Abstract
Background
New-onset postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery and is associated with increased long-term stroke and mortality. Anticoagulation has been suggested as a potential therapy, but data on safety and efficacy are scant.
Objectives
To determine the association between anticoagulation for POAF and long-term outcomes.
Methods
Adult patients with POAF after isolated coronary artery bypass surgery (CABG) were identified through the Society of Thoracic Surgeons Adult Cardiac Surgery Database and linked to the Medicare Database. Propensity-matched analyses were performed for all-cause mortality, stroke, myocardial infarction, and major bleeding for patients discharged with or without anticoagulation. Interaction between anticoagulation and CHA2DS2-VASc score was also assessed.
Results
Of 38,936 patients, 9861 (25%) were discharged on oral anticoagulation. After propensity score matching, discharge anticoagulation was associated with increased mortality (hazard ratio [HR] 1.16, 95% confidence interval [CI] 1.06–1.26). There was no difference in ischemic stroke between groups (HR 0.97, 95% CI 0.82–1.15), but there was significantly higher bleeding (HR 1.60, 95% CI 1.38–1.85) among those discharged on anticoagulation. Myocardial infarction was lower in the first 30 days for those discharged on anticoagulation, but this effect decreased over time. The incidence of all complications was higher for patients with CHA2DS2-VASc scores ≥5 compared to patients with scores of 2–4. Anticoagulation did not appear to benefit either subgroup.
Conclusion
Anticoagulation is associated with increased mortality after new-onset POAF following CABG. There was no reduction in ischemic stroke among those discharged on anticoagulation regardless of CHA2DS2-VASc score.
Keywords: Postoperative atrial fibrillation, Anticoagulation, Coronary artery bypass surgery, Arrhythmia, Cardiac surgery
Graphical abstract

Key Findings.
-
▪
Patients with new-onset atrial fibrillation after coronary artery bypass surgery who are discharged on anticoagulation have higher mortality than similar patients discharged without anticoagulation.
-
▪
Stroke rates are lower than predicted by traditional risk scores for patients with new-onset atrial fibrillation after coronary artery bypass surgery.
-
▪
Patients with new-onset atrial fibrillation after coronary artery bypass surgery with a higher CHA2DS2-VASc score do not appear to benefit more from anticoagulation on discharge than patients with a lower CHA2DS2-VASc score.
Introduction
New-onset postoperative atrial fibrillation (POAF) occurs in 25%–40% of patients undergoing cardiac surgery, making it the most commonly associated complication, and affects 60,000–70,000 patients annually.1, 2, 3 Historically, POAF has been considered self-limited, but several studies show a high incidence of recurrence in this patient population.4, 5, 6, 7 Furthermore, POAF heralds poor postoperative outcomes and is a strong independent predictor of long-term stroke and all-cause mortality.7, 8, 9, 10
Optimal management of POAF has not been defined. Many strategies for prophylaxis or rate/rhythm control, including β-blockers, antiarrhythmics, statins, anti-inflammatory medications, renin-angiotensin system inhibitors, magnesium, atrial pacing, and posterior pericardiotomy, have been studied. No interventions besides statins have been shown to decrease stroke or mortality in POAF patients, and it is unclear whether the benefit of statins pertains to the atrial fibrillation (AF) itself.11, 12, 13, 14, 15, 16 Another proposed approach for POAF management is anticoagulation, but few data are available regarding its role in this patient population and results are mixed.17, 18, 19 As a result, guideline recommendations for anticoagulation in POAF remain vague.20, 21, 22, 23, 24, 25, 26 For example, some guidelines recommend anticoagulation for POAF lasting >48 hours while others recommend 72 hours. Yet others recommend anticoagulation based on risk factors regardless of duration, and many guidelines differ with respect to what risk factors should be considered. Furthermore, risk scores for stroke, such as CHA2DS2-VASc, have not been validated in the postsurgical setting. It is, therefore, not surprising that attitudes toward anticoagulation in POAF vary substantially among providers.27 The purpose of our study was to assess the role of anticoagulation in POAF and whether it varies by CHA2DS2-VASc score.
Methods
Study population
We queried the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database for all patients undergoing isolated coronary artery bypass surgery (CABG) from July 2011 through December 2016. We excluded patients with a history of AF or flutter, left atrial appendage removal or ligation, emergent procedures or reoperations, cardiogenic shock or inotrope requirement, mechanical circulatory support, preoperative anticoagulation, and dialysis. We also excluded patients with in-hospital deaths, stroke, or transient ischemic attack, to avoid procedural complications, and those not receiving anticoagulation despite a current indication (Figure 1). We linked eligible patients to the Centers for Medicare and Medicaid Services Database for long-term outcomes based on previously described probabilistic matching algorithms.28 We divided the study population into cohorts by anticoagulant prescription at discharge (warfarin, direct thrombin inhibitors, or factor Xa inhibitors vs no oral anticoagulation).
Figure 1.
Flow diagram of study inclusions and exclusions. AF = atrial fibrillation; CABG = coronary artery bypass graft; CMS = Centers for Medicare and Medicaid Services; LAA = left atrial appendage; POAF = postoperative atrial fibrillation; TIA = transient ischemic attack.
Outcomes
Our primary outcome of interest was all-cause mortality after index discharge, ascertained using the linked Medicare Denominator file. We defined secondary outcomes using Medicare Part A data to identify rehospitalizations with a primary diagnosis for the following endpoints: thromboembolism (composite of ischemic stroke, transient ischemic attack, or systemic embolism), major bleeding, and myocardial infarction (MI). All analyses started from the date of index discharge. A complete list of ICD codes used is provided in Supplemental Appendix A.
Propensity score matching
We computed propensity scores for anticoagulation using a multivariate logistic model that included 77 covariates adapted from the previously validated STS CABG mortality predictive model (Supplemental Appendix B).29 We used a 1:1 optimal propensity matching approach to overcome differences in potential confounders between the 2 cohorts. To assess balance in the matched cohort, we compared the distribution of baseline characteristics before and after matching using standardized differences (absolute value <10% suggests adequate balance by convention). In addition, to account for possible residual confounding, we performed sensitivity analysis using multivariable Cox proportional hazard regression analysis in the matched sample (same covariates as STS CABG mortality model). We evaluated differences in baseline characteristics between cohorts before and after matching using Wilcoxon and Pearson χ2 tests.
Statistical analyses
We used time-to-event analysis to compare long-term survival and secondary outcomes between anticoagulation cohorts. For survival, we censored patient follow-up at the end of study period (January 1, 2017). We computed product-limit Kaplan-Meier survival estimates for each cohort in the unmatched and matched samples and compared with log-rank tests. We used Cox proportional hazard regression models to compute hazard ratios (HR) for anticoagulation in both samples. To account for hospital clustering of patients, we used a robust sandwich variance estimator and computed 95% confidence intervals (CI) accordingly. We tested the proportional hazards assumption using log-log survival plots (log(-log) survival vs log-time) and interactions between study groups and log-time.
For nonfatal secondary outcomes, death was considered a competing risk. We censored follow-up at date of death, end of Medicare fee-for-service date, or end of study period, whichever came first. For regression analysis, we used the Fine-Gray method to calculate subdistribution HR. We tested the proportional hazards assumption (accounting for competing risk of death) by plotting Schoenfeld residuals for each treatment cohort vs log-time and also with interaction terms between study cohorts and log-time in regression models.30,31 For outcomes whose results suggested a violation of the assumption of proportionality, we performed a landmark analysis with follow-up divided into 3 periods (<30 days, 30–180 days, and >180 days). For consistency, we also computed cumulative incidence function curves and subdistribution HR for each period. As a sensitivity analysis, we calculated cause-specific HR.
Finally, we evaluated for a possible interaction between anticoagulation and CHA2DS2-VASc scores on primary and secondary outcomes. We classified patients into moderate (scores of 2–4) and high (scores of 5–9) risk groups. We confirmed balance between anticoagulation cohorts in each risk group in the matched cohort with standardized differences. We computed Kaplan-Meier curves and log-rank tests for mortality and cumulative incidence curves and Gray’s tests for nonfatal outcomes to compare anticoagulation cohorts in each risk group.
We performed all analyses using SAS (version 9.4; SAS Institute, Cary, NC). We used 2-sided tests for all analyses and considered a P value of <.05 statistically significant. The Duke Clinical Research Institute, the data warehouse of the STS database, has received Institutional Review Board approval from Duke University. Informed consent was waived based on the de-identified retrospective nature of this study.
Results
Demographics
We identified 768,277 patients undergoing isolated CABG without a history of AF or flutter. Overall, 181,042 (24%) had new-onset POAF. After exclusions and database linkage, we included 38,936 patients in our analysis (Figure 1). Of these, 9861 (25%) were discharged on anticoagulation. Baseline patient characteristics can be found in Table 1. Distribution of CHA2DS2-VASc scores, prescription of discharge anticoagulation type and hospital, and additional demographic details can be found in Supplemental Appendix C. After propensity matching, 19,722 patients remained for adjusted analyses. Standardized differences after propensity matching were less than ±10% for all variables.
Table 1.
Characteristics of study population
| Variable | Overall (N = 38,936) | No AC (N =29,075) | AC (N = 9861) | P value |
|---|---|---|---|---|
| Age | 73 (69-77) | 73 (68-77) | 73 (69-78) | <.0001† |
| Male sex | 30,099 (77.30) | 22,292 (76.67) | 7,807 (79.17) | <.0001‡ |
| BMI | 28.72 (25.68-32.46) | 28.58 (25.53-32.23) | 29.27 (26.11-33.18) | <.0001† |
| Hypertension | 35,460 (91.07) | 26,395 (90.78) | 9,065 (91.91) | <.001‡ |
| Diabetes | 16,190 (41.58) | 11,924 (41.01) | 4,266 (43.26) | <.001‡ |
| Ejection fraction (%) | 55 (50-60) | 55 (50-60) | 55 (48-60) | <.0001† |
| Prior Stroke or TIA | 4,416 (11.34) | 3,281 (11.28) | 1,135 (11.51) | NS‡ |
| PVD | 6,451 (16.31) | 4,662 (16.03) | 1,689 (17.13) | <.05‡ |
| Sleep apnea | 4,892 (12.56) | 3,494 (12.02) | 1,398 (14.18) | <.0001‡ |
| Smoking | 15,398 (39.55) | 11,279 (38.79) | 4,119 (41.77) | <.0001‡ |
| CHA2DS2-VASc | 4 (4-5) | 4 (3-5) | 4 (4-5) | <.0001† |
Values are n (%) for categorical variables or median (interquartile range) for continuous variables.
AC = anticoagulation; BMI = body mass index; PVD = peripheral vascular disease; TIA = transient ischemic attack.
P values are based on χ2 rank based group means score statistics for all continuous/ordinal row variables.
P values are based on Pearson χ2 tests for all categorical row variables.
Outcomes
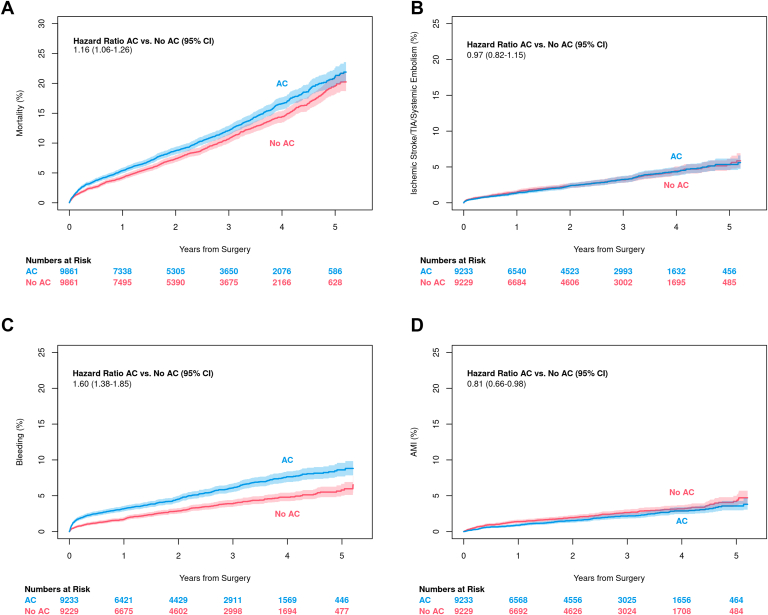
In matched patients, those discharged on anticoagulation experienced higher short- (within 30 days) and long-term mortality (HR 1.16, 95% CI 1.06–1.26; Figure 2A). This effect was proportional over 5 years of follow-up. There was no difference between anticoagulation cohorts in the combined thromboembolism endpoint (HR 0.97, 95% CI 0.82–1.15; Figure 2B). Readmission for bleeding was higher for those discharged on anticoagulation (HR 1.60, 95% CI 1.38–1.85; Figure 2C). Results demonstrated a violation of the proportional hazards assumption (P < .0001), suggesting that difference in bleeding was not uniform over the 5-year follow-up period. The difference in bleeding rates was largest in the first 30 days after discharge, but persisted over the duration of follow-up (Supplemental Appendix D). Patients discharged on anticoagulation had fewer readmissions for MI (HR 0.81, 95% CI 0.66–0.98; Figure 2D). Again, our results showed a violation of the proportional hazards assumption and demonstrated that this effect did not persist over the entire follow-up period (Supplemental Appendix D). Results were virtually the same on sensitivity analysis, indicative of successful matching, and are not shown here.
Figure 2.
Outcomes by anticoagulation (AC) status. A: Mortality. B: Readmission for thromboembolism. C: Readmission for bleeding. D: Readmission for myocardial infarction.
Effect of CHA2DS2-VASc score
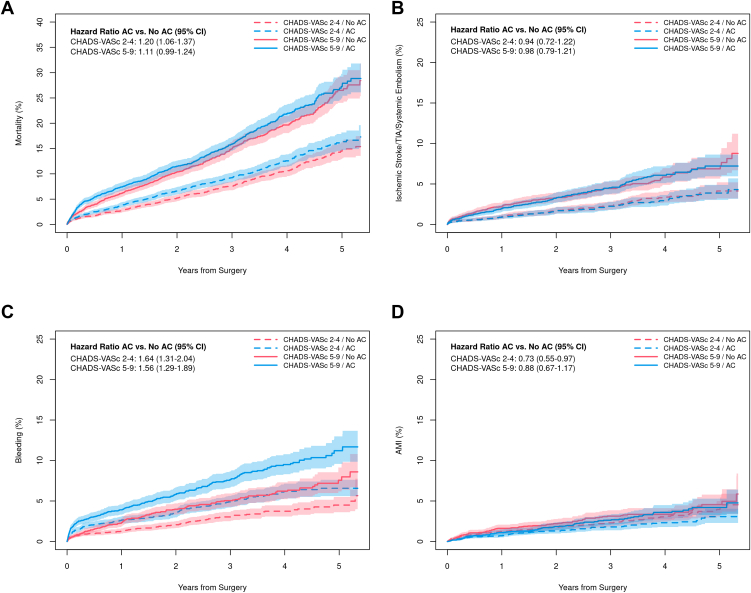
As expected, patients with high CHA2DS2-VASc scores (5–9) experienced more adverse outcomes compared to patients with moderately elevated scores (2–4). Within each CHA2DS2-VASc group, however, the results remain unchanged from the overall population. Higher mortality (HR 1.11, 95% CI 0.99–1.24 for high and HR 1.20, 95% CI 1.06–1.37 for moderately elevated scores; Figure 3A) was observed among patients discharged on anticoagulation in both risk groups. There was no interaction between anticoagulation cohort and CHA2DS2-VASc group for the primary outcome (P = .348 for interaction) or any secondary outcomes. There was no significant difference in thromboembolism irrespective of CHA2DS2-VASc group (HR 0.98, 95% CI 0.79–1.21 and HR 0.94, 95% CI 0.72–1.22, respectively; P = .809; Figure 3B). Readmission for bleeding was higher in the anticoagulation cohort in both CHA2DS2-VASc groups (HR 1.56, 95% CI 1.29–1.89 and HR 1.64, 95% CI 1.31–2.04, respectively; P = .753; Figure 3C). Lower rates of readmission for MI were observed in both CHA2DS2-VASc groups (HR 0.88, 95% CI 0.67–1.17 and HR 0.73, 95% CI 0.55–0.97, respectively; P = .360; Figure 3D). Results were virtually the same on sensitivity analysis and are not shown here.
Figure 3.
Outcomes by anticoagulation (AC) status and CHA2DS2-VASc score. A: Mortality. B: Readmission for thromboembolism. C: Readmission for bleeding. D: Readmission for myocardial infarction.
Discussion
POAF is a common postsurgical complication with high morbidity and mortality, but optimal management is unknown.7, 8, 9, 10 It is well accepted that anticoagulation decreases stroke for patients with AF at the cost of increased bleeding, but its role in provoked or “secondary” AF is controversial.32, 33, 34 It is unclear in the current literature if POAF should be treated the same as nonvalvular AF or provoked AF or as its own category of disease. Most guidelines endorse anticoagulation for patients with risk factors for stroke, but specifics vary between guidelines, reflecting the lack of robust data and mixed results for the role of anticoagulation in this patient population.17, 18, 19, 20, 21, 22, 23, 24, 25, 26,35 Furthermore, there are no data to support the use of risk scores such as CHA2DS2-VASc in these patients. Given the elevated risk of bleeding in this population, the potential benefits of anticoagulation therapy must also be carefully weighed against the possible risks.36
For the first time, our data demonstrate an increased mortality associated with anticoagulation for POAF after isolated CABG. We find that mortality curves separate early and continue to diverge over 5 years of follow-up. We also demonstrate that thromboembolic events were not decreased in patients receiving anticoagulation, irrespective of CHA2DS2-VASc score. Finally, we demonstrate an increased rate of readmission for bleeding in POAF patients discharged on anticoagulation.
Current literature shows an increased incidence of late AF recurrence as well as increased stroke and mortality for POAF patients, suggesting that POAF patients may benefit from long-term anticoagulation, as recommended by several guidelines.4, 5, 6, 7, 8, 9, 10,20,22,25,26 Our findings run counter to these observations, however, as we observe no reduction in stroke for POAF patients discharged on anticoagulation. One possible explanation is that anticoagulation use may have diminished over time, mitigating any potential benefit for stroke prevention. Nevertheless, in understanding these results, it is also important to note that the yearly risk of stroke in our study population (1%–2% per year) was substantially lower than that predicted for nonvalvular AF patients using the CHA2DS2-VASc score (over 8% per year).37 This finding has been validated in the literature and points toward a decrease in the potential benefit of anticoagulation in this population.19 Furthermore, surgical patients may have an increased risk of bleeding in the postoperative phase, pushing the risk-benefit ratio less in favor of anticoagulation in this population. Indeed, our data demonstrate an increased rate of readmission for bleeding as well as increased mortality in the anticoagulation group. Although data on cause of death were not available, it is plausible that the increased mortality is at least partly due to bleeding events. Readmissions for bleeding also have negative repercussions beyond mortality, including impact on patient quality of life, healthcare resource utilization, and quality outcomes. Taken together, the lower incidence of stroke along with the higher risk of bleeding highlight the need for proper patient selection before anticoagulation is prescribed for POAF patients.
It is commonly accepted that AF patients with higher CHA2DS2-VASc scores are at higher risk for stroke and may therefore derive more benefit from anticoagulation.37,38 Traditionally, this rationale has also been applied to POAF patients, as reflected in some guidelines.22 This translates into real-world practice, as most surveyed practitioners report using the CHA2DS2-VASc score to guide anticoagulation prescription in POAF patients.27 Interestingly, our data also do not support this notion. We again demonstrate a lower than predicted yearly incidence of stroke and no apparent stroke reduction for those receiving anticoagulation even in the higher CHA2DS2-VASc group. This suggests that traditional risk factors such as CHA2DS2-VASc may not be applicable in the POAF population.
Taken together, our findings highlight the need for a better understanding of POAF and a disease-tailored approach toward patient selection for anticoagulation. We propose an exploration of risk factors specific to POAF that reflect disease severity and chronicity as targets for future research. Three such risk factors could potentially include POAF duration and frequency as well as rhythm at discharge. There is evidence from the nonsurgical AF literature that suggests AF duration may be a relevant factor for stroke risk.39 This observation may be relevant particularly in the POAF population, where not all patients will go on to develop chronic AF. Incessant or frequently recurring POAF may also reflect greater disease severity, which could potentially influence stroke risk. The relationship between these risk factors and stroke in POAF has not been well studied, but these considerations are sometimes used for clinical decision making and even referenced in guidelines regarding POAF, highlighting the need for further investigation of their clinical utility.21, 22, 23,25, 26, 27 Our findings also highlight the need for a team-based approach to POAF management with careful consideration given to the potential risks and benefits of treatment as well as re-evaluation in the outpatient setting.
Limitations
Our data are limited by the retrospective nature of our study, which may not completely account for confounding factors despite rigorous statistical approaches to minimize bias. Outcomes data were derived from the Centers for Medicare and Medicaid Services Database and not individually adjudicated. Although performance of this database has been previously reported to be reliable, such data may over- or underestimate true outcomes.40, 41, 42 Data on POAF duration/recurrence and rhythm at discharge were not available to us but may bias the prescribing patterns of some providers. Previous work suggests that these are not driving factors in POAF management.27 Data on cause of death, duration of anticoagulation therapy, and new prescription of anticoagulation after discharge were also unavailable. Our data demonstrate a higher risk of bleeding in the anticoagulation group that persists over the course of the study, suggesting a persistent difference in anticoagulation practices between the 2 groups. This is further validated by the decreased incidence of MI throughout the study, which has been shown to be associated with anticoagulation use. Finally, we were unable to account for surgical factors that may have influenced the decision for anticoagulation, such as difficulty with hemostasis and bleeding prior to discharge.
Conclusion
Overall, our findings demonstrate higher mortality and bleeding for POAF patients discharged on anticoagulation, regardless of CHA2DS2-VASc score. No difference in thromboembolism was identified. We suggest further investigation into several risk factors that are commonly used in clinical decision making or referenced in clinical guidelines but that have limited data in support of their utility, such as POAF duration/frequency and rhythm at discharge. We also highlight the need for a team-based approach to POAF management, with communication between the surgical team, inpatient providers, and outpatient cardiology to carefully weigh the risks and benefits of therapy as well as to provide continuity of care across the inpatient and outpatient settings. Ultimately, prospective, randomized trials, including the ongoing PACES trial (https://clinicaltrials.gov/ct2/show/NCT04045665), will be necessary in identifying the optimal treatment strategies for this patient population.
Acknowledgments
Funding Sources
Data and statistical analysis for this research were provided through a grant by The Society of Thoracic Surgeons’ National Database Access and Publications Research Program, Chicago, Illinois, and performed at the Duke Clinical Research Institute, Durham, North Carolina.
Disclosures
The authors report the following industry relationships: Sundaram – Janssen Pharmaceuticals; Jacobs – SpecialtyCare; Thourani – Abbott Vascular, Atricure, Boston Scientific, Edwards Lifesciences, Gore Vascular, JenaValve, Shockwave; Vemulapalli – Abbott, ACP, Boston Scientific, HeartFlow, Janssen; Waldo – Biosense Webster, Bristol-Myers Squibb, Cardiac Insight, Pfizer; Sabik – Abbott, Edwards Lifesciences, Medtronic; All others – none.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed consent was waived based on the de-identified retrospective nature of this study.
Ethics Statement
The research reported in this study was conducted according to the principles of the Declaration of Helsinki. The Duke Clinical Research Institute, the data warehouse of the STS database, has received Institutional Review Board approval from Duke University.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.06.003.
Appendix. Supplementary data
References
- 1.Bowdish M.E., D'Agostino R.S., Thourani V.H., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2020 update on outcomes and research. Ann Thorac Surg. 2020;109:1646–1655. doi: 10.1016/j.athoracsur.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Nazeri A., Razavi M., Elayda M.A., Lee V.V., Massumi A., Wilson J.M. Race/ethnicity and the incidence of new-onset atrial fibrillation after isolated coronary artery bypass surgery. Heart Rhythm. 2010;7:1458–1463. doi: 10.1016/j.hrthm.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Echahidi N., Pibarot P., O’Hara G., Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Lowres N., Mulcahy G., Jin K., Gallagher R., Neubeck L., Freedman B. Incidence of postoperative atrial fibrillation recurrence in patients discharged in sinus rhythm after cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2018;26:504–511. doi: 10.1093/icvts/ivx348. [DOI] [PubMed] [Google Scholar]
- 5.Ayoub K., Habash F., Almomani A., et al. Long term risk of recurrent atrial fibrillation and ischemic stroke after post-operative atrial fibrillation complicating cardiac and non-cardiac surgeries. J Atr Fibrillation. 2018;10:1660. doi: 10.4022/jafib.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melduni R.M., Schaff H.V., Bailey K.R., et al. Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am Heart J. 2015;170:659–668. doi: 10.1016/j.ahj.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Ahlsson A., Fengsrud E., Bodin L., Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–1359. doi: 10.1016/j.ejcts.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Woldendorp K., Farag J., Khadra S., Black D., Robinson B., Bannon P. Postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg. 2021;112:2084–2093. doi: 10.1016/j.athoracsur.2020.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Lin M.H., Kamel H., Singer D.E., Wu Y.L., Lee M., Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50:1364–1371. doi: 10.1161/STROKEAHA.118.023921. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg J.W., Lancaster T.S., Schuessler R.B., Melby S.J. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52:665–672. doi: 10.1093/ejcts/ezx039. [DOI] [PubMed] [Google Scholar]
- 11.Arsenault K.A., Yusuf A.M., Crystal E., et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S., Acou W.J., Kiuchi M.G., et al. Association of preoperative renin-angiotensin system inhibitors with prevention of postoperative atrial fibrillation and adverse events: a systematic review and meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lertsburapa K., White C.M., Kluger J., Faheem O., Hammond J., Coleman C.I. Preoperative statins for the prevention of atrial fibrillation after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2008;135:405–411. doi: 10.1016/j.jtcvs.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Girerd N., Pibarot P., Daleau P., et al. Statins reduce short- and long-term mortality associated with postoperative atrial fibrillation after coronary artery bypass grafting: impact of postoperative atrial fibrillation and statin therapy on survival. Clin Cardiol. 2012;35:430–436. doi: 10.1002/clc.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan W., Pintar T., Anton J., Lee V.V., Vaughn W.K., Collard C.D. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:II45–II49. doi: 10.1161/01.CIR.0000138316.24048.08. [DOI] [PubMed] [Google Scholar]
- 16.Gillinov A.M., Bagiella E., Moskowitz A.J., et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. doi: 10.1056/NEJMoa1602002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Chami M.F., Kilgo P., Thourani V., et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–1376. doi: 10.1016/j.jacc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S., Cybulsky I., Delaney J. Anticoagulation for stroke prevention in new atrial fibrillation after coronary artery bypass graft surgery. Thromb Res. 2015;135:841–845. doi: 10.1016/j.thromres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Butt J.H., Xian Y., Peterson E.D., et al. Long-term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3:417–424. doi: 10.1001/jamacardio.2018.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.January C.T., Wann L.S., Alpert J.S., et al. ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. Erratum in: Circulation 2014;130:e270–e271. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhof P., Benussi S., Kotecha D., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 22.Frendl G., Sodickson A.C., Chung M.K., et al. American Association for Thoracic Surgery 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148:e153–e193. doi: 10.1016/j.jtcvs.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernando H.C., Jaklitsch M.T., Walsh G.L., et al. The Society of Thoracic Surgeons practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: executive summary. Ann Thorac Surg. 2011;92:1144–1152. doi: 10.1016/j.athoracsur.2011.06.104. [DOI] [PubMed] [Google Scholar]
- 24.Epstein A.E., Alexander J.C., Gutterman D.D., Maisel W., Wharton J.M. American College of Chest Physicians. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:24S–27S. doi: 10.1378/chest.128.2_suppl.24s. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell L.B., CCS Atrial Fibrillation Guidelines Committee Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol. 2011;27:91–97. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Doost A., Alasady M., Scott P. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2019;28:e106–e107. doi: 10.1016/j.hlc.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Riad F.S., German K., Deitz S., Sahadevan J., Sundaram V., Waldo A.L. Attitudes toward anticoagulation for postoperative atrial fibrillation: a nationwide survey of VA providers. Pacing Clin Electrophysiol. 2020;43:1295–1301. doi: 10.1111/pace.14095. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs J.P., Edwards F.H., Shahian D.M., et al. Successful linking of the Society of Thoracic Surgeons adult cardiac surgery database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg. 2010;90:1150–1156. doi: 10.1016/j.athoracsur.2010.05.042. discussion 1156–1157. [DOI] [PubMed] [Google Scholar]
- 29.Shahian D.M., Jacobs J.P., Badhwar V., et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 1-background, design considerations, and model development. Ann Thorac Surg. 2018;105:1411–1418. doi: 10.1016/j.athoracsur.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 31.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 32.Aguilar M.I., Hart R., Pearce L.A. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;3:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Quon M.J., Behlouli H., Pilote L. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC Clin Electrophysiol. 2018;4:386–393. doi: 10.1016/j.jacep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Lubitz S.A., Yin X., Rienstra M., et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131:1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butt J.H., Olesen J.B., Havers-Borgersen E., et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol. 2018;72:2027–2036. doi: 10.1016/j.jacc.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 36.DiMarco J.P., Flaker G., Waldo A.L., et al. AFFIRM Investigators. Factors affecting bleeding risk during anticoagulant therapy in patients with atrial fibrillation: observations from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:650–656. doi: 10.1016/j.ahj.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Friberg L., Rosenqvist M., Lip G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 38.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 39.Healey J.S., Connolly S.J., Gold M.R., et al. ASSERT Investigators Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. Erratum in: N Engl J Med 2016;374:998. [DOI] [PubMed] [Google Scholar]
- 40.Lowenstern A., Lippmann S.J., Brennan J.M., et al. Use of medicare claims to identify adverse clinical outcomes after mitral valve repair. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiyota Y., Schneeweiss S., Glynn R.J., Cannuscio C.C., Avorn J., Solomon D.H. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Kumamaru H., Judd S.E., Curtis J.R., et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with Medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7:611–619. doi: 10.1161/CIRCOUTCOMES.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.