Abstract
Pinostrobin is a flavanone isolated from Renealmia alpinia (Rottb.) Maas, which is used to treat painful diseases and ailments; indigenous peoples use it as plasters. Different plant species have been reported as a source of this flavonoid, among which are: Boesenbergia rotunda, Cajanus cajan, Piper ecuadorense, Piper hispidum, Teloxys graveolens, Kaempferia pandurata, among others.
Pinostrobin expresses potentially useful biological activities such as antioxidant, analgesic, and dermal anti-inflammatory, at low levels nonetheless due to its low solubility. The formation of inclusion complexes deems a good strategy to improve the pharmacologic effects of many substances.
In the present work, we evaluated the dermal toxicity, analgesic and dermal anti-inflammatory activity of pinostrobin included in cyclodextrins, to improve those effects on experimental animals. To include pinostrobin, we used two of beta cyclodextrin (βCD) and hydroxypropil beta cyclodextrin (HPβCD) complexes using two methods developed by Benesi-Hildebrand and Higuchi-Connors. Dermal anti-inflammatory activity was evaluated in experimental mice by inhibiting the edema generated by 12-O-tetradecanoylforbol-13-acetate (TPA). Analgesic activity was evaluated by inducing chemical pain by means of a Siegmund test. Antioxidant activities were measured with two in vitro tests.
Analgesic and dermal anti-inflammatory activities of pinostrobin, as included in control and experimental complexes, showed comparatively better effects than pinostrobin without inclusion complexes. Our results indicate that both beta cyclodextrin (βCD) and hydroxypropyl beta cyclodextrin (HPβCD) enhance the different effects of pinostrobin, which may indicate greater bioavailability.
Keywords: Pinostrobin, Acute dermal toxicity, Cyclodextrins, Antioxidant, Analgesic, And anti-inflammatory activity
Graphical abstract

Pinostrobin, Acute dermal toxicity, Cyclodextrins, Antioxidant, Analgesic, And anti-inflammatory activity.
1. Introduction
Inflammation is a response to a set of actions derived from the immune response to a stimulus that can be infectious, physical, chemical, or autoimmune. Although inflammation may be a natural process, its exaggerate and chronic expression and prevalence (per million) are present in different diseases such as rheumatoid arthritis (54.4; 2013–2015), osteoarthritis (30.8; 2008–2011), obstructive pulmonary disease (15.5; 2015), and cancer (5.2; 2010–2015), in the United States only [1, 2, 3, 4]. Different drug types are used to treat these diseases, including non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit the cyclooxygenase 1 and 2 enzymes (COX-1 and COX-2). As COX-1 is constitutive, its inhibition leads to the manifestation of different undesirable side effects such as gastric ulcers and increased blood pressure in hypertensive patients [5]. In these patients, particularly, the use of oral NSAIDs is avoided due to their systemic side effects, including nausea, cardiotoxicity, vomiting, dyspepsia, diarrhea, kidney failure, and nephrotic syndrome [6, 7].
Although drugs such as sulfasalazine, chloroquine, and hydroxychloroquine are also used as inhibitors of prostaglandins and cytokines, they trigger side effects such as headache, fever, gastric problems, and skin rash. The use of monoclonal antibodies has been introduced to treat rheumatoid arthritis, which exerts their effect through inhibition mechanisms of the immune system. However, there may persist risks such as anemia, nausea, headache, hypotension, and serious infections such as tuberculosis, hepatitis, cardiotoxicity, and opportunistic diseases [8]. For these reasons, it is essential to research safer drugs to minimize these side effects.
Pinostrobin is a flavonoid that has been shown to have anti-inflammatory activity. It is found in different plant species, including Cajanus cajan, Boesenbergia rotunda, Populus tomentosa Carr, Piper ecuadorense Sodiro, Piper hispidum Kunth, Kaempferia pandurate, Corymbia torellian, Teloxys graveolens, Polygonum ferrugineum [9, 10, 11], which have been traditionally used for the treatment of stomach ailments, dysentery, fever, antifungal, gastric ulcer, and inflammation [12, 13, 14]. Another plant from which pinostrobin has been isolated from is Renealmia alpinia, (Rottb.) Maas. This plant species is administered orally and dermally by the Emberá Katíos indigenous community in order to treat snakebite [15]. Pinostrobin anti-inflammatory activity has been verified by in vitro, in vivo hemolysis, coagulation, and proteolysis studies [16, 17, 18]. Other pharmacological activities, including both extracts and pinostrobin, have exhibited analgesic and anti-inflammatory activities by oral administration [19]. Essential oils from this plant have also been found to have analgesic activity [20].
Moreover, pinostrobin, when isolated from Cajanus cajan (L.) Millsp, has been found to inhibit edema when administered orally; it also inhibits the production of tumor necrosis factor-alpha (TNF-α) and interleukin one beta (IL-1β) in cells. Macrophages of murine origin also show a slight inhibition of COX-1 and COX-2 cyclooxygenases at a concentration of 100 μg/mL [21].
The biological activity of pinostrobin is mainly related to its antioxidant effects, which are generated by the possibility of delocalizing electrons in its structure. Free radicals can activate transcription factors that cause cytokines and other molecules that induce the inflammatory process. Some studies have shown that flavonoids exhibit analgesic activity due to the inhibition of cytokines, prostaglandins, and nitric oxide (NO) radicals [22, 23]. By counteracting the production of free radicals in the inflammatory cascade, the expression of cytokines as TNF-α, interleukins, and cyclooxygenases is reduced, which reduces the inflammatory effect. These synergistic effects by which flavonoids modulate the expression of proinflammatory molecules, having probably not a single mechanism but acting on multiple sites of the cellular machinery, motivate the search for flavonoids with a potency comparable to NSAIDs, which would allow to reduce the effects associated with the use of NSAIDs such as gastric ulcers, increased cardiovascular risk, and related vascular activity. This search is ongoing [23, 24, 25].
One of the main drawbacks in the study of pinostrobin pharmaceutical applications is its low solubility in aqueous media. Cyclodextrins are cyclic oligosaccharides that result from the microbiological degradation of starch, among which are those formed by six (α−), seven (β−) or eight (γ– cyclodextrin) glucopyranose units, linked by a bond α [1–4] glycosidic, which have been used in the pharmaceutical, cosmetic and food industries, as well as in a diverse range of commercial presentations such as tablets, eye drops and ointments [26, 27]. There are also synthetic derivatives of cyclodextrins, such as hydroxypropyl -b-cyclodextrin, which is the product of the condensation of beta cyclodextrin with propylene oxide. This modification significantly improves the dissolution makes it 13 times more soluble than beta cyclodextrin. Due to the characteristics of these cyclodextrins and to the reports in which it is mentioned how the solubility of the molecules is improved, it is interesting to evaluate how the complexes interact in the different tests that were performed in this study [25, 26, 27, 28].
Therefore, our goal in this study is to evaluate the antioxidant, analgesic, and anti-inflammatory activity of pinostrobin (dermal route). On the other hand, due to its low solubility in aqueous media, it is desired to verify if pharmacological activity improves when pinostrobin form inclusion complex in cyclodextrin.
2. Methodology
2.1. Reagents
Pinostrobin and 12-O-tetradecanoylphorbol-13-acetate (TPA) were purchased from Carbosynth Limited (Compton, Berkshire, UK), βCD and HPβCD, diphenyl-1-picrylhydrazil (DPPH), phenyl-1,4 benzoquinone, deuterium oxide, indomethacin, and ibuprofen were purchased from Sigma Aldrich Corporation (Saint Louis, Missouri, United States). A detailed list of reagents can be found in the appendix.
2.2. Determination of solubility diagrams
Methods developed by Benesi-Hildebrand and Higuchi-Connors, were used. The method developed by Benesi-Hildebrand has, as its initial assumption, the formation of inclusion complexes with stoichiometry 1:1. The Higuchi-Connors method was used to corroborate it. The Benesi-Hildebrand method fixes ligand concentration (cyclodextrin) to different solutions with variable amounts of the substrate, from one to five mM. The absorbance of the free ligand is measured, and then subtracted from the rest of the solution's absorbances levels (ΔA). The inverse of ΔA is then plotted against the inverse of substrate concentration. After that, linear regression of data is performed. From (1), it is possible obtain (2), the intercept is divided by the slope found, thus obtaining the inclusion constant (K), in M−1 units [29].
| (1) |
| (2) |
where,
Eq. (1): Equation to find the inclusion constant by the method developed by Benesi-Hildebrand.
In the Higuchi-Connors method (Eq. 3), unlike the Benesi-Hildebrand method, the substrate concentration was fixed, and the amount of ligand was varied from one to seven mM. These solutions were then stabilized by shaking for three days and filtered with 0.45 μm acrodisc. The absorbance spectrum between 190–400 nm was measured to the resulting solution, and the maximum absorbance value was reported. Pinostrobin concentration was obtained from the calibration curve, and plotted against the concentration of β-CD, to obtain a Higuchi-Connors diagram. Finally, a linear adjustment was made, and the inclusion constant was obtained [31, 32].
| (3) |
where,
Eq. (3): Equation to find the inclusion constant by the method developed by Higuchi-Connors.
2.3. Preparation and characterization of inclusion complexes pinostrobin–cyclodextrin
Equimolar amounts of cyclodextrin-pinostrobin were mixed by stirring for 72 h at 25 °C. Then, the resulting substance was lyophilized in a FreeZone 6 Labconco (Missouri, United States) until a solid was obtained. Different techniques were used to characterize this solid. A nuclear magnetic resonance spectroscopy (NMR), was carried out, using a Bruker Ascend 400 MHZ equipment (Massachusetts, United States), thus obtaining 1H NMR spectra of the complex and cyclodextrin using deuterium oxide as solvent. Infrared (IR) spectra of the complexes, obtained in a Variant 640 equipment (California, United States). Using a Mettler Toledo DSC 3, (Ohio, United States) a Differential Scanning Calorimetry (DSC) was employed for thermal analysis. We weighed 1 mg of each sample. Then, we fabricated one tablet per sample using potassium bromide (KBr). The spectrum of each tablet was measured using Variant 640. We used MestReNova NMR software version 11.0.3 (Mestrelab Research, Spain) for NMR visualization, and for IR, DSC, data analysis Origin-8 from OriginLab Corporation (Norhampton, USA).
2.4. Experimental animals
Mice of the Swiss Webster strain, weighing between 20–25 g, were supplied by the vivarium of the Universidad de Antioquia's SIU (Sede de Investigación Universitaria), in Medellín, Colombia. They were housed in rooms with air exchange every 4 h, controlled temperature (25 ± 3 °C), light/dark cycles of 12 h, with access to water and food ad libitum. The procedures described are endorsed by the Universidad de Antioquia's Ethics Committee for animal experimentation, in proceedings number 99 and 100, and by the guidelines of the Canadian Council on Animal Care (CCAC) [32].
2.5. Toxicity evaluation, dermal of pinostrobin
The acute dermal toxicity test of pinostrobin was performed according to guide 434 of the Organization for Economic Cooperation and Development (OECD). After shaving 10% of the experimental animal's body area, pinostrobin was applied at dermal level on their back corresponding to 1000 mg/kg of pinostrobin and carried in carboxymethylcellulose, with a weight/volume ratio of 10. The guide suggests using this dose in absence of information about the substance tested. This preparation was fixed by surgical bandaging the test substance to the application area. During this procedure, the experimental animals were anesthetized by ketamine/xylazine (87.5 mg/kg/12.5 mg/kg).
Immediately after dose application, the animals were observed during the first 30 min and then periodically during the first 24 h (with special attention in the first 4 h), for a total of 14 days. Toxicity signs and possible deaths were recorded. Changes and damages observed on the skin, fur, eyes, and mucous membranes, body weight, as well as other possible toxicity signs were recorded.
Post-mortem visual and weight examinations of mice's internal organs were made immediately after the animals were sacrificed. Weight of each organ was expressed as percentage of body weight (% BW), as expressed in Eq. (4).
| (4) |
Eq. (4): Body weight percentage (% BW).
2.6. Measurement of antioxidant activity
Antioxidant activity was measured by ferric/reducing antioxidant power (FRAP) and 2,2-Diphenyl-1-picril-hydrazil (DPPH), according to Chrzczanowicz et alia [33]. FRAP methodology was adapted by mixing 30 μL of a sample (pinostrobin, complex or Trolox), mixed with 90 μL of deionized water and 900 μL of FRAP reagent (pre-incubated at 37 °C). FRAP reagent results from mixing 10 mL of a sodium-acetate buffer, 1mL of 2,4,6-tripyridyl-S-triazine (TPTZ) reagent with a concentration of 10 mM, and 1 mL of ferric chloride hexahydrate (FeCl3.6H2O) with a concentration of 20 mM. This mixture was left at 37 °C for 4 min reacting, and the absorbance at 593 nm was measured with a Shimadzu 1700 spectrophotometer (Osaka, Japan). Absorbance data were expressed as Trolox equivalents to determine antioxidant activity.
For the DPPH methodology, 5 μL of a 10 mM solution of the radical dissolved in methanol was taken, and it was diluted to 970 μL with methanol. After 3 min, 25 μL of a sample (pinostrobin, complex or Trolox) were added, incubated for 30 min, and the absorbance was measured at 517 nm. Absorbance data were expressed as Trolox equivalents, using a calibration curve. They were made in triplicates for each of the concentrations.
The antioxidant activity measurements were complemented with the determinating of the electrochemical index, using differential scanning voltammetry (DPV) by measuring the electrochemical index. The electrochemical index has the advantage of allowing the determination of different oxidation potentials in a sample, which can be compared with the oxidation potential of a standard. In this work, the sakuranetin electrochemical index obtained was compared concerning Trolox. The electrochemical behavior of different concentrations of Trolox and pinostrobin was measured by differential scanning voltammetry (DPV). For this purpose, three-electrode cells were used, using glassy carbon electrode (GCE) as working electrode, platinum as a counter electrode, and silver/silver chloride (Ag|AgCl|KClsat) as reference. Phosphate buffer (PBS) was used as a solvent. The GCE working electrode was cleaned by polishing using alumina powder (1 μm, 0.3 μm, and 0.05 μm) for 2 min each and then washed with plenty of water and electrochemically activated (−0.6 V–1 V, vs, Ag/AgCl reference electrode) until a constant response was obtained. The electrochemical index (EI) was defined as the division of the peak current (I) and its potential (P), EI = I/P.
2.7. Determination of the in vivo anti-inflammatory activity of pinostrobin
The topical anti-inflammatory activity of pinostrobin and pinostrobin included in cyclodextrins was evaluated by inducing the inflammatory response through dermal TPA [34].
Initially, all mice groups were treated with 20 μL of TPA at a concentration of 0.125 μg/μL After 30 min, the test substances were applied. The substances were dissolved in a 50:50 acetone-water volume fraction and supplied to groups of three mice between 6–8 weeks of age, weighing 23–25 g, in the right ear of each animal, and the measurements were made every 2 h until complementing the first 6 h and then at 24 and 48 h, using a Fowler Ultra-Cal Mark III digital micrometer (Massachusetts, United States).
The substances under study were dissolved in acetone and then administered topically in a volume of 20 uL on each mouse's right ear. 13 working groups of three mice each were designed (n = 39). Three groups received pinostrobin at doses of 1.5, 2, and 3 mg; six other groups were treated with pinostrobin complexes with of 1.5, 2, and 3 mg. As a control group, two groups were tested, to which βCD and HPβCD were applied. Finally, two groups were evaluated: the dissolution vehicle and indomethacin, at a 3 mg/ear dose.
2.8. Analgesic activity of pinostrobin
The analgesic activity of pinostrobin and βCD and HPβCD based complexes were evaluated at a 20 mg/kg concentration, administered orally (p.o.), dissolved in phosphate buffer at pH 7.2, and 1% with DMSO. After 30 min, phenyl quinone at a 4 mg/kg concentration was administered intraperitoneally (i.p.). After 5 min, the writhes were recorded for 10 min. This procedure was modified by varying how phenyl quinone was administered after administering the test substance. For this study, the administration of phenyl quinone was modified to 15, 30, and 45 min to evaluate progressively the analgesic activity of pinostrobin in the complexes. Pure ibuprofen was included in the two cyclodextrins used as a positive control dose of 75 mg/kg [35].
2.9. Statistical analysis
The results were expressed as the mean ± standard deviation (SD); statistical significance between groups was analyzed using an ANOVA test, followed by a Dunnet comparison test. For the tests, a p-value ≤ 0.05 was as considered significant. This permitted to determine the statistical significance between groups. We use GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA) for these analyses.
3. Results
3.1. Study of complex formation
The inclusion constant (K) obtained by the Benesi-Hildebrand method was 276.5 M−1 for the pinostrobin-βCD and 302.0 M−1 for the pinostrobin-HPβCD complex. In the Higuchi-Connor method, the values of the inclusion constants were 2170.8 M−1 for pinostrobin-βCD complex and 497.2 M−1 for the pinostrobin-HPβCD complex. The linear solubility diagram for both methods showed that the relationship between cyclodextrin and flavonoid is equimolar. (see appendix A.1).
3.1.1. NMR spectra for ligand and cyclodextrins complex
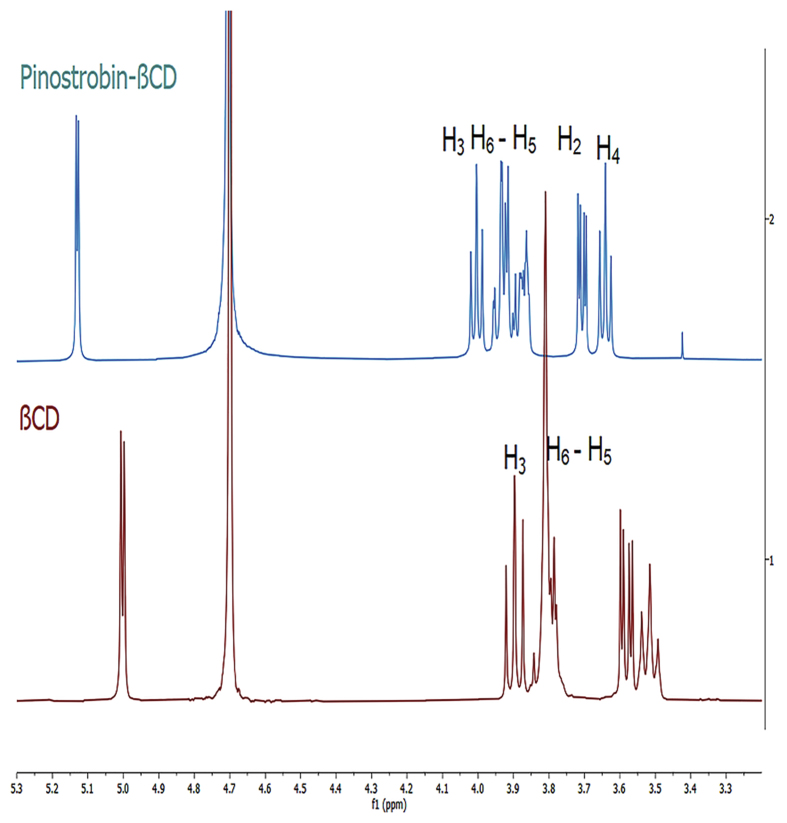
Figure 1 shows 1H hydrogen NMR for complex pinostrobin-βCD and pure βCD. Hydrogens 2, 3, 5, and 6 of the βCD displaced, this accounting for the formation of a complex. Table 1 summarizes, the most important signal shifts and observed displacements of the hydrogens in BCD and in the complex.
Figure 1.
1H shift: NMR spectrum pinostrobin-βCD compared to βCD.
Table 1.
Chemical shift in ppm of different hydrogens, βCD, and pinostrobin-βCD.
| H- βCD | Ϭ βCD (ppm) | Ϭ Complex (ppm) |
|---|---|---|
| H1 | 5.01 | 5.13 |
| H2 | 3.60 | 3.72 |
| H3 | 3.90 | 4.00 |
| H4 | 3.52 | 3.64 |
| H5 | 3.79 | 3.86 |
| H6 | 3.81 | 3.94 |
1H NMR (400 MHz, D2O) δ (βCD) 5.33, 5.01, 5.00, 4.70, 3.92, 3.90, 3.90, 3.90, 3.87, 3.85, 3.84, 3.81, 3.81, 3.80, 3.79, 3.79, 3.78, 3.60, 3.59, 3.57, 3.56, 3.54, 3.52, 3.49.
1H NMR (600 MHz, D2O) (Pinostrobin-βCD) δ 7.50, 7.50, 7.49, 7.49, 7.48, 7.45, 7.44, 5.57, 5.48, 5.13, 5.13, 4.92, 4.70, 4.02, 4.00, 3.99, 3.96, 3.96, 3.95, 3.94, 3.93, 3.92, 3.92, 3.90, 3.89, 3.88, 3.88, 3.88, 3.87, 3.87, 3.86, 3.86, 3.86, 3.73, 3.72, 3.71, 3.70, 3.70, 3.68, 3.66, 3.64, 3.63, 3.42.
3.1.2. IR spectra for ligand and cyclodextrins complex
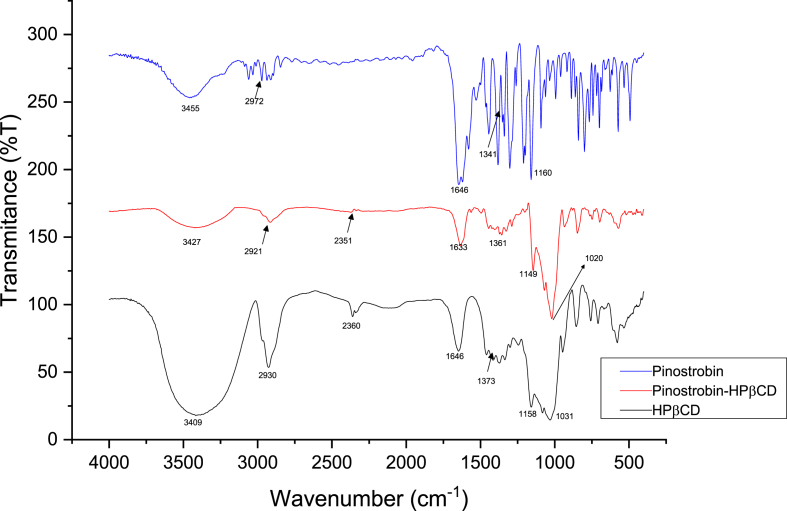
Infrared, NMR spectra, and thermal DSC experiments, showed evidence of a complex formed between pinostrobin-βCD and pinostrobin-HPβCD. Their interactions generated a complex of different physical-chemical properties from pure substances. Hydrogen displacement as mentioned earlier were observed in the RMN and the signal intensity differences. In the IR spectra, signal shifts were observed with respect to a pinostrobin pure at 1150 cm−1 for pinostrobin-βCD and 1160 cm−1 for the pinostrobin-HPβCD complex shown in Figures 2 and 3.
Figure 2.
Infrared spectra of the pinostrobin, pinostrobin-βCD and βCD.
Figure 3.
Infrared spectra of the pinostrobin, pinostrobin-HPβCD and HPβCD.
3.1.3. DSC for ligand and cyclodextrins complex
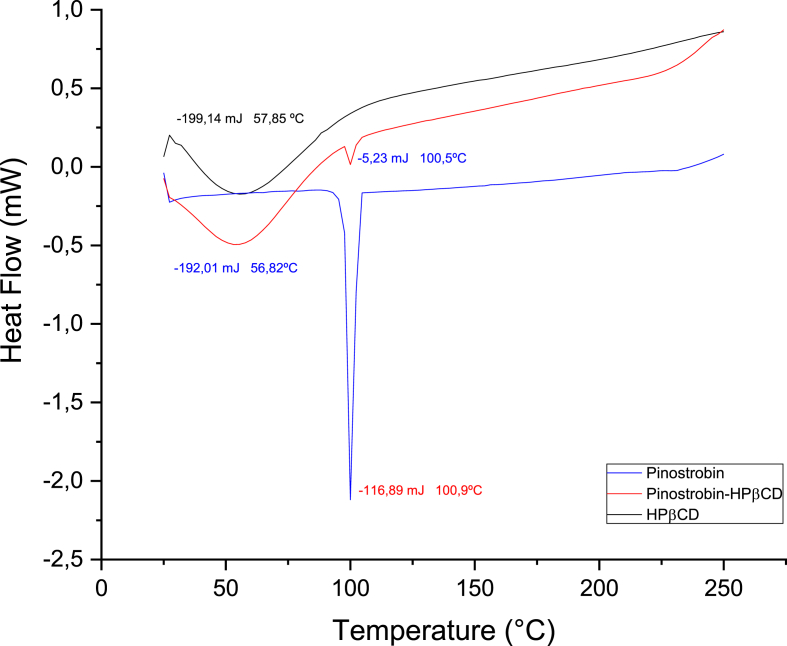
Figures 4 and 5 show the thermal events of the pinostrobin-βCD and pinostrobin-HPβCD complexes when performing a differential scanning calorimetry, with a temperature ramp rate of 5 °C/min, starting at 25 °C–250 °C. Endothermic events were observed as follows: for βCD it was presented at a temperature of 78.8 °C, while for the pinostrobin-βCD complex, it was observed at a lower intensity, at 78.5 °C. Pinostrobin has a melting temperature of 100.9 °C, while for pinostrobin-βCD, it was 99.5 °C. Figure 5 shows the same endothermic signals observed in Figure 4. However, due to probable interactions between water and βCD, some endothermic events were not observed as the intensity of endothermic heat by the HPβCD complex was lower than the signal observed in pinostrobin.
Figure 4.
Thermal events obtained by DSC of pinostrobin, pinostrobin-βCD and βCD.
Figure 5.
Thermal events obtained by DSC of pinostrobin, pinostrobin-HPβCD and HPβCD.
3.2. Acute dermal toxicity of pinostrobin
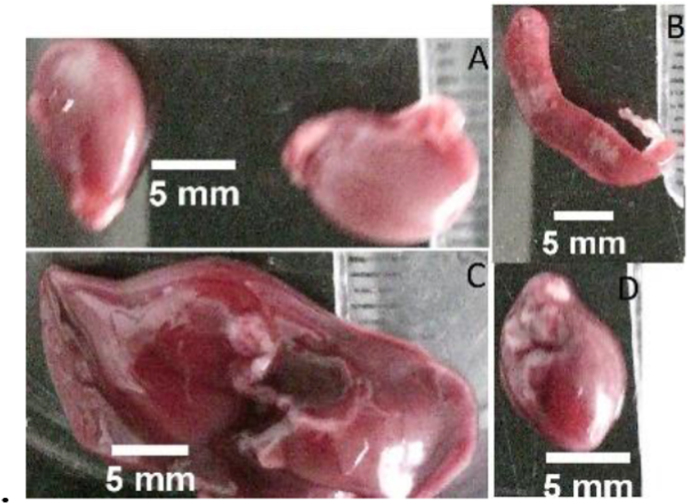
During the test, no death or signs of toxicity were observed in the subjects. Special attention was paid to any skin reactions such as dermatosis, dermatitis, ulcers, erythema, eczema, and piloerection. Other signs of toxicity were considered, such as the animal's behavior, posture, respiration, mucous coloration, shape, and color of feces, among others. After sacrifice, the state of internal organs was evaluated, which did not show macroscopic morphological alterations due to topical administration pinostrobin, a dose of 1000 mg/kg, as shown in Figure 6.
Figure 6.
Macroscopic image of main organs. (A-kidney, B- spleen, C-liver, D-heart).
The values of the organs compiled in Table 2 are the result of expressing the weight of each organ, as a percentage of body weight (% BW), according to Eq. (3).
Table 2.
Percentage average of weight organs respect body weight (% BW) of mice, pinostrobin dermal toxicity test.
| % BW (mean ± SD) | P | |
|---|---|---|
| Liver | 5.96 ± 0.65 | 0.87 |
| Kidney | 1.22 ± 0.06 | 0.13 |
| Heart | 0.41 ± 0.01 | 0.16 |
| Spleen | 0.62 ± 0.08 | 0.27 |
Student's t-test, (α = 0.05), significant differences are indicated for p < 0.05.
The animal body weight and target organ weight in the acute toxicity test were analyzed (liver, heart, kidney, and spleen). To evaluate the statistical significance of Tables 2 and 3, the data obtained were compared using a two-tailed t-student's test, for unpaired data, while considering data reported in the literature on body weight and organs [37, 38].
Table 3.
Body Weight of mice; pinostrobin toxicity test.
| 1 Day Body Weight (g) (mean ± SD) | 7 Day Body Weight (g) (mean ± SD) | 14 Days Body Weight (g) (mean ± SD) | |
|---|---|---|---|
| Average Weight Treated Animals 1000 mg/kg pinostrobin | 23.86 ± 1.00 | 25.44 ± 0.65 | 26.84 ± 0.57 |
| Mice | Weight day 14 (g) (mean ± SD) | P | |
| Animal 1 | 24.78 ± 1.09 | 0.45 | |
| Animal 2 | 25.72 ± 0.35 | ||
| Animal 3 | 26.04 ± 0.74 | ||
| Animal 4 | 25.40 ± 1.31 | ||
| Animal 5 | 25.78 ± 0.74 | ||
Student's t-test, (α = 0.05), significant differences are indicated for (P < 0.05).
3.3. Topical anti-inflammatory activity in experimental animals
Table 4 summarizes the behavior of the edema induced by TPA in experimental animals treated with pinostrobin at doses of 1.5, 2.0, and 3.0 mg/ear, and with pinostrobin included in the βCD and HPβCD complexes, recorded at 2, 4, 6, and 24 h. No significant differences were observed in the thickness of the ear between the groups in the 2 h timespan.
Table 4.
Anti-inflammatory activity pinostrobin and complexes of pinostrobin in βCD and HPβCD against the edema induced by TPA in experimental animals. Data obtained at 2, 4, 6, and 24 h.
| Compounds | Dose (mg) | 2 h | 4 h | 6 h | 24 h |
|---|---|---|---|---|---|
| Pinostrobin | 1.5 | 0.20 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.02 | 0.29 ± 0.02 |
| Pinostrobin | 2.0 | 0.20 ± 0.01 ∗ | 0.21 ± 0.01 ∗ | 0.26 ± 0.01 ∗ | 0.29 ± 0.01 ∗ |
| Pinostrobin | 3.0 | 0.19 ± 0.02 ∗∗ | 0.20 ± 0.01 ∗∗ | 0.25 ± 0.01 ∗∗ | 0.27 ± 0.01 ∗∗ |
| Pinostrobin-βCD | 1.5 | 0.18 ± 0.01 ∗∗∗ | 0.21 ± 0.01 ∗∗∗ | 0.24 ± 0.02 ∗∗∗ | 0.25 ± 0.01 ∗∗∗ |
| Pinostrobin-βCD | 2.0 | 0.17 ± 0.01 ∗∗ | 0.21 ± 0.02 ∗∗ | 0.24 ± 0.02 ∗∗ | 0.29 ± 0.02 ∗∗ |
| Pinostrobin-βCD | 3.0 | 0.18 ± 0.01 ∗∗∗ | 0.20 ± 0.02 ∗∗∗ | 0.21 ± 0.02 ∗∗∗ | 0.25 ± 0.02 ∗∗∗ |
| Pinostrobin-HPβCD | 1.5 | 0.18 ± 0.01 ∗∗∗ | 0.19 ± 0.02 ∗∗∗ | 0.2 ± 0.01 ∗∗∗ | 0.23 ± 0.01 ∗∗∗ |
| Pinostrobin-HPβCD | 2.0 | 0.18 ± 0.03 ∗∗∗ | 0.21 ± 0.01 ∗∗∗ | 0.21 ± 0.01 ∗∗∗ | 0.26 ± 0.01 ∗∗∗ |
| Pinostrobin-HPβCD | 3.0 | 0.18 ± 0.01 ∗∗∗ | 0.19 ± 0.02 ∗∗∗ | 0.20 ± 0.01 ∗∗∗ | 0.23 ± 0.01 ∗∗∗ |
| βCD | 13.0 | 0.22 ± 0.01 | 0.27 ± 0.01 | 0.35 ± 0.02 | 0.39 ± 0.0 |
| HPβCD | 15.0 | 0.23 ± 0.02 | 0.26 ± 0.01 | 0.32 ± 0.01 | 0.37 ± 0.01 |
| Indomethacin | 3.0 | 0.19 ± 0.00 ∗∗ | 0.20 ± 0.01 ∗∗ | 0.25 ± 0.01 ∗∗ | 0.27 ± 0.01 ∗∗ |
| Vehicle | 30 | 0.22 ± 0.01 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.40 ± 0.01 |
a. Units in percentage w/v.
Values as expressed how mean ± SD, p < 0.05 ∗, p < 0.01 ∗∗, p < 0.001 ∗∗∗, as compared to vehicle group. Two-way ANOVA and Dunnet's Post hoc test were performed. Pinostorobin-βCD: pinostrobin β-cyclodextrin complex, pinostrobin-HPβCD: Pinostrobin (2-Hydroxypropyl) -β-cyclodextrin complex, (n = 3).
At 4, 6, and 24 h, a statistically significant decrease (p < 0.001) concerning TPA was observed in the animals treated with pinostrobin and the inclusion complexes, at doses of 1.5, 2.0, and 3.0 mg per ear. Even though inflammation increases in all groups as time passes, the anti-inflammatory activity of the pinostrobin-treated groups continues to be statistically significant (p < .0.05) At 48 h, a slight decrease in inflammation levels, induced by TPA, was observed; however, the substance under study, alone and included in cyclodextrins, continues to show anti-inflammatory activity (p < 0.001).
Table 4 shows that the animal treated with pinostrobin alone showed higher levels of inflammation than those treated with pinostrobin included in both cyclodextrins at 4, 6, and 24 h. When comparing the edema measurements of the complexes obtained with the two cyclodextrins studied, it was observed that the mice treated with pinostrobin-HPβCD presented a statistically significant anti-inflammatory activity for the three doses used (p < 0.001).
Figure 7 contains information on the results of the percentage of edema obtained with respect to the TPA of the different pinostrobin samples and of the complexes formed between pinostrobin and cyclodextrins, at different concentrations, for 6 and 24 h, since at these hours a greater swelling caused by the TPA was observed. In the case of pure pinostrobin, at doses 1.5, 2.0, and 3.0 mg/ear, a dose-dependent behavior was observed, in which inflammation decreased as the dose increased, with inhibition percentages of 38.8%, 42.4%, and 43.6% respectively at 6 h and 46.1%, 21.4% and 56.6% at 24 h. This dose-dependent behavior was not observed when analyzing the complexes formed between pinostrobin and cyclodextrins.
Figure 7.
Percentual inhibition of inflammation. The values analyzed are 6 and 24 h after the administration of TPA (n = 3). Two Two-way ANOVA and Tukey's Post hoc test were performed. Values as expressed how, p < 0.05 ∗, p < 0.01 ∗∗, p < 0.001 ∗∗∗.
However, an increase in anti-inflammatory activity was shown by pinostrobin-βCD, with doses of 1.5 and 3 mg/ear, with 55.0% and 68.8%, respectively, 64.5% at 6 h, 64.7% at 24 h. This increased anti-inflammatory effect was not observed with the 2 mg dose of pinostrobin-βCD.
Pinostrobin-HPβCD at doses 1.5, 2.0, and 3.0 mg/ear was observed with percentages of 76.1, 64.3%, and 80.1% at 6 h and 71.9%, 55.8%, and 73.5%, at 24 h. The analysis highlighted the inhibition percentages achieved at 6 h by pinostrobin-βCD at the highest dose of 3 mg (68.8%) and pinostrobin- HPβCD 1.5 mg, at 6 and 24 h, with percentages of 76.1% and 71.9%, respectively.
Figure 8 shows the groups that showed statistical significance when comparing the average thickness of inflammation between 2–24 h. The difference in the averages between groups, showed that the thickness of the groups treated with the complex had a lower inflammation than the free molecule. Please see appendix section 4.
Figure 8.
Anti-inflammatory activity pinostrobin and complexes of pinostrobin in βCD and HPβCD against the edema induced by TPA in experimental animals. Two-way ANOVA and Tukey's Post hoc test were performed. Values as expressed how, p < 0.05 ∗, p < 0.01 ∗∗, p < 0.001 ∗∗∗.
3.4. Antioxidant activity
The antioxidant activity of the flavonoids under study was determined by radical scavenging of 2,2-diphenyl-1-picrylhydracil (DPPH). Evidence suggests that pinostrobin exhibits antioxidant activity, which varies between 977.33–1001.64 mM Trolox equivalent, in a pinostrobin concentration range between 100 mM–1000 mM pinostrobin (Table 5). The complexes also presented antioxidant activity; however, it was lower for Pinostrobin- HPβCD, which in Trolox equivalent varied between 940.53–948.58 mM and 948.86 mM–1003.58 mM for pinostrobin-βCD. The behavior of antioxidant activity was given by the different inclusion constants of the complexes, so it is possible to assert that the molecule was released differently from the cyclodextrin cavity at the moment of the assay.
Table 5.
Calculation of in vitro antioxidant activity by the DPPH method, expressed as a Trolox equivalent.
| Concentration (mM) | Pinostrobin (mM) (mean ± SD) | Pinostrobin-βCD (mM) (mean ± SD) | Pinostrobin- HPβCD (mM) (mean ± SD) |
|---|---|---|---|
| 100 | 977.3 ± 0.42∗∗∗ | 948.9 ± 1.73∗∗∗ | 940.5 ± 1.73∗∗∗ |
| 300 | 982.4 ± 0.48∗∗∗ | 959.9 ± 0.48∗∗∗ | 944.7 ± 0.48∗∗∗ |
| 500 | 986.9 ± 0.83∗∗ | 964.1 ± 0.96∗∗ | 945.5 ± 0.48∗∗ |
| 700 | 991.1 ± 1.67∗ | 965.5 ± 0.48∗ | 947.5 ± 0.48∗ |
| 1000 | 1001.6 ± 1.27 | 1003.6 ± 0.83 | 948.6 ± 0.83 |
ANOVA, Dunnet's Post hoc p < 0.05 ∗, p < 0.01 ∗∗, p < 0.001 ∗∗∗, compared with concentration of 1000 mM.
When analyzing Table 6 data of the antioxidant activity with the FRAP method. pinostrobin in the concentration range between 100–1000 mM was equivalent between 11.8 mM–28.8 mM in Trolox equivalent, and pinostrobin-HPβCD and pinostrobin-βCD were equivalent between 12.6–27.4 mM and 7.00 mM–54.4 mM, respectively.
Table 6.
Calculation of in vitro antioxidant activity FRAP method. Result expressed as Trolox Equivalent (mM) × SD ± SD.
| Concentration (mM) | Pinostrobin (mM) (mean ± SD) | Pinostrobin-βCD (mM) (mean ± SD) | Pinostrobin-HPβCD (mM) (mean ± SD) |
|---|---|---|---|
| 100 | 11.81 ± 1.70∗∗ | 12.56 ± 1.11∗∗ | 7.00 ± 1.11∗∗ |
| 300 | 21.07 ± 0.64∗ | 15.52 ± 1.28∗ | 15.52 ± 1.28∗ |
| 500 | 22.56 ± 2.22 | 23.67 ± 1.11 | 28.11 ± 1.11 |
| 700 | 25.89 ± 1.11 | 27.00 ± 1.11 | 32.93 ± 1.11 |
| 1000 | 28.11 ± 1.11 | 27.37 ± 3.39 | 54.41 ± 3.39 |
ANOVA, Dunnet's Post hoc p < 0.05 ∗, p < 0.01 ∗∗, compared with concentration of 1000 mM.
3.5. Analgesic activity
The influence of the cyclodextrins used in this work was tested, inclusion complexes between ibuprofen and cyclodextrins, to determine their behavior. The evaluation of analgesic activity after counting the stretching caused by the effect of phenyl quinone for 10 min for each group was found in Figure 9.
Figure 9.
Average number of writhes for ibuprofen and pinostrobin at doses of 75 mg/kg and 20 mg/kg, respectively. ANOVA is performed with a Dunnet post hoc where, P < 0.05 ∗, P < 0.01 ∗∗, P < 0.001 ∗∗∗, compared to control group (A).
It was observed that the stretching number of hind limbs for the ibuprofen (C) a dose of 75 mg/kg and pinostrobin (B) a dose of 20 mg/kg group, as free molecules at 30 min, were 15.0 and 21.8, stretching, respectively. For complexes with pinostrobin βCD (E), and HPβCD (J) 17.0, and 10.0, stretches were accounted for phenyl quinone is administered via i.p. 30 minutes after administering the test substance via p.o. a dose of 20 mg/kg. In this same time interval, the average number of stretches of the experimental animals' hind limbs was treated with the complexes formed between ibuprofen-βCD (F) and HPβCD (K), which were 10.5 and 3.0, stretching respectively a dose of 20 mg/kg.
Figure 10 shows the results of calculating the percentage of inhibition of the different groups with respect to the vehicle (A). At 15 min, stretches percentage calculated after the administration of phenyl quinone for the groups of animals treated with pinostrobin βCD (D) and HPβCD (I) was 9.7% and 1.8% a dose of 20 mg/kg. For 45 min, the percentage of stretches was 21.9% for pinostrobin-βCD (G) and 7.0% in the case of pinostrobin-HPβCD (L) a dose of 20 mg/kg. At 60 min, the complexes pinostrobin βCD (H) and HPβCD (M) 29.8% and 10.0% a dose of 20 mg/kg. Two-way ANOVA was performed to compare the effect on inhibiting the number of writhes between the free molecule and the complexes. Significant differences were found for the complexes formed with BCD at 15 min and HPBCD at 15, 20, and 30 min. Furthermore, the difference between the groups' means was much more significant for the complex groups (see appendix).
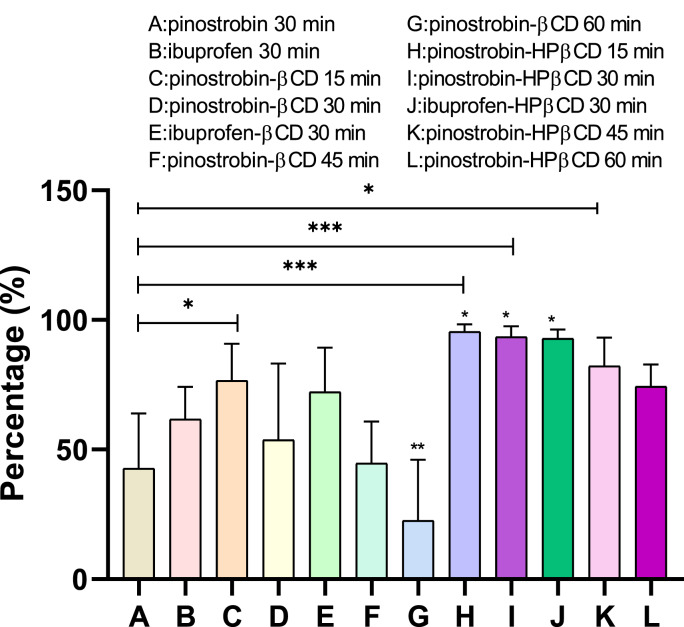
Figure 10.
Percentage of analgesia inhibition, for pinostrobin at a dose of 20 mg/kg with respect to the vehicle. An ANOVA test was performed with a Dunnet post hoc where, P < 0.05 ∗, P < 0.01 ∗∗, P < 0.001 ∗∗∗, compared to ibuprofen 30 min (C).
4. Discussion
Different analyses were carried with the complexes, including of NMR, IR, and DSC assays. The NMR spectra showed the formation of a complex due to the observed shifts of the hydrogens within the cyclodextrin. The H1 and H2 hydrogens are the signals with the largest displacement. This may be due to atoms with high electronegativity, thus causing shifts to higher fields due to van der Waals type interactions or hydrogen bonds. The IR spectra (Figures 2 and 3) show that the signals corresponding to the C–H and C–C stretching of aromatics, corresponding to 3061 cm−1 and 1400 cm−1 -500 cm−1, do not appear in the spectra of the complexes. Besides, the signals corresponding to the carbonyl group stretching at 1643 cm−1 have a different intensity in the IR of the complexes. These results suggest the interaction of pinostrobin with the cyclodextrin cavity upon complex formation.
After the DSC analysis (Figures 4 and 5), it can be observed that the hydroxyl group of carbon 5 may be responsible for the endothermic event occurring between 35–90 °C along with the loss of water and the melting process of pinostrobin at 100.9 °C. Water release from cyclodextrin was not observed. Nonetheless, a change in the temperature at which the endothermic event occurs at approximately 100 °C was observed for cyclodextrin and pinostrobin in the complex, which may be due to interactions between the respective binary systems' components. It may also indicate the amorphization of pinostrobin, which may be also due to the formation of the inclusion complex. In the DSC, analysis, the formation of an inclusion complex due to the interaction difference could be verified by comparing the thermal events' intensities observed for pinostrobin and the complex. Changes in the thermal stability of pinostrobin and cyclodextrin were observed due to the change of the melting temperature from 99 °C to 100.9 °C for pinostrobin-βCD and pinostrobin, and from 78.87 °C to 78.57 °C for βCD. Similar behavior was observed when analyzing the DSC of pinostrobin-HPβCD. This similarity suggests the formation of inclusions complex between cyclodextrins and pinostrobin.
No sign of toxicity was observed when pinostrobin was administered dermally at the concentration of 1000 mg/kg. Macroscopic observation of organs (Figure 6) such as heart, liver, spleen, and kidney showed no ulcer formation, swelling, or any other toxicity signs. Bodyweight statistical comparisons of the different animals do not show any significant anomalies with the literature reported. All the animals at the end of the test had a gaining of weight. It can be concluded that pinostrobin does not interfere in the homeostasis of experimental animals, nor does it affect nutrient absorption or other factors that could interfere in the correct functioning of different organ systems allowing healthy conditions [38]. The report on main organs weight did not significantly differ from data reported in the literature for animals of the same strain and weaning time between 7-8 weeks. All this evidence leads to the conclusion that pinostrobin at a dose of 1000 mg/kg does not exhibit toxicity. The maximum dose recommended by the guidelines of 2000 mg/kg was not tested since analgesic and anti-inflammatory activities were obtained with doses of 20 mg/kg and 3 mg/ear, respectively. Also, for the development of this work, not much pinostrobin was obtained, which would allow performing the acute toxicity test at higher doses. Previous toxicity studies of cyclodextrins have been reported, and they are also widely used as excipients in different pharmaceutical formulations [39]. The doses of cyclodextrins used in this work do not exceed 9 mg/kg, which are well below the concentrations at which signs of toxicity were observed in different studies. No signs of toxicity caused by supplying pinostrobin complexes in cyclodextrins were observed in the experiments.
When evaluating the antioxidant activity of pinostrobin at a 100 mM–1000 mM concentration, it was found that this range is equivalent to 977.33 mM–1003.58 mM in Trolox for pinostrobin DPPH and 11.81 mM–54.41 mM by the FRAP method (Tables 5 and 6). No changes in the absorbances generated by cyclodextrin were observed [40]. An improvement in the antioxidant capacity of pinostrobin was observed when complexes formed with cyclodextrin were evaluated. Significant differences were found when comparing the highest concentration of samples with the rest of the concentrations for the DPPH method. When the same comparison was made for the FRAP method, significant differences were only observed for the 100 mM and 300 mM concentration samples. The observed differences in the free molecule's antioxidant activity and the complex may be explained by the characteristics of the test, which did not allow the release of pinostrobin. Therefore, it would be necessary for future studies to carry out the antioxidant measurement in an in vivo model to determine the liberation levels of pinostrobin.
TPA's inflammatory response generated significant edema 4 h after administration, with maximal activity at 24 h. There was a statistically significant decrease in inflammation after 2 h, indicating that pinostrobin inhibits the inflammatory process at three different doses. When the pinostrobin complexes were applied, its anti-inflammatory activity increased, especially in the pinostrobin-HPβCD complex at the 3.0 mg dose with 80% and 73% inhibition at 6 h and 24 h, respectively. The complexation process generated some changes in the anti-inflammatory activity, which may be linked to a better bioavailability of the pinostrobin molecule [33, 34]. The anti-inflammatory activity of pinostrobin might be explained by a synergistic process in which flavonoids intervene in different active sites, producing an anti-inflammatory and analgesic response, among which we can mention the following, the inhibition of pinostrobin on COX-2 and COX-1 and the role of antioxidants in the anti-inflammatory process activity it exhibits [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. When an inflammatory process is triggered, a broad immune response is generated by a wide variety of cells such as lymphocytes, neutrophils, macrophages, leukocytes, and macrophages, which release a significant number of inflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukins such as IL-1, IL-6, IL-8, IL-12, and interferon (IFN-γ). Thereby, cytokines regulate the production of reactive nitrogen species and prostaglandins through the enzyme inducible nitric oxide synthase (iNOS), cyclooxygenases, and another pathway that can also generate all this type of response due to the action of leukotrienes. Leukotrienes are produced by the action of the enzyme lipoxygenase, which oxidizes arachidonic acid (AA). Then, cPLA2 is required to release AA from membrane phospholipids [36]. These responses and interactions may suggest that pinostrobin complexes could be part of future studies to develop a possible treatment for both pain and inflammation. The anti-inflammatory effect observed in this study may also explain the traditional medicinal use of the plant Renealmia alpinia, (Rottb.) Maas, by the Emberá Katíos indigenous community for snakebite treatment, since in previous studies, it has been found that methanol and dichloromethane extracts of this plant contain pinostrobin as its primary component [15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43].
When analyzing Figure 10, it can be inferred that when administering the complexes at different times, analgesic activity improves. The tests in which pinostrobin-HPβCD was administered were significant, mainly the test carried out 15 min after the administration of the complex formed with pinostrobin-HPβCD, showing analgesia inhibition of approximately 98%.
5. Conclusions
As concluding remarks, it was possible to establish a protocol that allows obtaining a solid complex, which consists of shaking equimolar amounts of cyclodextrin and pinostrobin for 72 h, at room temperature, and then lyophilizing. The antioxidant capacity of pinostrobin may be one of the mechanisms of action by which the inhibition of enzymes that participate in inflammation and analgesia, such as prostaglandins and COX 1 and COX 2, occurs. This would explain the anti-inflammatory and analgesic effects of pinostrobin, which was enhanced by improving its solubility, using inclusion complexes with cyclodextrins. This study has proven that substances with antioxidant activity can affirmatively interact with reactive oxygen species, which can, in turn, act as mediators of the activation of cytokines responsible for the immune response.
Declarations
Author contribution statement
Alejandro Serna González; Víctor H. Soto Tellini; Dora María Benjumea Gutiérrez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Mr. Alejandro Serna Gonzalez was supported by Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) [Beca posgrados 727].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at [URL].
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Barbour K.E., Helmick C.G., Boring M., Brady T.J. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable Activity limitation — United States, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017;66(9):246–253. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention Osteoarthritis (OA) | basics | arthritis | CDC. https://www.cdc.gov/arthritis/basics/osteoarthritis.htm
- 3.Centers for Diseases Control and Prevention (CDC) United States cancer statistics: data visualizations. https://gis.cdc.gov/Cancer/USCS/DataViz.html
- 4.Croft J.B., Wheaton A.G., Liu Y., et al. Urban-rural county and state differences in chronic obstructive pulmonary disease — United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2018;67(7):205–211. doi: 10.15585/mmwr.mm6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pountos I., Georgouli T., Bird H., Giannoudis P.V. Nonsteroidal anti-inflammatory drugs: prostaglandins, indications, and side effects. Int J Interf. 2011;3:19–27. [Google Scholar]
- 6.McPherson M.L., Cimino N.M. Topical NSAID formulations. Pain Med. 2013;14(suppl 1):S35–S39. doi: 10.1111/pme.12288. [DOI] [PubMed] [Google Scholar]
- 7.Pereira-Leite C., Figueiredo M., Burdach K., Nunes C., Reis S. Unraveling the role of drug-lipid interactions in NSAIDs-induced cardiotoxicity. Membranes. 2020;11(1):24. doi: 10.3390/membranes11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smedegård G., Björk J. Sulphasalazine: mechanism of action in rheumatoid arthritis. Rheumatol (United Kingdom) 1995;34:7–15. [PubMed] [Google Scholar]
- 9.Patel N.K., Bhutani K.K. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-alpha and IL-1beta production: in vitro and in vivo experimentation. Phytomedicine. 2014;21(7):946–953. doi: 10.1016/j.phymed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Kong Y., Zu Y.G., Fu Y.J., et al. Optimization of microwave-assisted extraction of cajaninstilbene acid and pinostrobin from pigeonpea leaves followed by RP-HPLC-DAD determination. J. Food Compos. Anal. 2010;23(4):382–388. [Google Scholar]
- 11.Yap A., Ching L., Wah T.S., Sukari M.A., Ee G., Lian C. Characterization of flavonoid derivatives from Boesenbergia rotunda (L.) Malaysian J Anal Sci. 2007;11(1):154–159. [Google Scholar]
- 12.López S.N., Furlan R.L.E., Zacchino S.A. Detection of antifungal compounds in Polygonum ferrugineum Wedd. extracts by bioassay-guided fractionation. Some evidences of their mode of action. J. Ethnopharmacol. 2011;138(2):633–636. doi: 10.1016/j.jep.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Sudsai T., Prabpai S., Kongsaeree P., Wattanapiromsakul C., Tewtrakul S. Anti-inflammatory activity of compounds from Boesenbergia longiflora rhizomes. J. Ethnopharmacol. 2014;154(2):453–461. doi: 10.1016/j.jep.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Betancur I., Benjumea D. Traditional use of the genus Renealmia and Renealmia alpinia (Rottb.) Maas (Zingiberaceae)-a review in the treatment of snakebites. Asian Pac J Trop Med. 2014;7S1:S574–S582. doi: 10.1016/S1995-7645(14)60292-3. [DOI] [PubMed] [Google Scholar]
- 15.Otero R., Núñez V., Jiménez S.L., et al. Snakebites and ethnobotany in the northwest region of Colombia. Part I: neutralization of lethal and enzymatic effects of Bothrops atrox venom. J. Ethnopharmacol. 2000;71(3):505–511. doi: 10.1016/s0378-8741(99)00197-x. [DOI] [PubMed] [Google Scholar]
- 16.Otero R., Núñez V., Jiménez S., et al. Snakebites and ethnobotany in the northwest region of Colombia Part II: neutralization of lethal and enzymatic effects of Bothrops atrox venom. J. Ethnopharmacol. 2000;71(3):505–511. doi: 10.1016/s0378-8741(99)00197-x. [DOI] [PubMed] [Google Scholar]
- 17.Otero R., Núñez V., Jiménez S., et al. Snakebites and ethnobotany in the northwest region of Colombia, Part I: traditional use of plants. J. Ethnopharmacol. 2000;71(3):505–511. doi: 10.1016/s0378-8741(99)00197-x. [DOI] [PubMed] [Google Scholar]
- 18.Otero R., Núñez V., Barona J., et al. Snakebites and ethnobotany in the northwest region of Colombia. Part III: neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol. 2000;73(1-2):233–241. doi: 10.1016/s0378-8741(00)00321-4. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Betancur I., Benjumea D., Patiño A., Jiménez N., Osorio E. Inhibition of the toxic effects of Bothrops asper venom by pinostrobin, a flavanone isolated from Renealmia alpinia (Rottb.) MAAS. J. Ethnopharmacol. 2014;155(3):1609–1615. doi: 10.1016/j.jep.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Betancur I., Cortés N., Benjumea D., et al. Antinociceptive activity of extracts and secondary metabolites from wild growing and micropropagated plants of Renealmia alpinia. J. Ethnopharmacol. 2015;165:191–197. doi: 10.1016/j.jep.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel N.K., Jaiswal G., Bhutani K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016;30(18):2017–2027. doi: 10.1080/14786419.2015.1107556. [DOI] [PubMed] [Google Scholar]
- 22.Panthong A., Kanjanapothi D., Tuntiwachwuttikul P., Pancharoen O., Reutrakul V. Antiinflammatory activity of flavonoids. Phytomedicine. 1994;1(2):141–144. doi: 10.1016/S0944-7113(11)80032-2. [DOI] [PubMed] [Google Scholar]
- 23.Kumar M., Thangavel C., Becker R.C., Sadayappan S. Monoclonal antibody-based immunotherapy and its role in the development of cardiac toxicity. Cancers. 2020;13(1):86. doi: 10.3390/cancers13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayre C.L., Alrushaid S., Martinez S.E., Anderson H.D., Davies N.M. Pre-clinical pharmacokinetic and pharmacodynamic characterization of selected chiral flavonoids: pinocembrin and pinostrobin. J. Pharm. Pharmaceut. Sci. 2015;18(4):368–396. doi: 10.18433/j3bk5t. [DOI] [PubMed] [Google Scholar]
- 25.Verri W.A., Vicentini F.T.M.C., Baracat M.M., et al. Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms. Stud. Nat. Prod. Chem. 2012;36:297–330. Elsevier B.V. [Google Scholar]
- 26.Villers G., Fougere Y. first ed. Nova Science Publishers Inc.; 2013. Kaempferol Chemistry, Natural Ocurrences and Health Benefits. [Google Scholar]
- 27.Carneiro S.B., Duarte F.Í.C., Heimfarth L., et al. Cyclodextrin-drug inclusion complexes: in vivo and in vitro approaches. Int. J. Mol. Sci. 2019;20(3):1–23. doi: 10.3390/ijms20030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitha J., Milecki J., Fales H., Pannell L., Uekama K. Hydroxypropyl-β-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 1986;29(1):73–82. [Google Scholar]
- 29.Connors K.A. Binding Constants. Wiley & Sons; 1987. Optical absorption spectroscopy; p. 411. [Google Scholar]
- 30.Higuchi K.A.C. Phase solubility techniques. Adv. Analyt. Chem. Instrument. 1965;4:117–212. [Google Scholar]
- 31.Jover A., Meijide F., Mosquera V., Tato J.V. A step-by-step dilution-extraction method for laboratory experiments. J Chem. Educ. 1990;67(6):530–532. [Google Scholar]
- 32.Olfert E. Guide to the Care and use of experimental animals. Olfert E.D.B.M.C.A.A.M., editor. LABORATORY MICE. 1984;2:XIX. http://www.ccac.ca [Google Scholar]
- 33.Chrzczanowicz J., Gawron A., Zwolinska A., et al. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity - possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 2008;46(3):342–349. doi: 10.1515/CCLM.2008.062. [DOI] [PubMed] [Google Scholar]
- 34.Park B.K., Heo M.Y., Park H., Kim H.P. Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. Eur. J. Pharmacol. 2001;425(2):153–157. doi: 10.1016/s0014-2999(01)01187-6. [DOI] [PubMed] [Google Scholar]
- 35.Siegmund E., Cadmus R., Lu go. A method for evaluating both non-narcotic and narcotic analgesics. Proc Soc Exp Biol Med. 1957;95(4):729–731. doi: 10.3181/00379727-95-23345. [DOI] [PubMed] [Google Scholar]
- 36.Charles river. Swiss Webster (CFW) mouse - growth chart | charles river. https://www.criver.com/products-services/find-model/swiss-webster-cfw-mouse?region=3611
- 37.Martey O., Nimick M., Taurin S., et al. Styrene maleic acid-encapsulated RL71 micelles suppress tumor growth in a murine xenograft model of triple negative breast cancer. Int. J. Nanomed. 2017;12:7225–7237. doi: 10.2147/IJN.S148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahyuningsih M.D., Agustina D.W., Widyarti S., Soewondo A., Tsuboi H., Rifa’i M. Noni (Morinda citrifolia L.) fruit extract potentially maintain the immune system homeostasis of Balb/C mice from DMBA and cigarette smokes exposure. J. Microbiol. Biotechnol. Food Sci. 2020;9(6):1119–1125. [Google Scholar]
- 39.Medicines Agency E . 2014. Background Review for Cyclodextrins Used as Excipients.www.ema.europa.eu/contact Published online. [Google Scholar]
- 40.Pápay Z.E., Sebestyén Z., Ludányi K., et al. Comparative evaluation of the effect of cyclodextrins and pH on aqueous solubility of apigenin. J. Pharm. Biomed. Anal. 2016;117:210–216. doi: 10.1016/j.jpba.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Kicuntod J., Sangpheak K., Mueller M., et al. Theoretical and experimental studies on inclusion complexes of pinostrobin and β-cyclodextrins. Sci. Pharm. 2018;86(1):5. doi: 10.3390/scipharm86010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baskar R., Lavanya R., Mayilvizhi S., Rajasekaran P. Free radical scavenging activity of antitumour polysaccharide fractions isolated from ganoderma lucidum (Fr.) P. Karst. Indian J. Nat. Prod. Resour. 2008 [Google Scholar]
- 43.Bakhouche I., Aliat T., Boubellouta T., Gali L., Şen A., Bellik Y. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and anti-inflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. South Afr. J. Bot. 2021;139:42–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.