Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system leading to demyelination and axonal damage with severe neurological impairment.1 Ocrelizumab, a humanized anti-CD20 monoclonal antibody, has been approved for the treatment of MS in Europe since 2018.2 Although much has been learned about the safety profile of ocrelizumab in MS in preceding phase II and phase III trials, there still is a paucity of real-world safety data regarding rare adverse events. We describe a patient with MS who developed vulvovaginal pyoderma gangrenosum (PG) secondary to ocrelizumab treatment, a side effect which has been reported only recently.3
PG is an inflammatory ulceration of the skin, which in half of the cases is associated with a systemic condition such as inflammatory bowel disease or rheumatoid arthritis. It is assumed that PG is caused by a dysregulation of neutrophil granulocytes and pro-inflammatory cytokines.4
Case
We describe a 23-year-old Caucasian woman with the first onset of relapsing-remitting MS in January 2018 presenting with sensory spinal syndrome. Other differential diagnoses were ruled out by laboratory findings and neuroimaging. The diagnosis of relapsing-remitting MS was made according to the 2017 revised McDonald criteria.5 Treatment with natalizumab was initiated but had to be discontinued after the repeated occurrence of infusion-related dyspnea and drug eruption. Due to repeated relapses, therapy was changed to ocrelizumab in June 2018 and administered 5 times within 2.5 years. During ocrelizumab treatment, there were no relapses or signs of focal magnetic resonance imaging activity. Laboratory tests revealed mild hypogammaglobulinemia, which is a common side effect upon ocrelizumab treatment. First symptoms of vulvovaginal lesions appeared in July 2020, in which histological evidence of granuloma was found and misinterpreted as an abscess. Ocrelizumab treatment was discontinued after the last application in November 2020. After numerous operative revisions including a pedicled gracilis and a reversed dermis flap, which left the patient with a bladder catheter and an artificial bowel outlet, she was transferred to our clinic for dermatology in December 2021.
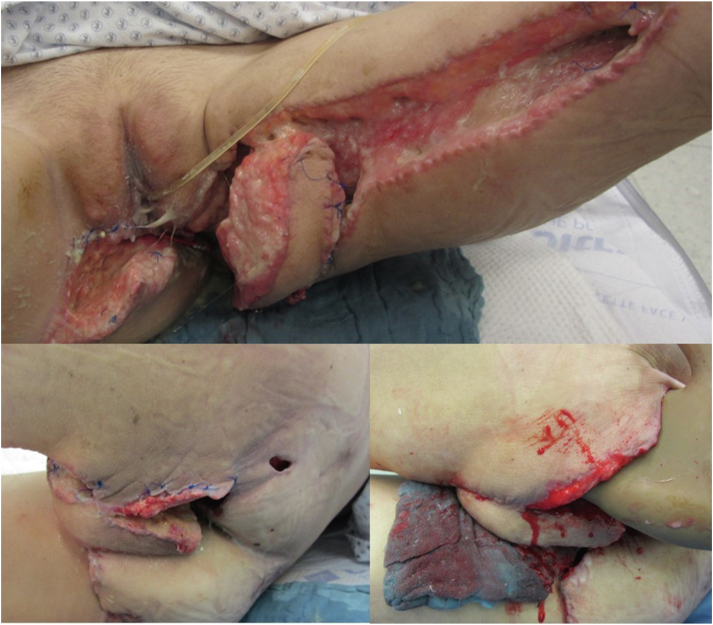
On admission, the patient presented with painful, inflamed ulcerations extending from the vaginal labium to the perianal and gluteal area (Fig 1).
Fig 1.
Admission findings with extensive wound areas, perianal cavity, and gluteal undermining.
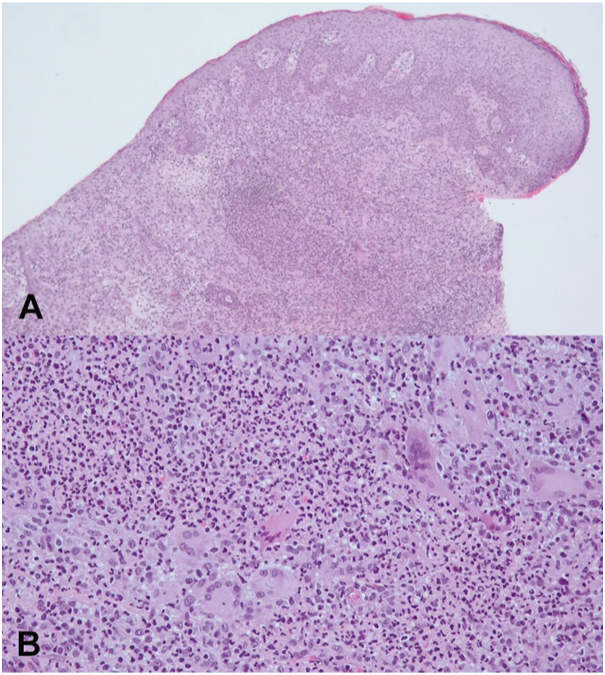
To confirm the suspected diagnosis of an autoinflammatory process, several tissue biopsies were obtained and showed histological signs of an inflammatory reaction with small granulomas including neutrophilic granulocytes and multinucleated giant cells (Fig 2).
Fig 2.
Tissue biopsy showed histological signs of an inflammatory reaction supporting diagnosis of pyoderma gangrenosum. A, Ulceration with diffuse mixed dermal infiltrates rich in neutrophils, hematoxylin-eosin stain, magnification, 50×. B, Superficial dermal accumulation of neutrophils surrounded by histiocytes and some giant cells, Hematoxylin-eosin stain, magnification, 200×.
Given the clinical presentation including the persistent pain, the preceding therapy with a CD20 antibody, and the rapid deterioration of the wound situation after multiple surgical interventions, we favored the diagnosis of PG. The laboratory parameters of immunoglobulins and protein fractions were decreased.
Due to the pre-existing MS, the choice of possible immunosuppressants was limited. Treatment with intravenous immunoglobulin (2 g/kg divided over 5 days every 4 weeks) and cyclosporine (200 mg/day) was initiated immediately and lead to pain reduction and incipient healing in 2 weeks.
Surgical treatment included careful debridement and—after significant granulation—gradual coverage using the already prepared pudendal thigh flap as well as several soft tissue flaps from the gluteal region as well as the thighs.
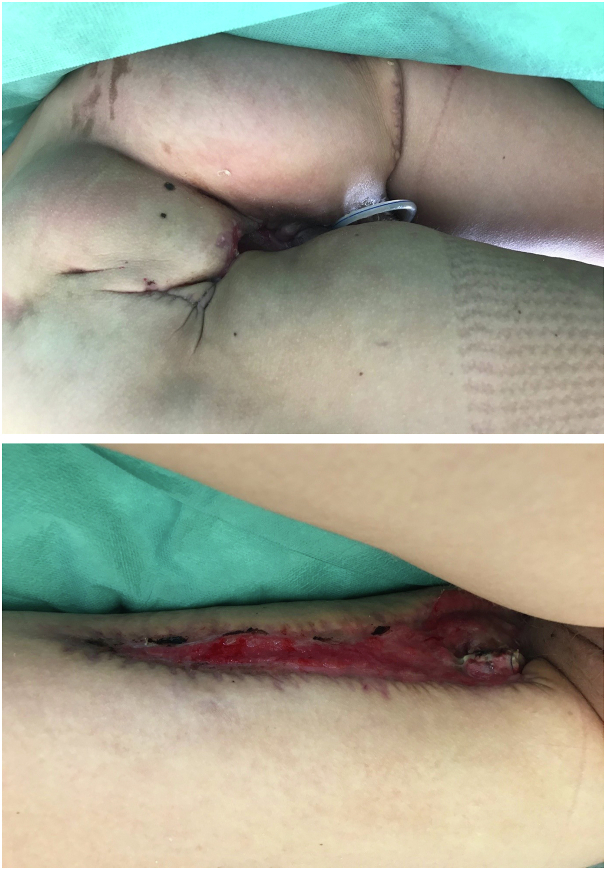
Six weeks after admission, the genital wounds were almost completely closed (Fig 3).
Fig 3.
Findings at discharge: significant reduction of the wound areas with adhesion of the wound cavities and secondary wound healing.
Discussion
The occurrence of vulvovaginal PG has been reported several times after therapy with rituximab, a chimeric monoclonal antibody targeting the CD20 antigen on B lymphocytes.6 The affected patients received the drug for B-cell systemic non-Hodgkin lymphoma6 or anti-neutrophilic cytoplasmic antibody-positive granulomatosis with polyangiitis.7
There are several hypotheses regarding the mechanisms underlying the development of PG after rituximab, including the activation of neutrophilic granulocytes and a dysregulated cytotoxic T-cell response due to B-cell depletion.7 Ocrelizumab shares the mechanism of action with rituximab but is humanized to reduce immunogenicity.
In patients who received treatment with a CD20 antibody and present with genital wounds that are inflamed, painful, and worsen with surgical therapy, physicians should be aware of PG. PG still is a diagnosis of exclusion, but the case conformed to the recent diagnostic criteria developed by the Delphi consensus.8 There are no uniform treatment recommendations for PG, but topical and systemic corticosteroids, various other immunosuppressants, and biologicals have shown good results.9 If PG is associated with ocrelizumab, therapy with intravenous immunoglobulins as well as cyclosporine may be an effective treatment option.
Ocrelizumab cannot be identified with absolute certainty as the trigger of PG, especially because this is only the second reported case as of yet. Both the underlying disease MS and the previous therapy with natalizumab might have had an influence on the development of the lesions as well. The occurrence of PG in close temporal relation to ocrelizumab administration and the improvement of the disease after discontinuation of the medication suggest a causal relationship. In the light of analyses of the US Food and Drug Administration’s Adverse Event Reporting System that found a significant signal between rituximab use and the adverse event by PG, an association between ocrelizumab use and PG in our patient seems plausible.10
Conclusion
This report extends the spectrum of adverse events of ocrelizumab by PG. Although PG is a rare skin disorder unfamiliar to many neurologists, it should be considered in the setting of a CD20 antibody treatment. Diagnosis can be challenging, but once it has been established, surgical measures should be used with restraint. In the present case, the combined use of immunoglobulins and ciclosporin followed by reconstructive surgery contributed to a good patient outcome.
Conflicts of interest
Luessi received consultancy fees from Roche and support with travel cost from Teva Pharma.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Patient consent: Written informed consent for the submission of this case report was obtained by the authors and included at the time of article submission to the journal stating that the patient gave consent with the understanding that this information may be publicly available.
References
- 1.Kamm C.P., Uitdehaag B.M., Polman C.H. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72(3-4):132–141. doi: 10.1159/000360528. [DOI] [PubMed] [Google Scholar]
- 2.Montalban X., Hauser S.L., Kappos L., et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 3.Breneman A.N., Eber A.E., Haque H., Levine L. Vulvovaginal pyoderma gangrenosum in a patient treated with ocrelizumab for multiple sclerosis. J Low Genit Tract Dis. 2022;26(2):189–191. doi: 10.1097/LGT.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 4.Ahronowitz I., Harp J., Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191–211. doi: 10.2165/11595240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Thompson A., Brenda B., Barkhof F., Carroll W. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 6.Georgakopoulos J.R., Rohekar G., Lovegrove F.E. A case of rituximab-induced pyoderma gangrenosum. JAAD Case Rep. 2018;4(10):979–981. doi: 10.1016/j.jdcr.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vikse J., Rygh A., Kaisen K., Omdal R. Life-threatening Rituximab-induced pyoderma gangrenosum successfully treated with intravenous immunoglobulin. Scand J Rheumatol. 2017;46:413–414. doi: 10.1080/03009742.2016.1241298. [DOI] [PubMed] [Google Scholar]
- 8.Maverakis E., Ma C., Shinkai K., et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi Consensus of International Experts. JAMA Dermatol. 2018;154(4):461. doi: 10.1001/jamadermatol.2017.5980. [DOI] [PubMed] [Google Scholar]
- 9.Quist S.R., Kraas L. Treatment options for pyoderma gangrenosum. J Dtsch Dermatol Ges. 2017;15(1):34–40. doi: 10.1111/ddg.13173. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal P. Pyoderma gangrenosum adverse event with rituximab use: a postmarketing pharmacovigilance analysis. Dermatol Ther. 2020;33(2) doi: 10.1111/dth.13221. [DOI] [PubMed] [Google Scholar]