Abstract
Background/Aim: Previous evaluation of the safety and clinical efficacy of re-irradiation for pelvic recurrence of rectal cancer after preoperative chemoradiotherapy (PCRT) and rectal surgery is insufficient. We evaluated the safety and efficacy of re-irradiation with carbon-ion radiotherapy (C-ion RT) for pelvic recurrence of rectal cancer after PCRT.
Patients and Methods: We reviewed the medical records of patients treated with C-ion RT between August 2011 and December 2021 and analyzed the data of seven consecutive patients. The probabilities of overall survival (OS), local control (LC), and progression-free survival (PFS) were calculated using the Kaplan-Meier method. Toxicities were classified using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Results: The median follow-up duration after C-ion RT initiation was 30.9 months. Five patients received 73.6 Gy [relative biological effectiveness (RBE)] in 16 fractions, and two patients received 57.6 Gy (RBE) in 12 fractions. All patients completed C-ion RT as scheduled. Two-year estimated OS, LC, and PFS rates after C-ion RT initiation were 100%, 83.3%, and 28.6%, respectively. No patients developed grade ≥3 acute toxicity. Regarding late toxicities, one patient who received Gore-Tex sheets as a spacer before C-ion RT developed grade 3 colon perforation, and then developed a grade 3 urinary tract disorder. One patient developed grade 2 peripheral neuropathy.
Conclusion: C-Ion RT showed favorable local efficacy with minimal toxicity. C-Ion RT might be an effective treatment option for pelvic recurrence of rectal cancer after PCRT even when re-irradiation of the pelvis is required.
Keywords: Rectal cancer, pelvic recurrence, carbon-ion radiotherapy, radiotherapy, re-irradiation
Preoperative chemoradiotherapy (PCRT) for rectal cancer is a feasible treatment option that achieves higher local control (LC) and anorectal preservation rates and omits prophylactic lateral lymph node dissection (1-3). However, up to 10% of patients develop pelvic recurrence after PCRT and rectal surgery (3-5), and chemotherapy alone is insufficient to achieve complete cure. Pelvic exenteration may be indicated, but such surgery is highly invasive and creates difficulties in postoperative management (6,7). Although X-ray radiotherapy (RT) is one of the treatment options, rectal cancer is known to be a radiation-resistant tumor, and dose constraints of the surrounding gastrointestinal (GI) tract might be exceeded because the pelvic GI tract was already irradiated during PCRT. Therefore, sufficient dose administration with conventional X-ray RT is difficult. Recently, clinical outcomes of re-irradiation with stereotactic body radiotherapy (SBRT) for postoperative pelvic recurrence of rectal cancer have been reported, and 2-year overall survival (OS) rates are 78.8-84.4% and LC rates are 68.2-69.0%, with a grade ≥3 toxicity rate of 3.3-10.7% (8,9). These clinical outcomes are unsatisfactory, even with advanced SBRT treatment.
Carbon-ion radiotherapy (C-ion RT) has physical and biological advantages compared with X-ray RT, in terms of higher dose localization properties and higher cell-killing effects. Higher dose localization properties enable higher dose administration to tumors whilst sparing normal tissues. Additionally, this might enable re-irradiation of pelvic recurrence of rectal tumors (10,11). With higher cell-killing effects, C-ion beams have high linear energy transfer, demonstrating superior ability to induce cell death in radioresistant and hypoxic cells compared with X-rays. Although rectal cancer involves large hypoxic components that exhibit radiation resistance (12), C-ion RT might overcome hypoxia given the biological nature (13). In the past decade, favorable clinical outcomes of C-ion RT for pelvic recurrence of rectal cancer have been reported, wherein 3-year LC and OS rates were 85-93% and 73-92%, respectively (14-16). However, these reports analyzed cohorts wherein patients did not receive other RT.
To date, reports of C-ion RT in patients with pelvic recurrence of rectal cancer who had previous irradiation to the pelvis are limited (17,18). Hence, we retrospectively analyzed the safety and efficacy of C-ion RT for unresectable pelvic recurrence of rectal cancer after PCRT and surgery.
Patients and Methods
Patients. In this retrospective study, we reviewed the medical records of patients with postoperative pelvic recurrence of rectal cancer treated by C-ion RT at Gunma University Heavy Ion Medical Center between August 2011 and December 2021. Overall, 84 patients received C-ion RT during the study period, and seven consecutive patients met the following criteria for enrollment: pelvic recurrence of rectal cancer without distant metastasis, confirmed by histology or diagnostic imaging, with radiographically measurable tumor, received PCRT and underwent curative resection for rectal cancer. In contrast, patients with direct bladder or GI tract invasion, intractable infection in the target area, or with another active malignancy were excluded. The clinical stage was determined based on the eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM staging system (19).
This study was reviewed and approved by the Gunma University Institutional Review Board (approval number: HS2019-130), and all patients signed an informed consent form before treatment initiation.
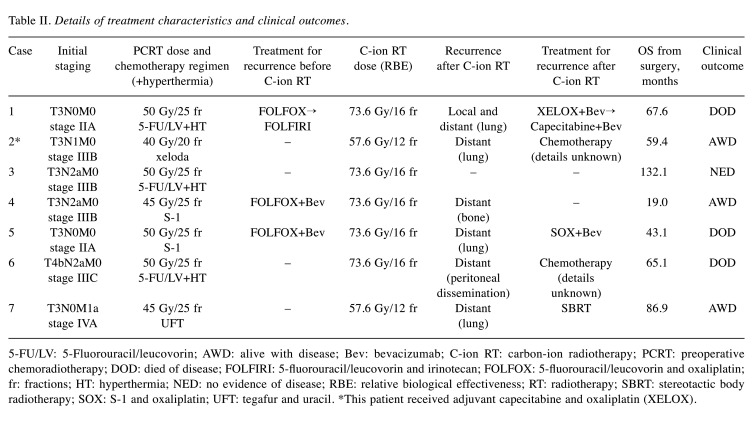
Carbon-ion radiotherapy. C-ion beams were generated by a synchrotron at Gunma University Heavy Ion Medical Center. The passive scattering technique was applied and beam energies of 290, 380, or 400 MeV/u were selected according to tumor depth. The dose-calculating system of XiO-N, which is an XiO (Elekta)-based software program incorporating a dose engine for ion-beam RT (K2dose), is used in our facility (20). The C-ion RT dose was expressed in Gy [relative biological effectiveness (RBE)], defined as the physical dose multiplied by the RBE of carbon ions (20). The preparation and target delineation of C-ion RT for postoperative pelvic recurrence of rectal cancer is similar, even for post-PCRT recurrence, and has been reported elsewhere (15). In the daily patient position matching, digital orthogonal radiographic images and digitally reconstructed computed tomography images for treatment planning were used. Patients received C-ion RT once daily, 4 days a week (Tuesday to Friday). Doses of C-ion RT were 73.6 Gy (RBE) administered in 16 fractions over 4 weeks for standard cases or 57.6 Gy (RBE) in 12 fractions over 3 weeks for close-to-GI tract cases which were not suitable for spacer placement surgery. Dose constraints were defined as the mean dose (Dmean) of <50 Gy (RBE) and maximum dose (Dmax) of <60 Gy (RBE) to the GI tract and the dose delivered to a 1-cm3 volume of the bladder (D1cc) <60 Gy (RBE). Dose distribution and diagnostic imaging in a representative case of pelvic recurrence of rectal cancer post-PCRT before and after C-ion RT are shown in Figure 1.
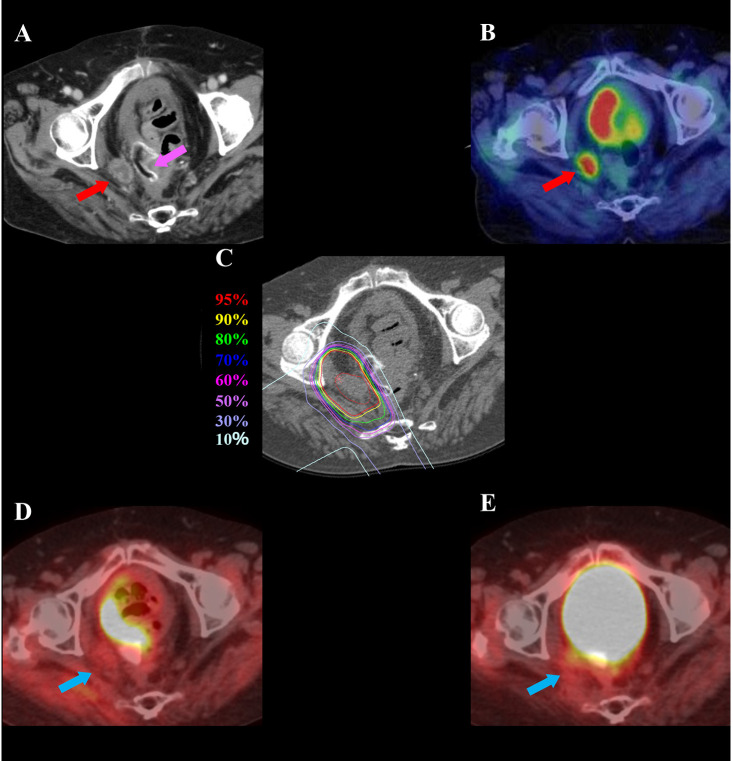
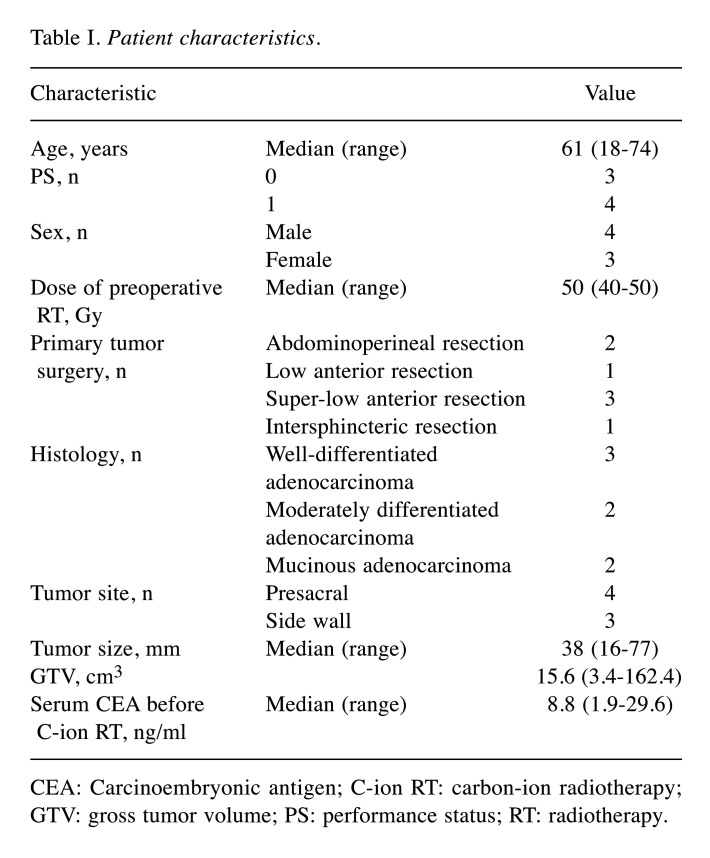
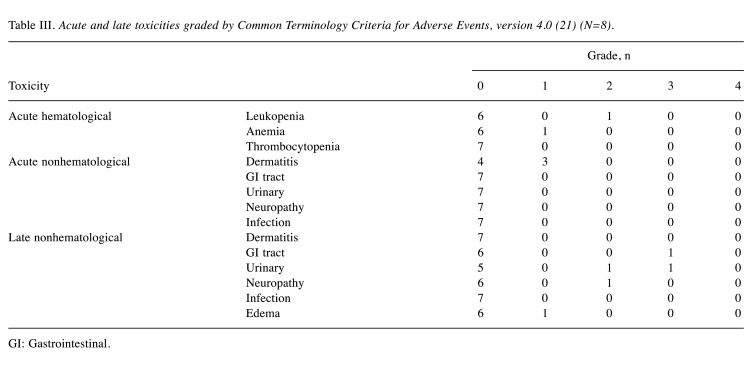
Figure 1. A 72-year-old female patient with pelvic recurrence of rectal cancer after preoperative chemoradiotherapy and rectal surgery treated with C-ion radiotherapy. A: Contrast-enhanced computed tomography image taken before treatment. The red arrow shows the tumor with contrast enhancement, and the magenta arrow shows the spacer. B: Fluorodeoxyglucose (FDG) positron-emission tomography (PET) taken before treatment. The red arrow shows the tumor with abnormal FDG uptake. C: Dose distribution on an axial computed tomography image. The area within the red outline is the gross tumor volume. Highlighted areas represent 95% (red), 90% (yellow), 80% (green), 70% (dark blue), 60% (magenta), 50% (purple), 30% (blue), and 10% (light blue) isodose curves; 100% was 73.6 Gy (relative biological effectiveness). D: FDG-PET taken 3 months after treatment. FDG uptake was reduced compared to before treatment (blue arrow). E: FDG-PET taken 21 months after treatment showing no FDG uptake (blue arrow). There was no evidence of local recurrence.
Evaluation during follow-up. Patients were observed on follow-up every 3 months after C-ion RT completion. Follow-up examinations comprised routine testing of blood cell counts and chemistry and diagnostic imaging using computed tomography, magnetic resonance imaging, or positron-emission tomography. LC was defined as no evidence of local progression with diagnostic imaging in the irradiated tumor bed, with or without continuous elevation of blood levels of tumor markers and progression-free survival (PFS) was defined as no progression of both locoregional and distant metastases. Acute and late toxicities were graded by the Common Terminology Criteria for Adverse Events, version 4.0, of the National Cancer Institute (21). Acute and late toxicities were evaluated as the highest grade of toxicity that occurred within 3 months and after 3 months, respectively, from C-ion RT initiation.
Statistical analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences software (version 25.0; IBM Inc., Armonk, NY, USA). OS was measured from the date of C-ion RT initiation or the date of rectal surgery to the date of death or most recent follow-up. LC was measured from the date of C-ion RT initiation to the date of observation of local progression or most recent follow-up. PFS was measured from the date of C-ion RT initiation to the date of observation of tumor progression, death from any cause, or most recent follow-up. The Kaplan-Meier method was used to calculate the probabilities of OS, LC, and PFS.
Results
Patients. Patient characteristics are summarized in Table I. Among seven patients, six developed pelvic recurrence within the previous radiation field and one developed pelvic recurrence at the edge of the previous radiation field. The median follow-up duration after C-ion RT initiation was 30.9 months (range=9.0-104.5 months) and that after the rectal surgery was 65.1 months (range=19.0-132.1 months). The median age at the time of registration for C-ion RT was 61 years (range=18-74 years) and the median tumor size was 38 mm in diameter (range=16-77 mm). The median time from rectal surgery to pelvic recurrence was 24.9 months (range=5.4-41.0 months) and the median time to C-ion RT initiation was 27.6 months (range=10.0-42.9 months). All patients were unsuitable for surgery. Before C-ion RT, two patients (cases 3 and 6 in Table II) received spacer placement surgery with Gore-Tex sheets (W. L. Gore and Associates, Newark, DE, USA). In terms of PCRT, all patients had received cytotoxic chemotherapy and 40-50 Gy of RT concurrently. Three patients had received hyperthermia five times during PCRT. All patients had undergone radical resection. In the postoperative adjuvant therapy, one patient had undergone cytotoxic chemotherapy and had recurrence during that period.
Table I. Patient characteristics.
CEA: Carcinoembryonic antigen; C-ion RT: carbon-ion radiotherapy; GTV: gross tumor volume; PS: performance status; RT: radiotherapy.
Table II. Details of treatment characteristics and clinical outcomes.
5-FU/LV: 5-Fluorouracil/leucovorin; AWD: alive with disease; Bev: bevacizumab; C-ion RT: carbon-ion radiotherapy; PCRT: preoperative chemoradiotherapy; DOD: died of disease; FOLFIRI: 5-fluorouracil/leucovorin and irinotecan; FOLFOX: 5-fluorouracil/leucovorin and oxaliplatin; fr: fractions; HT: hyperthermia; NED: no evidence of disease; RBE: relative biological effectiveness; RT: radiotherapy; SBRT: stereotactic body radiotherapy; SOX: S-1 and oxaliplatin; UFT: tegafur and uracil. *This patient received adjuvant capecitabine and oxaliplatin (XELOX).
Regarding the treatment for recurrence of rectal cancer before C-ion RT, three patients had received chemotherapy. Two had received bevacizumab combined with cytotoxic chemotherapy, and one had received cytotoxic chemotherapy alone.
Five patients received 73.6 Gy (RBE) in 16 fractions and two patients received 57.6 Gy (RBE) in 12 fractions. All patients completed C-ion RT as scheduled.
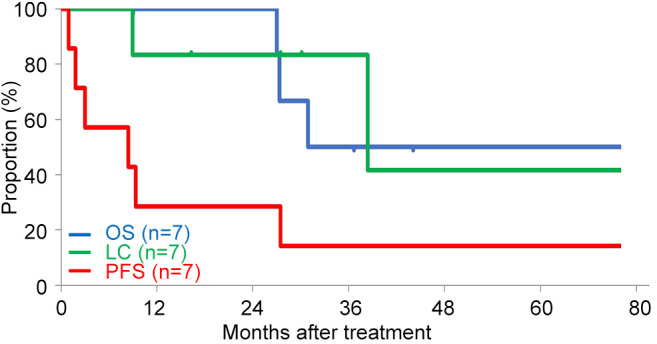
Survival and LC. The OS, LC, and PFS curves are shown in Figure 2. The 2-year estimated OS, LC, and PFS rates after C-ion RT initiation were 100%, 83.3%, and 28.6%, respectively. At the time of the analysis, recurrence after C-ion RT was observed in six patients. One patient had both local recurrence and distant metastases (lung), and the other five patients had distant metastases (three had lung metastases, one had bone metastases, and one had peritoneal dissemination). Three patients died of rectal cancer. Details of treatment characteristics and clinical outcomes are shown in Table II.
Figure 2. Kaplan-Meier curves for overall survival (blue line), local control (green line), and progression-free survival (red line). LC, Local control; OS, overall survival; PFS, progression-free survival.
Management of recurrences post-C-ion RT. Five out of six patients underwent salvage treatment for recurrence after C-ion RT. One patient with local recurrence and distant metastases received cytotoxic chemotherapy with bevacizumab. Three patients with distant metastases received cytotoxic chemotherapy with or without bevacizumab. The remaining patient with distant metastases of the lung alone received SBRT.
Toxicity. All the observed acute and late toxicities are listed in Table III. In terms of acute toxicity, none of the patients developed grade ≥3 toxicities. As for late toxicities, one patient who underwent spacer insertion before C-ion RT developed grade 3 colon perforation, and then developed grade 3 urinary tract disorders (case 3). This patient was a 61-year-old female with left side-wall recurrence of rectal cancer after Gore-Tex spacer insertion before C-ion RT. Although the pelvic tumor was controlled by C-ion RT, she developed colon perforation, which might have been caused by spacer-related infections 58 months after initiation of C-ion RT, necessitating surgical removal of the spacer, partial resection of the small intestine and colon, and colostomy. Additionally, she developed ureteral stenosis and received ureteral stenting 65 months after C-ion RT initiation. One patient developed grade 2 peripheral neuropathy.
Table III. Acute and late toxicities graded by Common Terminology Criteria for Adverse Events, version 4.0 (21) (N=8).
GI: Gastrointestinal.
Discussion
We show favorable clinical outcomes of C-ion RT for unresectable pelvic recurrence of rectal cancer after PCRT and rectal surgery, suggesting that C-ion RT is a highly safe and effective treatment, even when re-irradiation is needed. Additionally, our study suggests that C-ion RT can provide a high-dose administration to the recurrent tumor even in cases of re-irradiation, and thus favorable LC can be expected.
In the previous reports of SBRT as re-irradiation, the 2-year LC rate was 68.2-69.0% and 0-3% of patients developed grade 3 toxicities (8,9). In the current study, the 2-year LC rate for C-ion RT was 83.3% and one patient (14.3%) developed grade 3 toxicity. These results suggest C-ion RT in LC would be better than SBRT and the toxicity rate of C-ion RT compared to SBRT might be acceptable. In terms of OS, previous reports of SBRT showed 2-year OS rates of 78.8-84.4% (8,9) and the current study showed a 2-year OS rate of 100%, suggesting equal or better results in the current study. These results suggest that the higher LC rate due to high-dose administration with the physical and biological advantages of C-ion RT may contribute to improved OS. However, comparison of the OS for patients with recurrent tumor might be difficult owing to differences in patient backgrounds (such as whether the patient is in a general condition that allows them to receive chemotherapy) or how many chemotherapy regimens are left to be received after RT.
In a meta-analysis of re-irradiation for postoperative recurrence of rectal cancer, 2-year OS and LC rates were 71.8% and 63.8%, respectively in the RT with surgery group, and 34.2% and 54.8%, respectively in the RT alone group, with 25.5% with grade 3 or higher late toxicities (22). C-Ion RT in the current study led to favorable OS, LC, and toxicities compared to RT with/without surgery. These results suggest that C-ion RT might be a definitive local treatment option for patients with pelvic recurrence of rectal cancer who received prior pelvic RT.
Three-year LC rates in C-ion RT for pelvic recurrence of rectal cancer as an initial irradiation were 85-93%, with 2-7% with grade 3 toxicities, and 3-year LC rates in C-ion RT for re-irradiation were 52-90%, with 0-21% with grade 3 toxicities (14-18). In the current study, the 3-year LC rate was 83.3% (Figure 2). We consider that our LC results are comparable to those in the previous reports of C-ion RT as initial irradiation. This is because C-ion RT has high-dose localization properties, which enable higher dose administration to the tumor while sparing the surrounding normal tissue (e.g., GI tract) which was previously irradiated, and the same radical dose of C-ion RT can be administered in re-irradiation as was administered in the initial irradiation. Shinoto et al. (16) and Yamada et al. (18) reported that 3.1% and 16.9% of patients developed grade 3 late pelvic infection in the initial irradiation and re-irradiation of C-ion RT, respectively, and the current study showed that one patient (14.3%) developed a grade 3 colon perforation, which might have been caused by spacer-related pelvic infection. These results suggest that pelvic infections after C-ion RT can occur, even after initial treatment, and may occur with a high frequency in cases of re-irradiation. Therefore, more caution should be exercised regarding pelvic infection in re-irradiation cases than those in initial irradiation cases, especially in cases with placement of Gore-Tex sheet spacer.
Higher dose administration may improve LC. However, if the tumor is located close to the GI tract or other organ at risk (OAR), especially in re-irradiation cases, higher dose administration cannot be performed because of the high risk of severe toxicities. In these cases, a spacer that physically separates the tumor from the GI tract or other OARs may be useful (23,24). Using Gore-Tex sheets is one option in spacer placement but, recently, another option has emerged, use of a bioabsorbable polyglycolic acid (PGA) spacer which was developed in Japan (25,26). Using a Gore-Tex sheet as a spacer enables higher dose administration of C-ion RT for tumor control, even in cases of re-irradiation. However, there are risks of infection and adverse effects on the GI tract due to long-term spacer placement after C-ion RT. In the current study, spacer-related infection was observed, resulting in severe GI and urinary tract toxicities. In contrast, using a PGA spacer might be safer than Gore-Tex sheets because the PGA spacer is absorbed after C-ion RT, and the risk of infection is reduced. Therefore, spacer placement is considered an additional option in administering higher dose and improving LC in C-ion RT for patients with a tumor located close to the GI tract or other OARs, especially in re-irradiation cases where the GI tract has already been irradiated.
In pelvic re-irradiation with C-ion RT, Shiba et al. reported favorable clinical outcomes with LC and safety for lymph node recurrence of gynecological cancer (11). Additionally, in abdominal re-irradiation, Okamoto et al. reported re-irradiation with C-ion RT for local recurrence after C-ion RT of pancreatic cancer and demonstrated favorable LC and OS with tolerable toxicities (27). In proton beam therapy (PBT), a type of particle therapy that has high-dose localization properties, Berman et al. (28) reported that PBT for pelvic recurrence of rectal cancer produces a better dose distribution, particularly to the bowel, than that in intensity-modulated RT (IMRT). Furthermore, previous reports in physics showed that C-ion beams have sharper penumbra than the proton beams. Therefore, irradiation doses to the surrounding normal organs, such as the GI tract, can be further reduced (10,29). Thus, compared to X-ray RT including IMRT, PBT and C-ion RT, C-ion RT has higher dose localization properties than IMRT and PBT, and C-ion RT can deliver higher doses to tumors while sparing normal tissue, even in re-irradiation cases, and favorable LC can be expected. In contrast, there are no reports of direct comparison in the dose distribution and local effects of C-ion RT and PBT on pelvic recurrence of rectal cancer. Further studies should be conducted to determine the optimal treatment modality for each patient’s condition.
Our study had some limitations. Firstly, this was a single-institutional retrospective study with a small number of patients. Secondly, although this study showed favorable clinical outcomes, the evaluation of OS was difficult because patients had various backgrounds, such as initial staging before PCRT, the number of chemotherapy regimens received before C-ion RT initiation, and how many chemotherapy regimens were still to be received after C-ion RT. Therefore, further analysis of C-ion RT in a large number of patients with unresectable pelvic recurrence of rectal cancer after preoperative CRT is warranted.
In conclusion, C-ion RT for patients with unresectable pelvic recurrence of rectal cancer after PCRT showed favorable local efficacy with a minimal toxicity rate, although spacer-related grade 3 toxicity was observed in one patient. These results suggest that C-ion RT has potential as an effective treatment option for such patients, even when re-irradiation of the pelvic site is required.
Conflicts of Interest
Tatsuya Ohno received research funding from Hitachi. All other Authors declare they have no conflicts of interest.
Authors’ Contributions
Study conception and design was performed by S.S. and M.O. Data collection was performed by S.S. Analysis and Interpretation of results was performed by S.S. Statistical analyses were performed by S.S. All of the Authors contributed to the drafting and preparation of this article. All of the Authors reviewed the results and approve of the final version.
Acknowledgements
The Authors would like to thank all the patients who were involved in this study, our colleagues at Gunma University Heavy Ion Medical Center and Department of Radiation Oncology Gunma University Graduate School of Medicine, and Editage (www.editage.com) for English language editing.
References
- 1.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 2.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921 Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 3.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;(2):CD006041. doi: 10.1002/14651858.CD006041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 5.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 6.Steffens D, Solomon MJ, Young JM, Koh C, Venchiarutti RL, Lee P, Austin K. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open. 2018;2(5):328–335. doi: 10.1002/bjs5.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt E, Dovell G, Smolarek S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech Coloproctol. 2018;22(11):835–845. doi: 10.1007/s10151-018-1883-1. [DOI] [PubMed] [Google Scholar]
- 8.Smith T, O’Cathail SM, Silverman S, Robinson M, Tsang Y, Harrison M, Hawkins MA. Stereotactic body radiation therapy reirradiation for locally recurrent rectal cancer: outcomes and toxicity. Adv Radiat Oncol. 2020;5(6):1311–1319. doi: 10.1016/j.adro.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defoe SG, Bernard ME, Rwigema JC, Heron DE, Ozhasoglu C, Burton S. Stereotactic body radiotherapy for the treatment of presacral recurrences from rectal cancers. J Cancer Res Ther. 2011;7(4):408–411. doi: 10.4103/0973-1482.92000. [DOI] [PubMed] [Google Scholar]
- 10.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, Furusawa Y, Ando K, Suzuki M, Soga F, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44(1):201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 11.Shiba S, Okonogi N, Kato S, Wakatsuki M, Kobayashi D, Kiyohara H, Ohno T, Karasawa K, Nakano T, Kamada T. Clinical impact of re-irradiation with carbon-ion radiotherapy for lymph node recurrence of gynecological cancers. Anticancer Res. 2017;37(10):5577–5583. doi: 10.21873/anticanres.11991. [DOI] [PubMed] [Google Scholar]
- 12.Höckel M, Schlenger K, Höckel S, Aral B, Schäffer U, Vaupel P. Tumor hypoxia in pelvic recurrences of cervical cancer. Int J Cancer. 1998;79(4):365–369. doi: 10.1002/(sici)1097-0215(19980821)79:4<365::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki M, Morita S, Sato S, Oka K, Tsujii H. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res. 2006;12(7 Pt 1):2185–2190. doi: 10.1158/1078-0432.CCR-05-1907. [DOI] [PubMed] [Google Scholar]
- 14.Yamada S, Kamada T, Ebner DK, Shinoto M, Terashima K, Isozaki Y, Yasuda S, Makishima H, Tsuji H, Tsujii H, Isozaki T, Endo S, Takahashi K, Sekimoto M, Saito N, Matsubara H, Working Group on Locally Recurrent Rectal Cancer Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int J Radiat Oncol Biol Phys. 2016;96(1):93–101. doi: 10.1016/j.ijrobp.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Shiba S, Okamoto M, Kiyohara H, Ohno T, Kaminuma T, Asao T, Ojima H, Shirabe K, Kuwano H, Nakano T. Prospective observational study of high-dose carbon-ion radiotherapy for pelvic recurrence of rectal cancer (GUNMA 0801) Front Oncol. 2019;9:702. doi: 10.3389/fonc.2019.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinoto M, Yamada S, Okamoto M, Shioyama Y, Ohno T, Nakano T, Nemoto K, Isozaki Y, Kawashiro S, Tsuji H, Kamada T. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1404 Rectum. Radiother Oncol. 2019;132:236–240. doi: 10.1016/j.radonc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Barcellini A, Vitolo V, Cobianchi L, Peloso A, Vanoli A, Mirandola A, Facoetti A, Fiore MR, Iannalfi A, Vischioni B, Cuccia F, Ronchi S, Bonora M, Riva G, Petrucci R, D’Ippolito E, Mas FD, Preda L, Valvo F. Re-irradiation with carbon ion radiotherapy for pelvic rectal cancer recurrences in patients previously irradiated to the pelvis. In Vivo. 2020;34(3):1547–1553. doi: 10.21873/invivo.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Takiyama H, Isozaki Y, Shinoto M, Ebner DK, Koto M, Tsuji H, Miyauchi H, Sekimoto M, Ueno H, Itabashi M, Ikeda M, Matsubara H, Working Group on Locally Recurrent Rectal Cancer Carbon ion radiotherapy for locally recurrent rectal cancer of patients with prior pelvic irradiation. Ann Surg Oncol. 2022;29(1):99–106. doi: 10.1245/s10434-021-10876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobin LH, Gospodarowicz MK, Wittekind C, editors. Oxford, WileyBlackwell. 2017. International Union Against Cancer (UICC): TNM Classification of Malignant Tumours. Eighth Edition. [Google Scholar]
- 20.Kanematsu N. Dose calculation algorithm of fast fine-heterogeneity correction for heavy charged particle radiotherapy. Phys Med. 2011;27(2):97–102. doi: 10.1016/j.ejmp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Common terminology criteria for adverse events (CTCAE) version 4.0. Maryland, National Cancer Institute, 2010. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Last accessed on June 23, 2022]
- 22.Lee J, Kim CY, Koom WS, Rim CH. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: A meta-analysis and systematic review. Radiother Oncol. 2019;140:10–19. doi: 10.1016/j.radonc.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu S, Demizu Y, Sulaiman NS, Terashima K, Suga M, Kido M, Toyama H, Tokumaru S, Okimoto T, Sasaki R, Fukumoto T. Space-making particle therapy for sarcomas derived from the abdominopelvic region. Radiother Oncol. 2020;146:194–199. doi: 10.1016/j.radonc.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto T, Komatsu S, Hori Y, Murakami M, Hishikawa Y, Ku Y. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol. 2010;101(1):97–99. doi: 10.1002/jso.21417. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki R, Demizu Y, Yamashita T, Komatsu S, Akasaka H, Miyawaki D, Yoshida K, Wang T, Okimoto T, Fukumoto T. First-in-human Phase 1 study of a nonwoven fabric bioabsorbable spacer for particle therapy: Space-making particle therapy (SMPT) Adv Radiat Oncol. 2019;4(4):729–737. doi: 10.1016/j.adro.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiba S, Okamoto M, Tashiro M, Ogawa H, Osone K, Yanagawa T, Kohama I, Okazaki S, Miyasaka Y, Osu N, Chikuda H, Saeki H, Ohno T. Rectal dose-sparing effect with bioabsorbable spacer placement in carbon ion radiotherapy for sacral chordoma: dosimetric comparison of a simulation study. J Radiat Res. 2021;62(3):549–555. doi: 10.1093/jrr/rrab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto M, Shiba S, Okazaki S, Miyasaka Y, Shibuya K, Kiyohara H, Ohno T. Feasibility and safety of repeated carbon ion radiotherapy for locally advanced unresectable pancreatic cancer. Cancers (Basel) 2021;13(4):665. doi: 10.3390/cancers13040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman A, Both S, Sharkoski T, Goldrath K, Tochner Z, Apisarnthanarax S, Metz J, Plastaras J. Proton reirradiation of recurrent rectal cancer: Dosimetric comparison, toxicities, and preliminary outcomes. International Journal of Particle Therapy. 2017;1(1):2–13. doi: 10.14338/IJPT.13-00002.1. [DOI] [Google Scholar]
- 29.Winterhalter C, Lomax A, Oxley D, Weber DC, Safai S. A study of lateral fall-off (penumbra) optimisation for pencil beam scanning (PBS) proton therapy. Phys Med Biol. 2018;63(2):025022. doi: 10.1088/1361-6560/aaa2ad. [DOI] [PubMed] [Google Scholar]