Introduction
While novel immunotherapies, particularly immune checkpoint inhibitors, have improved outcomes in patients with melanoma, many patients have primary or secondary resistance to these treatments or develop substantial immune-related toxicities. Moreover, surgery is often impractical to control locally advanced disease. A variety of local therapeutic methods have previously been tried including intra-arterial regional perfusion therapy, intralesional interleukin 2, Bacille Calmette-Guerin vaccination, diphencyprone, CO2 laser, electrochemotherapy, and radiation therapy, all with varying degrees of success.1, 2, 3 Currently, the only Food and Drug Administration–approved intralesional treatment of cutaneous melanoma metastases is talimogene laherparepvec, a herpes simplex virus-1–derived oncolytic immunotherapy.4 Previously, a retrospective study demonstrated the effectiveness of imiquimod and cryotherapy in cutaneous melanoma metastases.5 Imiquimod is a potent modulator of innate and adaptive immune response by binding to Toll-like receptors 7 and 8 on antigen-presenting cells, improving T-cell priming, and stimulating tumor-specific T-cell responses.6 Topical application of imiquimod to unresectable cutaneous metastases may therefore improve response rates in patients receiving systemic immunotherapy.
Because advanced melanoma is thought to evade immune detection and destruction, immune checkpoint inhibitors represent a promising strategy to enhance the body’s natural antitumor response by releasing the brake(s) on key immunoregulatory mechanisms. Ipilimumab, nivolumab, and pembrolizumab are monoclonal antibodies that block inhibitory immune receptors. Ipilimumab targets cytotoxic T-lymphocyte–associated protein 4, while nivolumab and pembrolizumab target programmed cell death protein 1, leading to enhanced T-cell activation predominantly in lymph nodes and the tumor, respectively. Intratumoral imiquimod potentiated anticytotoxic T-lymphocyte–associated protein 4 therapy in animal models, in which systemic immunotherapy alone failed,7 suggesting that immunomodulation of the tumor microenvironment may increase susceptibility to immune blockade.4
We report 3 patients who achieved complete resolution of cutaneous metastases with concomitant use of imiquimod, cryotherapy, and immune checkpoint inhibitors. In the era of immune checkpoint inhibition for the treatment of advanced melanoma, locoregional treatments of cutaneous metastases offer regional control and may augment response to immunotherapy.
Cases
Case 1
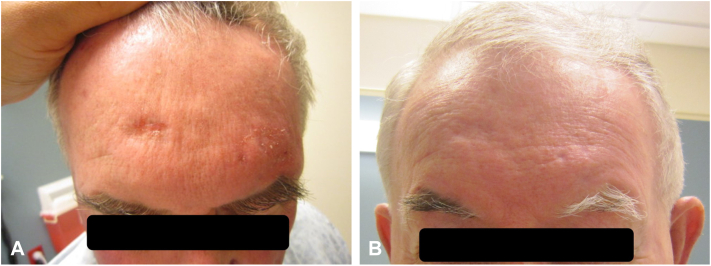
An 83-year-old Caucasian man with a remote history of uveal melanoma status post enucleation presented with a nonhealing papule on his scalp. Biopsy demonstrated cutaneous malignant melanoma, invasive to at least 3.0 mm with 12 mitoses per mm2 and ulceration (stage IIB [T3bNxMx]). He underwent wide local excision but declined sentinel node biopsy. Three months later, he re-presented with multiple nodules adjacent to the melanoma excision site consistent with in-transit metastases and widespread dermal lymphovascular invasion (BRAF wild type, c-Kit mutant exon 13). Potential skin graft failure and high likelihood of recurrence secondary to lymphovascular involvement precluded surgery. Five months later, he developed multiple new liver lesions on computed tomography (the largest measuring 1 cm) concerning for metastases for which ipilimumab (3 mg/kg) was started. He was then referred to dermatology for increasing locoregional disease (Fig 1, A). His left forehead was treated with cryotherapy followed by imiquimod 5% cream daily while continuing ipilimumab. Two weeks later, there was a dramatic reduction in the size of the scalp metastases (Fig 1, B) and resolution following 8 weeks of imiquimod application. Interestingly, while imiquimod was only applied to his left scalp, he also experienced resolution of metastatic lesions on his right scalp, suggesting a locoregional effect from the combination of cryotherapy and imiquimod.8 He completed 3 months total of daily imiquimod application. Clearance of in-transit metastatic foci was confirmed on biopsy 3 months after imiquimod cessation. His liver findings resolved upon follow-up computed tomography scan 2 months later. He remained disease-free for 6 years until imaging was performed for a mechanical fall demonstrating new adrenal nodules suspicious for metastatic disease. He opted for hospice and has deceased.
Fig 1.
Case 1 clinical photographs. A, Left frontal scalp in-transit metastatic melanoma lesions before application of topical imiquimod. B, Scalp lesions after 2 weeks of continuous imiquimod application.
Case 2
A 76-year-old man with a remote history of melanoma in situ of the left upper extremity developed a raised pigmented lesion on the left scalp. Biopsy showed malignant melanoma invasive to at least 8.1 mm with 5 mitoses per mm2 and no ulceration. The patient underwent wide re-excision with skin graft and left cervical sentinel lymph node biopsy, which was negative for malignancy (stage IIB [T4aN0Mx]). Seven months later, interval dermatologic screening revealed skin-colored subcutaneous nodules on the temples consistent with in-transit metastatic melanoma (BRAF wild type) (stage IIIC [T4aN1cM0]). These nodules were excised, but he developed new forehead nodules also consistent with in-transit metastases. Given wide distribution of in-transit metastases precluding surgery, cryotherapy was administered and imiquimod 5% cream was started, initially daily application with excellent response (Fig 2, A), then decreased to every other day at sites of exuberant inflammation. Eight weeks into treatment, imaging demonstrated new flurodeoxyglucose-avid right submandibular adenopathy, the largest measuring 2.3 × 2.0 cm with maximum standardized uptake value of 12.8, and a lymph node biopsy confirmed metastatic melanoma. Nivolumab monotherapy was started (3 mg/kg every 2 week for 20 cycles) (Fig 2, B), resulting in resolution of the flurodeoxyglucose-avid lymph node 3 months later (maximum standardized uptake value: 3.1). Interestingly, he developed unilateral poliosis of the left eyebrow corresponding to prior treatment with imiquimod (Fig 2, B). He has been disease-free for 6 years.
Fig 2.
Case 2 clinical photographs. A, Central forehead in-transit metastatic melanoma lesions after 3 months of topical imiquimod. B, Forehead lesions 4 months after initiation of nivolumab.
Case 3
A 60-year-old Caucasian man presented with a growing, black nodule on his right leg. Shave biopsy demonstrated malignant melanoma invasive to 2.1 mm with 4 mitoses per mm2 and no ulceration (stage IIIB [T3bN2aM0]). He underwent wide local excision with right femoral sentinel lymph node biopsy; 2/2 nodes were positive requiring lymphadenectomy. Positron emission tomography–computed tomography and magnetic resonance imaging were negative for distant metastases. Five months later, the patient developed skin-colored papules surrounding the excision site consistent with in-transit metastases (BRAF wild type) (Fig 3, A). The lesions clinically resolved after biopsy, but within 1 month, new nodules developed that were deemed unresectable. He was treated with cryotherapy followed by 3 months of topical imiquimod leading to complete resolution of the in-transit metastases (Fig 3, B). During treatment, flurodeoxyglucose-avidity was noted in the right external iliac region; however, all lymph nodes were too small to biopsy at that time and therefore he was monitored with imaging every 3 months. Two years later, he returned with a new right femoral soft tissue nodule (largest measuring 1.8 × 1.3 cm with maximum standardized uptake value of 7.5) that was biopsy-proven to be metastatic melanoma. He was treated with pembrolizumab (2 mg/kg) for 1 year leading to complete radiologic resolution of femoral lymphadenopathy. He has been disease-free for 6 years.
Fig 3.
Case 3 clinical photographs. A, Right leg in-transit metastatic melanoma lesions before application of topical imiquimod. B, Right leg lesions 6 months after imiquimod application.
Discussion
Advances in immunotherapy have changed the landscape of advanced melanoma treatment. While surgical excision is first-line treatment for discrete lesions, it is impractical in patients with multiple foci of disease and in areas where cosmetic/functional impact may be unacceptable. In such cases, therapies aimed at providing regional control may prove more effective and more durable.
Imiquimod, which mediates activation of antigen-presenting cells in the skin, indirectly stimulating release of immunomodulatory cytokines, has been employed in managing cutaneous melanoma metastases with mixed results.9 Recent guidelines highlight the importance of achieving inflammation with imiquimod application of up to 12 weeks.10 We typically start with daily application and titrate frequency based on degree of inflammation. These patients who achieved disease-free status with imiquimod/cryotherapy and systemic immunotherapy provide a potential proof of principle of the synergy between local immunomodulators and systemic immunotherapy in advanced melanoma, as has been reported by others.11 In all cases, imiquimod was applied after initial in-office cryotherapy, which could have acted as an additional stimulant of innate immunity or generator of new antigens. The immunomodulatory role of imiquimod/cryotherapy is further supported by the abscopal effect in case 1, in which the patient experienced resolution of melanoma metastases at a site distinct from the area of imiquimod application. It is possible that local imiquimod augmented the antitumor capacity of skin-resident CD8+ T cells beyond the threshold required to obtain a sustained and viable antitumor response.12, 13, 14Although it is unclear whether tumor regression was due to a delayed effect from ipilimumab or pseudoprogression from the addition of cryotherapy or imiquimod, the timing of tumor regression was clearly associated with intervention. Inflammation is a known effect of imiquimod, predicts response,15 and likely reflects the cytokine recruitment of cellular mediators. The lack of inflammation in case 1’s dramatic response, with tumor regression within 2 weeks, suggests that cytotoxic T-lymphocyte–associated protein 4 inhibition was critical, possibly by increasing the reservoir of tumor-specific T cells.
A similar abscopal effect has been reported with talimogene laherparepvec and pembrolizumab, possibly due to a combination of local granulocyte-macrophage colony-stimulating factor for dendritic cell recruitment and adaptive immune response.4 However, talimogene laherparepvec can be challenging to administer in areas like the scalp, making imiquimod/cryotherapy more feasible. Further investigation of topical imiquimod, with or without cryotherapy, together with systemic immunotherapy may provide mechanisms and into a better-tolerated, more affordable treatment option.
Understanding the mechanism by which imiquimod sensitizes metastatic melanoma cells to immune checkpoint inhibitors could predict patients likely to benefit from immunotherapy. Studies have shown that imiquimod enhances dendritic cell ability to activate CD8+ T cells.16 Moreover, a preexisting population of intratumor CD8+ T cells may be critical in predicting tumors likely to respond to immune checkpoint inhibition.17 Thus, imiquimod’s recruitment of tumor-reactive CD8+ cells may work synergistically with immune checkpoint blockade.
Immunotherapy has demonstrated benefit in treating advanced melanoma. For unresectable cutaneous melanoma metastases, topical immunomodulators like imiquimod and cryotherapy may provide a local antitumor immunologic “boost” that potentiates response to systemic immunotherapy. Such observations require further study as they may provide a rational strategy for patients with advanced melanoma.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Patient consent: All patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Abbott A.M., Zager J.S. Locoregional therapies in melanoma. Surg Clin North Am. 2014;94(5):1003–1015. doi: 10.1016/j.suc.2014.07.004. viii. [DOI] [PubMed] [Google Scholar]
- 2.Kidner T.B., Morton D.L., Lee D.J., et al. Combined intralesional Bacille Calmette-Guerin (BCG) and topical imiquimod for in-transit melanoma. J Immunother. 2012;35(9):716–720. doi: 10.1097/CJI.0b013e31827457bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damian D.L., Shannon K.F., Saw R.P., et al. Topical diphencyprone immunotherapy for cutaneous metastatic melanoma. Australas J Dermatol. 2009;50(4):266–271. doi: 10.1111/j.1440-0960.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A., Dummer R., Puzanov I., et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivas-Tolosa N., Ortiz-Brugues A., Toledo-Pastrana T., et al. Local cryosurgery and imiquimod: a successful combination for the treatment of locoregional cutaneous metastasis of melanoma: a case series. J Dermatol. 2016;43(5):553–556. doi: 10.1111/1346-8138.13197. [DOI] [PubMed] [Google Scholar]
- 6.Huang S.J., Hijnen D., Murphy G.F., et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129(11):2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M., Khong H., Dai Z., et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193(9):4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John H.E., Mahaffey P.J. Laser ablation and cryotherapy of melanoma metastases. J Surg Oncol. 2014;109(4):296–300. doi: 10.1002/jso.23488. [DOI] [PubMed] [Google Scholar]
- 9.Florin V., Desmedt E., Vercambre-Darras S., et al. Topical treatment of cutaneous metastases of malignant melanoma using combined imiquimod and 5-fluorouracil. Invest New Drugs. 2012;30(4):1641–1645. doi: 10.1007/s10637-011-9717-2. [DOI] [PubMed] [Google Scholar]
- 10.Swetter S.M., Tsao H., Bichakjian C.K., et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–250. doi: 10.1016/j.jaad.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 11.Joseph R.W., Cappel M., Tzou K., et al. Treatment of in-transit and metastatic melanoma in two patients treated with ipilimumab and topical imiquimod. Melanoma Res. 2016;26(4):409–412. doi: 10.1097/CMR.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 12.Ribas A., Medina T., Kummar S., et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a Phase Ib, multicenter study. Cancer Discov. 2018;8(10):1250–1257. doi: 10.1158/2159-8290.CD-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Campos J., Gallotta M., et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A. 2016;113(46):E7240–E7249. doi: 10.1073/pnas.1608555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X., Clark R.A., Liu L., et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papanikolaou M., Lawrence C.M. Long-term outcomes of imiquimod-treated lentigo maligna. Clin Exp Dermatol. 2019;44(6):631–636. doi: 10.1111/ced.13896. [DOI] [PubMed] [Google Scholar]
- 16.Fehres C.M., Bruijns S.C., van Beelen A.J., et al. Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8+ T-cell cross-priming. Eur J Immunol. 2014;44(8):2415–2424. doi: 10.1002/eji.201344094. [DOI] [PubMed] [Google Scholar]
- 17.Tumeh P.C., Harview C.L., Yearley J.H., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]