Abstract
Background
Malaria is extremely common in Ethiopia, and it is one of the country's most serious public health and economic problems. Traditional medicines have long been utilized in Ethiopia by people of various ethnic groups. As a result, the goal of this study is to record the use of Ethiopian medicinal herbs that have been used to treat malaria. Also, a critical review of the literature on the therapeutic properties of these and other Ethiopian medicinal plants that have been tested against Plasmodium spp. parasites was conducted with the goal of highlighting neglected studies and fostering further research in this area.
Methods
A comprehensive literature search was performed in Scopus, Web of Science Core Collection, PubMed, Science Direct, Google Scholar, and Scientific Electronic Library Online (SciELO) from August 2021 to October 2021. The study databases included original articles published in peer reviewed journals covering anti-malarial plants, dated until October 2021.
Results
The review looked at 51 plant species (28 families) that have been used to treat malaria in Ethiopia. The most often used ethnobotanical plant species for the treatment of malaria were Allium sativum, Croton macrostachyus, Carica papaya, and Lepidium sativum. Leaves were used more frequently as a therapeutic preparation than other parts. Plant extracts were found to have very good, good, and moderate anti-malarial activity in mice with rodent Plasmodium species. The most active species were Ajuga remota and Capsicum frufescens, which suppressed parasitaemia by 77.34% and 72.65%, respectively, at an oral dose of 100 mg/kg and an LD50 of above 2000 mg/kg. The compound Aloinoside reported from Aloe macrocarpa leave latex was the most potent; it suppressed parasitaemia by 100% at 400 mg/kg oral dose of Plasmodium berghei infected mice, and its LD50 was above 2000 mg/kg. Toxicity was shown to be safe in 84% of the plant extracts.
Conclusion
In Ethiopia, medicinal plants have a significant part in reducing the severity of malaria due to their widespread use. As a result, more studies are needed to identify and develop effective novel drugs that could be employed in broader malaria eradication efforts.
Keywords: Antimalarial, Ethnomedicine, Medicinal plants, Ethiopia
Background
Malaria is a disease caused by Plasmodium parasites and it is one of the primary causes of death and morbidity in many undeveloped countries [1]. Malaria affects an estimated 3.3 billion people globally [2], and it is a major public health issue in tropical and subtropical areas [3]. According to the World Health Organization (WHO), 229 million new cases of malaria were reported worldwide in 2019, and an estimated 409,000 people died from malaria in that period. The majority of malaria cases and resulting mortality occurred in the WHO African area (94%) [4]. Malaria causes major complications in infected people, such as severe anaemia, cerebral malaria, acute renal failure, and hypoglycaemia [5]. Five Plasmodium species are responsible for the disease [6] and four of these species occur in Ethiopia—Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae [7].
Plasmodium falciparum is the most severe Plasmodium species in terms of morbidity and mortality, followed by Plasmodium vivax with proportions of 60% and 40%, respectively [8]. Malaria is one of Ethiopia's most serious public health and economic issues. The prevalence of malaria in children and pregnant women are 0.6% and 16.3%, respectively [9, 10]. In Ethiopia, the transmission patterns and intensity vary greatly due to the large diversity in altitude, rainfall, and population movement, with areas below 2000 m being potentially malarious. Those areas are home to approximately 68% (52 million) of the Ethiopian population and cover almost 75% of the country's landmass, resulting in around 10 million clinical cases each year according to Ethiopian National Malaria Indicator survey of 2007 [11]. Ethiopia is one of the countries that have adopted the revised malaria control strategies. The most crucial in malaria prevention and control strategies are indoor residual spraying and long-lasting insecticidal nets. In Ethiopia, quick diagnostic tests are being introduced at the community level, as well as the adoption of artemisinin-based combination therapy (ACT). However, there have already been instances of increased treatment failure and probable resistance to certain combinations [12–14]. As a result, new medications as prospective substitutes for artemisinin-based combinations are urgently needed. Pharmaceutical firms, on the other hand, consider a large investment in the development of new (semi)synthetic anti-malarial medications to be a dangerous venture, because the populations of developing nations cannot afford to pay a high price for these drugs. There is a need to develop new cost effective anti-malarial drugs to assist in controlling malaria and reducing its impact in these areas until eradication programmes become realistic.
One approach to the development of novel anti-malarial drugs is to reinvestigate traditional medicines. In this context, Ethiopia possesses a diverse range of medicinal plants linked to a variety of traditional medical practices that vary by ethnic group [15]. Despite this, there is a paucity of well-documented ethnobotanical and ethnopharmacological literature on Ethiopian anti-malarial herbs. The review looked at the various ethnomedicinal studies that have been conducted, as well as the scientific validation of antiplasmodial activity, anti-malarial activity, toxicity, and phytochemistry of these plants utilized in Ethiopian traditional medicine. This review may open the way for additional supplementary research as well as the development of a number of readily available and affordable anti-malarial phytomedicines, in keeping with the goals of the WHO's "Traditional Medicine Strategy" [16].
Methods
A comprehensive literature search was performed in Scopus, Web of Science Core Collection, PubMed, Science Direct, Google Scholar, and Scientific Electronic Library Online (SciELO) from August 2021 to October 2021. The search was performed independently in all databases. The study databases included original articles published in peer reviewed journals covering anti-malarial plants, dated until October 2021.
Articles offering information on malaria or medicinal plants in Ethiopia were given utmost priority throughout all publishing years. As a result, references found in the returned results were evaluated for inclusion in this study, and further searches were conducted using more general search terms such as "Ethiopian," "medicinal plant," "traditional medicine," "ethnomedicine," "parasite," "malaria," "anti-malarial," and "antiplasmodial". The study was non-biased, with no preference for endemic species or taxonomic preference. The initial ethnobotanical literature search did not include scientific evidence to support traditional use, but it was added in subsequent searches to see if the traditional use had been validated. The search was restricted to studies that were written in English. Relevant articles were identified and the data extracted by the reviewers: plant species, plant family, parts of the plant used, methods of preparation, type of study (whether in vitro or in vivo), the extraction solvent used, IC50 or ED50 values, parasite suppression rate, isolated compounds, strain of Plasmodium tested and toxicity.
Categorization of anti-malarial and antiplasmodial activities
For in vitro investigations, antiplasmodial activity of extracts was rated very good if the IC50 was less than 5 μg/ml, good if the IC50 was greater than 5 μg/ml and less than 10 μg/ml, and moderate if the IC50 was 10 μg/ml ≤ IC50 < 20 μg/ml [17]. For in vivo investigations, an extract's anti-malarial activity is deemed very good if it suppresses malaria by ≥ 50% at 100 mg/kg body weight/day, good if it suppresses malaria by ≥ 50% at 250 mg/kg body weight/day, and moderate if it suppresses malaria by ≥ 50% at 500 mg/kg body weight/day [17].
Results and discussion
Ethiopian medicinal plants used traditionally to treat malaria
Ethiopia has a diverse flora, and some local people employ several of the plant species for medical purposes [18]. The widespread use of traditional medicines in Ethiopia can be linked to its cultural acceptability, efficacy against specific ailments, physical accessibility, and economic affordability when compared to modern medicine [19]. Traditional remedies are the most important and, in some cases, the only source of treatments for approximately 80% of Ethiopians, and 95% of the preparations are made from plants [19]. In different locations of Ethiopia, 51 plant species from 28 families were reported as being engaged in the treatment of malaria (Table 1). The following families account for 64% of the anti-malarial plant species documented across the country: Fabaceae has nine species, Asteraceae has five, Euphorbiaceae has four, Aloaceae has three, Solanaceae has three, Alliaceae has two, Urticaceae has two, and Meliaceae has two species. Allium sativum, Croton macrostachyus, Carica papaya, and Lepidium sativum were the most commonly employed ethnobotanical plant species for the treatment of malaria (Table 1).
Table 1.
Ethiopian medicinal plants used traditionally to treat malaria
| Familya | Plant species | Local name | Parts used | Methods of preparation | References |
|---|---|---|---|---|---|
| Alliaceae (2) | Allium sativum | Nech shinkurt (A) | Steams | Peeling the cover then eat with nutrient | [25] |
| Bulbs | The bulb, which is free of external scales, is crushed and blended with honey before being consumed on an empty stomach | [26] | |||
| Bulbs | Allium sativum bulb and Ginger officinale rhizome are pounded and eaten with honey | [27] | |||
| Fruits | Fresh or dry fruits is chewed orally | [28] | |||
| Bulbs | Before eating breakfast, take the bulb with Ethiopian traditional food 'injera' and Capsicum annuum for 5 days | [18] | |||
| Fruits | Crush the fruit and boil it, then drink it with much amount of milk for 1 day | [29] | |||
| Bulbs | Crush it and consume it alone or mixed with Lepidium sativum seeds | [30] | |||
| Allium cepa | Keye shinkurt (A) | Bulbs | Chew the bulbs and swallow it | [31] | |
| Aloaceae (3) | Aloe gilbertii | Kurunda (Had) | Leaves sap | Squeezed fresh leaves soup and taking the soup orally | [32] |
| Aloe sp. | Yeset qest (A) | Leaves | Fresh leaves were squeezed and diluted with water and drunk it. Syrup made from the plant's dried leaves, as well as those of Asparagus africanus and Senna italica, is also drunk | [33] | |
| Aloe megalacantha | Ere (T) | Leaves | Crush the leaves to get the juice, then filter and drink the filtrate | [30] | |
| Asteraceae (5) | Artemisia abyssinica | Aritimiza (Had) | Leaves | Fresh leaves were crushed and pounded with water, filtered and drunk until they were recovered | [34] |
| Vernonia amygdalina | Grawa (A) | Leaves and barks | For days, morning and evening, leaves and bark mixed with honey are consumed | [35] | |
| Leaves | Crushed leaves of Vernonia amygdalina concocted with leaves of Ruta chalepensis. One cup is served as a drink for 3–5 days with cold water in the morning | [22] | |||
| Artemisia afra | Chugughee (A) | Leaves | Powdered fresh/dry leaves mixed with butter is taken with coffee orally before breakfast for three days | [28] | |
| Leaves | Fresh leaves crushed and pounded with water and then filtered and drunk in one tea cup | [36] | |||
| Calpurnia aurea | Digita (A) | Leaves | Maceration, taken orally once daily for seven days | [37] | |
| Echnops kebericho | Kebericho (A) | Roots | Maceration; take orally once daily for seven days | [37] | |
| Boraginaceae (1) | Cordia africana | Wanza (A) | Roots and Barks | Decoction of roots and inner bark with ginger is consumed | [35] |
| Brassicaceae (1) | Lepidium sativum | Feto (A) | Fruits | Dried fruit is ground into powder, mixed with castor oil, and administered orally | [35] |
| Seeds | Pounded seeds mixed with Allium sativum bulbs and honey is taken orally for five days before breakfast After each dose, one glass of melted butter is recommended for immediate recovery | [28] | |||
| Seeds | The seed is powdered and made as Porridge with other grains | [38] | |||
| Capparidaceae (1) | Maerua oblongifolia | Ja”a (O) | Leaves | Pounded leaves boiled with goat milk and drunk. It is also taken in mixture with the leaves of Withania somnifera | [33] |
| Caricaceae (1) | Carica papaya | Papaya (A) | Leaves | Squeezed the fresh leaves juice and drunk | [39] |
| Leaves | The fresh leaves crush and drink with milk or without milk | [36] | |||
| Leaves | When the leaves become yellow, that means getting to dry, powdered and boiled in water and a cup of tea will be taken for 5 days | [18] | |||
| Leaves | Leaves are pounded and then boiled; the decoction is taken while cold | [38] | |||
| Caryophyllaceae (1) | Silene macrosolen | Saerosaero (T) | Roots | Crush and place it on fire for fumigation | [30] |
| Combretaceae (2) | Combretum molle | Agalo (A) | Leaves and Barks | Leaves and barks powder are mixed with tea or coffee and drunk | [35] |
| Terminalia brownie | Sebaea (T) | Barks | The fresh barks of Terminalia brownie pounding, homogenize with water and drink a bottle cup of the decant in the morning in empty stomach for 4 days | [40] | |
| Convolvulaceae (1) | Ipomoea kituiensis | Laalata (O) | Leaves | Juice of fresh leaves is drunk with coffee | [35] |
| Cucurbitaceae (1) | Lagenaria siceraria | Buqqe hadhaa (O) | Fruits | Ripe fruit of Lagenaria siceraria is bored rinsed with cold water, one glass is used as a drink early in the morning | [22] |
| Euphorbiaceae (4) | Croton macrostachyus | Bisana (A) | Leaves | Boil fresh leaf in water, filter, and drink with milk or tea | [36] |
| Leaves | Macerate with water; take two doses orally for one day | [37] | |||
| Leaves | Crushed/pounded fresh/dry leaves boiled with water is concocted with Allium sativum (bulb) roasted with butter and left over night outside home is taken orally at the morning | [28] | |||
| Leaves | Powdered leafy-stem of C. macrostachyus is mixed with H2O and butter and drank the filtrate part | [41] | |||
| Leaves | Crushing leaves and drinks with either Guizotia abyssinica or milk | [29] | |||
| Euphorbia abyssinica | Kulkual (A) | Latexs | Fresh latex of Euphorbia abyssinica eat bake with Eragrostis tef dough | [26] | |
| Jatropha curcas | Habet-muluk (So) | Leaves | The outer cover of the seed removed and the inside part swallowed with camel milk or chewed | [33] | |
| Euphorbia abyssinica | Kulkual (A) | Roots | Crushing the root and drink with milk | [29] | |
| Fabaceae (9) | Sesamum indcum | Eshkulubia (Ku) | Roots | Pounding the fresh root, mixed with boiled milk and drink a half cup of it in the morning and afternoon | [40] |
| Tephrosia gracilipes | Atotoka (Ku) | Roots | Crushing the dried root, homogenize with water and drink a bottle cup of it in the morning in empty stomach | [40] | |
| Acacia seyal | Tundukiyac (O) | Barks | Gum from bark is chewed | [35] | |
| Albizia amara | Ondoddee (O) | Barks | Bark is chewed | [35] | |
| Tamarindus indica | Mala (B) | Fruits | Chopped, dispersed in water and the suspension is drunk | [42] | |
| Cicer arietinum | Shinbira (A) | Seeds | The dried seeds germinate, then eat them with an Allium sativum bulb | [26] | |
| Entada abyssinica | Ambalta (O) | Barks | The bark ground along with rhizome of Zingiber officinale and bulb of Allium sativum and chewed once a day for few months | [22] | |
| Tamarindus indica | Ged-Kinin (So) | Fruit/pulp | Infusion of the fruit/pulp kept overnight and drunk after taking goat soup | [33] | |
| Senna italica | Salamaki (So) | Leaves | Dried leaves powdered and boiled with water and drunk after adding goat or camel milk | [33] | |
| Flacourtiaceae (1) | Flacourtia indica | Agnaneshewe (B) | Fruits | Eaten as it is | [42] |
| Lamiaceae (1) | Ajuga integrifolia | Anamuro (Hal) | Leaves | The fresh whole parts of plant crushed, the liquid is filtered & drunk it | [32] |
| Loganiaceae (1) | Buddleja polystachya | Amfar (A) | Leaves | Juice in empty stomach | [43] |
| Leaves | Maceration/decoction taken orally once daily for seven days | [37] | |||
| Melia azedarach | Almim (B) | Leaves | Leaves boiled with water and drunk | [42] | |
| Azadirachta indica | Neem (A) | Leaves | Fresh apical leaves (buds) are pounded and mixed with water (soaked) and the filtrate drunk. Lemon and salt and sometimes sugar are added | [33] | |
| Leaves | Grinding, chewing, boiling, liquid form | [44] | |||
| Skebergia capensis | Lol (A) | Barks | Infusion of fresh pulverized bark | [29] | |
| Moraceae (1) | Ficus sur | Oda’a (Had) | Fruits | Dry fruits pounded, powdered and then mixed with honey and taken orally twice | [34] |
| Myrtaceae (1) | Syzygium guineense | Duwancho (O) | Leaves | Well powdered leaves are taken with cold tea | [35] |
| Oleaceae (1) | Olea europaea | Awlie (T) | Bark | Boil it in water and drink the fluid | [30] |
| Phytolaccaceae (1) | Phytolacca dodecandra | Endod (A) | Roots | Fresh roots of Phytolacca dodecandra L’Herit. crush, squeeze then drink | [26] |
| Polygonaceae (1) | Rumex abysinicus | Mekimeko (A) | Roots | Dried roots of Rumex abysinicus boiled with butter and taken orally | [45] |
| Ranunculaceae (1) | Clematis simensis | Tauta (Ku) | Roots | Grinding fresh roots of Clematis simensis and giving a fingertip of this nasall | [40] |
| Rutaceae (1) | Ruta chalepensis | Xenadame (A) | Leaves | Leaves powder is mixed with water and drunk in the morning before breakfast for 3 days | [35] |
| Sapindaceae (1) | Dodonia angustifolia | kitkita (A) | Seeds | Grind and eat it with honey | [30] |
| Solanaceae (3) | Capsicum frutescens | Kariya (A) | Seeds | Eating the dry seeds mixing with foods | [32] |
| Datura stramonium | Manjii (O) | Fruits | Powdered fruit of Datura stramonium is mixed with honey and three to four spoons are eaten with pounded Allium sativum | [22] | |
| Withania somnifera | Gzawa (A) | Roots | Dried roots grounded and boiled and drunk after adding goat/camel milk | [33] | |
| Urticaceae (2) | Droguetia iners | Yewoba medihanit (Aari) | Leaves | Leaves chopped and mixed with Premna oligotricha and boiled together one glassful drank | [46] |
| Urtica simensis | Sama (A) | Roots | The crushed the roots and dried the mixed with fresh water, drink one glass of it and drink much amount of milk | [29] | |
| Verbenaceae (1) | Lantana trifolia | Yewoba medihanit (Aari) | Roots | Root chopped and soaked with water and mixed with local alcoholic drink (Areke) | [46] |
| Roots | Maceration, taken two doses orally for one day | [37] |
A Amhargna, O Oromigna, T Tigerigna, B Bertagna, Had Hadigna, So = Somaligna, Ku Kunamaigna, Hal Halabigna
aNumber of species studied by family in parentheses
The use of plant components and the manner in which they are prepared are limited by their availability and indigenous people's expertise [20]. According to the results of the analysis of the plant parts used, traditional healers used the leaves the most, accounting for around 42% of the total plant parts used (Fig. 1). Damage to medicinal plants caused by leaves harvesting is negligible when compared to other components [21]. The majority of these anti-malarial botanicals are employed as monotherapies, but others are utilized in combination therapies. The combination of Entada abyssinica barks, Zingiber officinale rhizomes, and Allium sativum bulbs chewed once a day for a few months to treat malaria is an example of a multi-herbal combination [22].
Fig. 1.
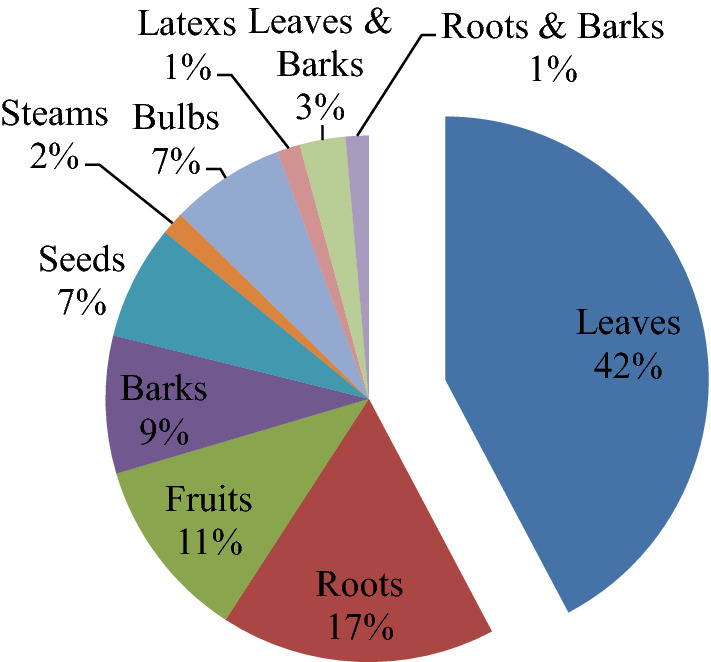
Frequency distribution of plant parts used to prepare remedies
According to the review, crushing, powdering, macerating and decoction were the most common remedy formulations, and oral administration was the most common mechanism of route of delivery. In the preparation of remedies, water and various ingredients such as honey, butter, salt, coffee, tea, and milk were frequently employed. It was believed that the additions would either reduce toxicity or increase flavor. A variety of plant species used to treat malaria in Ethiopia has also been found in other African countries. Anti-malarial such as Rumex abyssinicus and Vernonia amygdalina have been utilized in Kenya [23], Carica papaya and Azadirachta indica have been used in Southwest Nigeria [24]. Many similar examples exist amongst those documented in Table 1.
Scientific studies into the anti-malarial activity of Ethiopian medicinal plants
In vivo anti-malarial properties of extracts from 38 plant species studied in mice with rodent Plasmodium species were indicated for the treatment and/or prevention of malaria (Table 2). Seven studies (16%) indicated very good activity (suppression rate of 50% at 100 mg/kg body weight/day), sixteen studies (37%) reported good activity, and twenty studies (47%) found moderate activity. All in vivo experiments have been conducted using the 4-day suppressive test [47] and the Rane (curative) test [48]. Aloaceae and Asphodelacea were the most studied plant families. It is possible that this is an account of the Aloaceae family, which is found in every floristic region of the country [49]. Aerial parts, leaves, leaf latex, rhizomes, roots, stem bark, fruits, and seeds were among the plant parts examined for anti-malarial activity. The crude extract of the plants was employed in the majority of the studies (76%). Acanthus polystachyus [50, 51], Aloe debrana [52, 53], Combretum molle [54, 55], Croton macrostachyus [56, 57], Clerodendrum Myricoides [53, 58] and Dodonaea angustifolia [53, 59, 60] are examples of plant species that have been studied by more than one author. Echinops kebericho, Artemisia abyssinica, Aloe megalacantha, Carica papaya, Combretum molle, Croton macrostachyus, Ajuga remota, and Dodonaea angustifolia are some of the plant species listed in Table 2 that have anti-malarial activity, which supports the traditional uses indicated in Table 1. In experiments using a methanolic extract of the leaves, the most active species was Ajuga remota ('Akorarach' in Amharic; 'Etse-Libawit' in Ge'ez), which provided a high suppression of parasitaemia of 77.34% with an oral dose of 100 mg/kg [61]. Despite the fact that it is often suggested that more polar solvents such as water, methanol, and ethanol be used only in traditional preparations [62]. Surprisingly, the anti-malarial activity of most of the plant species studied matched to high polarity (methanol) plant extracts in most studies. According to Lipinski's laws of 5 [63], this is beneficial because it allows therapeutic components to absorb through the gut lumen into the circulatory system, where they are needed. As a result, active compounds work through cell surface receptors, with polar components providing clinically relevant potency in vivo.
Table 2.
in vivo anti-malarial activity of Ethiopian medicinal plants
| Familya | Plant species | Part (s) used | Parasitemia Inhibition with each extract and dose used for the treatment of the malaria infected mice (dose in mg/kg body weight) | Antimalarial activity | Strain of Plasmodium tested | Safe dose to non-infected mice (mg/kg body weight) | References |
|---|---|---|---|---|---|---|---|
| Acanthaceae (2) | Adhatoda schimperiana | Root | Hydroalcoholic crude extract, 53.6% at 600 mg/kg | Moderate | Pb | 2000 | [50] |
| Acanthus polystachyus | Leave | 80% methanol extract, 49.25% (400) | Moderate | Pb ANKA | 2000 | [51] | |
| Acanthus polystachyus | Root | 80% methanol, 51.48% (400) | Moderate | Pb ANKA | 2000 | [64] | |
| Aloaceae (5) | Aloe pirottae | Latex | 80% methanol extract, 47% % at 600 mg/kg | Moderate | Pb ANKA | 2000 | [65] |
| Aloe citrina | Leave latex | Latex extract, 60.59% (400) | Good | Pb ANKA | 5000 | [66] | |
| Aloe weloensis | leave latex | Leave latex extract, 66.84% at 400 mg/kg | Good | Pb | 2000 | [67] | |
| Aloe percrassa | Leave latex | Water extract, 73.6% (400) | Good | Pb ANKA | 5000 | [68] | |
| Aloe megalacantha | Leave | Leave latex extract, 56.4% (400 mg/kg) | Moderate | Pb ANKA | 2000 | [69] | |
| Anacardiaceae (1) | Schinus molle | Seed | Methanol crude extract,66.91% at 400 mg/kg | Good | Pb | 2000 | [70] |
| Asclepiadaceae (1) | Periploca linearifolia | Stem bark | Methanol extract, 56.98% (600 mg/kg) | Moderate | Pb ANKA | 2000 | [71] |
| Asphodelaceae (4) | Asparagus africanus | Leave | Leave latex crude extract, 60.70% at 300 mg/kg | Good | Pb | 1500 | [72] |
| Aloe debrana | Leave | Leave latex crude extract, 75.02% at 600 mg/kg | Good | Pb | 5000 | [52] | |
| Aloe macrocarpa | Leave | Leave exudate crude extract, 60% at 100 mg/kg, 67.8% at 200 mg/kg and 74.3% at 400 mg/kg | Very good | Pb ANKA | 2000 | [73] | |
| Aloe debrana | Leave | Methanol extract, 73.95% at 600 mg/kg | Good | Pb ANKA | 3000 | [53] | |
| Kniphofia foliosa | Rhizome | 80% methanol extract, 51.39% (200) and 61.52% (400) | Good | Pb ANKA | 2000 | [74] | |
| Asteraceae (3) | Echinops kebericho | Root | 70% ethanol, 57.29% (500 mg/kg) | Moderate | Pb ANKA | 5000 | [75] |
| Vernonia adoensis | Leave | methanol extract, 83.36% (600) | Moderate | Pb ANKA | 3000 | [76] | |
| Artemisia abyssinica | Aerial parts | 80% methanol, 64.7 and 82.4% at 200 and 400 mg/kg | Good | Pb | NE | [77] | |
| Balanitaceae (1) | Balanites rotundifolia | Leave | 80% methanol, 67% (400) | Moderate | Pb ANKA | 2000 | [78] |
| Brassicaceae (1) | Brassica nigra | Seed | 80% methanol extract, 50% (200) and 53.13% (400) | Good | Pb ANKA | NE | [79] |
| Caricaceae (1) | Carica papaya | Root & Fruit | Petroleum ether fraction of fruit rind extract, 61.78% (400 mg/kg) | Moderate | Pb ANKA | 2000 | [80] |
| Combretaceae (2) | Combretum molle | Stem bark | 80% methanolic extract, 59.7% at 400 mg/kg | Moderate | Pb | 2000 | [54] |
| Terminalia brownii | Bark | Methanol crude extract, 60.2% at 400 mg/kg | Good | Pb ANKA | 2000 | [81] | |
| Combretum molle | Seed | Methanol crude extract, 63.5% at 250 mg/kg | Moderate | Pb ANKA | NE | [55] | |
| Cucurbitaceae (1) | Zehenria scabra | Leave | 80% methanolic extract 62.5% (100 mg/kg), 72.85% (200 mg/kg) and 76.01% (400 mg/kg) | Very good | Pb | 2000 | [82] |
| Euphorbiaceae (1) | Croton macrostachyus | Leave | Methanol extract 91%, Chloroform fraction, 75.9% and methanol fraction, 64.2% at 600 mg/kg | Good | Pb ANKA | 5000 | [56] |
| Croton macrostachyus | Fruit & Root | 80% methanol fruit extract, 70% (400) and 87% (600), root extract, 75% (400) and 89% (600) | Moderate | Pb ANKA | 2000 | [57] | |
| Fabaceae (2) | Calpurnia aurea | Leaves | hydromethanolic leave extract, 51.15% (60 mg/kg) | Very good | Pb | LD50 > 300 | [83] |
| Indigofera spicata | Root | 80% methanol extract, 53.42% (600) | Moderate | Pb ANKA | NE | [84] | |
| Lamiaceae (2) | Clerodendrum Myricoides | Leaves | Methanol fraction, 77.24% and Ethyl acetate fraction 65.21% at 300 mg/kg | Good | Pb | NE | [58] |
| Clerodendrum myricoides | Leave | Methanol crude extract, 82.5% at 600 mg/kg | Good | Pb ANKA | 3000 | [53] | |
| Ajuga remota | Leave | methanol extract, 77.34% at 100 mg/kg | Very good | Pb ANKA | 2000 | [61] | |
| Strychnos mitis | Leave | Aqueous extract, 74.86% (400 mg/kg) and 95.5% (600 mg/kg), hydroalcoholic fraction, 81.49% (400) and 93.97% (600) | Moderate | Pb ANKA | 2000 | [85] | |
| Loganiaceae (1) | |||||||
| Menispermaceae (1) | Stephania abyssinica | Leave | 80% methanol crude extract, 45.60%, Ethyl acetate fraction, 51.44% and Chloroform fraction 55.80% at 400 mg/kg | Moderate | Pb ANKA | NE | [86] |
| Oleaceae (1) | Olea europaea | Stem bark | Methanol extract, 52.40% at 400 mg/kg | Moderate | Pb | 2000 | [87] |
| Rosaceae (1) | Hagenia abyssinica | Stem bark | Hydroalcoholic (80% methanol), 65.29% at 100 mg/kg | Very good | Pb, ANKA | 2000 | [88] |
| Rubiaceae (1) | Gardenia ternifolia | Stem bark | Methanol crude extract, 59.25% at 600 mg/kg | Moderate | Pb | 2000 | [89] |
| Rutaceae (1) | Fagaropsis angolensis | Stem bark | 80% methanol extract, 50.05% (200), 54.8% (400) and 59.7% (600) | Moderate | Pb ANKA | 2000 | [90] |
| Sapindaceae (1) | Dodonaea angustifolia | Root | n-butanol fraction of methanolic root extract, 67.51% (600) | Good | Pb | 2000 | [59] |
| Dodonea angustifolia | Root | Methanol crude extract, 84.52% at 600 mg/kg | Good | Pb ANKA | 3000 | [53] | |
| Dodonaea angustifolia | Leave | acetate soluble portion of the 80% aqueous MeOH extract, 80.28% at 150 mg/kg | Very good | Pb ANKA | NE | [60] | |
| Solanaceae (1) | Capsicum frutescens Var. Minima | Fruit | 80% methanolic crude fruit extract, 72.65 at 100 mg/kg | Very good | Pb | 2000 | [91] |
| Zygophyllaceae (1) | Balanites rotundifolia | Leave | Methanol extract, 60.59% at 500 mg/kg | Moderate | Pb | 5000 | [92] |
aNumber of species studied by family in parentheses, Pb = Plasmodium berghei
Toxicity of plants extract evaluated for their anti-malarial activity
In oral acute toxicity evaluation of the test extract, 36 studies studied toxicity assays out of 43 in vivo studies (Table 2), and 84% were found to be mortality or symptoms of toxicity was not observed, which could explain the plant's safe for folkloric use. In comparison to an in vitro investigation, the in vivo model was chosen because it takes into consideration any pro-drug effect and the likelihood of the immune system managing infection [53]. The leaves were the plant part that had the most toxicity reports. Toxicity tests have indicated that several plant species with various parts, such as Combretum molle stem barks and seeds [54, 55] and Croton macrostachyus leaves, fruits, and roots [56, 57], are harmless.
Reported compounds characterized as anti-malarial and antiplasmodial in Ethiopian medicinal plants
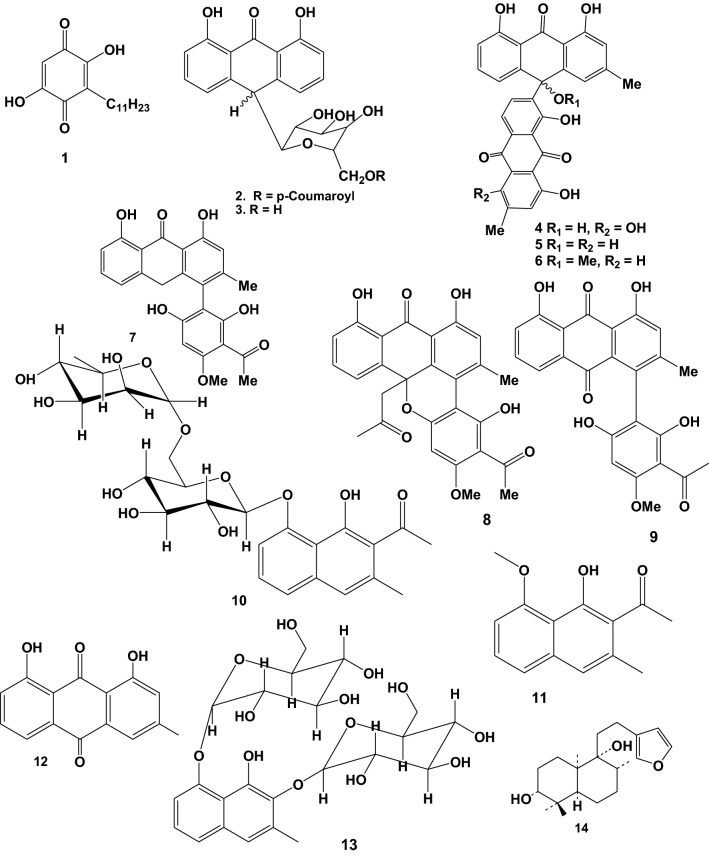
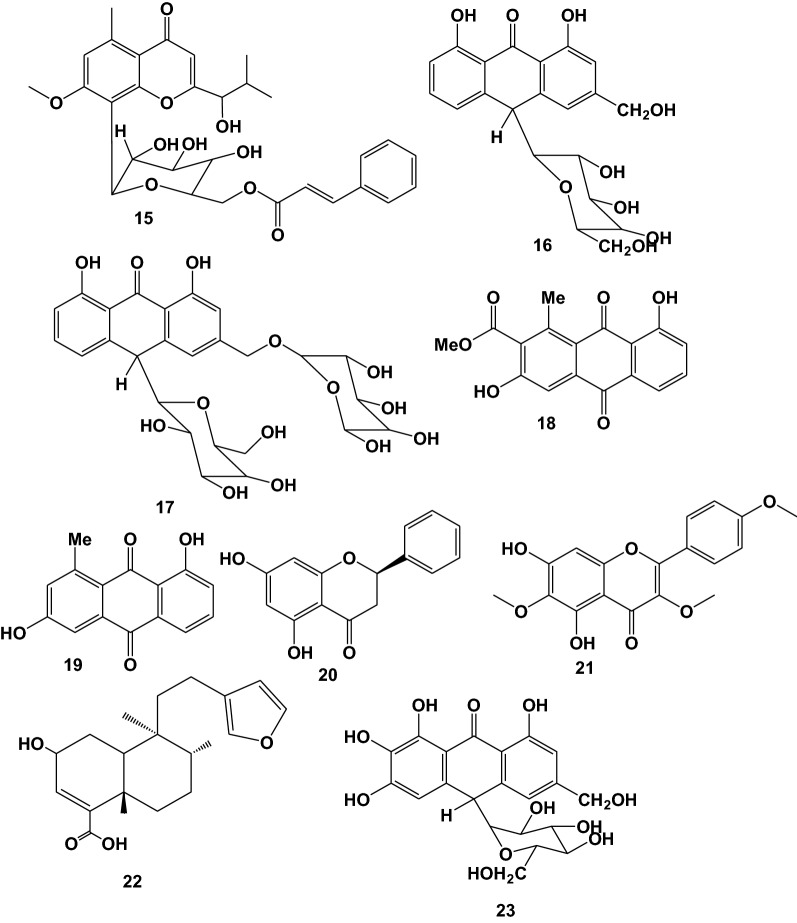
Ten plant species used in Ethiopian folkmedicine for malaria treatment have been shown to contain anti-malarial and antiplasmodial active compounds. The majority of the active compounds reported are anthraquinones, followed by naphthalene derivatives. Alkaloids are one of the most common types of compounds with anti-malarial activity. However, many naturally occurring nonalkaloidal compounds, such as terpenes, limonoids, chromones, xanthones, flavonoids, and anthraquinones, have anti-malarial activity when tested in various malarial models, according to a number of studies [93]. 14 (61%) of the reported compounds have been examined in vivo, whereas 9 (39%) have been examined in vitro against P. falciparum. There were 56% of very good, 35% of good, and 9% of moderate activity among the compounds reported. Details about these bioactive compounds are given below as well as in Table 3 and Fig. 2. The in vivo studies done by Melaku et al. [60], showed that three known compounds pinocembrin (20), flavanol santin (21) and clerodane diterpene 2-hydroxy-15, 16-epoxyceloda-3, 13, 14-trien-18-oic acid (22) were reported from Dodonaea angustifolia leaves and bio-assayed for their anti-malarial activities against Plasmodium berghei. According to the findings, compounds exhibited significant percent suppression of parasitaemia by 81% at 40 mg/kg, 80% at 50 mg/kg and 70% at 40 mg/kg, respectively in mice infected with P. berghei. Aloinoside (17) was reported from Aloe macrocarpa leave latex and evaluated for anti-malarial activity; it suppressed parasitemia by 100% at 400 mg/kg oral dose in P. berghei infected mice, and its LD50 was above 2000 mg/kg [73]. This suggests that this compound could be employed as an anti-malarial drug. Other phytochemicals, Aloin (16) reported in the latex of Aloe debrana leaves latex, inhibited infection by 78.3% at 100 mg/kg body weight and increased the survival time of mice infected with P. berghei [52].
Table 3.
Anti-malarial activity of compounds reported from Ethiopian medicinal plants
| Plant Species | Reported Compound (s) | Plant Part (s) used | IC50 or ED50 or Parasite suppression rate | Antiplasmodial/Anti-malarial activity | Strain of Plasmodium tested | Safe dose to non-infected mice (mg/kg body weight) | References |
|---|---|---|---|---|---|---|---|
| Embelia schimperi | Embelin (1) | Fruit | 54.8% at 400 mg/kg/day | Moderate | Pb | 2000 | [96] |
| Aloe percrassa | Microdontin A/B (2) | Leave latex | 61.4% at 400 mg/kg/day | Good | Pb | NE | [68] |
| Aloin A/B (3) | Leave latex | 66.8% at 400 mg/kg/day | |||||
| Kniphofia foliosa | Chryslandicin (4) | Rhizome | 2.1 and 1.5 μg/ml | Very good | Pb D6 and W2 respectively | NE | [97] |
| 10-Hydroxy-10 (chrysophanol-7’-yl)chrysophanol anthrone (5) | 1.7 and 0.7 μg/ml | ||||||
| 10-Methoxy-10-(chrysophanol-7’-yl)chrysophanol anthrone (6) | 4.1 and 1.2 μg/ml | ||||||
| Knipholone anthrone (7) | 4.1 and 3.6 μg/ml | ||||||
| 10-Acetonylknipholone cyclooxanthrone (8) | 4.4 and 3.1 μg/ml | ||||||
| Knipholone (9) | 55.14 and 60.2% at 100 and 200 m/kg/day | Good | Pb | 2000 | [74] | ||
| Dianellin (10) | 53.77 at 100 mg/kg/day | ||||||
| 2-acetyl-1-hydroxy-8-methoxy-3-methylnaphthalene (11) | Root | 15.4 μg/ml | Moderate | Pb 3D7 | NE | [98] | |
| 10-(chrysophanol-7′-yl)-10-(ξ)- hydroxychrysophanol-9-anthrone (12) | 0.260 μg/ml | Very good | |||||
| Aloe otallensis | 2,8-O,O-di(β-D-glucopyranosyl)-1,2,8-trihydroxy-3-methylnaphthalene (13) | Leave latex | 47.29% at 100 mg/kg/day | Moderate | Pb | NE | [72] |
| Otostegia integrifolia | Otostegindiol (14) | leave | 50.13, 65.58 & 73.16% at 25, 50 & 100 mg/kg/day | Very good | Pb ANKA | NE | [94] |
| Aloe debrana | (E)-2-(1-hydroxy-2-methylpropyl)-8-(6'-O-cinnamoyl)-β-D-glucopyranosyl-7-methoxy-5-Methylchromone (HCGMM) (15) | Leave latex | 63.13% at 100 mg/kg/day | Very good | Pb | 500 | [52] |
| Aloin (16) | 78.31% at 100 mg/kg/day | ||||||
| Aloe macrocarpa | Aloinoside (17) | Leave latex | 79.1, 90.9 & 100% at 100, 200 & 400 mg/kg/day | Very good | Pb ANKA | 2000 | [73] |
| Aloe pulcherrima | Aloesaponarin I (18) | Root | 7.8 μg/ml | Good | Pb D6 | NE | [99] |
| Aloesaponarin I (19) | 5.0 μg/ml | ||||||
| Dodonaea angustifolia | Pinocembrin (20) | Leave | 77.03% and 81.00% at 20 and 40 mg/kg/day | Very good | Pb | NE | [60] |
| Santin (21) | Leave | 80.95% and 85.50% at 50 and | |||||
| 100 mg/kg | |||||||
| (2-hydroxy-15,16-epoxyceloda-3,13,14-trien-18-oic acid) (22) | Leave | 60.35% and | |||||
| 70.81% at 20 and 40 mg/kg | |||||||
| Aloe pulcherrima | 7-hydroxyaloin (23) | Leave latex | 56.2% at 200 mg/kg/day | Good | Pb | 2000 | [100] |
Fig. 2.
Structures of reported anti-malarial compounds from plants used in Ethiopia for malaria treatment
In the studies with Otostegia integrifolia, very low doses of Otostegindiol (14), the active principle (25, 50, 100 mg/kg body weight), have been tested, resulting in chemosuppression of 50.13, 65.58 and 73.16%, respectively, in P. berghei (strain ANKA)-infected mice [94]. Because such low doses are clinically feasible for human use, efforts should be focused on the development of anti-malarial compounds with higher activity at low doses. However, because certain natural products are metabolized and the pharmacokinetics of individual natural products are frequently ignored, the likelihood that the in vitro data given (Table 3) in studies with some phytochemicals may be misleading cannot be overlooked. Compounds that are said to be active in vitro may be inactive in vivo. More pharmacokinetic studies using these phytochemicals would be tremendously beneficial, though it should be noted that most of the time, small quantities of these compounds are isolated, which limits in vivo studies. Moreover, some of the phytochemicals which have been reported to be active in vivo, exhibited such activities only at very high doses that may not have meaningful therapeutic use. Also, the toxicity of almost all of these purified compounds have not been be evaluated. This severely limits their potential as anti-malarial drugs in the future. Considering the importance of cytotoxicity tests, the selectivity index for all plant extracts (Table 2) and purified compounds (Table 3) has yet to be computed. The significance of the SI (CC50 value on cell lines/IC50 value against Plasmodium spp.) value in any study on herbal drugs and/or purified compounds is crucial for determining whether further works can be continued [95]. All these have brought limitations on some of the reported compounds being considered as lead molecules for anti-malarial drug development. Therefore, the purified compounds must be further investigated, taking into account the limitations in the development of new anti-malarial drugs and/or indicating the best anti-malarial remedies.
Conclusion
As a result of several ethnobotanical investigations conducted in Ethiopia, a great variety of plants utilized by indigenous people to treat various ailments, including malaria, have been described. The most often used ethnobotanical plant species for the treatment of malaria were Allium sativum, Croton macrostachyus, Carica papaya, and Lepidium sativum. Leaves were used more frequently as a therapeutic preparation than other parts. The anti-malarial activity of the species investigated, as well as their potential as sources of new anti-malarial compounds and toxicities, is reviewed here. The most active species were Ajuga remota, Capsicum frufescens, Hagenia abyssinica, Zehenria scabra and Aloe macrocapa, which suppressed parasitaemia by 77.34%, 72.65%, 65.29%, 62.5% and 60%, respectively, at an oral dose of 100 mg/kg and an LD50 of above 2000 mg/kg. These are herbs that have traditionally been used to treat malaria. The compound Aloinoside (17) reported from Aloe macrocarpa leave latex and evaluated for anti-malarial activity; it suppressed parasitaemia by 100% at 400 mg/kg oral dose of P. berghei infected mice, and its LD50 was above 2000 mg/kg. This suggests that this compound could be employed as an anti-malarial drug. Malaria control efforts and resources have expanded in Ethiopia, where the burden of malaria is the highest due to the country's vast population and geographical setting. In the light of these facts, this review focuses on Ethiopian medicinal plants used to treat malaria, as well as compounds purified from them, in the hope of helping eliminate the disease. Because it is hoped that the discovery of active compounds in plants would lead to the development of more effective drugs that are both economical and accessible to rural communities at the greatest risk of disease morbidity. However, no further investigation of the efficacy of several plant species that have been described as anti-malarial could be found. More studies are needed to identify and develop successful novel drugs that could be used in broader malaria eradication efforts.
Acknowledgements
Authors express their gratitude to Armauer Hansen Research Institute for providing access to various journal databases.
Author contributions
GN thought of the concept, gathered literature, drafted and edited the original paper, and wrote and edited the first draft of the manuscript. MW gathered literature, wrote the original paper, and proofread and corrected the final version. This manuscript's content was read by all writers, and they all agreed to take responsibility for it. All authors read and approved the final manuscript.
Funding
Nil.
Availability of data and materials
Nil.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cox FE. History of the discovery of the malaria parasites and their vectors. Parasit Vectors. 2010;3:5. doi: 10.1186/1756-3305-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nankabirwa JI, Yeka A, Arinaitwe E, Kigozi R, Drakeley C, Kamya MR, et al. Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J. 2015;14:528. doi: 10.1186/s12936-015-1056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghavendra K, Barik TK, Reddy BN, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108:757–759. doi: 10.1007/s00436-010-2232-0. [DOI] [PubMed] [Google Scholar]
- 4.WHO. World Malaria Report. Geneva, World Health Organization, 2020; Available from: https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 30 Sept 2021.
- 5.Choge JK, Ng’Wena GM, Akhwale W, Koech J, Ngeiywa MM, Oyoo-Okoth E, et al. Symptomatic malaria diagnosis overestimate malaria prevalence, but underestimate anaemia burdens in children: results of a follow up study in Kenya. BMC Public Health. 2014 doi: 10.1186/1471-2458-14-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne N. Urban malaria risk in sub-Saharan Africa: where is the evidence? Travel Med Infect Dis. 2007;5:135–137. doi: 10.1016/j.tmaid.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Bedane AS, Tanto TK, Asena TF. Malaria distribution in Kucha district of Gamo Gofa Zone, Ethiopia: a time series approach. Am J Theor Appl Stat. 2016;5:70–79. doi: 10.11648/j.ajtas.20160502.15. [DOI] [Google Scholar]
- 8.Ketema T, Bacha K, Birhanu T, Petros B. Chloroquine-resistant Plasmodium vivax malaria in Serbo town, Jimma zone, south-west Ethiopia. Malar J. 2009;8:177. doi: 10.1186/1475-2875-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsegaye AT, Ayele A, Birhanu S. Prevalence and associated factors of malaria in children under the age of five years in Wogera district, northwest Ethiopia: a cross-sectional study. PLoS ONE. 2021;16:e0257944. doi: 10.1371/journal.pone.0257944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gontie GB, Wolde HF, Baraki AG. Prevalence and associated factors of malaria among pregnant women in Sherkole district, Benishangul Gumuz regional state West Ethiopia. BMC Infect Dis. 2020;20:573. doi: 10.1186/s12879-020-05289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. World Malaria Report. World Health Organization. 2016. https://www.mmv.org/newsroom/publications/world-malaria-report. Accessed 18 Sept 2021.
- 12.Yewhalaw D, Kweka EJ. Insecticide resistance in East Africa—history, distribution and drawbacks on malaria vectors and disease control. In: Trdan S, editor. Insecticides resistance. London: IntechOpen; 2016. [Google Scholar]
- 13.Abate A, Hadis M. Susceptibility of Anopheles gambiae s.l. to DDT, malathion, permethrin and deltamethrin in Ethiopia. Trop Med Int Health. 2011;16:486–491. doi: 10.1111/j.1365-3156.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 14.Massebo F, Lindtjørn B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: a randomized trial. Malar J. 2013;12:319. doi: 10.1186/1475-2875-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigussie G. A review on traditionally used medicinal plants for scabies therapy in Ethiopia. Adv Tradit Med. 2021;21:199–208. doi: 10.1007/s13596-020-00453-7. [DOI] [Google Scholar]
- 16.WHO . Traditional medicine strategy. Geneva: World Health Organization; 2014. [Google Scholar]
- 17.Deharo E, Bourdy G, Quenevo C, Munoz V, Ruiz G, Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the anti-malarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77:91–98. doi: 10.1016/S0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 18.Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka district, east Welega zone of oromia regional state West Ethiopia. J Ethnobiol Ethnomed. 2013;9:68. doi: 10.1186/1746-4269-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nigussie G, Alemu M, Ibrahim F, Werede Y, Tegegn M, Neway S, et al. Phytochemistry, ethnomedicinal uses and pharmacological properties of Rhamnus prinoides: a review. Int J Second. 2021;8:136–151. [Google Scholar]
- 20.Umair M, Altaf M, Bussmann RW, Abbasi AM. Ethnomedicinal uses of the local flora in Chenab riverine area Punjab province Pakistan. J Ethnobiol Ethnomed. 2019;15:7. doi: 10.1186/s13002-019-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araya S, Abera B, Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray Ethiopia. J Ethnobiol Ethnomed. 2015;11:22. doi: 10.1186/s13002-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kebebew M. Diversity, knowledge and use of medicinal plants in Abay Chomen district, Horo Guduru Wollega zone, Oromia region of Ethiopia. J Med Plant Res. 2017;11:480–500. doi: 10.5897/JMPR2016.6274. [DOI] [Google Scholar]
- 23.Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County Kenya. J Ethnopharmacol. 2016;194:98–107. doi: 10.1016/j.jep.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olorunnisola O, Adetutu A, Balogun E, Afolayan A. Ethnobotanical survey of medicinal plants used in the treatment of malarial in Ogbomoso Southwest Nigeria. J Ethnopharmacol. 2013;150:71–78. doi: 10.1016/j.jep.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Woldeamanuel MM. Knowledge and use of medicinal traditional plant species ailments in Haramaya Ethiopia. J Environ Chem. 2019;3:18–23. [Google Scholar]
- 26.Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11:4. doi: 10.1186/1746-4269-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10:4. doi: 10.1186/1746-4269-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesfin F, Demissew S, Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR Ethiopia. J Ethnobiol Ethnomed. 2009;5:28. doi: 10.1186/1746-4269-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara Northwestern Ethiopia. BMC Res Notes. 2017;10:157. doi: 10.1186/s13104-017-2482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9:65. doi: 10.1186/1746-4269-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda Central Ethiopia. Afr J Plant Sci. 2014;8:366–379. doi: 10.5897/AJPS2013.1041. [DOI] [Google Scholar]
- 32.Regassa R, Bekele T, Megersa M. Ethnobotanical study of traditional medicinal plants used to treat human ailments by Halaba people, southern Ethiopia. J Med Plants Stud. 2017;5:36–47. [Google Scholar]
- 33.Mesfin A, Giday M, Animut A, Teklehaymanot T. Ethnobotanical study of anti-malarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J Ethnopharmacol. 2012;139:221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Kebebew M, Mohamed E. Indigenous knowledge on use of medicinal plants by indigenous people of Lemo district, Hadiya zone Southern Ethiopia. Int J Herb Med. 2017;5:124–135. [Google Scholar]
- 35.Mesfin F, Seta T, Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda Ethiopia. Ethnobot Res Appl. 2014;12:341–354. doi: 10.17348/era.12.0.341-354. [DOI] [Google Scholar]
- 36.Amsalu N, Bezie Y, Fentahun M, Alemayehu A, Amsalu G. Use and conservation of medicinal plants by indigenous people of Gozamin Wereda, East Gojjam Zone of Amhara region, Ethiopia: an ethnobotanical approach. Evid Based Complement Alternat Med. 2018;2018:1–23. doi: 10.1155/2018/2973513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragaw TJ, Afework DT, Getahun KA. Assessment of knowledge, attitude, and utilization of traditional medicine among the communities of Debre Tabor Town, Amhara Regional State, North Central Ethiopia: a cross-sectional study. Evid Based Complement Alternat Med. 2020;2020:6565131. doi: 10.1155/2020/6565131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia) Ethiopia. J Herbs Spices Med Plants. 2012;18:34–57. doi: 10.1080/10496475.2011.645188. [DOI] [Google Scholar]
- 39.Birhanu T, Abera D, Ejeta E, Nekemte E. Ethnobotanical study of medicinal plants in selected Horro Gudurru Woredas Western Ethiopia. J Biol Agricult Healthcare. 2015;5:83–93. [Google Scholar]
- 40.Gidey M, Beyene T, Signorini MA, Bruschi P, Yirga G. Traditional medicinal plants used by Kunama ethnic group in Northern Ethiopia. J Med Plant Res. 2015;9:494–509. doi: 10.5897/JMPR2014.5681. [DOI] [Google Scholar]
- 41.Gebre T, Chinthapalli B. Ethnobotanical study of the traditional use and maintenance of medicinal plants by the people of Aleta-Chuko Woreda South Ethiopia. Pharmacogn J. 2021;13:1097–1108. doi: 10.5530/pj.2021.13.142. [DOI] [Google Scholar]
- 42.Flatie T, Gedif T, Asres K, Gebre-Mariam T. Ethnomedical survey of Berta ethnic group Assosa Zone, Benishangul-Gumuz regional state, mid-west Ethiopia. J Ethnobiol Ethnomed. 2009;5:14. doi: 10.1186/1746-4269-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birhanu Z. Traditional use of medicinal plants by the ethnic groups of Gondar Zuria District North-Western Ethiopia. J Nat Remedies. 2013;13:46–53. [Google Scholar]
- 44.Tefera BN, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15:25. doi: 10.1186/s13002-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Getnet Z, Chandrodyam S, Masresha G. Studies on traditional medicinal plants in ambagiorgis area of Wogera District, Amhara Regional State Ethiopia. Int J Pure Appl Biosci. 2016;4:38–45. doi: 10.18782/2320-7051.2240. [DOI] [Google Scholar]
- 46.Tolossa K, Debela E, Athanasiadou S, Tolera A, Ganga G, Houdijk JG. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo Southern Ethiopia. J Ethnobiol Ethnomed. 2013;9:32. doi: 10.1186/1746-4269-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petres W. Drug resistance in Plasmodium berghei Vincke and Lips, 1948 I. Chloroquine resistance. Exp Parasitol. 1965;17:80–89. doi: 10.1016/0014-4894(65)90012-3. [DOI] [PubMed] [Google Scholar]
- 48.Ryley J, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 49.Oda BK, Erena BA. Aloes of Ethiopia: a review on uses and importance of Aloes in Ethiopia. Int J Plant Biol Res. 2017;5:1059. [Google Scholar]
- 50.Bobasa EM, Alemu BG, Berkessa ST, Gemechu MY, Fufa FG, Cari GZ, et al. Anti-malarial activity of selected Ethiopian medicinal plants in mice. J Pharm Pharmacogn Res. 2018;6:57–64. [Google Scholar]
- 51.Kifle ZD, Atnafie SA. Anti-oxidant potential and anti-malarial effects of Acanthus polystachyus Delile (Acanthaceae) against Plasmodium berghei: evidence for in vivo antimalarial activity. J Exp Pharmacol. 2020;12:575. doi: 10.2147/JEP.S282407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gemechu W, Bisrat D, Asres K. Anti-malarial anthrone and chromone from the leaf latex of Aloe Debrana chrstian. Ethiop Pharm J. 2014;30:1–9. doi: 10.4314/epj.v30i1.1. [DOI] [Google Scholar]
- 53.Deressa T, Mekonnen Y, Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24:25–29. [Google Scholar]
- 54.Mulaw T, Wubetu M, Dessie B, Demeke G, Molla Y. Evaluation of antimalarial activity of the 80% methanolic stem bark extract of combretum molle against Plasmodium berghei in mice. J Evid Based Integr Med. 2019 doi: 10.1177/2515690X19890866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anato M, Ketema T. Anti-plasmodial activities of Combretum molle (Combretaceae)[Zwoo] seed extract in Swiss albino mice. BMC Res Notes. 2018;11:312. doi: 10.1186/s13104-018-3424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo anti-malarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014 doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mekonnen LB. In vivo anti-malarial activity of the crude root and fruit extracts of Croton macrostachyus (Euphorbiaceae) against Plasmodium berghei in mice. J Tradit Complement Med. 2015;5:168–173. doi: 10.1016/j.jtcme.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebretsadik G, Mekonnen Y. In vivo antiplasmodial activity of fractions and chromatographic sub fractionions of ethanol extract of Clerodendrum myricoides leaves. J Drug Design Med Chem. 2016;2:60–64. doi: 10.11648/j.jddmc.20160206.11. [DOI] [Google Scholar]
- 59.Amelo W, Nagpal P, Makonnen E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice. BMC Complement Altern Med. 2014;14:462. doi: 10.1186/1472-6882-14-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melaku Y, Worku T, Tadesse Y, Mekonnen Y, Schmidt J, Arnold N, et al. Antiplasmodial compounds from leaves of Dodonaea angustifolia. Curr Bioact Compd. 2017;13:268–273. doi: 10.2174/1573407213666170403121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nardos A, Makonnen E. In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar J. 2017;16:25. doi: 10.1186/s12936-017-1677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willcox M. Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Med. 2011;77:662–671. doi: 10.1055/s-0030-1250548. [DOI] [PubMed] [Google Scholar]
- 63.Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Derebe D, Wubetu M. Anti-malarial activity of hydroalcoholic root extract of Acanthus polystachyus Delile (Acanthaceae) against Plasmodium berghei–infected mice. J Evid Based Integr Med. 2019 doi: 10.1177/2515690X19885322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dibessa TT, Engidawork E, Nedi T, Teklehaymanot T. Anti-malarial activity of the aqueous extract of the latex of Aloe pirottae Berger. (Aloaceae) against Plasmodium berghei in mice. J Ethnopharmacol. 2020;255:112763. doi: 10.1016/j.jep.2020.112763. [DOI] [PubMed] [Google Scholar]
- 66.Girma B, Bisrat D, Asres K. Anti-malarial evaluation of the leaf latex of Aloe citrina and its major constituent. Anc Sci Life. 2015;34:142. doi: 10.4103/0257-7941.157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teka T, Awgichew T, Kassahun H. Anti-malarial activity of the leaf latex of Aloe weloensis (Aloaceae) against Plasmodium berghei in mice. J Trop Med. 2020;2020:1397043. doi: 10.1155/2020/1397043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geremedhin G, Bisrat D, Asres K. Isolation, characterization and in vivo anti-malarial evaluation of anthrones from the leaf latex of Aloe percrassa Todaro. J Nat Remedies. 2014;14:119–125. [Google Scholar]
- 69.Hintsa G, Sibhat GG, Karim A. Evaluation of antimalarial activity of the leaf latex and TLC isolates from Aloe megalacantha Baker in Plasmodium berghei infected mice. Evid Based Complement Altern Med. 2019;2019:6459498. doi: 10.1155/2019/6459498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Habte G, Nedi T, Assefa S. Anti-malarial activity of aqueous and 80% methanol crude seed extracts and solvent fractions of Schinus molle Linnaeus (Anacardiaceae) in Plasmodium berghei-infected mice. J Trop Med. 2020;2020:9473250. doi: 10.1155/2020/9473250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belay WY, Endale Gurmu A, Wubneh ZB. Anti-malarial activity of stem bark of Periploca linearifolia during early and established Plasmodium infection in mice. Evid Based Complement Altern Med. 2018;2018:4169397. doi: 10.1155/2018/4169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paulos B, Bisrat D, Gedif T, Asres K. Anti-malarial and antioxidant activities of the leaf exudates and a naphthalene derivative from Aloe otallensis Baker. Ethiop Pharm J. 2011;29:100–107. [Google Scholar]
- 73.Tewabe Y, Assefa S. Anti-malarial potential of the leaf exudate of Aloe macrocarpa todaro and its major constituents against Plasmodium berghei. Clin Exp Pharmacol. 2018;8:1. doi: 10.4172/2161-1459.1000245. [DOI] [Google Scholar]
- 74.Alebachew Y, Bisrat D, Tadesse S, Asres K. In vivo anti-malarial activity of the hydroalcoholic extract of rhizomes of Kniphofia foliosa and its constituents. Malar J. 2021;20:3. doi: 10.1186/s12936-020-03552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toma A, Deyno S, Fikru A, Eyado A, Beale A. In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia. Malar J. 2015;14:196. doi: 10.1186/s12936-015-0716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zemicheal G, Mekonnen Y. Antiplasmodial activity of Vernonia adoensis aqueous, methanol and chloroform leaf extracts against chloroquine sensitive strain of Plasmodium berghei in vivo in mice. BMC Res Notes. 2018;11:736. doi: 10.1186/s13104-018-3835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adugna M, Feyera T, Taddese W, Admasu P. In vivo anti-malarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Global J Pharmacol. 2014;8:460–468. [Google Scholar]
- 78.Asrade S, Mengesha Y, Moges G, Gelayee DA. In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against plasmodium berghei. J Exp Pharmacol. 2017 doi: 10.2147/JEP.S130491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muluye AB, Melese E, Adinew GM. Anti-malarial activity of 80% methanolic extract of Brassica nigra (L.) Koch. (Brassicaceae) seeds against plasmodium berghei infection in mice. BMC Complement Altern Med. 2015 doi: 10.1186/s12906-015-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeleke G, Kebebe D, Mulisa E, Gashe F. In vivo anti-malarial activity of the solvent fractions of fruit rind and root of Carica papaya Linn (Caricaceae) against Plasmodium berghei in mice. Parasitol Res. 2017;2017:3121050. doi: 10.1155/2017/3121050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biruk H, Sentayehu B, Alebachew Y, Tamiru W, Ejigu A, Assefa S. In vivo anti-malarial activity of 80% methanol and aqueous bark extracts of Terminalia brownii Fresen. (Combretaceae) against plasmodium berghei in mice. Biochem Res Int. 2020 doi: 10.1155/2020/9749410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tesfaye WH, Alamneh EA. In vivo anti-malarial activity of the crude extract and solvent fractions of the leaves of Zehenria scabra (Cucurbitaceae) against Plasmodium berghei in mice. J Med Plant Res. 2014;8:1230–1236. [Google Scholar]
- 83.Eyasu M, Shibeshi W, Giday M. In vivo anti-malarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in mice infected with chloroquine sensitive Plasmodium berghei. Int J Pharmacol. 2013;2:131–142. [Google Scholar]
- 84.Birru EM, Geta M, Gurmu AE. Antiplasmodial activity of Indigofera spicata root extract against Plasmodium berghei infection in mice. Malar J. 2017;16:198. doi: 10.1186/s12936-017-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fentahun S, Makonnen E, Awas T, Giday M. In vivo anti-malarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017;17:13. doi: 10.1186/s12906-016-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zemene M, Geta M, Huluka SA, Birru EM. Anti-malarial activity of the 80% methanol leaf extract and solvent fractions of Stephania abyssinica (Dill. & A. Rich.) Walp. against Plasmodium berghei infection in mice. Ethiop Pharm J. 2020;36:109–120. doi: 10.4314/epj.v36i2.4. [DOI] [Google Scholar]
- 87.Hailesilase GG, Rajeshwar Y, Hailu GS, Sibhat GG, Bitew H. In vivo anti-malarial evaluation of crude extract, solvent fractions, and TLC-isolated compounds from Olea europaea Linn subsp. Cuspidata (Oleaceae) Evid Based Complement Altern Med. 2020 doi: 10.1155/2020/6731485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belete TM, Orijino TA. In-vivo anti-plasmodial activity of Hagenia abyssinica [family: Rosaceae] in Plasmodium berghei infected in mice. Int J Pharmacogn. 2019;6:66–74. [Google Scholar]
- 89.Assefa S, Nedi T, Engidawork E, Nureye D. In vivo anti-malarial activity of the 80 Methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complement Altern Med. 2018 doi: 10.1155/2018/9217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alemu BK, Misganaw D. Anti-malarial activity of Fagaropsis angolensis (Rutaceae) Crude extracts and solvent fractions of its stem bark against Plasmodium berghei in mice. J Exp Pharmacol. 2020;12:683. doi: 10.2147/JEP.S289478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Habte G, Assefa S. In vivo anti-malarial activity of crude fruit extract of Capsicum frutescens var. Minima (Solanaceae) against Plasmodium berghei-infected mice. Biomed Res Int. 2020 doi: 10.1155/2020/1320952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gebrehiwot S, Shumbahri M, Eyado A, Yohannes T. Phytochemical screening and in vivo anti-malarial activity of two traditionally used medicinal plants of Afar region, Ethiopia, against Plasmodium berghei in Swiss Albino mice. J Parasitol Res. 2019;2019:4519298. doi: 10.1155/2019/4519298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batista R, De Jesus Silva A, De Oliveira A. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules. 2009;14:3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Endale A, Bisrat D, Animut A, Bucar F, Asres K. In vivo anti-malarial activity of a labdane diterpenoid from the leaves of Otostegia integrifolia benth. Phytother Res. 2013;27:1805–1809. doi: 10.1002/ptr.4948. [DOI] [PubMed] [Google Scholar]
- 95.Sinha S, Batovska DI, Medhi B, Radotra B, Bhalla A, Markova N, et al. In vitro anti-malarial efficacy of chalcones: cytotoxicity profile, mechanism of action and their effect on erythrocytes. Malar J. 2019;18:421. doi: 10.1186/s12936-019-3060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bezu K, Bisrat D, Asres K. In vivo anti-malarial evaluation of embelin and its semi-synthetic aromatic amine derivatives. Pharmacogn J. 2015;7:305–310. doi: 10.5530/pj.2015.5.10. [DOI] [Google Scholar]
- 97.Induli M, Gebru M, Abdissa N, Akala H, Wekesa I, Byamukama R, et al. Antiplasmodial quinones from the rhizomes of Kniphofia foliosa. Nat Prod Commun. 2013 doi: 10.1177/1934578X1300800920. [DOI] [PubMed] [Google Scholar]
- 98.Wube AA, Bucar F, Asres K, Gibbons S, Rattray L, Croft SL. Anti-malarial compounds from Kniphofia foliosa roots. Phytother Res. 2005;19:472–476. doi: 10.1002/ptr.1635. [DOI] [PubMed] [Google Scholar]
- 99.Abdissa D, Geleta G, Bacha K, Abdissa N. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE. 2017;12:e0173882. doi: 10.1371/journal.pone.0173882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teka T, Bisrat D, Yeshak MY, Asres K. Anti-malarial activity of the chemical constituents of the leaf latex of Aloe pulcherrima Gilbert and Sebsebe. Molecules. 2016;21:1415. doi: 10.3390/molecules21111415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nil.