Alveolar macrophages (AMs) are not normally thought to be key culprits in cancer development, particularly given their location and primary function, which is to ensure proper gas exchange by removing excessive surfactant, cellular debris and inhaled pathogens. However, in Nature, Casanova-Acebes et al.1 show that in the context of non-small cell lung carcinoma (NSCLC), tumor-associated AMs alter their transcriptional profile to support tumor progression in the initial phases of the tumorigenic process.
The tumor microenvironment consists of cancerous and non-cancerous cells that support tumor growth, angiogenesis, invasion and metastasis. A variety of immune cells in the tumor microenvironment, including multiple types of tumor-associated macrophages (TAMs), confer both protection and vulnerability. Some TAM subsets clear tumor cells, whereas others release mediators that support tumor growth and induce an immunosuppressive environment. Targeting protumorigenic cells is a priority in cancer prevention and treatment, but in most tumor types and locations, it is unclear which TAM subsets have a protumorigenic role and when they should be targeted. Identifying the relevant TAM subsets and their functional roles in specific tumors is thus critical to allow for new therapies that employ cell-specific targeting.
The importance of defining macrophage diversity in the tumor microenvironment has been exemplified by research on other immune cell types, such as dendritic cells (DCs) and T cells, as a division of labor exists between various subsets. Similar to macrophages, T cells have either pro- or antitumorigenic properties, such as regulatory T (Treg) cells and cytotoxic T cells. It is possible that the pro- or antitumorigenic role of each subtype of macrophages in the tumor microenvironment may vary across different types of cancer and may need to be identified in the context of each particular cancer, tissue and organ.
There are two types of TAMs in the tumor microenvironment, tissue-resident and recruited macrophages. Recruited macrophages originate from circulating monocytes that infiltrate the tumor microenvironment as part of the body’s attempt to fight the tumor. Tissue-resident macrophages (TRMs) are mononuclear phagocytes with critical roles in tissue homeostasis and host defense. In the healthy lung, TRMs can be subdivided into tissue-specific AMs and interstitial macrophages (IMs)2-5, which differ in function, location, phenotype, transcriptional profile and origin. Embryonically-derived AMs self-maintain throughout life, while IMs are slowly replenished by circulating monocytes. While recruited macrophages are widely considered to be tumor suppressor cells, the role of AMs and IMs in the tumor environment has remained unclear.
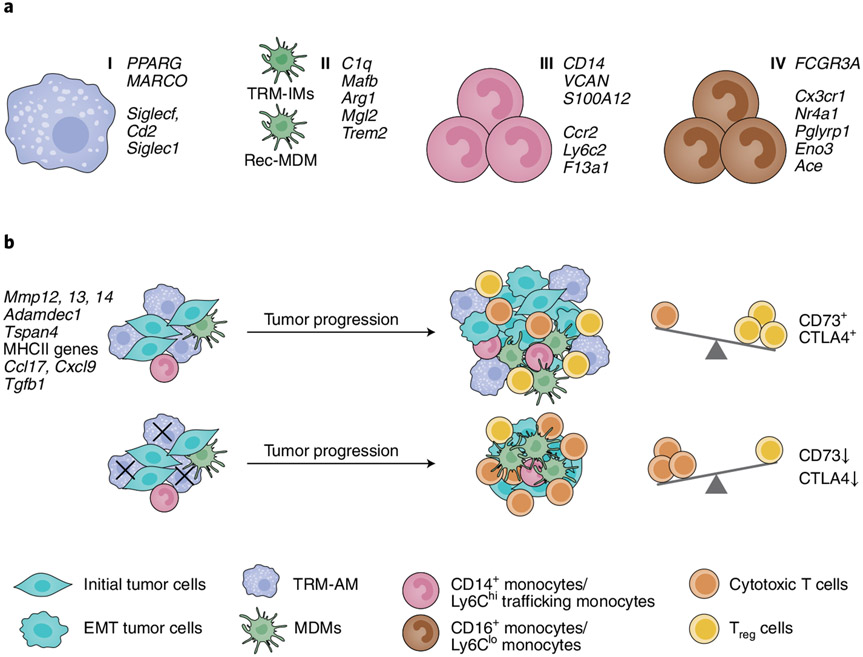
Using a series of converging experiments, Casanova-Acebes et al.1 show that AMs in the tumor alter their transcriptional profile to support early tumor progression. Single-cell RNA sequencing (scRNA-seq) in human NSCLC and mouse lung adenocarcinoma identified four major macrophage and monocyte clusters and aligned them across species. These clusters were designated as TRMs (group 1), monocyte-derived macrophages (MDMs, group 2), CD14+ monocytes (group 3) and CD16+ monocytes (group 4; Fig. 1a). Group 1 (TRMs) was identified as AMs expressing well-defined signature genes, such as PPARG, MARCO and FABP4. Group 2 (MDMs) included all of the other TAMs and represented a mix of IMs and recruited macrophages. Analysis of the scRNA-seq and bulk RNA-seq data in mice suggested that AMs in the early tumor microenvironment could be protumorigenic. Among the 1,322 genes that were differentially expressed between AMs in the healthy lung and the early tumor environment, genes such as Mmp12, Mmp14 and Adamdec1, which encode metalloproteinases, were specifically expressed in the tumor-associated AMs and may influence early tumor epithelial cells to acquire mesenchymal and invasive properties (Fig. 1b). Indeed, in a 3D-spheroid system, AMs induced the epithelial–mesenchymal transition in mouse lung epithelial tumor cells, characterized phenotypically by invasive protrusions, while bone marrow–derived monocytes or tumor-associated MDMs did not. In addition, AMs in early tumor lesions had increased expression of MHCII, CCL17 and TGF-β, which supported their ability to chemoattract CCR4-expressing T cells and induced the differentiation of naive CD4+ T cells into Treg cells (Fig. 1b). In vitro, AMs promoted the proliferation of Treg cells and the expression of the immunoinhibitory molecules CD73 and CTLA-4.
Fig. 1 ∣. TAM diversity and AM function in lung tumors in human and mouse.
a, Single-cell RNA sequencing identified four distinct macrophage and monocyte clusters in non-involved (naive) and tumor-bearing lungs in humans with NSCLC and in an orthotopic mouse model of NSCLC (KrasG12D and p53-deficient (KP) lung epithelial cells). TRM-AMs, group I; MDMs, group II; CD14+ monocytes, group III; and CD16+ monocytes, group IV. rec-MDM, recruited MDM. b, Selective depletion of CD169+ AMs in the early stages of tumor progression (day 0–3) induces changes in the ratio of CD8+ T cells to Treg cells in the tumor environment. EMT, epithelial–mesenchymal transition.
To directly assess the contribution of AMs to the early tumor microenvironment in vivo, and on the basis of the scRNA-seq data indicating that AMs express significantly more CD169 as compared to MDMs, the authors used CD169 as a marker to selectively target and deplete AMs. Administration of diphtheria toxin intranasally at days 0 and 3 post-tumor cell injection in CD169-DTR mice preferentially depleted the AMs and resulted in an increase in the ratio of IFN-γ+TNF+CD8+ T cells to CD4+ Treg cells in the tumors and a decrease in tumor size (Fig. 1b). Interestingly, this outcome was observed only when AMs were depleted early during tumor progression (day 0–3). Depletion of AMs after the establishment of tumor lesions (days 12 and 15) did not enhance antitumor immunity. Thus, this study highlights a previously unrecognized and critical role for AMs during early tumor progression.
Selective targeting of TAM subtypes represents a viable therapeutic option, and there is still much to learn. When and how to target TAMs and which subsets to target will most likely depend on the type of cancer and the growth stage. The diversity of potential targets is highlighted by previous studies that showed benefits in targeting other TAMs. For example, in a spontaneously metastasizing breast cancer model, lung-resident IMs support tumor growth, while recruited MDMs promote tumor spreading and tumor clearance6. Lineage tracing and selective depletion of embryonic-derived resident macrophages in parabiotic mice showed that tissue-resident macrophages of embryonic origin facilitate pancreatic ductal adenocarcinoma progression as compared to recruited monocyte-derived macrophages7. The study by Casanova-Acebes et al.1 now identifies AMs as a primary cell type to target during early stages of NSCLC lesion development. Excitingly, recent studies have characterized the extensive diversity within the human AM population in both naive and inflammatory settings, outlining with great granularity the presence of at least ten distinct AM subtypes, some of them with pro- or anti-inflammatory properties8-10. Based on the importance of AMs for optimal lung function, pan-targeting of AMs may not be indicated. Instead, it may be more advantageous to identify and target the particular subset within the AM population that contributes most to tumor growth.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Casanova-Acebes M et al. Nature 10.1038/s41586-021-03651-8 (2021). [DOI] [Google Scholar]
- 2.Gibbings SL et al. Am. J. Respir. Cell Mol. Biol 57, 66–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schyns J et al. Nat. Commun 10, 3964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakarov S et al. Science 363, eaau0964 (2019).30872492 [Google Scholar]
- 5.Ural BB et al. Sci. Immunol 5, eaax8756 (2020).32220976 [Google Scholar]
- 6.Loyher P-L et al. J. Exp. Med 215,2536–2553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y et al. Immunity 47, 323–338.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyfman PA et al. Am. J. Respir. Crit. Care Med 199, 1517–1536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant RA et al. Nature 590, 635–641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mould KJ et al. Am. J. Respir. Crit. Care Med 203, 946–956 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]