Research in cell membranes provides a fascinating intersection of the natural world, bioengineering, and fundamental physics. Model membranes form by the self-assembly of amphiphilic phospholipids into two-molecule-thick fluid sheets spanning up to tens of microns. Increasingly, model membranes include leaflet asymmetry, lipid phase separation, and spontaneous curvature to enable examination of the interplay of diverse physical characteristics that potentially govern complex natural behaviors. For example, lipid phase separation and leaflet asymmetry both encourage the formation of membrane curvature, but the degree to which living cells exploit these relationships is unknown.
Outstanding questions remain regarding how compositional heterogeneity, shape, and dynamics couple to reduce the demand for external energy sources (i.e., ATP) during the performance of complex behaviors. Further research is required to understand how cells regulate their highly dynamic and precisely controlled membrane shapes while ensuring membrane integrity. The results by Aleksanyan et al. reported in this issue of the Biophysical Journal (1) detail how leaflet asymmetry in the glycolipid composition encouraged membrane nanotube formation and limited pore resealing following electroporation.
Continuum models of membrane shape changes include parameterizing the membrane’s elasticity, bending modulus, spontaneous curvature, Gaussian curvature modulus, and tension to regulate its shape (2). These parameters are determined by the membrane composition, while the membrane responds to external forces such as osmotic pressure, cytoskeletal forces, and membrane-bound ATPases (3). Only when a membrane has an asymmetry in the composition of its two leaflets will it have a non-zero spontaneous curvature and form diverse tubules, vesicles, and cristae without large external forces.
Accordingly, live cells actively maintain their membrane composition, asymmetry, and shape. In addition to bulk vesicular trafficking of lipids, leaflet-specific membrane compositions are maintained with energy-dependent and lipid-specific protein mediators (i.e., flippases/floppases), energy-independent protein mediators (i.e., scramblase), protein-independent processes (i.e., spontaneous membrane absorption/desorption), and localized lipid metabolism (4). Similarly, cells employ diverse mechanisms of regulating membrane shapes with the binding of proteins that have membrane-inserting amphipathic alpha helices, intrinsically disordered regions, multivalent lipid binding, and/or a rigid molecular shape (5,6). The coupling of membrane shape with the local lipid composition is an area of active research in which the collective behavior of lipid phases may be necessary to concentrate <1-nm-wide lipids to curvatures of >30-nm radii (7). Throughout these dynamic membrane processes, any membrane pores that are formed must be quickly sealed to maintain cellular homeostasis. The pore edge tension and spontaneous sealing rate has been studied in theoretical and model membranes, but how cells balance tubule formation and osmotic balance while resealing membrane pores is largely unexplored.
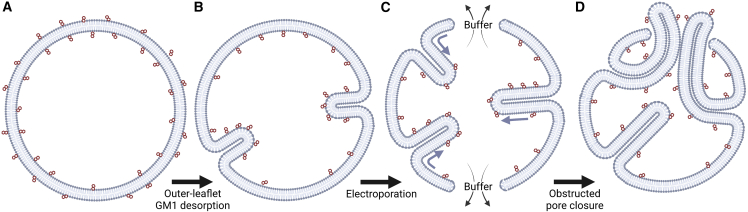
Toward these efforts, Aleksanyan et al. studied bilayer tubule formation and pore closure dynamics upon the electroporation of giant unilamellar vesicles with asymmetric GM1 content (Fig. 1) (1). Their newest publication builds upon their prior work, which demonstrated spontaneous GM1 desorption results in membrane asymmetry, spontaneous membrane curvature, and nanotubes formation (8). In this issue of the Biophysical Journal, Aleksanyan et al. reveal that leaflet asymmetry in membranes stabilized membrane pores through effectively lowering pore edge tension and the formation of nanotubes that obstructed the pores from closing.
Figure 1.
(A) Giant unilamellar vesicles initially contained symmetric GM1 (red dots) with no intrinsic curvature. (B) Upon dilution, GM1 desorbed from outer leaflet, and the vesicles exhibited inward tubules consistent with the asymmetric composition induced in the membrane. (C) Electroporation created pores in the vesicles, which allowed tubules to further form and extend while buffer escaped due to the Laplace pressure. (D) The nanotubes were pulled outward through the membrane pores, which obstructed the pore closing position and extended the vesicle leakage. To see this figure in color, go online.
Aleksanyan and colleagues have demonstrated three factors that impact pore stability in asymmetric membranes. First, the preexisting membrane tubules that formed due to membrane asymmetry were elongated, and new tubules were formed with a net outflow of buffer after electroporation. Second, the tubule growth effectively increased the pore tension, while the buffer exchange contributed to a loss of membrane asymmetry, which provided opposing influences for pore closure. Third, membrane viscosity and steric considerations resulted in dynamically limited changes to the membrane composition and shape. Aleksanyan et al. provided experimental evidence that GM1 concentration asymmetry was inversely proportional to pore edge tension and resulted in long-term vesicle permeability. Upon electroporation of the asymmetric vesicles, nanotubes formed and grew from the vesicle interior, extended through the vesicle pore, and limited resealing. These findings reveal new understandings of membrane shape and pore closure dynamics in the presence of membrane asymmetry and electroporation.
In summary, this work builds upon the authors’ body of work that provides a strong foundation to discern the interplay of membrane nanotubes, leaflet asymmetry, pore dynamics, and edge tension. Membrane pores are fundamentally important in cell biology and are used as pathway to inject natural and synthetic materials into cells for therapeutic and engineering applications. Aleksanyan et al. shed light on the diverse factors and mechanisms that influence stability of pore lifetime, which informs the development of tissue-specific electroporation methods and deepens our fundamental understanding of the diverse physical processes governing membrane structure and dynamics.
Acknowledgments
We are grateful to Richard J. Barber for financial support. This material is based upon work supported by the National Science Foundation under grant no. DMR-1652316. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK076629.
Declaration of interests
The authors declare no competing interests.
Editor: Andreas Janshoff.
References
- 1.Aleksanyan M., Lira R.B., et al. Dimova R. GM1 asymmetry in the membrane stabilizes pores. Biophys. J. 2022 doi: 10.1016/j.bpj.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deserno M. Fluid lipid membranes: from differential geometry to curvature stresses. Chem. Phys. Lipids. 2015;185:11–45. doi: 10.1016/j.chemphyslip.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Mahapatra A., Rangamani P. Formation of protein-mediated tubes is governed by a snapthrough transition. bioRxiv. 2022 doi: 10.1101/2022.06.07.494774. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prinz W.A. Lipid trafficking sans Vesicles: where, why, how? Cell. 2010;143:870–874. doi: 10.1016/j.cell.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabbani A.M., Raghunathan K., et al. Kelly C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA. 2020;117:14978–14986. doi: 10.1073/pnas.2001119117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeno W.F., Day K.J., et al. Stachowiak J.C. Principles and applications of biological membrane organization. Annu. Rev. Biophys. 2020;49:19–39. doi: 10.1146/annurev-biophys-121219-081637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward X., Javanainen M., et al. Kelly C.V. Nanoscale membrane curvature sorts lipid phases and alters lipid diffusion. bioRxiv. 2022 doi: 10.1101/2020.09.23.310086. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta R., Miettinen M.S., et al. Dimova R. The glycolipid GM1 reshapes asymmetric biomembranes and giant vesicles by curvature generation. Proc. Natl. Acad. Sci. USA. 2018;115:5756–5761. doi: 10.1073/pnas.1722320115. [DOI] [PMC free article] [PubMed] [Google Scholar]