrand E., Eliasson J., Karlsson H.K., et al. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006;136:269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- 135.Shimomura Y., Kitaura Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018;133:215–217. doi: 10.1016/j.phrs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 136.Berke J.D. What does dopamine mean? Nat. Neurosci. 2018;21:787–793. doi: 10.1038/s41593-018-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moreira da Silva Santos A., Kelly J.P., Dockery P., et al. Effect of a binge-like dosing regimen of methamphetamine on dopamine levels and tyrosine hydroxylase expressing neurons in the rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:303–309. doi: 10.1016/j.pnpbp.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 138.Belik J., Shifrin Y., Arning E., et al. Corrigendum: Intestinal microbiota as a tetrahydrobiopterin exogenous source in hph-1 mice. Sci. Rep. 2017;7 doi: 10.1038/srep39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y., Tong Q., Ma S.-R., et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct Target. Ther. 2021;6 doi: 10.1038/s41392-020-00456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Modoux M., Rolhion N., Mani S., et al. Tryptophan metabolism as a pharmacological target. Trends Pharmacol. Sci. 2021;42:60–73. doi: 10.1016/j.tips.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 141.Zhang J., Zhu S., Ma N., et al. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021;41:1061–1088. doi: 10.1002/med.21752. [DOI] [PubMed] [Google Scholar]
- 142.Jing W., Dong S., Luo X., et al. Berberine improves colitis by triggering AHR activation by microbial tryptophan catabolites. Pharmacol. Res. 2021;64 doi: 10.1016/j.phrs.2020.105358. [DOI] [PubMed] [Google Scholar]
- 143.Di Lorenzo F., De Castro C., Silipo A., et al. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019;43:257–272. doi: 10.1093/femsre/fuz002. [DOI] [PubMed] [Google Scholar]
- 144.Soares J.B., Pimentel-Nunes P., Roncon-Albuquerque R., et al. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hersoug L.G., Møller P., Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016;17:297–312. doi: 10.1111/obr.12370. [DOI] [PubMed] [Google Scholar]
- 146.Bianconi E., Piovesan A., Facchin F., et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013;40:463–471. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 147.Lozupone C.A., Stombaugh J.I., Gordon J.I., et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morgan X.C., Segata N., Huttenhower C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013;29:51–58. doi: 10.1016/j.tig.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan C., Guo Q., Lu N. Role of gut microbiota in the pharmacological effects of natural products. Evid. Based Complement. Alternat. Med. 2019;2019 doi: 10.1155/2019/2682748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan L., Wang Y., Ai G., et al. Dihydroberberine, a hydrogenated derivative of berberine firstly identified in Phellodendri Chinese Cortex, exerts anti-inflammatory effect via dual modulation of NF-κB and MAPK signaling pathways. Int. Immunopharm. 2019;75 doi: 10.1016/j.intimp.2019.105802. [DOI] [PubMed] [Google Scholar]
- 151.Bryant C., Hubbard L., McElroy W.D. Cloning, nucleotide sequence, and expression of the nitroreductase gene from Enterobacter cloacae. J. Biol. Chem. 1991;266:4126–4130. [PubMed] [Google Scholar]
- 152.Song J., Zhang S., Lu L. Fungal cytochrome P450 protein cyp51: What we can learn from its evolution, regulons and cyp51-based azole resistance. Fungal Biol. Rev. 2018;32:131–142. [Google Scholar]
- 153.Zhang Z.-W., Cong L., Peng R., et al. Transformation of berberine to its demethylated metabolites by the CYP51 enzyme in the gut microbiota. J. Pharm. Anal. 2021;11:628–637. doi: 10.1016/j.jpha.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu X.-T., Xu Y.-F., Huang Y.-F., et al. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu L., Gu P., Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharm. 2019;68:242–251. doi: 10.1016/j.intimp.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 156.Zhao Z., Wei Q., Hua W., et al. Hepatoprotective effects of berberine on acetaminophen-induced hepatotoxicity in mice. Biomed. Pharmacother. 2018;103:1319–1326. doi: 10.1016/j.biopha.2018.04.175. [DOI] [PubMed] [Google Scholar]
- 157.Abd El-Salama M., Mekky H., El-Naggar E.M.B., et al. Hepatoprotective properties and biotransformation of berberine and berberrubine by cell suspension cultures of dodonaea viscosa and ocimum basilicum. South Afr. J. Bot. 2015;97:191–195. [Google Scholar]
- 158.Liu Y.T., Hao H.P., Xie H.G., et al. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010;38:1779–1784. doi: 10.1124/dmd.110.033936. [DOI] [PubMed] [Google Scholar]
- 159.Wang X., Wang S., Ma J., et al. Pharmacokinetics in rats and tissue distribution in mouse of berberrubine by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2015;115:368–374. doi: 10.1016/j.jpba.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 160.Wu H., He K., Wang Y., et al. The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma Coptidis. Phytomedicine. 2014;21:1373–1381. doi: 10.1016/j.phymed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 161.Wang P., Gao X.Y., Yang S.Q., et al. Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Drug Des. Dev. Ther. 2019;13:2235–2247. doi: 10.2147/DDDT.S207315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hallajzadeh J., Maleki Dana P., Mobini M., et al. Targeting of oncogenic signaling pathways by berberine for treatment of colorectal cancer. Med. Oncol. 2020;37 doi: 10.1007/s12032-020-01367-9. [DOI] [PubMed] [Google Scholar]
- 163.Dong S.F., Hong Y., Liu M., et al. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur. J. Pharmacol. 2011;660:368–374. doi: 10.1016/j.ejphar.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 164.Shi R., Zhou H., Ma B., et al. Pharmacokinetics and metabolism of jatrorrhizine, a gastric prokinetic drug candidate. Biopharm Drug Dispos. 2012;33:135–145. doi: 10.1002/bdd.1779. [DOI] [PubMed] [Google Scholar]
- 165.Zhang M.Y., Yu Y.Y., Wang S.F., et al. Cardiotoxicity evaluation of nine alkaloids from Rhizoma Coptis. Hum. Exp. Toxicol. 2018;37:185–195. doi: 10.1177/0960327117695633. [DOI] [PubMed] [Google Scholar]
- 166.Li C., Ai G., Wang Y., et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020;152 doi: 10.1016/j.phrs.2019.104603. [DOI] [PubMed] [Google Scholar]
- 167.Dou Y., Huang R., Li Q., et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111312. [DOI] [PubMed] [Google Scholar]
- 168.Li C.L., Tan L.H., Wang Y.F., et al. Comparison of anti-inflammatory effects of berberine, and its natural oxidative and reduced derivatives from Rhizoma Coptidis in vitro and in vivo. Phytomedicine. 2019;52:272–283. doi: 10.1016/j.phymed.2018.09.228. [DOI] [PubMed] [Google Scholar]
- 169.Dey P. Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105135. [DOI] [PubMed] [Google Scholar]
- 170.Heinemann U., Schuetz A. Structural features of tight-junction proteins. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20236020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Slifer Z.M., Blikslager A.T. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bischoff S.C., Barbara G., Buurman W., et al. Intestinal permeability − a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14 doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schüppel V., Haller D. Intestinal microbiome in chronic diseases: Relevance of gut bacteria in inflammatory bowel diseases and metabolic disorders. Diabetologe. 2016;12:420–427. [Google Scholar]
- 174.Julio-Pieper M., Bravo J.A., Aliaga E., et al. Review article: Intestinal barrier dysfunction and central nervous system disorders – a controversial association. Aliment. Pharmacol. Ther. 2014;40:1187–1201. doi: 10.1111/apt.12950. [DOI] [PubMed] [Google Scholar]
- 175.Matuchansky C. Food and intestinal barrier function in irritable bowel syndrome. Neurogastroenterol. Motil. 2012;24 doi: 10.1111/j.1365-2982.2012.01978.x. [DOI] [PubMed] [Google Scholar]
- 176.Michielan A., D’Incà R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rogler G., Rosano G. The heart and the gut. Eur. Heart J. 2014;35:426–430. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 178.Meijers B., Farré R., Dejongh S., et al. Intestinal barrier function in chronic kidney disease. Toxins. 2018;10 doi: 10.3390/toxins10070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sharmila G.R., Venkateswaran G. Bacillus subtilis CFR5 isolated from fermented soybean attenuates the chronic pancreatitis. J. Funct. Foods. 2018;40:197–206. [Google Scholar]
- 180.Klein G.L., Petschow B.W., Shaw A.L., et al. Gut barrier dysfunction and microbial translocation in cancer cachexia: A new therapeutic target. Curr. Opin. Support. Palliat. Care. 2013;7:361–367. doi: 10.1097/SPC.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Natividad J.M., Verdu E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 182.Li J., Lin S., Vanhoutte P.M., et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in ApoE-/- mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 183.Bian X., Wu W., Yang L., et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Donohoe D.R., Garge N., Zhang X., et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kelly C.J., Zheng L., Campbell E.L., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu W., Luo X., Tang J., et al. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: By changing gut barrier. Eur. J. Nutr. 2020;60:2317–2330. doi: 10.1007/s00394-020-02431-w. [DOI] [PubMed] [Google Scholar]
- 187.Hegyi P., Maléth J., Walters J.R., et al. Guts and gall: Bile acids in regulation of intestinal epithelial function in health and disease. Physiol. Rev. 2018;98:1983–2023. doi: 10.1152/physrev.00054.2017. [DOI] [PubMed] [Google Scholar]
- 188.Scott S.A., Fu J., Chang P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U S A. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen Y.-Y., Li R.-Y., Shi M.-J., et al. Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-κB signaling and T-helper cell homeostasis. Inflamm. Res. 2017;66:187–196. doi: 10.1007/s00011-016-1005-3. [DOI] [PubMed] [Google Scholar]
- 190.Mollazadeh S., Sahebkar A., Hadizadeh F., et al. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018;214:118–123. doi: 10.1016/j.lfs.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 191.Silva R., Vilas-Boas V., Carmo H., et al. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015;149:1–123. doi: 10.1016/j.pharmthera.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 192.Sharom F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 193.Xie Q.-S., Zhang J.-X., Liu M., et al. Short-chain fatty acids exert opposite effects on the expression and function of P-glycoprotein and breast cancer resistance protein in rat intestine. Acta Pharmacol. Sin. 2021;42:470–481. doi: 10.1038/s41401-020-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang J., Xie Q., Kong W., et al. Short-chain fatty acids oppositely altered expressions and functions of intestinal cytochrome P4503A and P-glycoprotein and affected pharmacokinetics of verapamil following oral administration to rats. J. Pharm. Pharmacol. 2020;72:448–460. doi: 10.1111/jphp.13215. [DOI] [PubMed] [Google Scholar]
- 195.Mazzanti R., Fantappié O., Kamimoto Y., et al. Bile acid inhibition of P-glycoprotein-mediated transport in multidrug-resistant cells and rat liver canalicular membrane vesicles. Hepatology. 1994;20:170–176. doi: 10.1016/0270-9139(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 196.Zha W., Wang G., Xu W., et al. Inhibition of P-glycoprotein by HIV protease inhibitors increases intracellular accumulation of berberine in murine and human macrophages. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kumar A., Ekavali, Mishra J., et al. Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacology (Berl) 2016;233:137–152. doi: 10.1007/s00213-015-4095-7. [DOI] [PubMed] [Google Scholar]
- 198.Jandhyala S.M., Talukdar R., Subramanyam C., et al. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gagnière J., Raisch J., Veziant J., et al. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016;22:501–518. doi: 10.3748/wjg.v22.i2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lange K., Buerger M., Stallmach A., et al. Effects of antibiotics on gut microbiota. Dig. Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 201.Ramirez J., Guarner F., Bustos Fernandez L., et al. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yin J., Xing H., Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yue S.-J., Liu J., Wang W.-X., et al. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.109002. [DOI] [PubMed] [Google Scholar]
- 204.Wong B.S., Camilleri M., Carlson P., et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 2012;10 doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Börnigen D., Morgan X.C., Franzosa E.A., et al. Functional profiling of the gut microbiome in disease-associated inflammation. Genome Med. 2013;5 doi: 10.1186/gm469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Feng W., Liu J., Ao H., et al. Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs. Theranostics. 2020;10:11278–11301. doi: 10.7150/thno.47289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dey P. The pharmaco-toxicological conundrum of oleander: Potential role of gut microbiome. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110422. [DOI] [PubMed] [Google Scholar]
- 208.Wang Y., Tong Q., Shou J.W., et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics. 2017;7:2443–2451. doi: 10.7150/thno.18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang Z., Zhang H., Li B., et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014;5 doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 210.Turner N., Li J.-Y., Gosby A., et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 211.Zimmermann M., Zimmermann-Kogadeeva M., Wegmann R., et al. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science. 2019;363 doi: 10.1126/science.aat9931. [DOI] [PMC free article] [PubMed] [Google Scholar]