Abstract
The operation of a novel glycolytic pathway was demonstrated in nongrowing cells of Thermococcus zilligii by analysis of the isotopic enrichment in the end products derived from fermentation of 13C-labeled glucose. The new pathway involved the formation of formate, derived from C-1 in glucose, via cleavage of a six-carbon carboxylic acid.
Thermococcus zilligii, formerly designated Thermococcus strain AN1, is a hyperthermophilic archaeon with an optimum growth temperature of 75 to 80°C belonging to the order Thermococcales (9, 13). All the organisms of this order are hyperthermophiles and obligate anaerobes that grow heterotrophically by fermenting peptides and, in some cases, complex carbohydrates.
In recent years, several hyperthermophiles belonging to the domains Archaea and Bacteria were examined to identify their glycolytic pathways (3, 6, 11, 16–18, 21). This interest was fueled by the expectation that novel metabolic pathways and enzymes might operate under the extreme temperature conditions required for growth of these organisms. For example, a modified version of the Embden-Meyerhof (EM) glycolytic pathway, involving ADP-dependent hexokinase and phosphofructokinase, was found in the Thermococcus and Pyrococcus species investigated (6, 7, 17, 19).
The hyperthermophilic archaeon T. zilligii is an atypical member of the thermococci. Unlike the other members of the genus Thermococcus, this species is not a marine organism and has a relatively low tolerance for NaCl (200 mM); furthermore, it has the lowest optimal temperature for growth among the species of the same genus and an unusual lipid membrane composition (10); finally, glucose is a preferred substrate for growth as long as low levels of peptides are provided. These differences prompted us to select T. zilligii as a putatively interesting target to extend studies on carbohydrate metabolism in hyperthermophiles.
Here, we use 13C-labeling experiments with whole cells to demonstrate the operation of a novel glycolytic pathway that involves the cleavage of a six-carbon compound to yield formate.
Analysis of end products derived from the metabolism of [13C]glucose by cell suspensions.
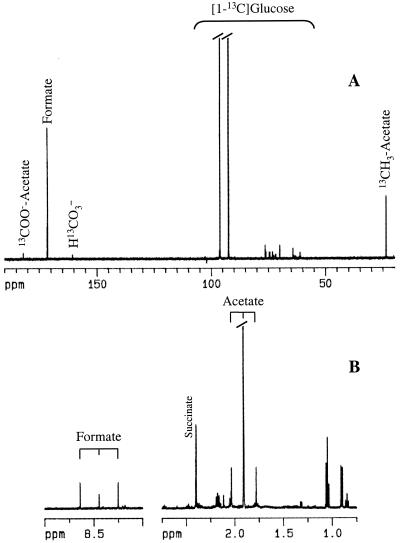
T. zilligii DSM2770 was grown at 75°C in a 5-liter fermentor in the medium described by Lanzotti et al. (10) using 10 g of tryptone (Difco, Detroit, Mich.) per liter as carbon source with continuous bubbling of nitrogen gas and stirring at 39 rpm. Cells were harvested by centrifugation (7,500 × g for 10 min at 27°C) at the end of the exponential phase and washed once with anaerobic 20 mM potassium phosphate buffer, pH 7.4, containing 2.5 g of NaCl per liter. The cells were then suspended in the same buffer, and 4.5 ml of cell suspension (5 to 10 mg of protein/ml) was incubated for 2 h at 75°C with selectively enriched [13C]glucose (15 mM) in closed serum bottles under an argon atmosphere. Samples were centrifuged, and the supernatants were frozen and stored until analyzed by 1H and 13C nuclear magnetic resonance (NMR). The major products, quantified by 1H NMR, were acetate (4 to 9 mM) and formate (0.5 to 2 mM). Succinate, isobutyrate, propionate, and isovalerate were also produced, but they were derived from internal reserves present in the cells, since these compounds were not isotopically enriched. Typically around 60% of acetate produced was derived from internal reserves. To identify the type of glycolytic pathway operating in this organism, six independent experiments were performed with glucose selectively labeled in each of the six carbon atoms. The isotopic enrichments determined in the end products, acetate and formate, are summarized in Table 1. The protein content in the sonicated cell suspension was determined by the Bradford method (2). The label in [1-13C]glucose ended primarily on formate (78% labeled) and to a smaller extent on the methyl group of acetate. The 13C NMR spectrum in Fig. 1 shows clearly the resonances due to formate and acetate at 171.4 and 23.6 ppm, respectively. The percentage of labeling in each compound was estimated from the 1H NMR spectrum of the same supernatant (Fig. 1, trace B). In each of the triplet of resonances, the central line (8.45 and 1.91 ppm for formate and acetate, respectively) is due to the unlabeled group, while the two lateral lines, with equal intensity, are due to the protons directly attached to 13C atoms (2JCH = 195 and 127 Hz for formate and acetate, respectively). A very low isotopic enrichment in formate (4%) was observed when [6-13C]glucose was metabolized, and no significant isotopic enrichment of formate was derived from any other carbon in glucose. Both carbon atoms in acetate were labeled from [2-13C]glucose, but the percentage of enrichment was about twofold higher on the methyl group than on the carboxylic group. On the other hand, acetate derived from [6-13C]glucose was labeled exclusively on the methyl group. Metabolism of [4-13C]glucose resulted in no significant incorporation of 13C label in the methyl group of acetate, and the enrichment on the carboxylic group was very low. Finally, the label ended on the carboxylic group of acetate when [3-13C]glucose or [5-13C]glucose was metabolized.
TABLE 1.
Percentage of 13C labeling on the fermentation products derived from the metabolism of [13C]glucose by cell suspensions of T. zilligii grown on tryptonea
| Substrate | Value for product:
|
||||
|---|---|---|---|---|---|
| Acetate
|
Formate
|
||||
| Total amt (μmol/mg of protein) | 13CH3COO− (%) | CH313COO− (%) | Total amt (μmol/mg of protein) | H13COO− (%) | |
| [1-13C]glucoseb | 0.94 ± 0.26 | 9 ± 1 | —e | 0.19 ± 0.06 | 78 ± 3 |
| [2-13C]glucosec | 0.93 ± 0.07 | 13 ± 2 | 6 ± 2 | 0.17 ± 0.04 | — |
| [3-13C]glucosec | 1.05 ± 0.10 | — | 18 ± 2 | 0.21 ± 0.04 | — |
| [4-13C]glucosec | 1.03 ± 0.07 | — | 2 ± 1 | 0.18 ± 0.03 | 1 ± 1 |
| [5-13C]glucosec | 1.01 ± 0.06 | 1 ± 1 | 12 ± 2 | 0.13 ± 0.03 | — |
| [6-13C]glucosed | 1.19 | 27 | — | 0.21 | 4 |
Cell suspensions were incubated with 15 mM glucose at 75°C for 2 h. Fermentation products were quantified in supernatants by proton NMR using formate as a concentration standard. Percentages of 13C labeling were calculated from the areas of the respective resonances in the 1H spectra. Isotopic enrichment on the carboxylic group of acetate was confirmed by comparing the intensity of the carboxylic resonance in the 13C spectra with that of the resonance of the methyl group and taking into account the enrichment of this group determined by 1H NMR. The natural abundance of 13C (1.1%) was subtracted, and therefore the values represent the excess of 13C in the metabolites.
Average values for five independent experiments.
Average values for two independent experiments.
Result from a single experiment.
—, not detected.
FIG. 1.
NMR spectra of the supernatant containing the end products derived from the fermentation of [1-13C]glucose by T. zilligii. The cell suspension (4.5 ml and 7-mg/ml protein concentration) was incubated with 15 mM [1-13C]glucose for 2 h at 75°C. (A) 13C NMR spectrum acquired on a Bruker DRX500 spectrometer using a 5-mm selective probe head (spectral width, 31 kHz; data size, 64,000; repetition delay, 61 s; pulse width, 7 μs). Proton decoupling was applied during the acquisition time (1 s). (B) 1H NMR spectrum acquired on the same spectrometer with water presaturation (spectral width, 5 kHz; data size, 64,000; repetition delay, 17 s; pulse width, 7 μs). In the 13C NMR spectrum, resonances arising from nonmetabolized [1-13C]glucose are apparent in the region between 60 and 100 ppm. In the representation of the 1H NMR spectrum, the strong intensity resonance due to the unlabeled methyl group of acetate has been truncated.
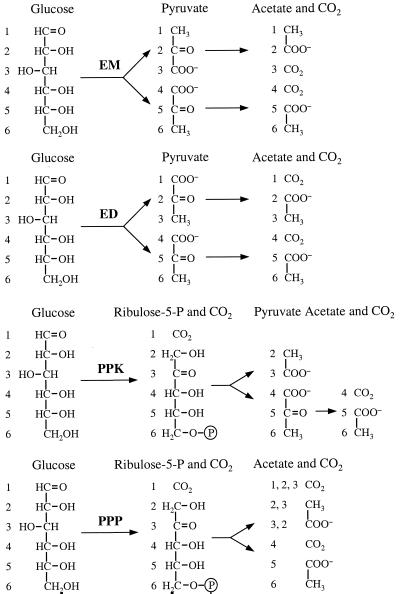
The labeling patterns of acetate expected when glucose is metabolized by well-known glycolytic pathways, the EM and Entner-Doudoroff (ED) pathways and the pentose phosphate pathway (PPP), are summarized in Fig. 2. The PPP is usually found to operate simultaneously with the EM or the ED pathway since it does not have a strictly catabolic role. The labeling pattern expected for the operation of the pentose phosphoketolase (PPK) pathway, a major glycolytic route in some lactic acid bacteria (5), has also been included in Fig. 2. The labeling in formate is not shown in Fig. 2, but if this product was derived from pyruvate by the action of pyruvate-formate-lyase, an enzyme responsible for the formation of formate in many anaerobic organisms, the label would originate from the carboxylic group of pyruvate (4).
FIG. 2.
Expected labeling pattern of acetate and CO2 from selectively labeled glucose via EM, ED, and PPK pathways and the PPP. The numbers show the fate of each carbon from glucose. The labeling on acetate derived from the metabolism of [2-13C]glucose and [3-13C]glucose via the PPP takes into account the scrambling of label due to eventual recycling of fructose-6-phosphate.
The utilization of an ED glycolytic pathway by T. zilligii was ruled out by the absence of labeling on the methyl group of acetate derived from the metabolism of [3-13C]glucose (Table 1 and Fig. 2). On the other hand, the detection of 13C enrichment on the methyl group of acetate derived from [1-13C]glucose showed that T. zilligii utilizes an EM glycolytic pathway. However, the operation of this pathway alone is not reconcilable with the labeling pattern observed on acetate derived from [2-13C]glucose or [3-13C]glucose, leading to the conclusion that at least two glycolytic pathways are operating. Metabolism of [2-13C]glucose led to the formation of a mixture of the two single-labeled isotopomers of acetate, 13CH3COOH and CH313COOH, whereas metabolism of [3-13C]glucose led to the formation of acetate labeled on the carboxylic group (Table 1). The operation of the EM pathway alone would lead to labeling exclusively on the carboxylic group of acetate derived from [2-13C]glucose and to complete loss of label from [3-13C]glucose (Fig. 2); therefore, the results cannot be explained by the operation of the EM pathway alone.
The labeling observed on the methyl group of acetate derived from [2-13C]glucose and on the carboxylic group of acetate from [3-13C]glucose is consistent with the cleavage of the C-2-C-3 bond in a five-carbon compound, a splitting pattern expected for the operation of either the PPP or the PPK pathway. However, a major contribution of the PPP can be ruled out because of the lack of labeling of the methyl group of acetate derived from [3-13C]glucose (Table 1 and Fig. 2). Altogether, the results described until now allow us to conclude that T. zilligii metabolizes glucose via the simultaneous operation of an EM pathway and a pathway that leads to a labeling pattern of acetate consistent with the operation of a PPK pathway.
Labeling experiments with [13C]pyruvate to elucidate the origin of formate.
Pyruvate-formate-lyase is a well-known enzyme that catalyzes the formation of formate from pyruvate. Therefore, it was conceivable that the labeled formate could derive from the metabolism of [1-13C]pyruvate, if this were an intermediate in the metabolism of [1-13C]glucose by the unknown glycolytic pathway. Experiments with pyruvate labeled on either C-1 or C-3 were carried out according to the procedure described above for glucose.
Pyruvate (15 mM) was metabolized at a much higher rate than was glucose by cell suspensions of T. zilligii (4.5 ml containing 6 mg of protein/ml), but a much lower proportion of formate was formed from pyruvate metabolism, suggesting that the activity of pyruvate-formate-lyase, if it exists, is very low. When [3-13C]pyruvate was supplied, 90% of the label provided was recovered in the methyl group of acetate and formate was not labeled (Table 2). On the other hand, when the label was provided in [1-13C]pyruvate, the pool of formate was partially labeled (20%), but the total label recovered in formate accounted for only 0.5% of the label utilized. Most probably, the remaining label in [1-13C]pyruvate was lost as 13CO2, due to the activity of pyruvate:ferredoxin oxidoreductase (1, 8), since no other labeled product was found. This high concentration of labeled 13CO2 led us to hypothesize that the labeling of formate in this experiment could be due to the activity of formate-hydrogen-lyase that interconverts CO2-H2 and formate. Evidence for the operation of this enzyme was obtained when cell suspensions were incubated with unlabeled glucose and labeled bicarbonate. Indeed, under these conditions the pool of formate was partially labeled (Table 2).
TABLE 2.
Percentage of 13C labeling on the fermentation products derived from the metabolism of different substrates by cell suspension of T. zilligii grown on tryptonea
| Substrate(s) | Value for product:
|
||||
|---|---|---|---|---|---|
| Acetate
|
Formate
|
||||
| Total amt (μmol/mg of protein) | 13CH3COO− (%) | CH313COO− (%) | Total amt (μmol/mg of protein) | H13COO− (%) | |
| [3-13C]pyruvate | 2.70 | 82 | — | 0.05 | — |
| [1-13C]pyruvate | 2.80 | — | — | 0.05 | 20 |
| Glucose and [1-13C]pyruvate | 3.13 | — | — | 0.13 | 23 |
| Glucose and [13C]bicarbonate | 1.2 | — | — | 0.23 | 9 |
Cell suspensions were incubated at 75°C for 2 h with the different substrates at a 15 mM concentration each. Fermentation products were quantified in supernatants by proton NMR using formate as a standard. Percentages of 13C labeling were calculated by integration of the respective resonances in the 1H spectra. The natural abundance of 13C (1.1%) was subtracted. —, not detected.
The observation that formate was a minor product of pyruvate metabolism (the acetate/formate ratio was approximately 50), in contrast to what was observed from glucose metabolism (the acetate/formate ratio was approximately 6), led us to carry out an experiment in which [1-13C]pyruvate would be cometabolized with unlabeled glucose to simulate the physiological conditions occurring during glucose metabolism. The production of formate doubled (the acetate/formate ratio was 25), but the isotopic enrichment of the formate pool remained low compared to the very high incorporation of 13C on formate derived from [1-13C]glucose (78%) (Table 2). Therefore, we concluded that pyruvate was not a precursor of the formate detected from the metabolism of glucose in T. zilligii.
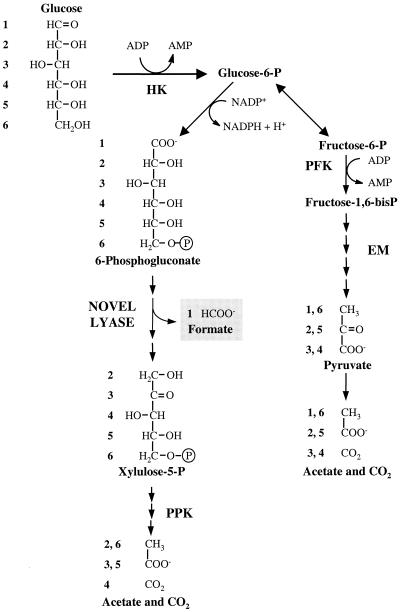
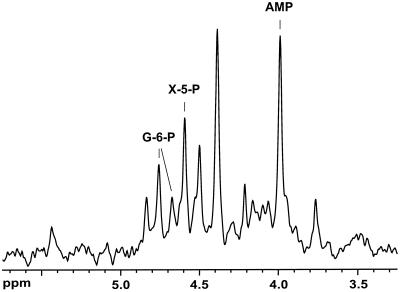
We propose that the metabolism of glucose in T. zilligii proceeds via the simultaneous operation of an EM pathway and a novel pathway involving cleavage of a six-carbon carboxylic acid to yield formate and a pentose phosphate, which is subsequently cleaved between C-2 and C-3 (Fig. 3). Further evidence for this pathway was obtained from the identification, by 31P NMR, of xylulose-5-phosphate as a major phosphorylated metabolite in ethanolic extracts of T. zilligii cells metabolizing glucose (Fig. 4). The proposed metabolic scheme is in complete agreement with the comprehensive set of 13C-labeling data; the low isotopic enrichment in formate derived from [6-13C]glucose and in the carboxyl group of acetate derived from [4-13C]glucose (Table 1) is explained by scrambling of label in the EM branch at the level of triose-phosphate isomerase and aldolase, as previously reported for other microorganisms (12, 20). The barely detectable isotopic enrichment in formate when [4-13C]glucose was metabolized resulted from the activity of formate-hydrogen-lyase upon labeled CO2 produced via the two glycolytic branches (Fig. 3).
FIG. 3.
Proposed glycolytic pathway in T. zilligii. HK, hexokinase; PFK, phosphofructokinase; P, phosphate. The numbers show the fate of each carbon from glucose.
FIG. 4.
Phosphomonoester region of the 31P NMR spectrum of an ethanol extract from T. zilligii. The cell suspension was incubated at 75°C with 15 mM glucose for 2 h and extracted for 30 min with 70% ethanol. The final pH of the sample was 6.9. The spectrum was acquired on a Bruker DRX500 spectrometer using a 10-mm quadruple nucleus probe head (repetition delay, 1.8 s; pulse width, 16 μs, corresponding to 55° flip angle; proton decoupling during acquisition only). Assignments were made by spiking the extracts with the pure compounds at pH 6.9 and 8.2. Abbreviations: X-5-P, xylulose-5-phosphate; G-6-P, glucose-6-phosphate.
The kinases in the EM glycolytic branch are ADP dependent.
Cell extracts of T. zilligii catalyzed the ADP-dependent phosphorylation of glucose and fructose-6-phosphate. The specific activities of the ADP-dependent hexokinase and phosphofructokinase in cell extracts of T. zilligii were 0.14 and 0.07 U/mg of protein, respectively (cell extract preparation and enzyme assays were performed as previously described [15, 17]. ADP-dependent kinases have been found in other members of the Thermococcales, and recently an ADP-dependent phosphofructokinase was purified from T. zilligii (14). At least, with respect to the ADP dependency of the kinases, the EM branch of T. zilligii is similar to the EM pathway described for Pyrococcus furiosus, Thermococcus litoralis, and Thermococcus celer (6, 17).
The relative contribution of the two glycolytic branches depends on the presence of glucose during growth.
All the experiments described above were carried out on cells grown on tryptone medium without glucose. Interestingly, the labeling pattern of end products derived from 13C-labeled glucose was different when glucose (5 g/liter) was added to the medium in addition to tryptone (5 g/liter) (Table 3). The relative fluxes of carbon metabolized via the two branches could be calculated from the ratio of the concentrations of the two acetate isotopomers, 13CH3COOH and CH313COOH, obtained from the experiments with [2-13C]glucose. In fact, the labeling on the methyl group is due to the operation of the novel pathway, whereas the labeling on the carboxylic group is due to metabolism via the EM pathway (Fig. 3). A relative contribution of 2:1 (novel pathway versus EM pathway) was calculated for cells grown on tryptone. When [2-13C]glucose was metabolized by cells grown in the presence of glucose (Table 3) the 13CH3COOH/CH313COOH ratio was approximately 0.5, indicating the inversion of the relative contributions of the two branches. The presence of glucose in the growth medium appears to repress the enzymes of the novel glycolytic pathway (also compare formate production in Tables 1 and 3). However, even under these growth conditions the contribution of this pathway is still important.
TABLE 3.
Percentage of 13C labeling on the fermentation products derived from the metabolism of [13C]glucose by cell suspensions of T. zilligii grown on glucosea
| Substrate | Value for product:
|
||||
|---|---|---|---|---|---|
| Acetate
|
Formate
|
||||
| Total amt (μmol/mg of protein) | 13CH3COO− (%) | CH313COO− (%) | Total amt (μmol/mg of protein) | H13COO− (%) | |
| [1-13C]glucoseb | 0.88 | 15 | —d | 0.05 | 66 |
| [2-13C]glucosec | 1.21 ± 0.38 | 7 ± 1 | 15 ± 2 | 0.09 ± 0.05 | — |
Cell suspensions were incubated with 15 mM glucose at 75°C for 2 h. Products were quantified by proton NMR using formate as a standard. Percentages of 13C labeling were calculated by integration of the respective resonances in the 1H spectra. The natural abundance of 13C (1.1%) was subtracted.
Result from a single experiment.
Average values for two independent experiments.
—, not detected.
Concluding remarks.
We demonstrate the operation of a novel glycolytic strategy in T. zilligii with two branches diverging at the level of glucose-6-phosphate (Fig. 3). Glucose is phosphorylated by an ADP-dependent hexokinase to glucose-6-phosphate which is subsequently degraded by two glycolytic branches: an EM-type glycolytic pathway and a new route where formate is produced by a reaction involving cleavage of the C-1 carboxylic group of a six-carbon compound to yield formate and a pentose phosphate. By analogy with the pyruvate-formate-lyase reaction, we suggest that the six-carbon compound is an α-ketoacid, such as 2-keto-3-deoxy-6-phosphogluconate, derived from 6-phosphogluconate. The contribution of the novel glycolytic branch was twice as high as that of the EM-type pathway when cells were grown on tryptone, and the inverse relationship was found for cells grown in the presence of glucose. This is the first report of a glycolytic pathway involving the formation of formate from C-1 in glucose. It is noteworthy that the most atypical member of the Thermococcales, T. zilligii, possesses also this unusual glycolytic feature.
Acknowledgments
This work was supported by the European Community Biotech Programme (Extremophiles as Cell Factories, BIO4-CT96-0488) and by PRAXIS XXI and FEDER, Portugal (PRAXIS/2/2.1/BIO/1109/95).
We thank Mónica Dias for performing some of the labeling experiments and Peter Schönheit for vivid discussions.
REFERENCES
- 1.Blamey J M, Adams M W W. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim Biophys Acta. 1993;1161:19–27. doi: 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.De Rosa M, Gambacorta A, Nicolaus B, Giardina P, Poerio E, Buonocore V. Glucose metabolism in the extreme thermoacidophilic archaebacterium Sulfolobus solfataricus. Biochem J. 1984;224:407–414. doi: 10.1042/bj2240407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottschalk G. Bacterial metabolism. 2nd ed. New York, N.Y: Springer-Verlag; 1986. [Google Scholar]
- 5.Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1983;49:208–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- 6.Kengen S W M, De Bok F A M, van Loo N-D, Dijkema C, Stams A J M, De Vos W M. Evidence for the operation of a novel Embden-Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus. J Biol Chem. 1994;269:17537–17541. [PubMed] [Google Scholar]
- 7.Kengen S W M, Tuininga J E, de Bok F A M, Stams A J M, de Vos W M. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1995;270:30453–30457. doi: 10.1074/jbc.270.51.30453. [DOI] [PubMed] [Google Scholar]
- 8.Kerscher L, Oesterhelt D. Pyruvate:ferredoxin oxidoreductase—new findings on ancient enzyme. Trends Biochem Sci. 1982;7:371–374. [Google Scholar]
- 9.Klages K U, Morgan H W. Characterization of a thermophilic sulphur-metabolizing archaebacterium belonging to the Thermococcales. Arch Microbiol. 1994;162:261–266. [Google Scholar]
- 10.Lanzotti V, Trincone A, Nicolaus B, Zillig W, De Rosa M, Gambacorta A. Complex lipids of Pyrococcus and AN1, thermophilic members of archaebacteria belonging to Thermococcales. Biochim Biophys Acta. 1989;1004:44–48. [Google Scholar]
- 11.Mukund S, Adams M W W. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1995;270:8389–8392. doi: 10.1074/jbc.270.15.8389. [DOI] [PubMed] [Google Scholar]
- 12.Neves A R, Ramos A, Nunes M C, Kleerebezem M, Hugenholtz J, de Vos W M, Almeida J, Santos H. In vivo NMR studies of glycolytic kinetics in Lactococcus lactis. Biotechnol Bioeng. 1999;64:200–212. doi: 10.1002/(sici)1097-0290(19990720)64:2<200::aid-bit9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Ronimus R S, Reysenbach A-L, Musgrave D R, Morgan H W. The phylogenic position of the Thermococcus isolate AN1 based on 16S rRNA gene sequence analysis: a proposal that AN1 represents a new species, Thermococcus zilligii sp. nov. Arch Microbiol. 1997;168:245–248. doi: 10.1007/s002030050495. [DOI] [PubMed] [Google Scholar]
- 14.Ronimus R S, Koning J, Morgan H W. Purification and characterization of an ADP-dependent phosphofructokinase from Thermococcus zilligii. Extremophiles. 1999;3:121–129. doi: 10.1007/s007920050107. [DOI] [PubMed] [Google Scholar]
- 15.Schäfer T, Schönheit P. Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Acetate formation from acetyl-CoA and ATP synthesis are catalyzed by an acetyl-CoA synthetase (ADP forming) Arch Microbiol. 1991;155:366–377. [Google Scholar]
- 16.Schäfer T, Xavier K B, Santos H, Schönheit P. Glucose fermentation to acetate and alanine in resting cell suspensions of Pyrococcus furiosus: proposal of a novel glycolytic pathway based on 13C labelling data and enzyme activities. FEMS Microbiol Lett. 1994;121:107–114. [Google Scholar]
- 17.Selig M, Xavier K B, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- 18.Siebers B, Hensel R. Glucose catabolism of the hyperthermophilic archaeum Thermoproteus tenax. FEMS Microbiol Lett. 1993;111:1–8. [Google Scholar]
- 19.Tuininga J E, Verhees C H, van der Oost J, Kengen S W, Stams A J, de Vos W M. Molecular and biochemical characterization of the ADP-dependent phosphofructokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1999;274:21023–21028. doi: 10.1074/jbc.274.30.21023. [DOI] [PubMed] [Google Scholar]
- 20.Ugurbil K, Brown T R, Den Hollander J A, Glynn P, Shulman R G. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci USA. 1978;75:3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood A P, Kelly D P, Norris P R. Autotrophic growth of four Sulfolobus strains on tetrathionate and the effect of organic nutrients. Arch Microbiol. 1987;146:382–389. [Google Scholar]