Abstract
Background
Interatrial conduction has been postulated to play an important role in atrial fibrillation (AF). The pathways involved in interatrial conduction during AF remain incompletely defined.
Objective
We recently showed physiological assessment of fibrillatory dynamics could be performed using renewal theory, which determines rates of phase singularity formation (λf) and destruction (λd). Using the renewal approach, we aimed to understand the role of the interatrial septum and other electrically coupled regions during AF.
Method
RENEWAL-AF is a prospective multicenter observational study recruiting AF ablation patients (ACTRN 12619001172190). We studied unipolar electrograms obtained from 16 biatrial locations prior to ablation using a 16-electrode Advisor HD Grid catheter. Renewal rate constants λf and λd were calculated, and the relationships between these rate constants in regions of interatrial connectivity were examined.
Results
Forty-one AF patients (28.5% female) were recruited. A positive linear correlation was observed between λf and λd (1) across the interatrial septum (λf r2 = 0.5, P < .001, λd r2 = 0.45, P < .001), (2) in regions connected by the Bachmann bundle (right atrial appendage–left atrial appendage λf r2 = 0.29, P = .001; λd r2 = 0.2, P = .008), and (3) across the inferior interatrial routes (cavotricuspid isthmus–left atrial septum λf r2 = 0.67, P < .001; λd r2 = 0.55, P < .001). Persistent AF status and left atrial volume were found to be important effect modifiers of the degree of interatrial renewal rate statistical correlation.
Conclusion
Our findings support the role of interseptal statistically determined electrical disrelation in sustaining AF. Additionally, renewal theory identified preferential conduction through specific interatrial pathways during fibrillation. These findings may be of importance in identifying clinically significant targets for ablation in AF patients.
Keywords: Renewal theory, Atrial fibrillation, Interatrial conduction, Interatrial septum, Bachmann’s bundle
Key Findings.
-
▪
Renewal rate constants provide a method to enable determination of areas with contiguous electrical conduction between the left and right atria during cardiac fibrillation.
-
▪
Positive linear correlations are observed between renewal rate constants in regions across the interatrial septum, the Bachmann bundle, and the inferior interatrial connecting routes.
-
▪
Atrial fibrillation (AF) progression to persistent AF and increased atrial size are associated with decreased levels of interatrial renewal rate constant correlation.
Introduction
A mechanistic role for interatrial connections in atrial fibrillation (AF) maintenance has been postulated.1, 2, 3, 4 However, to date, the precise mechanisms by which interatrial conduction plays a part in sustaining AF have not been fully defined.2,4 A potential barrier to delineating the role of interatrial conduction in AF perpetuation has been that it has been challenging to physiologically probe these connections during sustained AF owing to the turbulent nature of the fibrillatory process.
To date, interatrial conduction has been proposed to contribute to several divergent but important roles in sustaining AF. Mechanistically, interatrial connections have been suggested to be (1) potential sources of reentrant circuits1,5 and (2) potential sites of conduction block that could assist in sustaining AF.6,7 There has also been interest in a potential therapeutic effect of modifying interatrial conduction via catheter ablation, in both experimental1,3 and computational models.8
In this study, we sought to extend previous research by systematic evaluation of interatrial conduction during AF in humans using a renewal theory approach. Renewal theory provides a way to measure the continuous formation and destruction of unstable reentrant circuits in AF9, 10, 11, 12 and ventricular fibrillation.9 Unstable reentrant circuits, at present believed to be spiral vortices,13,14 have been consistently observed in AF research.1,15, 16, 17, 18, 19
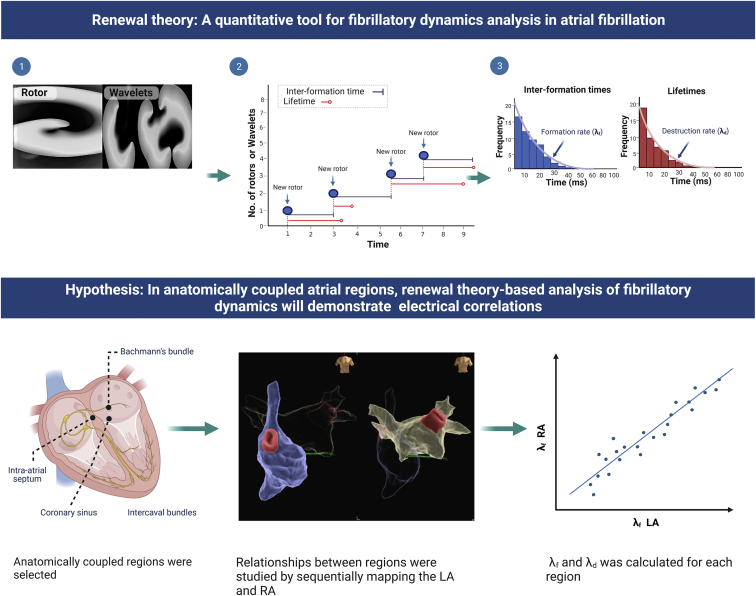
The repetitive creation and annihilation of spiral waves observed in AF is parallel to other turbulent systems in nature.20, 21, 22, 23 Theoretically, it has been proposed that in systems characterized by spatiotemporal turbulence, spiral dynamics will follow a common set of statistical laws,12,23 namely that (1) spiral lifetimes will follow exponential distributions23, 24, 25 and (2) spiral populations will follow a Poisson distribution.24,26 These predictions have been independently validated in diverse physical,21 chemical,22 and biological systems.20,23 In the context of fibrillation, exponential-type distributions have consistently been observed for phase singularity (PS) formation, as well as PS lifetimes in multiple laboratories.18,19,27, 28, 29 In computational, animal, and human models of cardiac fibrillation, using the renewal theory approach,9, 10, 11, 12,30 we confirmed these observations and demonstrated it could allow for accurate quantification of rates of PS formation (λf, pronounced as “lambda f”) and destruction of PS (λd, pronounced as “lambda d”)9,11,12,30 (Figure 1). We found that although the renewal rate constants scaled with catheter size,30 the renewal equations for population distribution remained internally consistent,30 in line with theoretical predictions about the effect of noise31 and 3-dimensional character of cardiac turbulence.32 A summary figure explaining renewal theory is provided in Figure 1.
Figure 1.
Introduction to renewal theory. (1) Renewal theory is based on the presence of unstable reentrant circuits, currently believed to be spiral waves in atrial fibrillation (AF). (2) The intervals between phase singularity (PS) formation events and the lifetimes of PS are measured, and distributions for these constructed. (3) These have been shown to be statistically independent and to form exponential distributions, implying a constant rate of PS formation (which we call λf and λd). The hypothesis evaluated in this study was that anatomically connected biatrial regions would show a linear correlation between λf and λd.
In the current study, we build on this earlier work by aiming to determine whether renewal theory could be applied to further understand the electrophysiological relationships between the left and right atria. We hypothesized that (1) atrial regions with anatomical contiguity across the interatrial septum should have a positive correlation between respective renewal rate constants and (2) different patterns of AF could potentially have variable renewal rate constants. We further applied renewal theory to analyze fibrillatory dynamics in other locations involved in interatrial conduction, such as the Bachmann bundle and the inferior interatrial route.
Methods
Study population
RENEWAL-AF was a multicenter prospective observational study involving 4 Australian hospitals (ACTRN 12619001172190p). Paroxysmal or persistent AF patients clinically indicated for AF ablation were enrolled. Ethics approval was by the Southern Adelaide Local Health Network Ethics Committee (HREC/19/SAC/292). This research adhered to Helsinki Declaration guidelines. All patients provided informed consent.
Electrophysiology study
Baseline demographics were obtained preprocedurally and documented in an electronic clinical record form (REDCap). Electrophysiologic studies were performed 5 half-lives free of antiarrhythmic drug therapy, except for patients taking amiodarone. Patients were mapped under spontaneous or induced AF using the EnSite Precision electroanatomic mapping system (Abbott Cardiovascular, Plymouth MN). Advisor™ HD Grid mapping catheter (Abbott Cardiovascular, Plymouth, MN) was used. This catheter has 16 electrodes in a square grid (13 × 13mm2 grid, 3 mm inter-spacing). Unipolar electrograms were recorded at 1000 Hz (bandpass filter 0.5–500 Hz). Electrograms and electrocardiogram tracings were recorded for 1 minute prior to ablation in 6 different right atrial (RA) intracardiac locations, sequentially superior vena cava (SVC)–RA junction, cavotricuspid isthmus (CTI), RA septum, RA lateral wall, RA appendage (RAA), and RA posterior; and 10 left atrial (LA) locations: left superior pulmonary vein, left inferior pulmonary vein, right superior pulmonary vein, right inferior pulmonary vein, left high posterior wall, left low posterior wall, left lateral region, LA appendage (LAA), LA anterior region, and LA septum (Figure 1).
Signal processing
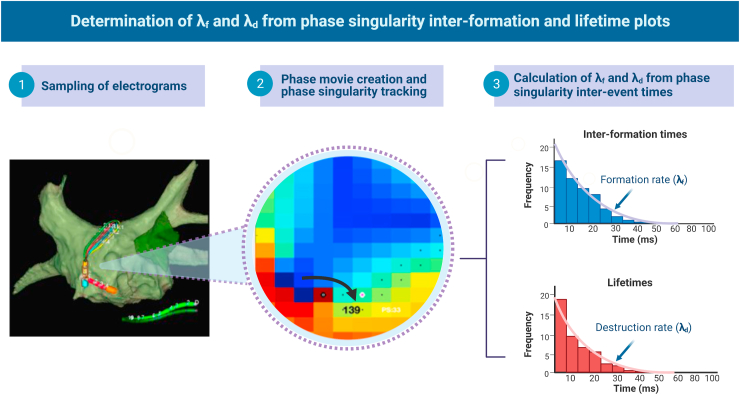
Unipolar electrogram signals were processed as described.9,11,30 QRS subtraction was performed and Butterworth filters applied.9,11,30 Sinusoidal recomposition was applied with the dominant frequency set as wavelet period and phase computed using the Hilbert transform to construct phase maps.9,11,28,30 In each phase map, PS were detected and tracked as previously described using a convolution kernel method based on topological charge.9,11,30 PS tracking enabled calculation of PS lifetimes and inter-formation times (times between consecutive PS formations), which also enabled construction of PS lifetime and inter-formation time distributions.9,11,30 PS distributions were fitted using maximum likelihood fitting to estimate the rate of PS formation (denoted as λf) and PS destruction (λd) (Figure 2).9,11,30 A description of the processing steps is provided in Figure 2.
Figure 2.
λf and λd were determined as follows: (1) Unipolar electrograms in atrial fibrillation were sampled preablation in 16 predefined biatrial segments (1-minute recordings) with an Advisor HD Grid catheter (Abbott Cardiovascular, Plymouth, MN). Phase movies were created (2), and renewal rate constants were calculated for formation and destruction (3).
Statistical analysis
Data were reported as the mean (standard deviation) or the median [interquartile range] for parametric and nonparametric data. Categorical variables were presented as n (%) and differences between groups were examined using the χ2 test. Two-group comparisons were analyzed using Student t test. Associations between different anatomical regions were evaluated using the Pearson correlation coefficient. Statistical analysis was performed using STATA 15.1 with α at P < .05.
Results
Patient characteristics
Patient characteristics are described in Table 1. Data from 41 AF patients who underwent AF ablation were available for analysis. Mean age (years) of patients recruited was 59.1 ± 9.4; 28.5% were female. Mean body mass index (kg/m2) was 31.2 ± 4.4, and 41% of patients had paroxysmal AF. Mean CHA2DS2-VASc score was 1.9 ± 1.5. Mean left atrial volume index (LAVi) was 42.9 ± 8.5 mL/m2 (Table 1).
Table 1.
Patient baseline demographics
| Baseline demographics (N = 41 patients) | Mean (SD) or n [%] |
|---|---|
| Age (years) | 59.1 (9.4) |
| BMI (kg/m2) | 31.2 (4.4) |
| Sex, female | 12 [28.5] |
| Diabetes mellitus | 3 [7] |
| Hypertension | 17 [40.5] |
| Vascular disease | 8 [19.5] |
| Hyperlipidemia | 13 [31] |
| OSA | 14 [34] |
| Heart failure | 17 [41.5] |
| CVA | 6 [14] |
| Smoking history | 10 [23.8] |
| Alcohol intake | 27 [64.3] |
| Alcohol standard drinks/week | 5.7 (11.7) |
| CHA2DS2-VASc score | 1.9 (1.5) |
| Paroxysmal AF | 17 [41] |
| LVEF | 57.5% (10.0%) |
| LAVi, mL/m2 | 42.9 (8.5) |
Data presented as mean (standard deviation) or n [%].
AF = atrial fibrillation, BMI = body mass index; CVA = cerebrovascular accident; LAVi = left atrial volume index; LVEF = left ventricular ejection fraction; OSA = obstructive sleep apnea.
Interatrial electrical relationships
Interatrial septum
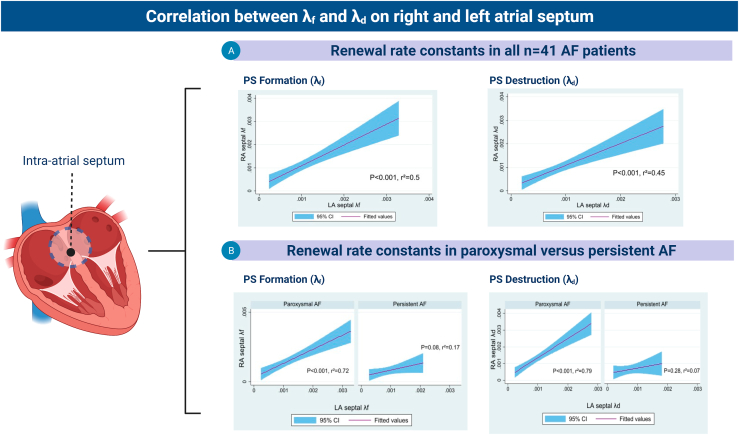
For this purpose, we compared λf and λd between RA and LA septal regions (Figure 3). Overall a positive correlation was observed between the λf (r2 = 0.5, P < .001) and λd (r2 = 0.45, P < .001) between both regions.
Figure 3.
Interseptal conduction showed a positive linear correlation between the right and left side of the interatrial septum, an effect that was diminished in patients with persistent atrial fibrillation (AF). PS = phase singularity.
Persistent AF status and LA volume were important effect modifiers. Paroxysmal AF showed stronger correlations across the LA and RA septal region (λf r2 = 0.72, P < .001; λd r2 = 0.79, P < .001) than persistent AF (λf r2 = 0.17, P = .08; λd r2 = 0.07, P = .28). LA volume index had a comparable effect (Figure 3). For patients with LA volume index <40 mL/m2, there was a stronger correlation than in patients with LA volume index ≥40 mL/m2 (LAVi <40 mL/m2: λf r2 = 0.68, P < .001, λd r2 = 0.68, P < .001; LAVi ≥40 mL/m2: λf r2 = 0.27, P = .03 and r2 = 0.14, P = .14) (Figure 3).
Bachmann bundle
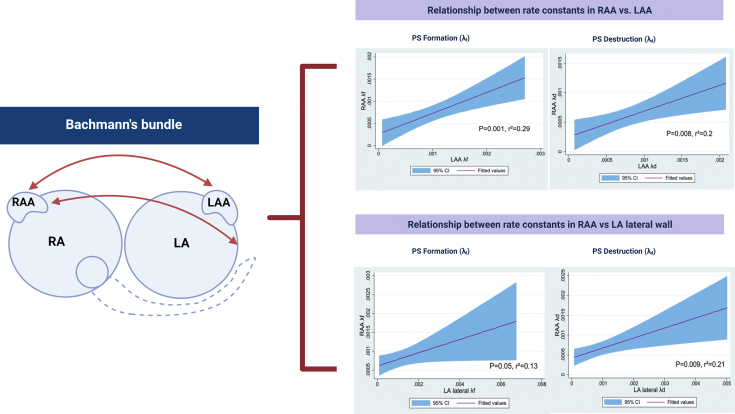
The Bachmann bundle is an epicardial muscular bundle that connects the left and right atrium, and the presence and the extent of conduction block in this structure has been associated with AF incidence and persistence.6,33 The Bachmann bundle rightward arm crosses the region of the cavoatrial junction superiorly around the region of the sagittal bundle, and inferiorly around the subepicardium of the RA vestibule (area between the RA appendage [RAA] orifice and the RA atrioventricular valve annulus).33,34 The Bachmann bundle then courses across the anterior interatrial groove and leftward to the LAA, with superficial circular muscular fiber projections around the left lateral wall.35,36 Other studies have suggested the role of high upper septum, which has been shown to form both structural and electrical connection to the mid portion of the Bachmann bundle.37 For this study, only endocardial mapping was performed in these patients. Hence, RAA, RA septum, and SVC-RA junction were used as surrogates for RA site attachments and LAA and LA lateral wall were used as surrogates for LA site attachments for the Bachmann bundle.
At the RAA to LAA and LA lateral wall, a modest linear correlation was observed between λf of the LAA and RAA (λf r2 = 0.29, P = .001) and λd of LAA vs λd of RAA (r2 = 0.2, P = .008) (Supplemental Figure 1). A modest linear correlation was also observed between λf of LA lateral wall and λf of RAA (r2 = 0.13, P = .05) and λd of LA lateral wall and λd of RAA (r2 = 0.21, P = .009) (Supplemental Figure 1).
At the SVC-RA junction to LAA and LA lateral wall, a modest linear correlation was seen between λf of the SVC-RA junction and LAA (λf r2 = 0.22, P = .003) and λd of LAA vs λd of the SVC-RA junction (r2 = 0.23, P = .002). A modest linear correlation was also observed between λf of LA lateral wall and λf of SVC-RA junction (r2 = 0.13, P = .05) and λd of LA lateral wall and λd of SVC-RA junction (r2 = 0.29, P = .001).
At the RA septal to LAA and LA lateral wall, a modest linear correlation was observed between RA septal and LAA (λf r2 = 0.16, P = .011) and λd of LAA vs λd of RA septal (r2 = 0.11, P = .04). No significant relationship was observed between λf of LA lateral wall and λf of RA septal (r2=0.02, p=0.35) in contrast with a stronger linear correlation observed between λd of LA lateral wall and λd of RAA (r2 = 0.51, P < .0001).
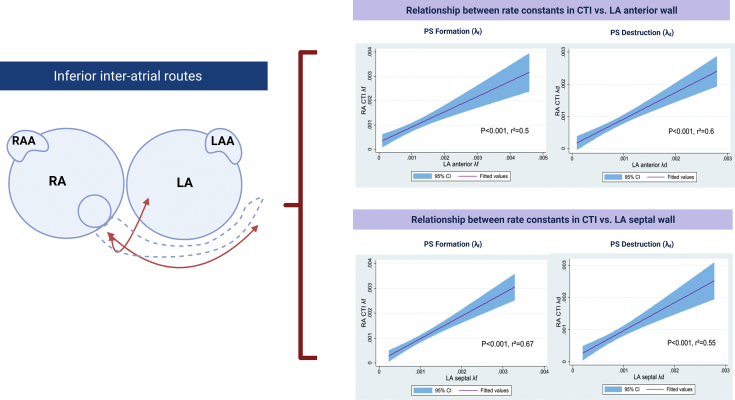
Inferior interatrial connections
In this study, the cavotricuspid isthmus region was used as indicator of potential interatrial conduction in the inferior region of the RA with all 6 LA atrial regions within the LA atrial body. Positive correlations between RA CTI and a variety of LA regions (λf CTI-LA septum r2 = 0.67, P < .001, CTI-LA-lateral wall r2 = 0.53, P < .001, CTI-LA anterior wall r2 = 0.5, P = .001, CTI-LA low posterior wall r2 = 0.42, P = .001, CTI-LA high posterior wall r2 = 0.28, P < .001. For λd, positive correlations were observed between CTI and LA anterior wall (r2 = 0.6, P < .001), CTI–LA septum (r2 = 0.55, P < .001), CTI–LA lateral wall (r2 = 0.53, P = .001), CTI–LA low posterior wall (r2 = 0.36, P < .001), and CTI–high posterior LA wall (r2 = 0.28, P = .001) (Supplemental Figure 2).
Relationship between spatial variation of global fibrillatory process in the left atrium with the right atrium
We then analyzed the relationship of all RA atrial locations with the mean LA λf. Mean LA λf was obtained by averaging λf of all 10 LA locations. Mean LA λf showed the highest relationship with CTI region (r2 = 0.75, P < .001), followed by RA septal wall (r2 = 0.6, P < .001), RA lateral wall (r2 = 0.5, P < .001), RAA (r2 = 0.34, P < .001), and SVC-RA junction (r2 = 0.32, P < .001) and RA posterior wall (r2 = 0.11, P = .038) (Table 2). Similar observations were made when the relationships between mean LA λd were analyzed with all 6 RA locations. Mean LA λd showed the highest relationship with CTI region (r2 = 0.76, P < .001), followed by RA septal wall (r2 = 0.58, P < .001), RAA (r2 = 0.52, P < .001), SVC-RA junction (r2 = 0.49, P < .001), RA lateral wall (r2 = 0.45, P < .001), and RA posterior wall (r2 = 0.12, P = .037) (Table 3).
Table 2.
Degree and significance, in descending order, of correlations between λf of measured right atrial locations with mean left atrial λf
| λf values of measured RA locations | Correlation with mean LA λf |
|---|---|
| CTI | r2 = 0.75, P < .001 |
| Septal RA | r2 = 0.6, P < .001 |
| Lateral RA | r2 = 0.5, P < .001 |
| RAA | r2 = 0.34, P < .001 |
| SVC-RA junction | r2 = 0.32, P < .001 |
| Posterior RA | r2 = 0.11, P = .038 |
CTI = cavotricuspid isthmus; LA = left atrial; RA = right atrial; RAA = right atrial appendage; SVC = superior vena cava.
Table 3.
Degree and significance, in descending order, of correlations between λd of measured right atrial locations with mean left atrial λd
| λd values of measured RA locations | Correlation with mean LA λd |
|---|---|
| CTI | r2 = 0.76, P < .001 |
| Septal RA | r2 = 0.58, P < .001 |
| RAA | r2 = 0.52, P < .001 |
| SVC-RA junction | r2 = 0.49, P < .001 |
| Lateral RA | r2 = 0.45, P < .001 |
| Posterior RA | r2 = 0.12, P = .037 |
CTI = cavotricuspid isthmus; LA = left atrial; RA = right atrial; RAA = right atrial appendage; SVC = superior vena cava.
When patients were examined according to their paroxysmal-persistent AF status, significant positive correlations were observed between mean LA λf and λf at the RA septal wall (r2 = 0.78, P < .001) and the CTI region (r2 = 0.8, P < .001) in patients with paroxysmal AF (Table 4). However, in those with persistent AF, only a modest correlation was observed in the CTI region (r2 = 0.54, P < .001), followed by RAA (r2 = 0.38, P = .004), RA posterior wall (r2 = 0.37, P = .003), SVC-RA junction (r2 = 0.26, P = .013), and RA septal wall (r2 = 0.22, P = .02) (Table 4). In persistent AF patients, no significant relationships were observed between mean LA λf and RA lateral wall (r2 = 0.1, P = .17) (Table 4).
Table 4.
Degree and significance of correlations between λf of measured right atrial locations with mean left atrial λf, according to the paroxysmal-persistent atrial fibrillation classification
| λf values of measured RA locations | Correlation with mean LA λf: paroxysmal AF (n = 18) | Correlation with mean LA λf: persistent AF (n=23) |
|---|---|---|
| CTI | r2 = 0.8, P < .001 | r2 = 0.54, P < .001 |
| Septal RA | r2 = 0.78, P < .001 | r2 = 0.22, P = .02 |
| Lateral RA | r2 = 0.5, P < .001 | r2 = 0.1, P = .17 |
| RAA | r2 = 0.3, P = .035 | r2 = 0.38, P = .004 |
| SVC-RA junction | r2 = 0.4, P = .007 | r2 = 0.26, P = .013 |
| Posterior RA | r2 = 0.04, P = .44 | r2 = 0.37, P = .003 |
CTI = cavotricuspid isthmus; LA = left atrial; RA = right atrial; RAA = right atrial appendage; SVC = superior vena cava.
Discussion
Role of interatrial conduction in atrial fibrillation
Anatomically, biatrial electrical propagation occurs via 3 main pathways: (1) the Bachmann bundle, (2) the interatrial septum, and (3) inferior interatrial connections via coronary sinus.4 In sinus rhythm, it has been argued that the Bachmann bundle plays the dominant role in interatrial electrical conduction,38,39 while other studies argue for the dominant role of the coronary sinus.40,41 However, there is limited evidence to date describing the contributions of these interatrial pathways to electrical propagation during fibrillation, using high-density mapping. To the best of our knowledge, this was a study performed by Kumagai and colleagues,1 who observed preferential conduction of fibrillation via the Bachmann bundle using sterile pericarditis canine models of AF using simultaneous electrogram recordings from 372 unipolar electrodes.
Interatrial conduction has been postulated to play a potentially important contribution in AF pathophysiology.1,2,6,7,42 Increased thickness of the interatrial septum has been linked to the presence of AF and an increased recurrence of AF post ablation.42,43 A highly significant contributory role for AF is as a site for critical conduction block leading to the development and perpetuation of AF, based on 185 cases mapped intraoperatively in sinus rhythm.2,6,7 Similarly, the presence of conduction block in the Bachmann bundle has also previously been linked to the initiation and perpetuation of fibrillation in human AF.6,7,44
Understanding the mechanisms of fibrillatory propagation across interatrial connections is crucial to define clinically significant atrial regions that could be targeted during catheter ablation in AF.2,4 Interatrial conduction has been suggested as a potential target for catheter ablation in AF.2 Early experimental studies in animal models suggested that AF dynamics could be modified by ablation in the septum.3,5 A potential mechanism for this effect was identified physiologically in more contemporary biatrial computational models, which demonstrated termination of AF with disconnection of the RA and LA in 80% of cases.8
Findings from RENEWAL-AF
Our study adds to the literature as the first to apply a statistical-based approach in a clinical setting to explore anatomical and electrical connections during sustained fibrillation. Renewal theory–based analysis of fibrillatory dynamics in the interatrial septum and other atrial regions involved with interatrial conductions revealed the following findings:
First, during fibrillation, statistically determined electrical disrelation between the LA and RA septum is dependent on paroxysmal-persistent status and LA size. Significant statistical electrical disrelation, measured by λf and λd, was observed in persistent AF patients compared to paroxysmal AF patients and in patients with larger LA size, suggesting interseptal statistical disrelation plays a role in AF persistence. This observation is in concordance with other studies investigating the associations between structural and electrical changes within the interatrial septum and AF presence, AF persistence, and known AF-related cardiac structural remodeling. Shin and colleagues45 observed thicker interatrial adipose tissue in patients with AF, measured using cardiac computed tomography when compared with control. In AF patients, degree of thickness of interatrial adipose tissue was closely linked with both AF persistence and LA volume. In another prospective study using transesophageal echocardiogram for quantification of interatrial septal thickness, interatrial septum was observed to be significantly thicker, independent of age, weight, and height, in AF patients, compared with controls.43 Histologically, interatrial septal biopsies obtained from AF patients have shown evidence of lymphomononuclear infiltrates, cardiomyocyte necrosis, and patchy fibrosis, when compared with patients without a history of AF.46 When histologic analysis of interatrial septal biopsies was compared between paroxysmal and persistent AF patients, a significantly higher burden of atrial tissue C-reactive protein was observed in patients with paroxysmal AF, suggesting local atrial inflammation in the interatrial septal region plays a crucial role in early stages of AF, which may then progress to structural changes suggestive of chronicity.46,47 Electrically, increased interatrial septal thickness has been linked with a significantly higher burden of complex fractionated electrograms and a lower procedural success rate post AF ablation.42 This observation is crucial, as complex fractionated electrograms have previously been associated with atrial regions of conduction slowing or block and sites of wavefront collisions.48,49
Second, varying statistical correlations of fibrillatory processes were observed in other anatomically and electrically connected LA and RA regions, likely owing to the varying contributions of interatrial conduction to RA fibrillatory processes.
Third, highest statistical correlations between rates of PS formation and destruction between the RA and LA during fibrillation were observed in the cavotricuspid region, a surrogate for inferior interatrial conduction, followed by interatrial septum and RAA. This suggests that during fibrillation, there is either a preferential conduction through the inferior interatrial routes or presence of conduction slowing in the Bachmann bundle or interatrial septum.
Rationale for using renewal theory approach in assessing interatrial conduction
The renewal theory approach used in the current study is useful because it provides a conceptual connection between AF and other systems in nature characterized by the repetitive regeneration of PS. The repetitive generation of PS occurs in biological,20,23 physical,21,50 and chemical22 systems throughout nature. Renewal theory allows the development of statistical approaches to understand the formation and destruction of PS. Importantly, the distributions identified for PS lifetimes and population distributions have been shown to be equivalent for AF and ventricular fibrillation9,11,30 and these other natural systems, suggesting thematic similarities in terms of the underlying processes sustaining spatiotemporal turbulence in these.
Renewal theory: An alternative, statistical-based approach to demonstrate electrical dyssynchrony
The presence of electrical dyssynchrony between the endocardium and epicardium has also been observed and is hypothesized to play a crucial role in AF persistence.51,52 In animal AF models, endo-epicardial dyssynchrony (EED) has been shown to be present in both acute and persistent forms of AF.53 In persistent human AF models, the presence of EED has been defined either as differences of endo-epicardial activation times of ≥15 ms, differences in wavefront directionality or the frequency of epicardial breakthroughs.22,52,54,55 However, a major obstacle remains in determining the optimal cutoff difference of endo-epicardial activation times to demonstrate electrical dissociation. While de Groot and colleagues52 used a cutoff of ≥15 ms as a marker for dyssynchronous electrical activation, Parameswaran and colleagues55 and Walters and colleagues56 more recently used a stringent cutoff of ≥20 ms in studies involving high-density mapping of swine models and human persistent AF.55,56 The use of renewal theory to demonstrate electrical dyssynchrony provides a robust statistical-based approach that complements currently used quantitative methods by, firstly, demonstrating the presence of statistically determined electrical disrelation between 2 atrial regions; and, secondly, in those determined to have statistically determined electrical disrelation, further quantifying the degree of electrical dissociation in this cohort. An area currently under active investigation is the application of a renewal-based approach to demonstrate EED, using simultaneous endo-epicardium HD Grid recordings in AF patients undergoing cardiac surgery (ACTRN12621000684820).
Limitations
There were a few limitations to this study. Firstly, this study involves a relatively small number of patients. However, the methodology described based on renewal theory could be applied in larger studies involving more atrial regions to improve atrial surface coverage. Secondly, sampling of the interatrial septal region was limited to the endocardium and performed sequentially. However, sequential mapping is likely to be reasonable with the renewal theory approach, as (1) the renewal rate constants are known to be temporally stable for sustained periods of time and (2) this study provides indirect evidence of temporal stability of renewal constants by showing that physiological correlations exist between anatomically connected regions. Finally, we acknowledge that all groups studying AF do not universally accept the notion of PS mapping.57 However, PS dynamics are widely used to understand cardiac fibrillation and comparably spatiotemporally turbulent systems throughout nature.20, 21, 22, 23
Conclusion
Using a renewal-based approach for fibrillatory dynamic analysis, we observed varying statistically determined electrical relationships between fibrillatory processes in RA regions to electrically and anatomically connected LA regions. Identification of atrial regions that have significant electrical disrelation could be clinically important to select a subset of AF patients who would potentially benefit from targeted ablation to these areas.
Acknowledgments
Funding Sources
National Heart Foundation Vanguard Grant 102650.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided informed consent.
Ethics Statement
Ethics approval was by the Southern Adelaide Local Health Network Ethics Committee (HREC/19/SAC/292). This research adhered to Helsinki Declaration guidelines.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2022.05.007.
Appendix. Supplementary data
Supplemental Figure 1.
Supplemental Figure 2.
References
- 1.Kumagai K., Khrestian C., Waldo A.L. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model. Insights into the mechanism of its maintenance. Circulation. 1997;95:511–521. doi: 10.1161/01.cir.95.2.511. [DOI] [PubMed] [Google Scholar]
- 2.Kharbanda R.K., Özdemir E.H., Taverne Y.J.H.J., et al. Current concepts of anatomy, electrophysiology, and therapeutic implications of the interatrial septum. JACC Clin Electrophysiol. 2019;5:647–656. doi: 10.1016/j.jacep.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Tondo C., Scherlag B.J., Otomo K., et al. Critical atrial site for ablation of pacing-induced atrial fibrillation in the normal dog heart. J Cardiovasc Electrophysiol. 1997;8:1255–1265. doi: 10.1111/j.1540-8167.1997.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 4.Platonov P.G. Interatrial conduction in the mechanisms of atrial fibrillation: from anatomy to cardiac signals and new treatment modalities. Europace. 2007;9(Suppl 6):vi10–vi16. doi: 10.1093/europace/eum201. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima H., Kumagai K., Urata H., et al. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 6.Teuwen C.P., Yaksh A., Lanters E.A.H., et al. Relevance of conduction disorders in Bachmann bundle during sinus rhythm in humans. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003972. [DOI] [PubMed] [Google Scholar]
- 7.Campenhout MJHv, Yaksh A., Kik C., et al. Bachmann bundle. Circ Arrhythm Electrophysiol. 2013;6:1041–1046. doi: 10.1161/CIRCEP.113.000758. [DOI] [PubMed] [Google Scholar]
- 8.Lim B., Park J.-W., Hwang M., et al. Electrophysiological significance of the interatrial conduction including cavo-tricuspid isthmus during atrial fibrillation. J Physiol. 2020;598:3597–3612. doi: 10.1113/JP279660. [DOI] [PubMed] [Google Scholar]
- 9.Dharmaprani D., Jenkins E., Aguilar M., et al. M/M/Infinity birth-death processes – a quantitative representational framework to summarize and explain phase singularity and wavelet dynamics in atrial fibrillation. Front Physiol. 2021;11 doi: 10.3389/fphys.2020.616866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quah J.X., Dharmaprani D., Lahiri A., Tiver K., Ganesan A.N. Reconceptualising atrial fibrillation using renewal theory: a novel approach to the assessment of atrial fibrillation dynamics. Arrhythm Electrophysiol Rev. 2021;10:77–84. doi: 10.15420/aer.2020.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmaprani D., Schopp M., Kuklik P., et al. Renewal theory as a universal quantitative framework to characterize phase singularity regeneration in mammalian cardiac fibrillation. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007569. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins E.V., Dharmaprani D., Schopp M., et al. Understanding the origins of the basic equations of statistical fibrillatory dynamics. Chaos. 2022;32 doi: 10.1063/5.0062095. [DOI] [PubMed] [Google Scholar]
- 13.Comtois P., Kneller J., Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace. 2005;7(Suppl 2):10–20. doi: 10.1016/j.eupc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Nattel S., Xiong F., Aguilar M. Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms. Nat Rev Cardiol. 2017;14:509–520. doi: 10.1038/nrcardio.2017.37. [DOI] [PubMed] [Google Scholar]
- 15.Konings K.T., Kirchhof C.J., Smeets J.R., et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 16.Nademanee K., McKenzie J., Kosar E., et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 17.Lee G., Kumar S., Teh A., et al. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 18.Child N., Clayton R.H., Roney C.R., et al. Unraveling the underlying arrhythmia mechanism in persistent atrial fibrillation: results from the STARLIGHT study. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005897. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Mandapati R., Berenfeld O., et al. Dynamics of wavelets and their role in atrial fibrillation in the isolated sheep heart. Cardiovasc Res. 2000;48:220–232. doi: 10.1016/s0008-6363(00)00177-2. [DOI] [PubMed] [Google Scholar]
- 20.Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- 21.Ecke R.E., Hu Y. Spiral defect chaos in Rayleigh-Bénard convection: defect population statistics. Phys A Stat Mech Appl. 1997;239:174–188. [Google Scholar]
- 22.Qiao C., Wang H., Ouyang Q. Defect-mediated turbulence in the Belousov-Zhabotinsky reaction. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79 doi: 10.1103/PhysRevE.79.016212. [DOI] [PubMed] [Google Scholar]
- 23.Tan T.H., Liu J., Miller P.W., et al. Topological turbulence in the membrane of a living cell. Nat Phys. 2020;16:657–662. [Google Scholar]
- 24.Vidmar D., Rappel W.-J. Extinction dynamics of spiral defect chaos. Phys Rev E. 2019;99 doi: 10.1103/PhysRevE.99.012407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aron M., Herzog S., Parlitz U., Luther S., Lilienkamp T. Spontaneous termination of chaotic spiral wave dynamics in human cardiac ion channel models. PloS One. 2019;14 doi: 10.1371/journal.pone.0221401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil L., Lega J., Meunier J.L. Statistical properties of defect-mediated turbulence. Phys R. 1990;41:1138–1141. doi: 10.1103/physreva.41.1138. [DOI] [PubMed] [Google Scholar]
- 27.Christoph J., Chebbok M., Richter C., et al. Electromechanical vortex filaments during cardiac fibrillation. Nature. 2018;555:667. doi: 10.1038/nature26001. [DOI] [PubMed] [Google Scholar]
- 28.Kuklik P., Zeemering S., van Hunnik A., et al. Identification of rotors during human atrial fibrillation using contact mapping and phase singularity detection: technical considerations. IEEE Trans Biomed Eng. 2017;64:310–318. doi: 10.1109/TBME.2016.2554660. [DOI] [PubMed] [Google Scholar]
- 29.Rogers J.M., Huang J., Smith W.M., Ideker R.E. Incidence, evolution, and spatial distribution of functional reentry during ventricular fibrillation in pigs. Circ Res. 1999;84:945–954. doi: 10.1161/01.res.84.8.945. [DOI] [PubMed] [Google Scholar]
- 30.Dharmaprani D., Jenkins E.V., Quah J.X., et al. A governing equation for rotor and wavelet number in human clinical ventricular fibrillation: implications for sudden cardiac death. Heart Rhythm. 2022;19:295–305. doi: 10.1016/j.hrthm.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Wang H. Statistics of defect-mediated turbulence influenced by noise. Phys Rev Lett. 2004;93 doi: 10.1103/PhysRevLett.93.154101. [DOI] [PubMed] [Google Scholar]
- 32.Davidsen J., Zhan M., Kapral R. Filament-induced surface spiral turbulence in three-dimensional excitable media. Phys Rev Lett. 2008;101 doi: 10.1103/PhysRevLett.101.208302. [DOI] [PubMed] [Google Scholar]
- 33.van Campenhout M.J.H., Yaksh A., Kik C., et al. Bachmann’s bundle. Circ Arrhythm Electrophysiol. 2013;6:1041–1046. doi: 10.1161/CIRCEP.113.000758. [DOI] [PubMed] [Google Scholar]
- 34.Hołda J., Słodowska K., Tyrak K., et al. Topographical anatomy of the right atrial appendage vestibule and its isthmuses. J Cardiovasc Electrophysiol. 2020;31:3199–3206. doi: 10.1111/jce.14767. [DOI] [PubMed] [Google Scholar]
- 35.Ho S.Y., Anderson R.H., Sánchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res. 2002;54:325–336. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera J.A., Ho S.Y., Climent V., Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 37.Knol W.G., Teuwen C.P., Kleinrensink G.J., et al. The Bachmann bundle and interatrial conduction: comparing atrial morphology to electrical activity. Heart Rhythm. 2019;16:606–614. doi: 10.1016/j.hrthm.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Lemery R., Soucie L., Martin B., et al. Human study of biatrial electrical coupling: determinants of endocardial septal activation and conduction over interatrial connections. Circulation. 2004;110:2083–2089. doi: 10.1161/01.CIR.0000144461.83835.A1. [DOI] [PubMed] [Google Scholar]
- 39.Tapanainen J.M., Jurkko R., Holmqvist F., et al. Interatrial right-to-left conduction in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2009;25:117–122. doi: 10.1007/s10840-008-9359-2. [DOI] [PubMed] [Google Scholar]
- 40.Betts T.R., Roberts P.R., Morgan J.M. High-density mapping of left atrial endocardial activation during sinus rhythm and coronary sinus pacing in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1111–1117. doi: 10.1046/j.1540-8167.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 41.Markides V., Schilling R.J., Ho S.Y., et al. Characterization of left atrial activation in the intact human heart. Circulation. 2003;107:733–739. doi: 10.1161/01.cir.0000048140.31785.02. [DOI] [PubMed] [Google Scholar]
- 42.Park Y.M., Park H.C., Ban J.-E., et al. Interatrial septal thickness is associated with the extent of left atrial complex fractionated atrial electrograms and acute procedural outcome in patients with persistent atrial fibrillation. Europace. 2015;17:1700–1707. doi: 10.1093/europace/euu403. [DOI] [PubMed] [Google Scholar]
- 43.López-Candales A., Grewal H., Katz W. The importance of increased interatrial septal thickness in patients with atrial fibrillation: a transesophageal echocardiographic study. Echocardiography. 2005;22:408–414. doi: 10.1111/j.1540-8175.2005.04088.x. [DOI] [PubMed] [Google Scholar]
- 44.Goyal S.B., Spodick D.H. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 45.Shin S.Y., Yong H.S., Lim H.E., et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:647–655. doi: 10.1111/j.1540-8167.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 46.Narducci M.L., Pelargonio G., Dello Russo A., et al. Role of tissue C-reactive protein in atrial cardiomyocytes of patients undergoing catheter ablation of atrial fibrillation: pathogenetic implications. Europace. 2011;13:1133–1140. doi: 10.1093/europace/eur068. [DOI] [PubMed] [Google Scholar]
- 47.Frustaci A., Chimenti C., Bellocci F., et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 48.Konings K.T., Smeets J.L., Penn O.C., Wellens H.J., Allessie M.A. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 49.Vaquero M., Calvo D., Jalife J. Cardiac fibrillation: from ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egolf D.A., Melnikov I.V., Pesch W., Ecke R.E. Mechanisms of extensive spatiotemporal chaos in Rayleigh–Bénard convection. Nature. 2000;404:733–736. doi: 10.1038/35008013. [DOI] [PubMed] [Google Scholar]
- 51.Verheule S., Eckstein J., Linz D., et al. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol. 2014;115:173–185. doi: 10.1016/j.pbiomolbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 52.de Groot N., van der Does L., Yaksh A., et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003648. [DOI] [PubMed] [Google Scholar]
- 53.Eckstein J., Maesen B., Linz D., et al. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res. 2010;89:816–824. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 54.Aronis K.N., Trayanova N.A. Endocardial-epicardial dissociation in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parameswaran R., Kalman J.M., Royse A., et al. Endocardial-epicardial phase mapping of prolonged persistent atrial fibrillation recordings. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008512. [DOI] [PubMed] [Google Scholar]
- 56.Walters T.E., Lee G., Lee A., et al. Site-specific epicardium-to-endocardium dissociation of electrical activation in a swine model of atrial fibrillation. JACC Clin Electrophysiol. 2020;6:830–845. doi: 10.1016/j.jacep.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Podziemski P., Zeemering S., Kuklik P., et al. Rotors detected by phase analysis of filtered, epicardial atrial fibrillation electrograms colocalize with regions of conduction block. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005858. [DOI] [PMC free article] [PubMed] [Google Scholar]