Abstract
Purpose
This study aimed at evaluating the effectiveness of a nutritional counselling intervention based on encouraging the consumption of unprocessed and minimally processed foods, rather than ultra-processed products, and the practice of physical activities to prevent excessive gestational weight gain in overweight pregnant women.
Methods
This was a two-armed, parallel, randomized controlled trial conducted in primary health units of a Brazilian municipality from 2018 to 2021. Overweight, adult pregnant women (n = 350) were randomly assigned to control (CG) or intervention groups (IG). The intervention consisted of three individualized nutritional counselling sessions based on encouraging the consumption of unprocessed and minimally processed foods rather than ultra-processed products, following the NOVA food classification system, and the practice of physical activities. The primary outcome was the proportion of women whose weekly gestational weight gain (GWG) exceeded the Institute of Medicine guidelines. Adjusted logistic regression models were employed.
Results
Complete data on weight gain were available for 121 women of the IG and 139 of the CG. In modified intention-to-treat analysis, there was a lower chance of the IG women having excessive GWG [OR 0.56 (95% CI 0.32, 0.98), p = .04], when compared to the CG. No between-group differences were observed for the other maternal outcomes investigated.
Conclusion
The present study was unprecedented in demonstrating that nutritional counselling based on the NOVA food classification system, together with encouraging the practice of physical activity, is effective in preventing excessive weight gain in overweight pregnant women.
Trial registration
Registered on July 30th 2018 at Brazilian Registry of Clinical Trials (RBR-2w9bhc).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-02995-9.
Keywords: Food processing, NOVA, Randomized controlled trial, Nutritional counselling, Overweight, Gestational weight gain
Introduction
The high prevalence of excess body weight in women of reproductive age is an alarming public health problem and may impact the health of the following two generations [1, 2]. Excessive gestational weight gain exposes the woman and the child to a higher risk of harmful health outcomes in the short and long term [3–6]. Therefore, sustainable and effective life-cycle interventions are of utmost relevance [1].
In a meta-analysis of 68 randomized controlled trials of nutritional interventions to prevent excessive gestational weight gain (GWG), a lower risk of excessive GWG in women of the intervention groups (IG) was found [RR 0.83 (95% CI 0.77, 0.89)]. However, limited efficacy was observed among those that are overweight before pregnancy [RR 0.91 (95% CI 0.78, 1.01)] [7]. The authors concluded that the effects on adequate weight gain are more pronounced for high intensity interventions [7], which might not be feasible to implement in developing countries, given the limited resources available, especially in public health systems. The number of studies conducted in less developed countries is considered insufficient, and the effect of lifestyle interventions adapted to the usual conditions of public health systems in these countries is unknown [7, 8].
In addition to the limited efficacy of lifestyle interventions conducted among overweight pregnant women [7], observational studies highlight the higher risk of excessive GWG among this specific group of women [9–11]. In Brazil, it is estimated that 33% of adult women are overweight [12]. Accordingly, intervention studies explicitly conducted for this group of women are needed.
Evidence suggests that the degree of industrial food processing plays a relevant role in the relationship between dietary intake and health outcomes [13–16]. Dietary patterns based on the substitution of meals made with unprocessed or minimally processed foods for the consumption of ultra-processed items can be partly blamed for the exponential global growth in the incidence of obesity [13–16].
Unprocessed foods, according to the NOVA (which is not an acronym) food classification system, are foods that have not been industrially processed, such as fresh fruits, beans, and fresh meats. Coffee, natural fruit juices, and pasteurized whole milk are examples of minimally processed foods that have been industrially processed but have not had any compounds added or ingredients removed. Soft drinks, sugar-sweetened beverages, crackers, biscuits, instant noodles and flavoured yogurts are examples of ultra-processed items created by the food industry containing additives [13, 14].
Observational studies suggest that a higher consumption of ultra-processed products during pregnancy is directly related to obesity [17], higher gestational weight gain [18–20], postpartum weight retention [20], and greater neonatal adiposity [18]. In addition to the implications for human health, the high consumption of ultra-processed products has a detrimental impact on the environment [14, 21], and distorts countries’ food cultures, which were originally based on freshly prepared dishes and meals using unprocessed and minimally processed foods [14]. However, no previous clinical trials investigating the effect of this strategy on GWG were found.
Evidence also suggests that the regular practice of physical activity might reduce maternal GWG. Data from a meta-analysis of 23 randomized controlled trials showed that physical activity interventions in pregnant women are effective in reducing the risk of excessive GWG, especially for those that exercise three times a week for 30–45 min [22].
The present study aimed to evaluate the effectiveness of a nutritional intervention based on encouraging the consumption of unprocessed and minimally processed foods rather than ultra-processed products, following the NOVA food classification system, together with the practice of physical activities, in preventing excessive weight gain in overweight, adult pregnant women receiving prenatal care in primary healthcare units of the public health system of a Brazilian city. The primary outcome was the proportion of women whose weekly GWG exceeded the Institute of Medicine (IoM) guidelines [23]. Additionally, the effect of the intervention on other maternal outcomes [gestational hypertension, gestational diabetes mellitus (GDM), preeclampsia, premature birth, and type of delivery] was also investigated.
Materials and methods
Study setting
This was a two-armed parallel randomized controlled trial conducted among overweight, pregnant women receiving prenatal care in seven primary health units of Ribeirão Preto, São Paulo state, Brazil, described in detail in the publication of Sartorelli et al. [24].
The potentially eligible women were interviewed by trained nutritionists at the time of the prenatal consultation. Women aged ≥ 18 years, with pre-gestational body mass index (BMI) between 25.0 and 29.9 kg/m2 and gestational age up to 15 weeks and 6 days were included. Those who reported previous diabetes or the current use of weight loss medications were excluded. The participants agreed to participate in the study by signing the consent form and were randomly allocated into the IG or control group (CG).
Interventions
The women allocated into the IG, in addition to the usual prenatal care, were invited to participate in three individualized nutritional counselling sessions conducted by trained nutritionists. The counselling sessions were planned to be all through face-to-face interviews. However, as a result of the social isolation imposed by the COVID-19 pandemic, face-to-face interviews were suspended between March and October 2020. The women who had already been included in the study at this time received nutritional counselling through video conversations.
Each session lasted approximately 30 min. In the first meeting of the intervention, the pregnant women were informed about the goals of the nutritional strategy (appropriate weight gain, daily consumption of unprocessed and minimally processed foods, including fruits and vegetables, and regular practice of 150 min of physical activities per week, avoiding the intake of ultra-processed products), adopting the recommendations of the Institute of Medicine [23], Food Guide for the Brazilian Population [25], and American College of Obstetricians and Gynecologists [26] as the theoretical reference.
Educational material, consisting of three folders, one for each meeting, with key messages and illustrative images related to the goals set, was used for the nutritional intervention strategy. All topics of the intervention were addressed in the three meetings, although different approaches were employed, appropriate for the gestational evolution. The folders were developed by the nutritionists of the research team and contained six key messages and photos. For the semantic validation of the material, focus groups were conducted with pregnant women and nutritionists with professional experience in nutritional counselling [27]. The folders are presented in supplementary material (in Portuguese).
The original study protocol [24] predicted that nutritional counselling sessions would take place at predetermined gestation periods: with the first meeting being conducted up to the 19th gestational week (GW), the second meeting between the 20th and 26th GW, and the third meeting between the 27th and 33rd GW. However, due to the high rate of absenteeism observed in the prenatal consultations of the women included in the study, it was decided to make the intervention period more flexible, maintaining the three sessions between the first and second study evaluations.
The women allocated into the CG received only the usual prenatal care. The routine prenatal care in the city includes only one nutritional counselling section with a nurse in the health care unit, which consists of the evaluation of the nutritional status, orientations about healthy weight gain, detection of possible dietary inadequacies, and clarification of misconceptions. After the birth, the participants of both groups received standardized nutritional counselling to assist them in recovering their pre-gestational weight.
Outcomes
The primary outcome was the proportion of women whose weekly GWG exceeded IoM guidelines [23]. Secondary outcomes included total and mean weekly GWG, and the maternal outcomes: gestational hypertension, GDM, preeclampsia, premature birth, and mode of delivery.
Weight measurements were performed by trained nutritionists during the first and second study evaluations using a portable digital scale (Tanita®, model HS 302). Secondary data regarding weight at each prenatal consultation were obtained through medical records. In a sample of 261 women with weight measurements performed by the research team and by the heath care unit on the same day, the Spearman correlation coefficient was of 0.99, p ≤ 0.001 (data not shown).
For the study inclusion criteria of being overweight in the pre-pregnancy period, the measurements made up to the 13th gestational week (GW) were considered to represent the pre-gestational weight. For the women included in the study in the 14th GW, a value of 0.45 kg was subtracted from the measured weight and for the women in the 15th GW a value of 0.91 kg was subtracted from their measured weight to estimate the pre-gestational BMI, similar to previous trials [28].
The weight measured in the second study evaluation, preferably between the 34th and 36th GW, and the first weight measured during the pregnancy were considered for the calculation of total GWG. The first weight measurement during the second trimester of pregnancy (≥ 12 GW) and the second between the 34th and 36th GW, divided by the number of weeks between the evaluations was used to estimate the rate of weight gain. If a weight measurement between the 34th and 36th GW was not available, the last weight measurement at the prenatal consultation prior to 36 GW was used.
Secondary data regarding blood pressure at each prenatal consultation were obtained through medical records. Women who self-reported previous hypertension, using antihypertensive medication or had blood pressure values greater than 140/90 mmHg at the first prenatal consultation were considered as having pre-gestational hypertension. Those who presented changes in blood pressure to above 140/90 mmHg or the use of hypertensive drugs in the following consultations were classified as having gestational hypertension [29].
Data on glucose levels at the oral glucose tolerance test, duration of the pregnancy, medical diagnosis of preeclampsia, and type of delivery were obtained through medical records. The World Health Organization criteria was adopted for the diagnosis of GDM [30], and births at less than 37 weeks of gestation were classified as preterm.
To evaluate compliance with the counselling recommendations, the women responded to a questionnaire on the frequency of the weekly consumption of foods of interest in the first and second evaluations of the study. The instrument used was adapted from the Surveillance System for Risk and Protective Factors for Chronic Diseases by Telephone Survey (VIGITEL) [31], previously validated for the Brazilian adult population [32].
The practice of walking for leisure, and physical exercise were assessed in the first and second study evaluations using a questionnaire that included information on performance and duration in the week prior to the interview, according to the questionnaire adapted from the VIGITEL, which was validated for the Brazilian adult population. [31, 33].
Covariates
Data on age, educational level of the women and head of the family, possession of items, employment at the moment of randomization, self-reported skin colour, marital status, use of medications and dietary supplements, obstetric history, self-reported hypertension, smoking habits, and other lifestyle data were obtained through structured questionnaires applied in the first and second evaluations. For the socioeconomic classification, the Brazilian Economic Classification Criteria were used, based on the possession of items and educational level of the head of the family, which categorize the socioeconomic status from class A (highest level) to class E (lowest level) [34].
Sample size
The sample size calculation was based on the primary outcome of the study, the proportion of women whose weekly GWG exceeded IoM guidelines. A 20% difference in the proportion of excessive weight gain between the treatment groups was expected [35]. A minimum significance level of 5% (α = 0.05), a power of 90% (β = 0.10) and a 40% loss in the follow-up were considered, indicating that a sample of 350 women would be sufficient.
Allocation
Randomization was performed using the Research Electronic Data Capture (REDCap) software [36, 37], with a spreadsheet of randomly generated numbers. Stratification between the groups was performed considering the prenatal care Health Unit. Given the nature of the intervention, both the study participants and the researchers were aware of the group allocation.
Data collection and management methods
The pregnant women of both groups underwent two evaluations in the health units during the pregnancy. The first assessment (baseline) was conducted prior to the 16th GW and the second preferably between the 34th and 36th GW.
Study data were collected and managed using the REDCap program [36, 37]. To ensure the quality of the data collected, the research group consisted of trained nutritionists. A guidance manual was prepared for use as a protocol for all study procedures. All data collected were verified by a researcher other than the one that entered the data into the system.
Statistical analyses
The analyses of this study followed the intention-to-treat principles (including all the randomized women), and modified intention-to-treat principles (in which the women of the IG who did not attend any of the nutritional counselling sections were excluded). The differences between groups were verified using Student’s t-test for independent variables, Mann–Whitney U test, or X2, as appropriate. The effect of the intervention on maternal outcomes was assessed using logistic regression models adjusted by maternal age (years), smoking (yes/no), parity (number of children), employment at the moment of randomization (yes/no), and antenatal care health unit. The same adjustment variables were considered in the linear regression models used to explore the effect of the intervention on total weight gain and on the rate of weight gain. To analyze total weight gain from baseline to 34–36 GW, only women who had their body weight evaluated after the 33rd GW were considered. For weight gain outcomes, the models were further adjusted for GW of the last evaluation of body weight in pregnancy.
The adjustment for the pregnant woman's employment status was used as a proxy for socioeconomic status since it is considered more sensitive than possession of items, and indicates the woman's economic autonomy. The pre-pregnancy BMI was not considered a confounder in the models because the sample was solely composed of overweight, pregnant women. The analyses were conducted using the SPSS® software (version 24), and p values < 0.05 were considered significant.
Results
Of the 350 pregnant women randomized, a total of 335 (166 of the CG, and 169 of the IG) completed the baseline evaluation. The median (P25, P75) age of the women was 27 (23, 32) years, and the median pre-pregnancy BMI was 27 (26, 28) kg/m2. At baseline, there were no differences in maternal characteristics between the groups (Table 1).
Table 1.
Baseline characteristics of the pregnant women according to treatment group
| Characteristics | Intervention (n = 169) | Control (n = 166) | p a |
|---|---|---|---|
| Median (P25, P75) | |||
| Maternal age, years | 27 (23, 31) | 27 (22, 32) | .39 |
| Gestational age at randomization, weeks | 11 (9, 13) | 11 (9, 12) | .86 |
| BMI at baseline, kg/m2 | 27.2 (26.2, 28.3) | 26.9 (25.9, 28.4) | .30 |
| Time sleeping per day, hours | 9 (7, 10) | 9 (8, 11) | .40 |
| n (%) | |||
| Married/living with partner | 132 (78.1) | 120 (72.3) | .22 |
| Self-reported skin colourb | .91 | ||
| White | 50 (30.3) | 53 (32.3) | |
| Black | 24 (14.6) | 26 (15.8) | |
| Mulatto | 87 (53.0) | 89 (53.9) | |
| Schooling, years | .61 | ||
| ≤ 8 | 36 (21.3) | 40 (24.1) | |
| 9–11 | 108 (63.9) | 107 (64.5) | |
| ≥ 12 | 25 (14.8) | 19 (11.4) | |
| Socioeconomic statusc | .39 | ||
| A + B | 32 (21.2) | 31 (21.1) | |
| C | 98 (64.9) | 87 (59.2) | |
| D + E | 21 (13.9) | 29 (19.7) | |
| Employed at randomization | 109 (64.5) | 90 (54.2) | .06 |
| Nulliparous | 130 (76.9) | 126 (75.9) | .83 |
| Use of dietary supplements | 152 (89.9) | 148 (89.2) | .81 |
| Smoking status | .56 | ||
| Never smoked | 50 (71.4) | 57 (71.3) | |
| Current smoker | 4 (5.7) | 8 (10.0) | |
| Ex-smoker | 16 (22.9) | 15 (18.8) | |
| Reported intake of alcohol over the last 30 days | 31 (18.3) | 34 (20.5) | .62 |
| Pre-pregnancy hypertensiond | 7 (4.2) | 5 (3.0) | .56 |
aAccording to Student’s t-test for independent samples or Chi-square test for difference between groups
bSelf-reported skin colour is used as proxy for ethnicity in Brazil. Only 5 women reported being yellow, and one refused to respond, with these not included in the analysis. No women reported being indigenous
cBased on the Brazilian Economic Classification Criteria, which categorize the socioeconomic status from class A (highest level) to class E (lowest level) [34]. Data available for 151 women of the intervention and 147 of control group, as 37 did not know the level of education of the head of the family
dData available for 167 women of the intervention and 166 of control group
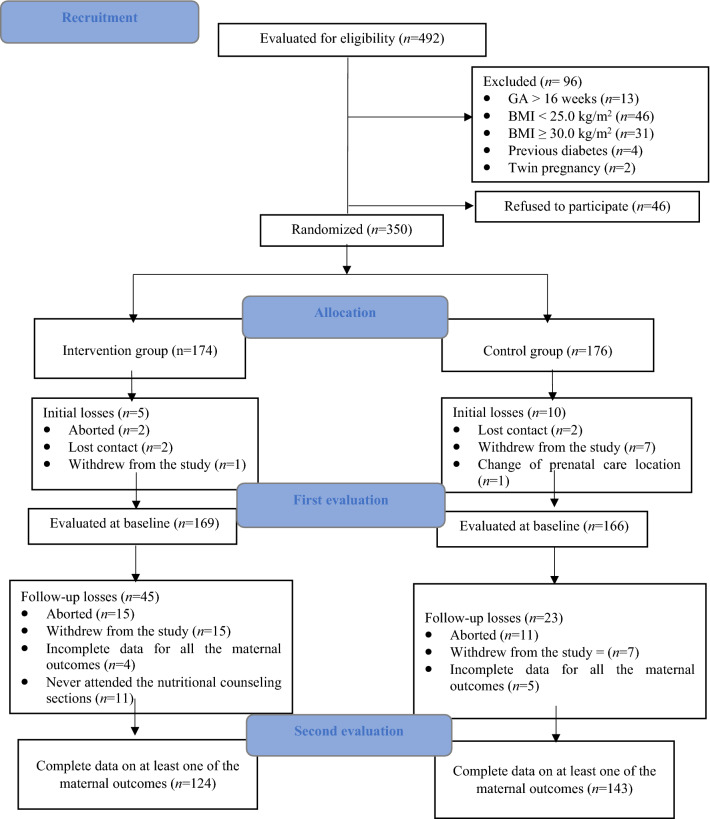
In the modified intention-to-treat analysis, complete data on at least one maternal outcome was available for 124 women in the IG and 143 women in the CG. Data on the rate of GWG per week was available for 121 women in the IG and 139 women in the CG. In both treatment groups, 70% of the women performed their last weight assessment between the 34th and 36th GW, while 30% performed their last weight measurement between the 18th and 33rd GW. Data to estimate total weight gain from baseline to 34–36 GW were available for 98 women in the IG and 112 women in the CG. The flow chart of the study is presented in Fig. 1.
Fig. 1.
Study flowchart.GA: gestational age, BMI: body mass index
Among the IG women with complete data for at least one maternal outcome (n = 124), eight attended one nutritional counselling section, 20 two sections, and 96 three sections.
In the modified intention-to-treat analysis, the IG presented a lower mean total and weekly GWG compared to the CG; however, neither was statistically significant. In the adjusted logistic regression models, a lower chance of women with excessive GWG was found in the IG compared with the CG. No between-group differences were observed for adequate GWG, insufficient GWG, gestational hypertension, GDM, preeclampsia, preterm birth, or caesarean section (Table 2).
Table 2.
Gestational weight gain and maternal outcomes according to treatment group
| Intervention | Control | p-valuea | β (95% CI)b | OR (95% CI)c | |
|---|---|---|---|---|---|
| Gestational weight gaind | |||||
| Rate of GWG per week, kg/week, mean (SD) | 0.43 (0.19) | 0.47 (0.21) | .09 |
– 0.04 (– 0.09, 0.01), p = .13 |
|
| Between baseline and 34–36 weeks of gestation, mean (SD)e | 8.9 (4.3) | 10.1 (4.6) | .07 |
– 1.22 (– 2.44, 0.003), p = .051 |
|
| GWG per week, n (%)d | |||||
| Excessive weight gain | 75 (62.0) | 102 (73.4) | .049 |
0.56 (0.32, 0.98), p = .04 |
|
| Adequate weight gain | 29 (24.0) | 25 (18.0) | .24 |
1.40 (0.74, 2.64), p = .29 |
|
| Insufficient weight gain | 17 (14.0) | 12 (8.6) | .17 |
1.92 (0.85, 4.34), p = .12 |
|
| Other maternal outcomes | |||||
| Gestational hypertensionf | 21 (16.9) | 33 (23.1) | .21 |
0.72 (0.36, 1.45), p = .36 |
|
| Gestational diabetes mellitusg | 14 (12.1) | 14 (11.1) | .82 |
0.95 (0.36, 2.49), p = .91 |
|
| Premature birthh | 3 (3.3) | 3 (2.8) | .83 |
1.38 (0.25, 7.49), p = .71 |
|
| Caesarean deliveryi | 46 (47.4) | 43 (39.1) | .23 |
1.35 (0.76, 2.39), p = .31 |
|
| Preeclampsial | 1 (4.2) | 4 (14.3) | .22 |
0.33 (0.03, 4.04), p = .38 |
Following the modified intention-to-treat principles
OR odds ratio, GWG gestational weight gain
aAccording to Student’s t-test for independent samples or Chi-Square test for differences between groups
bEstimated by linear regression models adjusted by maternal age (years), smoking (yes/no), parity (number of children), employed at randomization (yes/no), health care unit, and gestational week of the last evaluation of body weight in pregnancy (for weight gain outcomes)
cEstimated by logistic regression models adjusted by maternal age (years), smoking (yes/no), parity (number of children), employed at randomization (yes/no), health care unit, and gestational week of the last evaluation of body weight in the pregnancy (for weight gain outcomes)
dData available for 121 women of the intervention, and 139 of control group
eOnly women with a gestational week of 33 or more at the time of the last evaluation of body weight in the pregnancy were included in the analysis, with 98 women in the intervention group and 112 in the control group
fData available for 124 women of the intervention, and 143 of control group, after the exclusion of those with pre-pregnancy hypertension
gData available for 36 women of the intervention, and 35 of control group
hData available for 91 women of the intervention, and 108 of control group
iData available for 97 women of the intervention, and 110 of control group
jData available for 97 women of the intervention, and 110 of control group
At baseline, no differences between groups were found for the frequency of intake of minimally processed foods, vegetables, fruits, ultra-processed food products, and sugar sweetened beverages. At the second evaluation, a higher proportion of women in the IG reported the daily intake of minimally processed foods and vegetables at lunch time when compared to the CG; however, this was not statistically significant. No between-group difference was found for the practice of physical activity either at baseline or at the second evaluation (Table 3).
Table 3.
Compliance with the intervention recommendations by the pregnant women, according to treatment group
| Intervention | Control | p-valuea | |
|---|---|---|---|
| Daily consumption of a minimally processed food-based meal at lunch time on the last weekb | |||
| ≤ 16 weeks of gestation | 111 (65.7) | 108 (65.1) | .91 |
| 34–36 weeks of gestation | 76 (78.4) | 67 (66.3) | .06 |
| Daily consumption of vegetables at lunch time in the previous week c | |||
| ≤ 16 weeks of gestation | 74 (43.8) | 73 (44.0) | .97 |
| 34–36 weeks of gestation | 43 (44.3) | 32 (31.7) | .07 |
| Daily consumption of fruits in the previous week | |||
| ≤ 16 weeks of gestation | 81 (47.9) | 87 (52.4) | .41 |
| 34–36 weeks of gestation | 48 (49.5) | 43 (42.6) | .33 |
| Frequency of intake of ultra-processed food products | |||
| ≤ 16 weeks of gestation | .13 | ||
| ≤ 2 times/week | 120 (71.0) | 109 (65.7) | |
| 3–4 times/week | 33 (19.5) | 29 (17.5) | |
| ≥ 5 times/week | 16 (9.5) | 28 (16.9) | |
| 34–36 weeks of gestation | .14 | ||
| ≤ 2 times/week | 73 (75.3) | 63 (62.4) | |
| 3–4 times/week | 9 (9.3) | 16 (15.8) | |
| ≥ 5 times/week | 15 (15.5) | 22 (21.8) | |
| Frequency of intake of sugar sweetened beverages | |||
| ≤ 16 weeks of gestation | .23 | ||
| ≤ 2 times/week | 88 (52.1) | 78 (47.0) | |
| 3–4 times/week | 28 (16.6) | 40 (24.1) | |
| ≥ 5 times/week | 53 (31.4) | 48 (28.9) | |
| 34–36 weeks of gestation | .27 | ||
| ≤ 2 times/week | 37 (38.1) | 48 (47.5) | |
| 3–4 times/week | 30 (30.9) | 22 (21.8) | |
| ≥ 5 times/week | 30 (30.9) | 31 (30.7) | |
| Physical activity for ≥ 150 min per week | |||
| ≤ 16 weeks of gestation | 11 (6.5) | 12 (7.2) | .79 |
| 34–36 weeks of gestation | 18 (18.6) | 16 (15.8) | .61 |
Data were available for 169 women of the intervention and 166 of the control group at baseline, and for 97 of the intervention and 101 of control group at 34–36 weeks of gestation
aAccording to Chi-Square test for the difference between the groups
bLunch is the main meal in the country
Following the intention-to-treat principles (GI n = 135, GC n = 143), no effect of the intervention was found for the rate of GWG per week [β – 0.04 (95% CI – 0.09, 0.01), p = 0.15], GWG from baseline to 34–36 weeks [β – 1.11 (95% CI – 2.33, 0.10), p = 0.07], excessive GWG [OR 0.59 (95% CI 0.34, 1.03), p = 0.06], adequate GWG [OR 1.36 (95% CI 0.73, 2.54), p = 0.34], insufficient GWG [OR 1.82 (95% CI 0.80, 4.10), p = 0.15], gestational hypertension [OR 0.59 (95% CI 0.32, 1.09), p = 0.09], GDM [OR 1.21 (95% CI 0.55, 2.66), p = 0.63], premature birth [OR 1.45 (95% CI 0.52, 4.07), p = 0.48], caesarean delivery [OR 1.29 (95% CI 0.78, 2.12), p = 0.32], and preeclampsia [OR 0.32 (95% CI 0.05, 2.02), p = 0.23] in adjusted models (data not shown). Data on the compliance with the intervention recommendations are the same as the presented in the Table 3.
Discussion
This clinical trial was unprecedented in demonstrating the effectiveness of a nutritional counselling based on the NOVA food classification system, together with encouraging the practice of physical activity, in the prevention of excessive weight gain in overweight pregnant women. Among the IG women, the chance of excessive GWG was 44% lower, when compared to the CG. No effect of the intervention was found for gestational hypertension, GDM, preterm birth, caesarean delivery, or preeclampsia.
The effectiveness of the intervention in limiting excessive GWG in the present study [OR 0.56 (95% CI 0.32, 0.98)] is in line with data from previous studies conducted among pregnant women with excessive body weight, such as the GLOW study [39] [RR 0.70 (95% CI 0.59, 0.83)], and the LIFE-Moms meta-analysis [38] [OR 0.52 (95% CI 0.40, 0.67)], which evaluated the results of seven clinical trials conducted in overweight and obese women. However, the LIFE-Moms studies used mostly moderate (4–11 sessions) to high (12 or more sessions) intensity interventions [28]. The behavioural intervention applied in the GLOW study consisted of two in-person and 11 telephone sessions [39]. The one adopted in the present study (3 sessions) is of low-cost and can be feasibly implemented in the public health system of less developed countries.
The nutritional intervention of the present study has some similarities with previous lifestyle intervention studies, such as encouraging physical activity, fruit and vegetable consumption, and increased the water intake, while reducing the intake of sugar sweetened beverages and cookies [28, 39]. However, in the present study, the recommendation of food consumption focused on the degree of industrial processing, rather than the nutrient content of the food. For example, according to the NOVA food classification, it is better to eat a homemade, low-fibre, white flour bread than a high-fibre, whole grain bread that has been industrially produced and contains additives, such as colorants, flavours and taste enhancers, emulsifiers, and non-sugar sweeteners [40].
A higher chance of insufficient weight gain among women enrolled in nutritional intervention studies was previously reported. A meta-analysis study, which included 65 randomized controlled trials that tested the effectiveness of nutritional interventions on excessive GWG, reported that those in the IGs had a higher risk of insufficient GWG when compared to the women of the CGs [RR 1.14, 95% CI 1.02–1.27] [8]. In the present study, no effect of the intervention was found for insufficient weight gain.
A high prevalence of gestation hypertension (20.2%) was found in the present study, probably related to the women being overweight. Data from studies on the prevalence of gestational hypertension in Brazil vary widely, from 11% in the South of the country [41] to 23% in the Southeast [42]. Gestational hypertensive syndromes and their complications are the leading causes of maternal death in Brazil [43]. The intervention applied in the present study was not effective in preventing this outcome.
No effect of the intervention was found for the other maternal outcomes investigated. It is possible that the sample size was insufficient to estimate the impact of the intervention on outcomes with lower prevalence, such as premature birth and preeclampsia. High rates of caesarean delivery are observed in the country [44], and a lifestyle intervention probably has a limited power to change this tendency. Furthermore, the period between the first nutritional counselling session and the GW recommended for the oral glucose tolerance test was possibly too short to prevent GDM.
Regarding compliance with the intervention recommendations, although there was a tendency for a higher frequency of consumption of minimally processed foods and vegetables at the main meal in the IG, the intervention was not effective in reducing the overall intake of ultra-processed food products and sugar sweetened beverages. The high frequency of intake of ultra-processed food products and sugar sweetened beverages in the sample evaluated should be highlighted. At the second evaluation, 15.5% of the IG and 21.8% of the CG reported consuming ultra-processed food products on more than 5 days of the week, and 30.9% of the IG and 30.7% of the CG reported consuming sugar sweetened beverages on more than 5 days of the week.
Increasing evidence from observational studies suggests detrimental effects on the health of adults and pregnant women due to the high consumption of ultra-processed products [17, 18, 45–49]. Ultra-processed products have low nutritional quality, high energy density and are rich in sugar, fat and salt, characteristics that make them hyperpalatable, resulting in impaired regulation of the appetite [50, 51]. In contrast, a higher intake of homemade meals, prepared using unprocessed or minimally processed, nutrient-balanced and satiating foods, leads to a lower chance of obesity [50, 51]. Furthermore, encouraging the consumption of unprocessed and minimally processed foods helps to revive the country’s traditional food culture [14].
The high intake of ultra-processed foods also exerts harmful effects on the environment. The packaging of ultra-processed products creates large amounts of waste, their manufacture and distribution involve long transport routes, and their ingredients are produced through monocultures, contributing to climate disruption, air pollution and loss of water [14, 21].
Only one previous clinical trial has investigated the effect of the consumption of ultra-processed products when compared to diets composed of minimally processed foods. This inpatient randomized controlled cross-over trial of ad libitum food intake conducted among American adults (n = 20) supported the hypothesis of the deleterious effect of the consumption of ultra-processed foods on body weight control [50]. In the study by Hall and colleagues [50], all meals were provided to the study participants, who did not face obstacles related to food acquisition and preparation, steps necessary to adopt a diet based on the consumption of unprocessed or minimally processed food.
Ultra-processed products are offered widely, through various marketing and advertising strategies, which influences the food choices of free-living individuals [13, 45]. The control of marketing and higher taxes for sugar sweetened beverages and other ultra-processed products might be needed to control the excessive intake of these products [52].
The present study intervention was not effective in the promotion of the practice of physical activity. Evidence suggest that the regular practice of physical activity is effective in preventing adverse maternal outcomes, such as excessive GWG [22]. Sedentary behaviour among Brazilian pregnant women has been previously reported [53, 54], and more intensive interventions might be necessary to promote changes in this lifestyle behaviour.
The fact that the study is unprecedented and the feasibility of the implementation of the strategy in public health services of less developed countries can be considered important strengths. The nutritional counselling was based on the NOVA food classification system, which advocates a more sustainable diet, driven by the traditional food culture of the country [14].
The study presents some limitations. The women included in the study lived in areas of high social vulnerability, which was aggravated by the financial crisis generated by the COVID-19 pandemic. In 2020, it was estimated that in 55.2% of Brazilian households the inhabitants lived with food insecurity [55]. The low access to a healthy and varied diet may have directly influenced the adherence to the food consumption goals of the pregnant women. In addition, the imposed social isolation may also have reduced the time spent practicing physical activity. Therefore, the results presented may underestimate the potential of the strategy employed. Furthermore, as a result of the pandemic, in-person data collection was interrupted for seven months, and some of the consultations and evaluations of the study were carried out remotely. Also, the questionnaires on the frequency of the food intake and practice of physical activity were not validated for use with pregnant women and data for some of the health outcomes were obtained from medical records, which may not have been collected following the strict criteria of the research protocol. More intensive nutritional interventions might be necessary to promote the practice of physical activity, and to reduce the intake of ultra-processed food products and sugar sweetened beverages.
The present trial was conducted only among women who were overweight before pregnancy and the effect of the intervention on women of different BMI categories remains unknown. As described in the study protocol [24], the effect of the intervention on birth weight, neonatal adiposity, and the child’s weight and height at 6, 12 and 24 months of age will be further investigated.
Conclusions
The present study was unprecedented in demonstrating that a nutritional counselling intervention based on the NOVA food classification system, together with the practice of physical activity, is effective in preventing excessive GWG in overweight pregnant women. Future studies are needed to investigate the effectiveness of this strategy among women of other BMI categories, as well as the effect of the intervention on infant outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The research was funded by the São Paulo Research Foundation (FAPESP 2017/15386–2, 2017/18980–2, 2021/06586-3, and 2021/06486-9), the National Council for Scientific and Technological Development (CNPq 406000/2018–2 and 302487/2018–2), the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Foundation for Support to Teaching, Research and Assistance of the Clinical Hospital, Ribeirão Preto Medical School, University of São Paulo (FAEPA 1039/2018, 1114/2018, 61/2019, 62/2019 and 754/2021). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the Research Ethics Committee of the Centre for School Health, Ribeirão Preto Medical School, University of São Paulo (CAAE: 69997717.6.0000.5414). Written consent was obtained from all participants.
References
- 1.Hanson M, Gluckman P, Bustreo F. Obesity and the health of future generations. Lancet Diabetes Endocrinol. 2016;4(12):966–967. doi: 10.1016/S2213-8587(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masho SW, Urban P, Cha S, Ramus R. Body mass index, weight gain, and hypertensive disorders in pregnancy. Am J Hypertens. 2016;29(6):763–771. doi: 10.1093/ajh/hpv184. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald SC, Bodnar LM, Himes KP, Hutcheon JÁ. Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology. 2017;28(3):419–427. doi: 10.1097/EDE.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sridhar SB, Xu F, Hedderson MM. Trimester-specific gestational weight gain and infant size for gestational age. Krukowski RA, ed. PLoS ONE. 2016;11(7):e0159500. doi: 10.1371/journal.pone.0159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siega-Riz AM, Viswanathan M, Moos MK, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201(4):339.e1–339.e14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Cantor A, Jungbauer RM, McDonagh MS, et al (2021). Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US). http://www.ncbi.nlm.nih.gov/books/NBK571093/. Accessed 23 Aug 2021 [PubMed]
- 8.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Pregnancy and Childbirth Group, ed. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godoy A, Nascimento S, Surita F. A systematic review and meta-analysis of gestational weight gain recommendations and related outcomes in Brazil. Clinics. 2015;70(11):758–764. doi: 10.6061/clinics/2015(11)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Carvalhaes MABL, de Gomes CB, Malta MB, Papini SJ, de Parada CMGL. Sobrepeso pré-gestacional associa-se a ganho ponderal excessivo na gestação. Rev Bras Ginecol E Obstetr. 2013;35(11):523–529. doi: 10.1590/S0100-72032013001100008. [DOI] [PubMed] [Google Scholar]
- 11.Benham JL, Booth JE, Donovan LE, Leung AA, Sigal RJ, Rabi DM. Prevalence of and risk factors for excess weight gain in pregnancy: a cross-sectional study using survey data. CMAJ Open. 2021;9(4):E1168–E1174. doi: 10.9778/cmajo.20200276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasil. Instituto Brasileiro de Geografia e Estatística - IBGE. Pesquisa Nacional de Saúde 2019: Atenção Primária à Saúde e Informações Antropométricas. IBGE; 2020. https://biblioteca.ibge.gov.br/visualizacao/livros/liv101758.pdf Accessed 20 June 2022
- 13.Monteiro CA, Cannon G, Levy R, et al (2016).NOVA. The star shines bright. World Nutr. 7(1–3):28–38. https://worldnutritionjournal.org/index.php/wn/article/view/5 Accessed 3 Sep 2019
- 14.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125(3):308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauber F, Chang K, Vamos EP, et al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr. 2021;60(4):2169–2180. doi: 10.1007/s00394-020-02367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartorelli DS, Crivellenti LC, Zuccolotto DCC, Franco LJ. Relationship between minimally and ultra-processed food intake during pregnancy with obesity and gestational diabetes mellitus. Cad Saúde Pública. 2019 doi: 10.1590/0102-311x00049318. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi KW, Tinius RA, Cade WT, Steele EM, Cahill AG, Parra DC. Relationships between consumption of ultra-processed foods, gestational weight gain and neonatal outcomes in a sample of US pregnant women. PeerJ. 2017;5:e4091. doi: 10.7717/peerj.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Gomes CB, Malta MB, Benício MHD, de Carvalhaes MABL. Consumption of ultra-processed foods in the third gestational trimester and increased weight gain: a Brazilian cohort study. Public Health Nutr. 2021;24(11):3304–3312. doi: 10.1017/S1368980020001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings JR, Lipsky LM, Schwedhelm C, Liu A, Nansel TR. Associations of ultra-processed food intake with maternal weight change and cardiometabolic health and infant growth. Int J Behav Nutr Phys Act. 2022;19(1):61. doi: 10.1186/s12966-022-01298-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seferidi P, Scrinis G, Huybrechts I, Woods J, Vineis P, Millett C. The neglected environmental impacts of ultra-processed foods. Lancet Planet Health. 2020;4(10):e437–e438. doi: 10.1016/S2542-5196(20)30177-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Wen D, Liu X, Liu Y. Impact of exercise on maternal gestational weight gain: An updated meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98(27):e16199. doi: 10.1097/MD.0000000000016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen KM, Yaktine AL, Institute of Medicine (U.S.), eds (2009). Weight Gain during Pregnancy: Reexamining the Guidelines. Yaktine AL, editors. Washington (DC): National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK32813/Accessed 20 June 2022 [PubMed]
- 24.Sartorelli DS, Crivellenti LC, Manochio-Pina MG, et al. Study Protocol effectiveness of a nutritional intervention based on encouraging the consumption of unprocessed and minimally processed foods and the practice of physical activities for appropriate weight gain in overweight, adult, pregnant women: a randomized controlled trial. BMC Pregnancy Childbirth. 2020;20(1):24. doi: 10.1186/s12884-019-2672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica (2014). Guia Alimentar Para a População Brasileira. Brasília: Ministério da Saúde. https://bvsms.saude.gov.br/bvs/publicacoes/guia_alimentar_populacao_brasileira_2ed.pdf Accessed 20 June 2022
- 26.ACOG Committee on Obstetric Practice Committee opinion #267: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171–173. doi: 10.1016/S0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 27.Manochio-Pina M, Crivellenti LC, Sartorelli DS, Diez-Garcia R. Educational instrument for intervention in the lifestyle of overweight pregnant women. Braz J Mother Child Health. 2022 doi: 10.1590/1806-9304202200020022. [DOI] [Google Scholar]
- 28.Clifton RG, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity: lifestyle Interventions in Pregnancy. Obesity. 2016;24(2):305–313. doi: 10.1002/oby.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sociedade Brasileira de Cardiologia (2016). 7a Diretriz Brasileira de hipertensão arterial. Arq Bras Cardiol. 107(3 (Suppl. 3)):1–83. http://publicacoes.cardiol.br/2014/diretrizes/2016/05_HIPERTENSAO_ARTERIAL.pdf Accessed 20 June 2022
- 30.World Health Organization Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção da Saúde (2018). Vigitel Brasil 2017: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no distrito federal em 2017. Brasília: Ministério da Saúde. https://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2017_vigilancia_fatores_riscos.pdf Accessed 20 June 2022
- 32.Monteiro CA, Moura EC, Jaime PC, Claro RM. Validade de indicadores do consumo de alimentos e bebidas obtidos por inquérito telefônico. Rev Saúde Públ. 2008;42(4):582–589. doi: 10.1590/S0034-89102008000400002. [DOI] [PubMed] [Google Scholar]
- 33.Moreira AD, Claro RM, Felisbino-Mendes MS, Velasquez-Melendez G. Validade e reprodutibilidade de inquérito telefônico de atividade física no Brasil. Rev Bras Epidemiol. 2017;20(1):136–146. doi: 10.1590/1980-5497201700010012. [DOI] [PubMed] [Google Scholar]
- 34.ABEP (Associação Brasileira de Empresas de Pesquisa) (2012) Critério de Classificação Econômica Brasil. https://www.abep.org/criterio-brasil Accessed 20 June 2022
- 35.Pocock SJ. Clinical trials: a practical approach. Chichester: Wiley; 1983. [Google Scholar]
- 36.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peaceman AM, Clifton RG, Phelan S, et al. Lifestyle interventions limit gestational weight gain in women with overweight or obesity: life-moms prospective meta-analysis. Obesity. 2018;26(9):1396–1404. doi: 10.1002/oby.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara A, Hedderson MM, Brown SD, et al. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020;8(6):490–500. doi: 10.1016/S2213-8587(20)30107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteiro CA, Astrup A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? YES. Ludwig DS, ed. Am J Clin Nutr. 2022 doi: 10.1093/ajcn/nqac122. [DOI] [PubMed] [Google Scholar]
- 41.De Franceschi KG, Melere C. Prevalência de síndromes hipertensivas gestacionais em usuárias de um hospital no sul do Brasil. Rev Cuid. 2017;8(3):1899. doi: 10.15649/cuidarte.v8i3.454. [DOI] [Google Scholar]
- 42.Morais ACFD, Silva ARD, Nazareth DC, et al. Prevalência de doença hipertensiva específica da gestação em um hospital de ensino de Juiz de Fora. MG Braz J Dev. 2020;6(10):79242–79251. doi: 10.34117/bjdv6n10-379. [DOI] [Google Scholar]
- 43.Pacagnella R, Nakamura-Pereira M, Gomes-Sponholz F, et al. Maternal mortality in Brazil: proposals and strategies for its reduction. RBGO Gynecol Obstet. 2018;40(09):501–506. doi: 10.1055/s-0038-1672181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascarello KC, Matijasevich A, dos Santos I da S, Silveira MF. Complicações puerperais precoces e tardias associadas à via de parto em uma coorte no Brasil. Rev Bras Epidemiol. 2018 doi: 10.1590/1980-549720180010. [DOI] [PubMed] [Google Scholar]
- 45.da Louzada MLC, Canella DS, Jaime PC, Monteiro CA. Alimentação e Saúde: A Fundamentação Científica Do Guia Alimentar Para a População Brasileira. Universidade de São Paulo, Faculdade de Saúde Pública; 2019. [Google Scholar]
- 46.de Mendonça R, D, Pimenta AM, Gea A,, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 47.Monteiro CA, Moubarac JC, Levy RB, Canella DS, da Louzada MLC, Cannon G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21(1):18–26. doi: 10.1017/S1368980017001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018;120(1):90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 49.Nardocci M, Leclerc BS, Louzada ML, Monteiro CA, Batal M, Moubarac JC. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019;110(1):4–14. doi: 10.17269/s41997-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Louzada ML, C, Baraldi LG, Steele EM,, et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev Med. 2015;81:9–15. doi: 10.1016/j.ypmed.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Moodie R, Stuckler D, Monteiro C, et al. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. The Lancet. 2013;381(9867):670–679. doi: 10.1016/S0140-6736(12)62089-3. [DOI] [PubMed] [Google Scholar]
- 53.da Silva CM, da Perdoná GSC, Sartoreli DS. Behavior of pregnant women regarding physical activity in gestational diabetes mellitus: secondary analysis of a descriptive cross-sectional study. J Matern Fetal Neonatal Med. 2021 doi: 10.1080/14767058.2021.1946778. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira SC, dos Imakawa T, DuarteQuintanaMoisés GSMECD. Do the body mass index and the diagnosis of gestational diabetes mellitus influence the level of physical activity during pregnancy and postpartum? Hill B, ed. PLoS ONE. 2019;14(8):e0220947. doi: 10.1371/journal.pone.0220947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rede Brasileira de Pesquisa em Soberania e Segurança Alimentar e Nutricional. VIGISAN. Inquérito Nacional Sobre Insegurança Alimentar No Contexto Da Pandemia Da Covid-19 No Brasil. Rede Penssan; 2021. http://olheparaafome.com.br/Accessed 20 June 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.