Abstract
Honey bees are the most efficient pollinators of several important fruits, nuts and vegetables and are indispensable for the profitable production of these crops. Health and performance of honey bee colonies have been declining for decades due to a combination of factors including poor nutrition, agrochemicals, pests and diseases. Bees depend on a diversity of plants for nutrition as pollen is the predominant protein and lipid source, and nectar, the source of carbohydrates for larval development. Additionally, pollen and nectar also contain small amounts of plant secondary metabolites or phytochemicals that are primarily plant defense compounds. Bees have coevolved to benefit from these compounds as seen by the improved longevity, pathogen tolerance and gut microbiome abundance in worker bees whose diets were supplemented with select phytochemicals. Here we investigate the impact of four phytochemicals, known to benefit bees, – caffeine, kaempferol, gallic acid and p-coumaric acid, on hypopharyngeal gland (HPG) size of nurse bees. Newly emerged bees were provided with 25 ppm of each of the four phytochemicals in 20% (w/v) sucrose solution and the size of HPGs were measured after a 10 d period. Bees that received p-coumaric acid or kaempferol showed a significant increase in HPG size. A significant decrease in HPG size was seen in bees receiving caffeine or gallic acid. The implication of our findings on worker bee ontogeny, transitioning from nurses to foragers and relevance to foraging related competencies are discussed. It is critical that bees have access to phytochemicals to ensure colony health and performance. Such access could be through natural habitats that provide a diversity of pollen and nectar sources or through dietary supplements for bee colonies.
Keywords: Caffeine, Gallic acid, Honey bees, Hypopharyngeal glands (HPG), Kaempferol, p-coumaric acid, Phytochemicals
Caffeine, Gallic acid, Honey bees, Hypopharyngeal glands (HPG), Kaempferol, p-coumaric acid, Phytochemicals.
1. Introduction
Managed honey bee colonies are primary pollinators promoting production and yield in several major insect-pollinated crops (Hristov et al., 2020). However, global honey bee colony numbers have not kept pace with the growing demand (Aizen and Harder, 2009). Colony losses have been exacerbated by several interacting biotic and abiotic factors (Neumann and Blacquière, 2017; van Engelsdorp et al., 2012). Habitat loss and intensified agriculture have compromised access to healthy and diverse forage (Potts et al., 2010) while simultaneously increasing exposure to potentially harmful agrochemicals (Doublet et al., 2015; Johnson et al., 2010). Poor nutrition has escalated the susceptibility of bees to pests and pathogens (Berenbaum, 2015; Simone-Finstrom et al., 2016). Ongoing changes to climate further compromise the availability of nutritive flowers, as plants respond adversely to stressful growing conditions (Arathi et al., 2018; Phillips et al., 2018). Additionally, widespread prevalence of Varroa destructor, an ectoparasitic mite, mite-vectored viruses, and the resurgence of fungal and bacterial brood diseases have augmented colony stress (Dainat et al., 2012; Goulson et al., 2015; Le Conte et al., 2010), necessitating the need for integrative colony management strategies that focus on strengthening individual worker bees to promote colony performance.
Nutrition is key to the ability of an organism to withstand challenges during its lifespan. Optimal nutrition during colony growth, either through access to diverse natural forage (Smart et al., 2018) or as nutritional supplements (Fedoriak et al., 2021), determines the survival trajectory of a colony (Di Pasquale et al., 2013). While macronutrient (carbohydrate, protein and lipid) needs of honey bees are fairly well understood (Brodschneider and Crailsheim, 2010), we are now beginning to understand the importance of minerals, vitamins and plant secondary metabolites (Bernklau et al., 2019; Boncristiani et al., 2021; Geldert et al., 2021; Johnson et al., 2012; Liao et al., 2017; Mao et al., 2015). For the development of optimal dietary supplements to maintain colony health and productivity (Negri et al., 2019; Tauber et al., 2019), a deeper understanding of all compounds contributing to honey bee diet is critical. Healthy colony growth results from increased brood production which in turn depends on the presence of a healthy population of nurse bees within the colony to produce adequate brood food via glandular secretions (Döke et al., 2015; Lass and Crailsheim, 1996). Glands that produce food provisioned for brood development are the mandibular and hypopharyngeal glands (HPGs) (Brouwers, 1983; Wang and Li-Byarlay, 2015). The size of HPGs has long been used as a reliable marker of honey bee nutritional status (DeGrandi-Hoffman et al., 2010). Here, we determine the HPG size in nurse bees supplemented with each of the four dietary phytochemicals, p-coumaric acid, caffeine, gallic acid or kaempferol. Previous studies have shown that these phytochemicals, at low doses (25 ppm), improved longevity, pathogen tolerance (Bernklau et al., 2019), and gut microbiomes (Geldert et al., 2021). In addition, other studies suggest that such a dose (25 ppm) is within the range of concentration of phytochemicals in floral nectar (Kretschmar and Baumann, 1999; Palmer-Young et al., 2019). Here, we investigate the impact of the same phytochemicals at the same low dose on HPG size, a physiological trait responsible for brood food production, and discuss our findings in the context of targeted nutritional supplements for healthy honey bees.
2. Materials and methods
2.1. Bee rearing and feeding assay
The experiment was conducted at the Harry H. Laidlaw Jr. Honey bee Research facility at University of California, Davis (Davis, CA). Source colonies of Apis mellifera ligustica (Jackie Park-Burris Queens Inc., Palo Cedro, CA) and A. mellifera caucasica (Can-Am Apiaries, Orland, CA) headed by naturally mated queens, were used to obtain newly emerged worker bees. Frames of capped brood from three source colonies of respective sub-species were placed in wooden brood boxes stored in an incubator at 34.5 °C and ∼50% relative humidity until emergence (Niño et al., 2013). Groups of 17–19 one-day old workers from the same source colony were placed in individual cages fashioned out of plastic cups (Bernklau et al., 2019; Evans et al., 2009). They were given ad libitum access to one of the four phytochemicals: caffeine, gallic acid, kaempferol or p-coumaric acid, at 25 ppm in 20% sucrose (w/v), and a uniform piece of Ultra Bee pollen patty as protein (Mann Lake, Ltd., Woodland, CA). Test compounds (97.5% purity) were obtained from Sigma-Aldrich (St. Paul, MN, USA; Catalog numbers: kaempferol K0133, p-coumaric acid C9008, gallic acid G7384, caffeine C0750). Control bees received 20% (w/v) sucrose solution and pollen patty. 9–10 replicate cages per treatment were kept in an incubator at 34.5 °C and ∼50% relative humidity, daily bee mortality was recorded, and dead bees were removed. On day 10, four bees were collected from each of the cages and immediately stored at −80 °C and used later for dissection.
2.2. Hypopharyngeal gland (HPG) measures
Dissection and measurement of HPGs were completed following the protocol described in Corby-Harris and Snyder (2018). Worker heads and bodies were separated, and heads transferred into individually labeled microcentrifuge capsules over a bed of dry ice using sterile dissection tools. HPGs were then dissected, imaged under an Olympus SZX10 microscope (Olympus IE, Waltham, MA) and an Olympus SC50 microscope camera (Olympus IE, Waltham, MA). Figure 1 shows well developed (A) and poorly developed (B) HPGs. For each head, the diameters of 10 randomly selected acini, whose borders were clearly in focus, were measured (Figure 1C) in pixels and converted to millimeters using the 5.667937:1 pixel-millimeter conversion ratio. The averages of the 10 individual acini measured per bee head were used for statistical analysis.
Figure 1.
Hypopharyngeal glands (HPGs) made up of acini serve as the site for glandular secretions in nurse bees that produce brood food fed to developing larvae. The images below depict (A) well-developed acini, (B) poorly developed acini and (C) an enlarged acinus with the red measurement line. Four bees per cage per treatment were dissected and 10 acini were measured per bee for use in statistical analysis. This image is in color. HPG are white and are pictured as they appear on dissection. Figure 1C is in color as seen by the red line indicating the measurement of HPG.
2.3. Statistical Analysis
Average measurements of 10 acini from four bees per cage, confirmed for normality, were compared using a univariate analysis of variance with dietary phytochemical treatment and sub-species as independent variables (IBM Statistics SPSS 28) and the average acini size as the dependent variable followed by Tukey's post-hoc multiple comparisons to compare averages across treatments.
3. Results
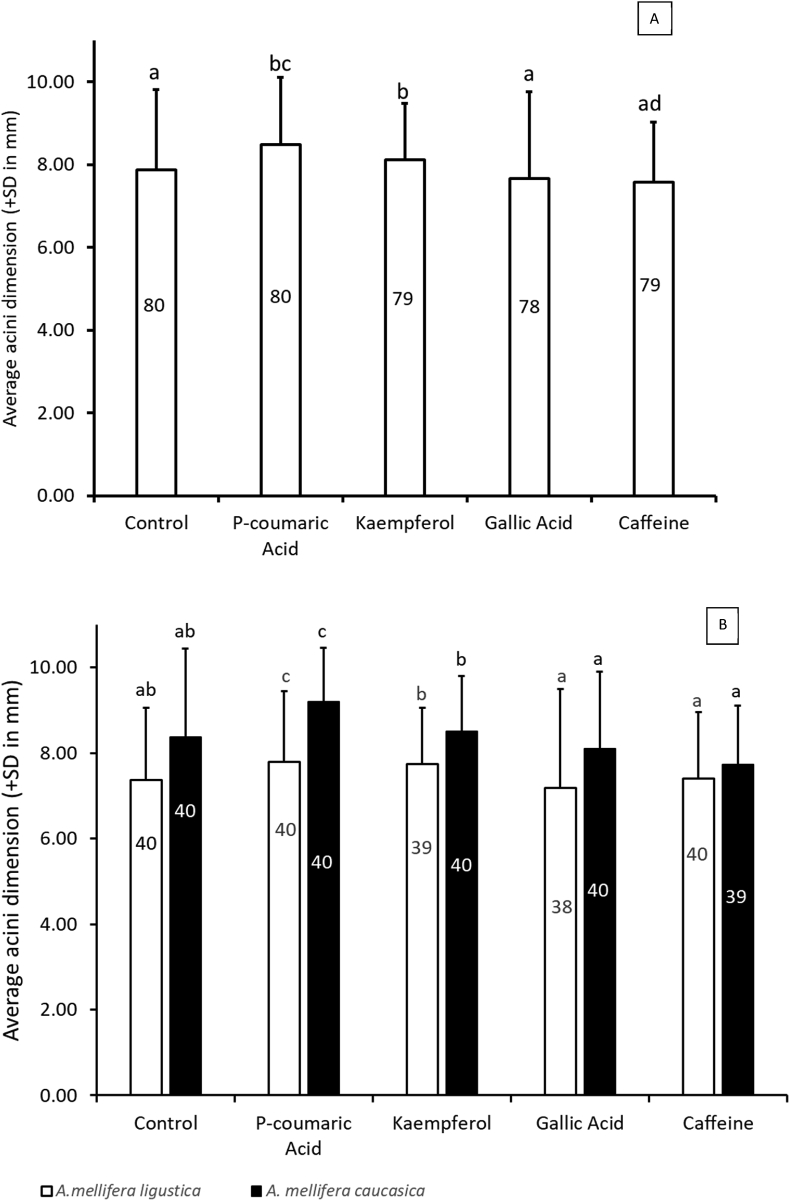
Phytochemical supplementation altered HPG development in worker bees that received 25 ppm of one of the four phytochemicals, caffeine, kaempferol, gallic acid or p-coumaric acid. Univariate analysis of variance indicated a significant effect of treatment diet on the average acini size (Table 1). Tukey's post-hoc mean comparison (Figure 2A) indicated that, while average acini sizes were significantly larger than that of the control (7.87 ± 1.94; n = 80) in bees receiving p-coumaric acid (8.49 ± 1.62; n = 80) or kaempferol (8.13 ± 1.36; n = 79), the average acini sizes were significantly smaller in bees that received caffeine (7.57 ± 1.46; n = 79) or gallic acid (7.65 ± 2.1; n = 78).
Table 1.
Univariate analysis of variance showing that the average acini measurements were significantly influenced by dietary phytochemicals.
| Source | MSS | Df | F | P |
|---|---|---|---|---|
| Dietary phytochemical treatment | 11.21 | 4 | 4.06 | 0.003 |
| Sub-species | 76.85 | 1 | 27.81 | <0.001 |
| Dietary phytochemical treatment x Sub-species | 2.99 | 4 | 1.09 | 0.36 |
| Error | 2.76 | 386 |
Figure 2.
(A) Acini sizes of worker bees fed on dietary phytochemicals. Statistically significant differences following Tukey's post-hoc analysis. Numbers within the bars indicate sample sizes for each treatment. Bars with different letters are significantly different average measurements at p < 0.0001. (B) Acini sizes for the different sub-species. Numbers within the bars indicate sample sizes for each sub-species. Bars with different letters show significantly different average measurements within the relevant sub-species at p < 0.0001.
The univariate analysis also showed a significant effect of sub-species, and a non-significant interaction effect of treatment and sub-species, on the resulting acini size following dietary phytochemical supplementation (F(1,386) = 27.81, p < 0.001; Table 1). Accordingly, the response trends for each phytochemical were similar across the two sub-species, and A. m. caucasica exhibited higher responses than A. m. ligustica. Figure 2B shows the average acini sizes in response to phytochemical supplementation in the two sub-species.
4. Discussion
Dietary supplementation with phytochemicals caffeine, kaempferol, gallic acid and p-coumaric acid has been shown to improve longevity, pathogen tolerance, pesticide resilience and gut microbiome abundance (Arathi and Bernklau, 2021; Bernklau et al., 2019; Geldert et al., 2021). Our results provide further evidence for the benefits provided by these specific phytochemicals. Improved HPG size with p-coumaric acid consumption are consistent with trends in previous reports. P-coumaric acid, is reported to being an up-regulator of detoxification and immunity genes in the P450 enzyme superfamily (Liao et al., 2017; Mao et al., 2013). Finding that HPG size decreased with caffeine consumption is novel and may relate to the role caffeine plays in promoting learning and memory in foragers (Wright et al., 2013). Caffeine has been suggested to act as an adenosine receptor antagonist through potentiated responses of mushroom body neurons involved in olfactory learning and memory. The differential effect of caffeine and gallic acid in relation to other phytochemicals tested suggests that the beneficial impacts of these phytochemicals could be age dependent.
Dietary phytochemicals have also shown to benefit Bombus impatiens infected with Crithidia bombi (Richardson et al., 2015) and honey bees infected with Nosema ceranae (Bernklau et al., 2019). While pathogen tolerance is not an age-independent benefit, HPG size is important for younger, nurse-aged bees that perform brood care. Cognitive capacities such as learning and memory are important for older, forager-aged bees. Age-related changes to hormones have been shown to drive the transitioning from nursing to foraging behaviors in honey bees (Pankiw et al., 1998; Robinson, 1992). Our results showing that caffeine reduces HPG size suggests that such a reduction maybe a precursor to the onset of foraging. Therefore, caution may be recommended for colony level supplementation with caffeine to avoid precocious onset of foraging behavior.
Enhanced HPG development in nurse-age workers receiving p-coumaric acid aligns with its established role in honey bee ontogeny. Mao et al. (2015) demonstrated that dietary p-coumaric acid reduced ovarian development in workers and may regulate caste determination via methylation. This complements the fact that queen mandibular pheromone and (E)-βocimene larval pheromones both suppress ovariole activation and promote HPG development in workers (Huang et al., 1989; Mohammedi et al., 1996; Traynor et al., 2014). Thus, in addition to reinforcing the reproductive monopoly of the queen, dietary p-coumaric acid may act in tandem with queen and/or brood pheromones to enforce division of labor by supporting healthy HPG development necessary for functioning nurses (Crailsheim, 1991; Naiem et al., 1999; Pankiw et al., 1998).
While there are no studies so far demonstrating and comparing the sensitivity to diet between two sub-species of the western honey bee, a few others have investigated physiological variability amongst these. In a study by Al-Ghamdi et al., (2011), there is evidence for HPG variation among subspecies such that nurse-aged (9 days) A. m. carnica workers developed larger HPGs and more acini secretory cells than A. m. jemenitica, the Arabian honey bee. Our findings are significant in that dietary regimes could be tailored to unique subspecies that could help optimize resource allocation in commercial settings.
The findings reported here support the importance of diverse nutritional sources for healthy honey bee populations. Diverse pollen and nectar sources provide a supply of micronutrients and phytochemicals that are important for normal physiological and behavioral ontogeny of worker bees. Loss of natural habitats that provide diversity in nutritional components for bees necessitates the need for supplemental feeding to ensure healthy colony growth. Phytochemicals in low doses could be developed as supplemental nutrients to ensure healthy colonies that can provide much needed pollination services and ensure agricultural productivity. Future research is necessary to evaluate the benefits of colony level supplementation and to develop appropriate application regimes.
Declarations
Author contribution statement
Elina L. Niño, Arathi H.S. (Arathi Seshadri): Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Seiji Yokota, William H. O. Stacy: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Arathi H.S. was supported by Foundation for Food and Agriculture Research (549029) and USDA (2030-21000-055-000D); Elina L. Niño was supported by California State Beekeepers Association and Foundation for Food and Agriculture Research (549032).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The statements made in the article represent authors' views and should not be interpreted as endorsement from their respective employers or the funding agencies. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA) or University of California. USDA is an equal opportunity provider, employer, and lender.
Contributor Information
Elina L. Niño, Email: elnino@ucdavis.edu.
H.S. Arathi, Email: arathi.seshadri@usda.gov.
References
- Aizen M.A., Harder L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009;19:915–918. doi: 10.1016/j.cub.2009.03.071. [DOI] [PubMed] [Google Scholar]
- Al-Ghamdi A.A., Al-Khaibari A.M., Omar M.O.M. Effect of honeybee race and worker age on development and histological structure of hypopharyngeal glands of honeybee. Saudi J. Biol. Sci. 2011;18:113–116. doi: 10.1016/j.sjbs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arathi H.S., Bernklau E. Context-dependent effect of dietary phytochemicals on honey bees exposed to a pesticide, thiamethoxam. J. Insect Sci. 2021;21 doi: 10.1093/jisesa/ieab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arathi H.S., Bjostad L., Bernklau E. Metabolomic analysis of pollen from honey bee hives and from canola flowers. Metabolomics. 2018;14:86. doi: 10.1007/s11306-018-1381-5. [DOI] [PubMed] [Google Scholar]
- Berenbaum M.R. Does the honey bee “risk cup” runneth over? Estimating aggregate exposures for assessing pesticide risks to honey bees in agroecosystems. J. Agric. Food Chem. 2015 doi: 10.1021/acs.jafc.5b01067. [DOI] [PubMed] [Google Scholar]
- Bernklau E., Bjostad L., Hogeboom A., Carlisle A., Arathi H.S. Dietary phytochemicals, honey bee longevity and pathogen tolerance. Insects. 2019;10:14. doi: 10.3390/insects10010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncristiani D.L., Tauber J.P., Palmer-Young E.C., Cao L., Collins W., Grubbs K., Lopez J.A., Meinhardt L.W., Nguyen V., Oh S., Peterson R.J., Zamora H., Chen Y., Evans J.D. Impacts of diverse natural products on honey bee viral loads and health. Appl. Sci. 2021;11 [Google Scholar]
- Brodschneider R., Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010;41:278–294. [Google Scholar]
- Brouwers E.V.M. Activation of the hypopharyngeal glands of honeybees in winter. J. Apicult. Res. 1983;22:137–141. [Google Scholar]
- Corby-Harris V., Snyder L.A. JoVE; 2018. Measuring Hypopharyngeal Gland Acinus Size in Honey Bee (Apis mellifera) Workers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crailsheim K. Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J. Comp. Physiol. B. 1991;161:55–60. [Google Scholar]
- Dainat B., Evans J.D., Chen Y.P., Gauthier L., Neumann P. Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2012;78:981–987. doi: 10.1128/AEM.06537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandi-Hoffman G., Chen Y., Huang E., Huang M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.) J. Insect Physiol. 2010;56:1184–1191. doi: 10.1016/j.jinsphys.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G., Salignon M., Le Conte Y., Belzunces L.P., Decourtye A., Kretzschmar A., Suchail S., Brunet J.-L., Alaux C. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One. 2013;8 doi: 10.1371/journal.pone.0072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döke M.A., Frazier M., Grozinger C.M. Overwintering honey bees: biology and management. Curr. Opin. Insect Sci. 2015;10:185–193. doi: 10.1016/j.cois.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Doublet V., Labarussias M., Miranda J.R.d., Moritz R.F.A., Paxton R.J. Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2015;17:969–983. doi: 10.1111/1462-2920.12426. [DOI] [PubMed] [Google Scholar]
- Evans J.D., Chen Y.P., diPrisco G., Pettis J., Williams V. Bee cups: single-use cages for honey bee experiments. J. Apicult. Res. Bee World. 2009;48:300–302. [Google Scholar]
- Fedoriak M., Kulmanov O., Zhuk A., Shkrobanets O., Tymchuk K., Moskalyk G., Olendr T., Yamelynets T., Angelstam P. Stakeholders’ views on sustaining honey bee health and beekeeping: the roles of ecological and social system drivers. Landsc. Ecol. 2021;36:763–783. [Google Scholar]
- Geldert C., Abdo Z., Stewart J.E., Arathi H.S. Dietary supplementation with phytochemicals improves diversity and abundance of honey bee gut microbiota. J. Appl. Microbiol. 2021;130:1705–1720. doi: 10.1111/jam.14897. [DOI] [PubMed] [Google Scholar]
- Goulson D., Nicholls E., Botías C., Rotheray E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347 doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- Hristov P., Shumkova R., Palova N., Neov B. Factors associated with honey bee colony losses: a mini-review. Veterin. Sci. 2020;7:166. doi: 10.3390/vetsci7040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.-Y., Otis G.,W., Teal P.,E.A. Nature of brood signal activating the protein synthesis of hypopharyngeal gland in honey bees, Apis mellifera (Apidae : hymenoptera) Apidologie. 1989;20:455–464. [Google Scholar]
- Johnson R.M., Ellis M.D., Mullin C.A., Frazier M. Pesticides and honey bee toxicity — USA. Apidologie. 2010;41:312–331. [Google Scholar]
- Johnson R.M., Mao W., Pollock H.S., Niu G., Schuler M.A., Berenbaum M.R. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmar J.A., Baumann T.W. Caffeine in citrus flowers. Phytochemistry. 1999;52:19–23. [Google Scholar]
- Lass A., Crailsheim K. Influence of age and caging upon protein metabolism, hypopharyngeal glands and trophallactic behavior in the honey bee (Apis mellifera L.) Insectes Soc. 1996;43:347–358. [Google Scholar]
- Le Conte Y., Ellis M., Ritter W. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 2010;41:353–363. [Google Scholar]
- Liao L.H., Wu W.Y., Berenbaum M.R. Impacts of dietary phytochemicals in the presence and absence of pesticides on longevity of honey bees (Apis mellifera) Insects. 2017;8 doi: 10.3390/insects8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W., Schuler M.A., Berenbaum M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. National Acad. Sci. USA. 2013;110:8842–8846. doi: 10.1073/pnas.1303884110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W., Schuler M.A., Berenbaum M.R. A dietary phytochemical alters caste-associated gene expression in honey bees. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammedi A., Crauser D., Paris A., Le Conte Y. Effect of a brood pheromone on honeybee hypopharyngeal glands. C R Acad Sci III. 1996;319:769–772. [PubMed] [Google Scholar]
- Naiem E.S., Hrassnigg N., Crailsheim K. Nurse bees support the physiological development of young bees (Apis mellifera L.) J. Comp. Physiol. B. 1999;169:271–279. [Google Scholar]
- Negri P., Villalobos E., Szawarski N., Damiani N., Gende L., Garrido M., Maggi M., Quintana S., Lamattina L., Eguaras M. Towards precision nutrition: a novel concept linking phytochemicals, immune response and honey bee health. Insects. 2019;10:401. doi: 10.3390/insects10110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P., Blacquière T. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evolutionary Appl. 2017;10:226–230. doi: 10.1111/eva.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño E.L., Malka O., Hefetz A., Tarpy D.R., Grozinger C.M. Chemical profiles of two pheromone glands are differentially regulated by distinct mating factors in honey bee queens (Apis mellifera L.) PLoS One. 2013;8 doi: 10.1371/journal.pone.0078637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Young E.C., Farrell I.W., Adler L.S., Milano N.J., Egan P.A., Irwin R.E., Stevenson P.C. Secondary metabolites from nectar and pollen: a resource for ecological and evolutionary studies. Ecology. 2019;100 doi: 10.1002/ecy.2621. [DOI] [PubMed] [Google Scholar]
- Pankiw T., Huang Z.Y., Winston M.L., Robinson G.E. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Phillips B.B., Shaw R.F., Holland M.J., Fry E.L., Bardgett R.D., Bullock J.M., Osborne J.L. Drought reduces floral resources for pollinators. Global Change Biol. 2018;24:3226–3235. doi: 10.1111/gcb.14130. [DOI] [PubMed] [Google Scholar]
- Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Richardson L.L., Adler L.S., Leonard A.S., Andicoechea J., Regan K.H., Anthony W.E., Manson J.S., Irwin R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. Royal Soc. B. 2015;282 doi: 10.1098/rspb.2014.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Simone-Finstrom M., Li-Byarlay H., Huang M.H., Strand M.K., Rueppell O., Tarpy D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016;6 doi: 10.1038/srep32023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart M., Otto C., Cornman R., Iwanowicz D. Using colony monitoring devices to evaluate the impacts of land use and nutritional value of forage on honey bee health. Agriculture. 2018;8:2. [Google Scholar]
- Tauber J.P., Collins W.R., Schwarz R.S., Chen Y., Grubbs K., Huang Q., Lopez D., Peterson R., Evans J.D. Natural product medicines for honey bees: perspective and protocols. Insects. 2019;10:356. doi: 10.3390/insects10100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor K.S., Le Conte Y., Page R.E., Jr. Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav. Ecol. Sociobiol. 2014;68:2059–2073. doi: 10.1007/s00265-014-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelsdorp D., Caron D., Hayes J., Underwood R., Hensen M., Rennich K., Spleen A., Andree M., Snyder R., Lee K., Roccasecca K., Wilson M., Wilkes J., Lengerich E., Pettis J. A national survey of managed honey bee 2010-2011 winter colony losses in the USA: results from the Bee Informed Partnership. J. Apicult. Res. 2012;51:115–124. [Google Scholar]
- Wang Y., Li-Byarlay H. In: Adv. Insect Physiol. Jurenka R., editor. Academic Press; 2015. Chapter two - physiological and molecular mechanisms of nutrition in honey bees; pp. 25–58. [Google Scholar]
- Wright G.A., Baker D.D., Palmer M.J., Stabler D., Mustard J.A., Power E.F., Borland A.M., Stevenson P.C. Caffeine in floral nectar enhances a pollinator's memory of reward. Science. 2013;339:1202–1204. doi: 10.1126/science.1228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.