Abstract
Background
Lactate dehydrogenase (LDH) has been reported in multiple heart diseases. Herein, we explored the prognostic effects of preoperative LDH on adverse outcomes in cardiac surgery patients.
Methods
Retrospective data analysis was conducted from two large medical databases: Medical Information Mart for Intensive Care (MIMIC) III and MIMIC IV databases. The primary outcome was in-hospital mortality, whereas the secondary outcomes were 1-year mortality, continuous renal replacement therapy, prolonged ventilation, and prolonged length of intensive care unit and hospital stay.
Results
Patients with a primary endpoint had significantly higher levels of LDH (p < 0.001). Multivariate regression analysis presented that elevated LDH was independently correlated with increased risk of primary and secondary endpoints (all p < 0.001). Subgroup analyses showed that high LDH was consistently associated with primary endpoint. Moreover, LDH exhibited the highest area under the curve (0.768) for the prediction of primary endpoint compared to the other indicators, including neutrophil–lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), platelet–lymphocyte ratio (PLR), lactate, and simplified acute physiology score (SAPS) II. The above results were further confirmed in the MIMIC IV dataset.
Conclusions
Elevated preoperative LDH may be a robust predictor of poor prognosis in cardiac surgery patients, and its predictive ability is superior to NLR, LMR, PLR, lactate, and SAPS II.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02848-7.
Keywords: Cardiac surgery, Lactate dehydrogenase, Mortality, Complications, Medical Information Mart for Intensive Care
Introduction
In the United States, approximately 300,000 people undergo cardiac surgery each year, of which over 80% are coronary artery bypass graft (CABG) and valve surgeries [1]. Although mortality after cardiac surgery has decreased significantly over previous deCHDes due to the progress of cardiopulmonary bypass (CPB) and surface hypothermia [2–4], postoperative complications are still high [5]. Acute kidney injury is one of the most frequent complications of cardiac surgery, with an incidence rate ranging from 5 to 50% depending on variable definitions [6, 7]. In addition, low cardiac output syndrome after cardiac surgery has a high prevalence, with varying occurrences ranging from 10 to 20% [8]. Evidence suggests that these organ damages probably linked to systemic inflammatory response and ischemia–reperfusion injury caused by CPB [9]. Previous studies have shown that inflammatory markers, such as lymphocyte-to-monocyte ratio (LMR) and neutrophil–lymphocyte ratio (NLR), are connected with unfavorable outcomes in patients undergoing cardiac surgery [10, 11].
Long-term ischemia leads to insufficient oxygen supply and hypoxia. Lactate dehydrogenase (LDH) is a significant intracellular enzyme in energy production, which catalyzes pyruvate to lactate under anaerobic conditions [12]. Initially, the increase of LDH can reflect cardiac damage and is used to diagnose acute myocardial infarction [13]. Subsequently, LDH has been found to be elevated in patients with valve heart disease, heart failure, and coronary heart disease [14, 15]. However, no study has investigated the relationship between LDH and poor prognosis of patients undergoing cardiac surgery. Thus, we attempted to explored the prognostic impact of LDH at admission on poor prognosis in patients after cardiac surgery.
Materials and methods
Study design
This was a retrospective cohort study based on two large publicly available critical care databases the Medical Information Mart for Intensive Care III version 1.4 (MIMIC III v 1.4) and MIMIC IV v 0.4 [16, 17]. MIMIC-III covers over 40,000 intensive care unit (ICU) admissions at the Beth Israel Deaconess Medical Center in Boston between 2001 and 2012. MIMIC-IV is an update to MIMIC-III. We passed the Protecting Human Research Participants exam and obtain the seniority to access these databases. Data were extracted by authors YZ, YYL and YHZ.
Population selection criteria
Adult patients who underwent cardiac surgery and were admitted to the ICU for the first time were included in the study. The following exclusion criteria were applied: (1) missing LDH data at admission, and (2) missing data > 5%.
Data extraction
The extracted data contained age, gender, marital status, ethnicity, body mass index (BMI), heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), simplified acute physiology score (SAPS) II, sequential organ failure assessment (SOFA) score, comorbidities, laboratory parameters and outcomes. Comorbidities comprised hypertension, diabetes, coronary heart disease (CHD), valve disease, heart failure (HF), chronic obstructive pulmonary disease (COPD), and chronic kidney disease (CKD). Laboratory parameters included LDH, white blood cell (WBC) count, platelet (PLT) count, blood urea nitrogen (BUN), serum creatine (SCr), sodium, potassium, glucose, neutrophil count, lymphocyte count, monocyte count, and lactate. We took the preoperative laboratory indicators from the first results after admission. The NLR, LMR, and platelet–lymphocyte ratio (PLR) were calculated as follows: NLR = neutrophil/lymphocyte counts, LMR = lymphocyte/ monocyte counts, and PLR = platelet/lymphocyte counts. Cardiac surgical procedures included CABG and/or heart valve surgery. The primary endpoint was in-hospital mortality, whereas the secondary endpoints were 1-year mortality, continuous renal replacement therapy (CRRT), prolonged ventilation, and prolonged length of ICU and hospital stay. 1-year mortality referred to the time from admission to mortality from any cause within one year. Prolonged ventilation was defined as the need for mechanical ventilation for more than 24 h. Prolonged length of stay was defined as any stay beyond the 75th percentile for the total study population. Moreover, prolonged length of ICU and hospital stays were length of stay longer than 6 days and 15 days, respectively.
Statistical analysis
MIMIC III was regarded as the training cohort which was used to investigate whether LDH is a poor prognostic factor for patients undergoing cardiac surgery. We performed external validation in MIMIC IV to confirm the results obtained from MIMIC III. A total of 2325 patients were enrolled from MIMIC III, whereas 1387 patients were enrolled from the MIMIC IV (Additional file 1: Figure S1). Next, we compared the prognostic power of LDH with other indicators in patients undergoing cardiac surgery, including NLR, LMR, PLR, lactate and SAPS II. These indicators have previously been shown to be strong prognostic biomarkers in cardiac surgery patients. After excluding patients with missing NLR, LMR, PLR, lactate, and SAPS II data, there were 830 patients left in MIMIC III and 731 patients in MIMIC IV who were then subjected to further analysis (Additional file 1: Figure S1).
The relationship between categorical variables were examined by Pearson χ2 tests and reported as counts (percentage). Continuous variables were presented as means (standard deviation) or medians (range), and differences between values were examined by independent t-test or Mann–Whitney U test. Receiver operating characteristic (ROC) curve was applied to determine the best cut-off point of LDH for predicting in-hospital mortality. We performed Kaplan–Meier curves with the log-rank test to asses the one-year survival rate between groups based on the optimal cut-off value of LDH. Multivariate logistic regression analysis and Cox proportional hazards regression analysis were used to explore the predictive value of LDH for poor outcomes. Notably, LDH was tested both as a continuous and a categorical variable, and NLR, LMR, PLR, and lactate were not entered into the multivariate analysis because more than 20% of the data was missing. Significant factors associated with primary endpoint from univariate analyses were included in multivariate analysis. The following covariates in MIMIC III were adjusted as potential confounders: ethnicity, SBP, heart rate, hypertension, CHD, heart failure, BUN, SCr, sodium, potassium, glucose, SAPS II, and SOFA score (Additional file 2: Table S1). The following covariates in MIMIC IV were adjusted: hypertension, heart failure, CKD, WBC, BUN, SCr, sodium, potassium, and SAPS II (Additional file 2: Table S1). Results were expressed with odds ratios (OR) for logistic regression analysis or hazard ratio (HR) for Cox proportional hazards analysis, and their 95% confidence intervals (95% CI). To evaluate the consistency of the prognostic impact of LDH on primary endpoint, we conducted stratified analyses in different groups of gender, age, hypertension, diabetes, CHD, CKD, heart failure, and valve disease. Interaction tests between each subgroup were analyzed. We used ROC curve based on DeLong’s test to compare the predictive ability of LDH with other prognostic indicators, including NLR, LMR, PLR, lactate, and SAPS II. To assess correlations between LDH and these indicators, Pearson or Spearman analyses were performed where appropriate. All statistical analyses were examined using packages implemented in R software (version 3.6.3) and MedCalc version 19.1 (MedCalc Software, Belgium). A p < 0.05 was considered as statistically significant.
Results
Baseline characteristics of the study population
Additional file 2: Table S2 shows comparisons between baseline characteristics of patients in MIMIC III and MIMIC IV databases. Some degree of heterogeneity between the two datasets was observed. Table 1 presents the baseline characteristics of the study patients grouped by in-hospital death. In both datasets, LDH was remarkably higher in patients with in-hospital death compared to those without. Patients with in-hospital death in MIMIC III tended to be older, had faster heart rate, higher BUN, SCr, SAPS II, and SOFA score, and higher prevalence of heart failure. In addition, there were fewer white people, lower SBP, DBP, sodium, and lower prevalence of hypertension and CHD. Patients with a primary endpoint in MIMIC IV had higher proportions of heart failure and CKD, increased WBC, BUN, SCr, and SAPS II, but decreased levels of sodium and lower proportions of hypertension.
Table 1.
Baseline clinical characteristics of patients based on in-hospital mortality
| Variables | MIMIC III | MIMIC IV | ||||
|---|---|---|---|---|---|---|
| Survivors | Non-survivors | P | Survivors | Non-survivors | P | |
| N = 2247 | N = 78 | N = 1352 | N = 35 | |||
| Age, years | 69.2 (60.4–77.5) | 73.6 (65.0–78.9) | 0.023 | 69.0 (61.0–77.0) | 75.0 (58.5–82.0) | 0.104 |
| Male, n (%) | 1516 (67.5) | 50 (64.1) | 0.617 | 945 (69.9) | 22 (62.9) | 0.479 |
| Marital, n (%) | 0.098 | 0.052 | ||||
| Married | 1349 (60.0) | 46 (59.0) | 782 (57.8) | 16 (45.7) | ||
| Un-married | 810 (36.0) | 25 (32.1) | 500 (37.0) | 14 (40.0) | ||
| Unknown | 88 (3.9) | 7 (9.0) | 70 (5.2) | 5 (14.3) | ||
| Ethnicity, n (%) | 0.043 | 0.193 | ||||
| White | 1603 (71.3) | 48 (61.5) | 941 (69.6) | 22 (62.9) | ||
| Non-white | 234 (10.4) | 7 (9.0) | 185 (13.7) | 3 (8.6) | ||
| Unknown | 410 (18.2) | 23 (29.5) | 226 (16.7) | 10 (28.6) | ||
| BMI, kg/m2 | 27.6 (24.6–31.6) | 28.4 (25.0–32.8) | 0.218 | 28.8 (25.5–33.0) | 30.8 (25.3–35.9) | 0.152 |
| SBP, mmHg | 115 (102–128) | 108 (96–126) | 0.018 | 122 (104–140) | 133 (98–153) | 0.720 |
| DBP, mmHg | 59 (52–67) | 56 (48–62) | 0.009 | 65 (54–90) | 66 (52–90) | 0.979 |
| Heart rate, bpm | 84 (77–90) | 88 (80–99) | < 0.001 | 80 (69–90) | 80 (60–103) | 0.706 |
| Hypertension, n (%) | 1330 (59.2) | 30 (38.5) | < 0.001 | 803 (59.4) | 13 (37.1) | 0.014 |
| Diabetes, n (%) | 753 (33.5) | 25 (32.1) | 0.883 | 479 (35.4) | 11 (31.4) | 0.757 |
| CHD, n (%) | 1749 (77.8) | 50 (64.1) | 0.007 | 1059 (78.3) | 25 (71.4) | 0.442 |
| Valve disease, n (%) | 1109 (49.4) | 45 (57.7) | 0.183 | 680 (50.3) | 22 (62.9) | 0.195 |
| Heart failure, n (%) | 841 (37.4) | 51 (65.4) | < 0.001 | 463 (34.2) | 26 (74.3) | < 0.001 |
| COPD, n (%) | 27 (1.2) | 2 (2.6) | 0.254 | 12 (0.9) | 0 (0.0) | 1.000 |
| CKD, n (%) | 282 (12.6) | 15 (19.2) | 0.118 | 236 (17.5) | 16 (45.7) | < 0.001 |
| LDH, u/l | 228 (184, 320) | 436 (278–966) | < 0.001 | 206 (171–256) | 299 (231–458) | < 0.001 |
| WBC, k/ul | 8.6 (6.8–11.6) | 9.7 (7.3–13.3) | 0.088 | 7.8 (6.4–9.7) | 9.5 (7.3–13.1) | 0.006 |
| PLT, k/u | 206 (161–257) | 205 (148–257) | 0.477 | 211 (172–262) | 231 (176–293) | 0.243 |
| BUN, mg/dl | 19 (15–26) | 27 (18–42) | < 0.001 | 19 (15–26) | 29 (19–43) | < 0.001 |
| SCr, mg/dl | 1.0 (0.8–1.3) | 1.3 (1.0–1.8) | < 0.001 | 1.0 (0.9–1.3) | 1.4 (1.1–1.9) | < 0.001 |
| Sodium, mEq/l | 139 (137–141) | 138 (136–140) | 0.002 | 139 (137–141) | 138 (135–140) | 0.013 |
| Potassium, mEq/l | 4.1 (3.9–4.5) | 4.2 (3.9–4.9) | 0.059 | 4.1 (3.9–4.4) | 4.2 (3.9–4.8) | 0.052 |
| Glucose, mg/dl | 119 (101–151) | 124 (105–177) | 0.092 | 116 (99–152) | 120 (105–155) | 0.234 |
| SAPS II | 36 (30–44) | 46 (36–56) | < 0.001 | 36 (29–43) | 46 (37–52) | < 0.001 |
| SOFA score | 5 (3–7) | 8 (5–10) | < 0.001 | 2 (1–4) | 3 (1–4) | 0.397 |
MIMIC Medical Information Mart for Intensive Care, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CHD coronary heart disease, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, LDH lactate dehydrogenase, WBC white blood cell count, PLT platelets, BUN blood urea nitrogen, SCr serum creatine, SAPS simplified acute physiology score, SOFA sequential organ failure assessment
Association between LDH and outcomes
The ROC curve presented that the optimum cut-off point of LDH for predicting in-hospital mortality in MIMIC III was > 328u/l (sensitivity 65.4%, specificity 76.6%), with an area under the curve (AUC) of 0.795 (Additional file 1: Figure S2). Next, patients were stratified into two groups (low and high LDH levels) based on the optimum LDH cut-off value. In MIMIC III, patients in the elevated LDH group showed a significantly higher risk of in-hospital and 1-year deaths, higher rates of mechanical ventilation and CRRT, and longer length of ICU and hospital stays compared to patients in the reduced LDH group (all p < 0.001). The results were verified in MIMIC IV (Table 2).
Table 2.
Clinical outcomes between study cohorts
| Outcomes | Low LDH (≤ 328) | High LDH (> 328) | P |
|---|---|---|---|
| MIMIC III | N = 1749 | N = 576 | |
| In-hospital mortality, n (%) | 27 (1.5%) | 51 (8.9%) | < 0.001 |
| 1-year mortality, n (%) | 131 (7.5%) | 107 (18.6%) | < 0.001 |
| Prolonged ventilation, n (%) | 222 (12.7) | 207 (35.9%) | < 0.001 |
| CRRT, n (%) | 86 (4.9%) | 77 (13.4%) | < 0.001 |
| ICU stay > 6 days, n (%) | 307 (17.6%) | 264 (45.8%) | < 0.001 |
| Hospital stay > 15 days, n (%) | 356 (20.4%) | 242 (42.0%) | < 0.001 |
| MIMIC IV | N = 1196 | N = 191 | P |
|---|---|---|---|
| In-hospital mortality, n (%) | 20 (1.7%) | 15 (7.8%) | < 0.001 |
| 1-year mortality, n (%) | 36 (3.0%) | 18 (9.4%) | < 0.001 |
| Prolonged ventilation, n (%) | 181 (15.1%) | 85 (44.5%) | < 0.001 |
| CRRT, n (%) | 67 (5.6%) | 35 (18.3%) | < 0.001 |
| ICU stay > 6 days, n (%) | 114 (9.5%) | 52 (27.2%) | < 0.001 |
| Hospital stay > 15 days, n (%) | 160 (13.4%) | 83 (43.5%) | < 0.001 |
MIMIC Medical Information Mart for Intensive Care, LDH lactate dehydrogenase, ICU intensive care units, CRRT continuous renal replacement therapy
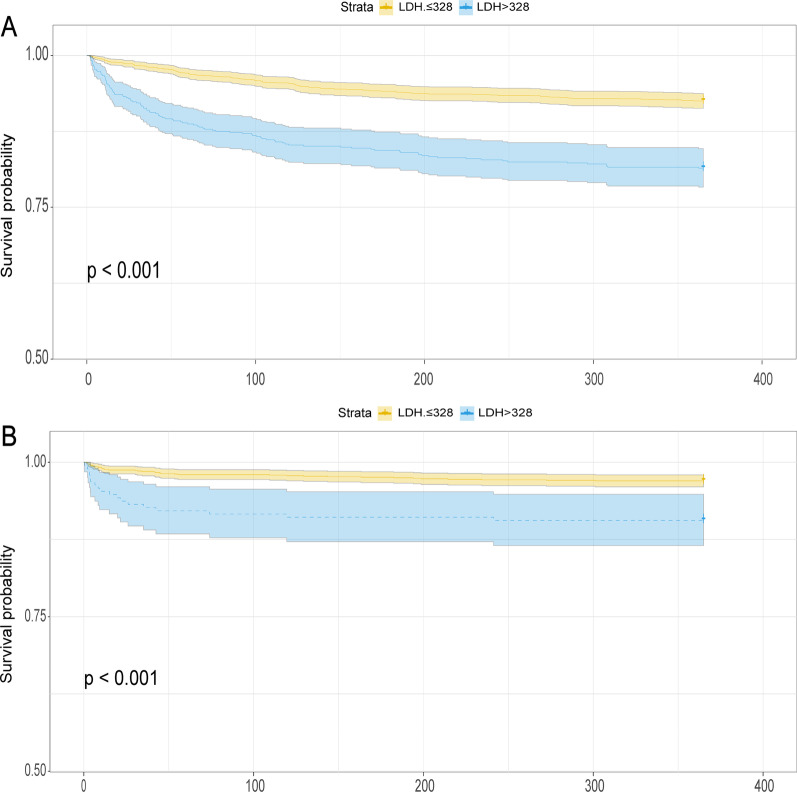
Figure 1 presents the Kaplan–Meier curves for the 1-year cumulative survival rate according to the optimal LDH cut-off value. In MIMIC III, the incidence of 1-year death in the increased LDH (> 328) group was remarkably higher than in the decreased LDH (≤ 328) group (log-rank p < 0.001, Fig. 1A). Similarly, data obtained from MIMIC IV database showed that the cumulative risk of death by one year was remarkably higher in patients with high LDH (LDH > 328) than in patients with low LDH (LDH ≤ 328) (log-rank p < 0.001, Fig. 1B).
Fig. 1.
Kaplan Meier curves for 1-year mortality stratified by high and low LDH. A Kaplan Meier curves for 1-year mortality in MIMIC III dataset. B Kaplan Meier curves for 1-year mortality in MIMIC IV dataset. LDH lactate dehydrogenase, MIMIC Medical Information Mart for Intensive Care
Multivariate regression analyses were carried out to examine the predictive potential of LDH for primary and secondary endpoints. In MIMIC III, multivariate logistic regression analyses revealed that elevated LDH was a significant independent risk factor for the occurrence of in-hospital death (p < 0.001, Table 3). Continuous variables of the LDH were also significantly correlated with in-hospital death in all models, despite accounting for confounding variables (p < 0.001, Table 3). Multivariate Cox/logistic regression analysis further demonstrated that elevated LDH were associated with an increased risk of secondary endpoints [HR (95% CI): 2.08 (1.59–2.73), p < 0.001 for 1-year mortality; OR (95% CI): 3.02 (2.38–3.84), p < 0.001 for prolonged ventilation; OR (95% CI): 2.84 (1.85–4.36), p < 0.001 for CRRT; OR (95% CI): 3.22 (2.58–4.02), p < 0.001 for ICU stay > 6 days; and OR (95% CI): 2.24 (1.80–2.80), p < 0.001 for hospital stay > 15 days, Table 4]. Similarly, the predictive value of LDH for adverse outcomes was verified in MIMIC IV. After accounting for confounding factors, high LDH was still a significant predictor of in-hospital mortality, whether treated LDH as a nominal or continuous variable (Table 3). Multivariate regression analysis revealed that high LDH was remarkably associated with increased risk of 1-year mortality, prolonged ventilation, CRRT, ICU stay > 6 days, and hospital stay > 15 days (all p < 0.05, Table 4).
Table 3.
Predictive value of LDH for in-hospital mortality in different models
| Unadjusted | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| OR (95% Cl) | P | OR (95% Cl) | P | OR (95% Cl) | P | |
| MIMIC III* | ||||||
| LDH as continuous variablea | 1.00 (1.00–1.00) | < 0.001 | 1.00 (1.00–1.00) | < 0.001 | 1.00 (1.00–1.00) | < 0.001 |
| LDH as nominal variableb | 6.20 (3.85–9.98) | < 0.001 | 4.79 (2.93–7.83) | < 0.001 | 4.90 (2.96–8.09) | < 0.001 |
| MIMIC IV** | ||||||
| LDH as continuous variablea | 1.00 (1.00–1.00) | < 0.001 | 1.00 (1.00–1.00) | 0.002 | 1.00 (1.00–1.00) | 0.004 |
| LDH as nominal variableb | 5.01 (2.52–9.97) | < 0.001 | 3.29 (1.54–7.06) | 0.002 | 3.80 (1.82–7.95) | < 0.001 |
aThe OR was examined by per 1-point increase of LDH
bThe OR was examined regarding the low LDH as reference
*MIMIC III
Model 1: adjusted for ethnicity, SBP, heart rate, hypertension, CHD, heart failure
Model 2: adjusted for BUN, SCr, sodium, potassium, glucose, SAPS II, SOFA score
**MIMIC IV
Model 1: adjusted for hypertension, heart_failure, CKD, WBC
Model 2: adjusted for BUN, SCr, sodium, potassium, SAPS II
OR odds ratio, 95% CI 95% confidence interval, MIMIC Medical Information Mart for Intensive Care, LDH lactate dehydrogenase
Table 4.
Predictive value of LDH for secondary endpoints
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| OR/HR (95% Cl) | P | OR/HR(95% Cl) | P | |
| MIMIC IIIa | ||||
| 1-year mortality | 2.69 (2.08–3.47) | < 0.001 | 2.08 (1.59–2.73) | < 0.001 |
| Prolonged ventilation | 3.86 (3.09–4.81) | < 0.001 | 3.02 (2.38–3.84) | < 0.001 |
| CRRT | 2.98 (2.16–4.12) | < 0.001 | 2.84 (1.85–4.36) | < 0.001 |
| ICU stay > 6 days | 3.97 (3.24–4.88) | < 0.001 | 3.22 (2.58–4.02) | < 0.001 |
| Hospital stay > 15 days | 2.84 (2.32–3.47) | < 0.001 | 2.24 (1.80–2.80) | < 0.001 |
| MIMIC IVb | ||||
| 1-year mortality | 3.27 (1.86–5.76) | < 0.001 | 1.99 (1.05–3.76) | 0.034 |
| Prolonged ventilation | 4.50 (3.25–6.23) | < 0.001 | 2.77 (1.90–4.04) | < 0.001 |
| CRRT | 3.78 (2.43–5.88) | < 0.001 | 2.92 (1.57–5.40) | 0.001 |
| ICU stay > 6 days | 3.55 (2.45–5.15) | < 0.001 | 2.37 (1.55–3.63) | < 0.001 |
| Hospital stay > 15 days | 4.98 (3.57–6.93) | < 0.001 | 3.05 (2.09–4.46) | < 0.001 |
The OR/HR was examined regarding the low LDH as reference
OR odds ratio, HR hazard ratio, 95% CI 95% confidence interval, MIMIC Medical Information Mart for Intensive Care, LDH lactate dehydrogenase, ICU intensive care units, CRRT continuous renal replacement therapy
aThe baseline model includes variables that are significant in univariate logistic proportional hazard analysis in MIMIC III, including ethnicity, SBP, heart rate, hypertension, CHD, heart failure, BUN, SCr, sodium, potassium, glucose, SAPS II, SOFA score(details shown in Additional file 2: Table S1)
bThe baseline model includes variables that are significant in univariate logistic proportional hazard analysis in MIMIC IV, including hypertension, heart failure, CKD, WBC, BUN, SCr, sodium, potassium, SAPS II (details shown in Additional file 2: Table S1)
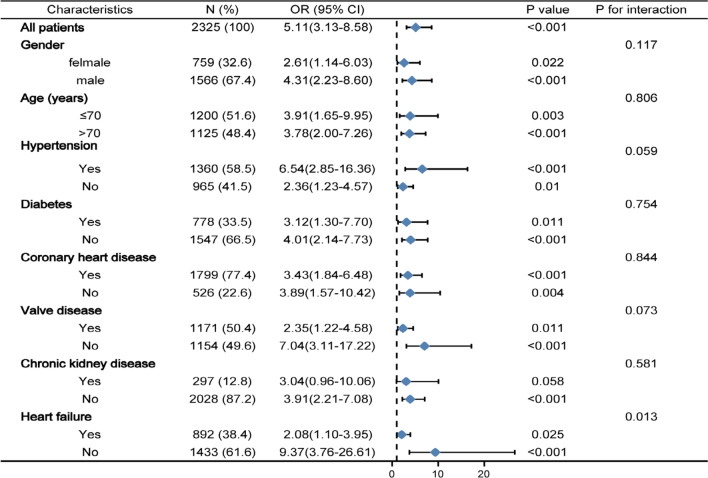
Subgroup analyses
Further analyses was performed to identify risk stratification of LDH for primary endpoint in various subgroups (Fig. 2). Elevated LDH was consistently correlated with primary endpoint in various subgroups, including female or male, age ≤ 70 or > 70 years, with or without hypertension, diabetes, CHD, CKD, heart failure, and valve disease. It is worth noting that the predictive implication of LDH seemed to be more prominent in patients without heart failure (Pinteraction = 0.013).
Fig. 2.
Logistic regression analysis evaluating prognostic value of LDH in various subgroups in MIMIC III. The OR was examined regarding the low LDH as reference. LDH lactate dehydrogenase, OR odds ratio, 95% CI 95% confidence interval, MIMIC Medical Information Mart for Intensive Care
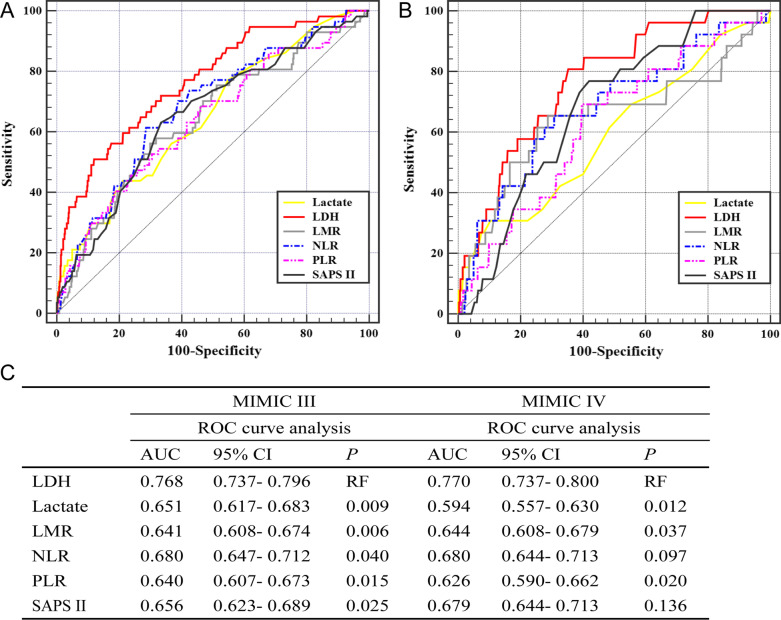
Comparison with other indicators
The C-statistic was performed to compare the predictive power of various indicators for in-hospital mortality. Results showed that LDH yielded the highest AUC values compared to other indicators in both MIMIC III and IV (Fig. 3A, B). The Delong test found a significant difference between the AUCs of LDH and other indicators, including NLR, LMR, PLR, lactate, and SAPS II in MIMIC III (all p < 0.05, Fig. 3C). Comparison of the AUCs between LDH and PLR, LMR, and lactate showed significant differences in MIMIC IV (p < 0.05, Fig. 3C). Moreover, Spearman or Pearson tests were implemented to assess the correlation between LDH and the above indicators (Additional file 2: Table S3). Results indicated that LDH was positively related to lactate, NLR, and SAPS II, but negatively related to LMR.
Fig. 3.
Predictive performance of various indicators for in-hospital mortality. A ROC curve of LDH, Lactate, LMR, NLR, PLR and SAPS II in MIMIC III dataset for predicting in-hospital mortality. B ROC curve of LDH, Lactate, LMR, NLR, PLR and SAPS II in MIMIC IV dataset for predicting in-hospital mortality. C The predictive power of LDH was compared with the other objective indices using ROC curve analysis. ROC receiver operator characteristic, AUC area under the curve, 95% CI 95% confidence interval, LDH lactate dehydrogenase, NLR neutrophil-lymphocyte ratio, LMR lymphocyte-monocyte ratio, PLR platelet-lymphocyte ratio, SAPS simplified acute physiology score, MIMIC Medical Information Mart for Intensive Care
Discussion
We are the first to investigate the prognostic value of LDH for poor prognosis in cardiac surgery patients. The following are the major findings of the study: (1) patients in the high LDH group had significantly higher incidence of primary and secondary endpoints compared to patients in the low LDH group; (2) elevated LDH at admission was independently correlated with increased risk of primary and secondary endpoints, and this finding persisted even after adjusting for confounding risk factors; and (3) LDH showed better predictive value for primary endpoint than other indicators, including NLR, LMR, PLR, lactate, and SAPS II. Notably, the above findings were fully demonstrated in the MIMIC IV database, implying the reliability of our results.
Previous studies have detected that elevated LDH level is correlated with unfavorable outcomes in patients with acute decompensated heart failure, acute aortic syndromes, and acute aortic dissection [18–20]. Moreover, higher LDH was found to be related to increased risk of cardiovascular mortality in patients with chronic arsenic exposure [21] or incident dialysis [22]. However, little is known regarding LDH and cardiac surgery. Our study demonstrated that elevated LDH at admission was a vital marker for predicting mortality in cardiac surgery patients. Previous investigations demonstrated that complications after cardiac surgery are related to increased risk of operative death and prolonged hospital stay [5, 23]. Thus, to better elucidate the correlation between LDH and unfavorable outcomes in cardiac surgery patients, we set CRRT, prolonged ventilation, and prolonged length of ICU and hospital stay as the secondary endpoints. Multivariate analysis results also revealed that LDH was an independent predictor of secondary endpoints.
Next, we compared the prognostic value of NLR, LMR, PLR, lactate, SAPS II, and LDH for predicting in-hospital death in cardiac surgery patients. NLR and LMR have previously been shown to be strong prognostic biomarkers in cardiac surgery patients [10, 11, 24]. To date, there is no direct evidence to prove that PLR is correlated with cardiac surgery prognosis. However, a previous study found that PLR is correlated with increased surgical risk in patients undergoing transcatheter aortic-valve replacement [25]. Studies have also revealed that there is an important relationship between PLR and clinical prognosis in patients with CHD [26] or acute coronary syndrome [27]. These findings may potentially suggest that there are certain associations between PLR and cardiac surgery prognosis. Prior evidences have demonstrated that elevated lactate levels are prominently correlated with increased risk of unfavorable outcomes after cardiac surgery [28, 29]. Schoe et al. [30] found that SAPS II had better discriminatory power than SOFA score for predicting mortality in cardiac surgery patients. Herein, LDH showed a better performance for predicting in-hospital mortality in cardiac surgery patients compared to the above prognostic indicators.
The underlying mechanisms that may account for the relationship between LDH and adverse cardiac surgery outcomes may be attributed to the following reasons: 1) LDH is involved in anaerobic glycolysis and 2) LDH is a marker of inflammation. On the one hand, CHD is closely related to insufficient supply of blood to the myocardium, which leads to cardiomyocytes ischemia and hypoxia. Moreover, there may be a mutual cause–effect association between hypoxia and heart valve disease: mid to late valve disease is accompanied by intracardiac hypoxia; in turn, hypoxia further aggravates the progression of disease [31]. In ischemic tissues, LDH dependent glycolysis is the main source of ATP in cells [32]. Previous studies revealed that hypoxia and apoptosis of cardiomyocytes can induce LDH expression [12, 33]. Our analysis also found that LDH had a positive relationship with SAPS II, which suggests that the LDH level represents disease severity. Inflammation might be additional possible explanation for the association between increased LDH level and adverse prognosis in patients after cardiac surgery. Accumulating evidence revealed that inflammatory biomarkers, such as NLR [34, 35] and CRP [36], have been reported to be correlated with adverse outcomes of CHD or acute myocardial infarction. There is a paucity of reports about the prognostic effect of inflammatory markers in valve disease. Previous studies have provided evidences of LDH as an inflammation indicator in lung diseases [37], acute pancreatitis [38], and cancer[39]. Song et al. [40] found that inhibiting the expression of LDH had an anti-inflammatory effect through the downregulation of inflammatory mediators. Collectively, these findings suggest that serum LDH is closely related to inflammation. Besides, this study found that LDH showed a positive association with inflammatory markers, including LMR and NLR.
However, this study was subject to some limitations that must be acknowledged. First, since the half-life of LDH is long, it not reflects the dynamic changes of the disease. Second, we did not include NLR, LMR, PLR, and lactate into multivariate regression analysis due to missing data. Third, MIMIC database lacks some important information, including intra- and post-operative information, EuroSCORE II and types of surgery, which have been demonstrated to influence the rate of operative mortality after cardiac surgery.
Conclusion
Elevated preoperative LDH may be a robust predictor of poor prognosis in cardiac surgery patients, and provides a more powerful value to predict in-hospital mortality than NLR, LMR, PLR, lactate, and SAPS II.
Supplementary Information
Additional file 1. Supplementary Figure 1. Flow chart of the study population enrollment.
Additional file 2. Supplementary table 1. Univariate regression analyses for in-hospital mortality in MIMIC III and MIMIC IV. Supplementary table 2. Characteristics of the study population. Supplementary table 3. Correlation between LDH and other prognostic indicators.
Acknowledgements
None.
Abbreviations
- AUCs
Area under curves
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CABG
Coronary artery bypass graft
- CHD
Coronary heart disease
- CI
Confidence interval
- CKD
Chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- CPB
Cardiopulmonary bypass
- CRRT
Continuous renal replacement therapy
- DBP
Diastolic blood pressure
- HF
Heart failure
- HR
Hazard ratio
- ICU
Intensive care unit
- LMR
Lymphocyte-monocyte ratio
- MIMIC IV
Medical Information Mart for Intensive Care IV
- NLR
Neutrophil–lymphocyte ratio
- OR
Odds ratio
- PLR
Platelet–lymphocyte ratio
- PLT
Platelet
- ROC
The receiver operating characteristic curves
- SAPS
Simplified acute physiology score
- SBP
Systolic blood pressure
- SCr
Serum creatine
- SOFA
The sequential organ failure assessment
- WBC
White blood cell
Author contributions
YZ, SRD, YHZ and YW conceived and designed the study. YZ, YYL and YHZ collected the data. YZ and RYZ performed the statistical analysis. HY and MZ wrote the first draft of the paper. YW revised the article. This manuscript was read and approved by all credited authors.
Funding
This project was supported by Chongqing Science and Technology Bureau (cstc2021jcyj-msxmX0601).
Availability of data and materials
All data in this study was extracted from MIMIC III (https://mimic.mit.edu/docs/iii/) and MIMIC IV (https://mimic.mit.edu/docs/iv/) databases. The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in line with the principles of the Helsinki Declaration. Since MIMIC databases have been approved by the institutional review boards of Massachusetts Institute of Technology and all data were deidentified, this study no longer needs additional ethical approval.
Consent for publication
Not applicable.
Competing interests
All authors declare no potential competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Zeng and Yuhe Zhao contributed equally to this work and should be considered as co-first authors.
References
- 1.Bowdish ME, D'Agostino RS, Thourani VH, et al. The society of thoracic surgeons adult cardiac surgery database: 2020 update on outcomes and research. Ann Thorac Surg. 2020;109(6):1646–1655. doi: 10.1016/j.athoracsur.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Stoney WS. Evolution of cardiopulmonary bypass. Circulation. 2009;119(21):2844–2853. doi: 10.1161/CIRCULATIONAHA.108.830174. [DOI] [PubMed] [Google Scholar]
- 3.Bigelow WG. Application of hypothermia to cardiac surgery. Minn Med. 1954;37(3):181–5. [PubMed] [Google Scholar]
- 4.Gibbon JJ. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37(3):171–85. [PubMed] [Google Scholar]
- 5.Crawford TC, Magruder JT, Grimm JC, et al. Complications after cardiac operations: all are not created equal. Ann Thorac Surg. 2017;103(1):32–40. doi: 10.1016/j.athoracsur.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Lagny MG, Jouret F, Koch JN, et al. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. Bmc Nephrol. 2015;16:76. doi: 10.1186/s12882-015-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 8.Lomivorotov VV, Efremov SM, Kirov MY, et al. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31(1):291–308. doi: 10.1053/j.jvca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Salameh A, Dhein S. Strategies for pharmacological organoprotection during extracorporeal circulation targeting ischemia-reperfusion injury. Front Pharmacol. 2015;6:296. doi: 10.3389/fphar.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Liang M, Wu H, et al. Preoperative lymphocyte-to-monocyte ratio as a prognostic predictor of long-term mortality in cardiac surgery patients: a propensity score matching analysis. Front Cardiovasc Med. 2021;8:639890. doi: 10.3389/fcvm.2021.639890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberman S, Abu-Yunis U, Tauber R, et al. Neutrophil-lymphocyte ratio: prognostic impact in heart surgery early outcomes and late survival. Ann Thorac Surg. 2018;105(2):581–6. doi: 10.1016/j.athoracsur.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Cobben NA, Drent M, Schols AM, et al. Serum lactate dehydrogenase and its isoenzyme pattern in ex-coalminers. Respir Med. 1997;91(10):616–623. doi: 10.1016/s0954-6111(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald RP, Simpson JR, Nossal E. Serum lactic dehydrogenase; a diagnostic aid in myocardial infarction. J Am Med Assoc. 1957;165:35–40. doi: 10.1001/jama.1957.02980190037009. [DOI] [PubMed] [Google Scholar]
- 14.Piper C, Horstkotte D, Bock AK, et al. Myocardial lactate dehydrogenase patterns in volume or pressure overloaded left ventricles. Eur J Heart Fail. 2002;4(5):587–591. doi: 10.1016/s1388-9842(02)00088-0. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Lu C, Pan N, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep. 2021;11(1):12997. doi: 10.1038/s41598-021-92430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson A, Bulgarelli L, Pollard T, et al. MIMIC-IV (version 0.4). PhysioNet (2020)
- 18.Yamaguchi S, Abe M, Arakaki T, et al. Prognostic value of lactate dehydrogenase for mid-term mortality in acute decompensated heart failure: a comparison to established biomarkers and brain natriuretic peptide. Heart Lung Circ. 2020;29(9):1318–1327. doi: 10.1016/j.hlc.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Morello F, Ravetti A, Nazerian P, et al. Plasma lactate dehydrogenase levels predict mortality in acute aortic syndromes: a diagnostic accuracy and observational outcome study. Medicine. 2016;95(6):e2776. doi: 10.1097/MD.0000000000002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, Chai X, Zhou Y, et al. Association of lactate dehydrogenase with in-hospital mortality in patients with acute aortic dissection: a retrospective observational study. Int J Hypertens. 2020;2020:1347165. doi: 10.1155/2020/1347165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao YT, Chen CJ, Li WF, et al. Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern Taiwan. Toxicol Appl Pharmacol. 2012;262(3):232–237. doi: 10.1016/j.taap.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Ryu SY, Kleine CE, Hsiung JT, et al. Association of lactate dehydrogenase with mortality in incident hemodialysis patients. Nephrol Dial Transplant. 2021;36(4):704–712. doi: 10.1093/ndt/gfaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seese L, Sultan I, Gleason TG, et al. The impact of major postoperative complications on long-term survival after cardiac surgery. Ann Thorac Surg. 2020;110(1):128–135. doi: 10.1016/j.athoracsur.2019.09.100. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Li J, Wang X. The neutrophil-lymphocyte ratio is associated with postoperative mortality of cardiac surgery. J Thorac Dis. 2021;13(1):67–75. doi: 10.21037/jtd-20-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Condado JF, Junpaparp P, Binongo JN, et al. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) can risk stratify patients in transcatheter aortic-valve replacement (TAVR) Int J Cardiol. 2016;223:444–449. doi: 10.1016/j.ijcard.2016.08.260. [DOI] [PubMed] [Google Scholar]
- 26.Qiu Z, Jiang Y, Jiang X, et al. Relationship between platelet to lymphocyte ratio and stable coronary artery disease: meta-analysis of observational studies. Angiology. 2020;71(10):909–915. doi: 10.1177/0003319720943810. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Zhou Y, Ma Y, et al. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: a systematic review and meta-analysis. Kardiol Pol. 2017;75(7):666–673. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 28.Hajjar LA, Almeida JP, Fukushima JT, et al. High lactate levels are predictors of major complications after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146(2):455–460. doi: 10.1016/j.jtcvs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Demers P, Elkouri S, Martineau R, et al. Outcome with high blood lactate levels during cardiopulmonary bypass in adult cardiac operation. Ann Thorac Surg. 2000;70(6):2082–2086. doi: 10.1016/s0003-4975(00)02160-3. [DOI] [PubMed] [Google Scholar]
- 30.Schoe A, Bakhshi-Raiez F, de Keizer N, et al. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. Bmc Anesthesiol. 2020;20(1):65. doi: 10.1186/s12871-020-00975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapp MC, Krishnamurthy VK, Puperi DS, et al. Differential cell-matrix responses in hypoxia-stimulated aortic versus mitral valves. J R Soc Interface. 2016;13:5. doi: 10.1098/rsif.2016.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opie LH. Metabolism of the heart in health and disease. II. Am Heart J. 1969;77:100–122. doi: 10.1016/0002-8703(69)90135-5. [DOI] [PubMed] [Google Scholar]
- 33.Colgan SM, Mukherjee S, Major P. Hypoxia-induced lactate dehydrogenase expression and tumor angiogenesis. Clin Colorectal Cancer. 2007;6(6):442–446. doi: 10.3816/CCC.2007.n.014. [DOI] [PubMed] [Google Scholar]
- 34.Liu GQ, Zhang WJ, Shangguan JH, et al. Association of derived neutrophil-to-lymphocyte ratio with prognosis of coronary heart disease after PCI. Front Cardiovasc Med. 2021;8:705862. doi: 10.3389/fcvm.2021.705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji Z, Liu G, Guo J, et al. The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovasc Med. 2021;8:706852. doi: 10.3389/fcvm.2021.706852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mincu RI, Janosi RA, Vinereanu D, et al. Preprocedural C-reactive protein predicts outcomes after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction a systematic meta-analysis. Sci Rep. 2017;7:41530. doi: 10.1038/srep41530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faruqi S, Wilmot R, Wright C, et al. Serum LDH in chronic cough: a potential marker of airway inflammation. Clin Respir J. 2012;6(2):81–87. doi: 10.1111/j.1752-699X.2011.00250.x. [DOI] [PubMed] [Google Scholar]
- 38.Tian F, Li H, Wang L, et al. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clin Chim Acta. 2020;510:665–670. doi: 10.1016/j.cca.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Manerba M, Di Ianni L, Govoni M, et al. Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Eur J Pharm Sci. 2017;96:37–44. doi: 10.1016/j.ejps.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Song YJ, Kim A, Kim GT, et al. Inhibition of lactate dehydrogenase A suppresses inflammatory response in RAW 264.7 macrophages. Mol Med Rep. 2019;19(1):629–37. doi: 10.3892/mmr.2018.9678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Figure 1. Flow chart of the study population enrollment.
Additional file 2. Supplementary table 1. Univariate regression analyses for in-hospital mortality in MIMIC III and MIMIC IV. Supplementary table 2. Characteristics of the study population. Supplementary table 3. Correlation between LDH and other prognostic indicators.
Data Availability Statement
All data in this study was extracted from MIMIC III (https://mimic.mit.edu/docs/iii/) and MIMIC IV (https://mimic.mit.edu/docs/iv/) databases. The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.