Abstract
Background/Aim: Few studies have examined the correlation between pyruvate kinase M2 (PKM2) overexpression and triple-negative breast cancer (TNBC). TNBC is considered incurable with the currently available treatments, highlighting the need for alternative therapeutic targets.
Materials and Methods: PKM2 expression was examined immunohistochemically in human breast tumor samples. Furthermore, we studied the effect of three PKM2 inhibitors (gliotoxin, shikonin, and compound 3K) in the MDA-MB-231 TNBC cell line.
Results: PKM2 overexpression correlates with TNBC. Interestingly, most TNBC tissues showed increased levels of PKM2 compared to those of receptor-positive breast cancer tissues. This suggests that PKM2 overexpression is an important factor in the development of TNBC. MDA-MB-231 TNBC cells are resistant to anticancer drugs, such as vincristine (VIC) compared to other cancer cells. We found that the recently developed PKM2 inhibitor gliotoxin sensitized MDA-MB-231 cells at a relatively low dose to the same extent as the known PKM2 inhibitor shikonin, suggesting that PKM2 inhibitors could be an effective treatment for TNBC. Detailed sensitization mechanisms were also analyzed. Both gliotoxin and shikonin highly increased late apoptosis in MDA-MB-231 cells, as revealed by annexin V staining. However, MDA-MB-231 cells with high cellular density inhibited the sensitizing effect of PKM2 inhibitors; therefore, we investigated ways to overcome this inhibitory effect. We found that gliotoxin+shikonin co-treatment highly increased toxicity in MDA-MB-231 cells with high density, whereas either VIC+gliotoxin or VIC+shikonin were not effective. Thus, combination therapy with various PKM2 inhibitors may be more effective than combination therapy with anticancer drugs. Gliotoxin+shikonin co-treatment did not increase S or G2 arrest in cells, suggesting that the co-treatment showed a high increase in apoptosis without S or G2 arrest. We confirmed that another recently developed PKM2 inhibitor compound 3K had similar mechanisms of sensitizing MDA-MB-231 cells, suggesting that PKM2 inhibitors have similar sensitization mechanisms in TNBC.
Conclusion: PKM2 is a regulator of the oncogenic function of TNBC, and combination therapy with various PKM2 inhibitors may be effective for high-density TNBC. Targeting PKM2 in TNBC lays the foundation for the development of PKM2 inhibitors as promising anti-TNBC agents.
Keywords: PKM2, triple-negative breast cancer, gliotoxin, shikonin, compound 3K
Among the different molecular subtypes of breast cancer, triple-negative breast cancer (TNBC) is an extremely aggressive subtype; it is associated with poor prognosis and high mortality rates, despite systemic therapy (1,2). TNBC is also a heterogeneous disease compared to the luminal and HER2-enriched subtypes (1-3). Chemotherapy and combination chemotherapy are the conventional treatments for TNBC; however, resistant types of TNBC are prevalent (3-6). Although several clinical trials targeting molecules specific to TNBC, including poly (ADP-ribose) polymerase (PARP), epidermal growth factor receptor (EGFR), protein kinase B (AKT), phosphoinositide 3-kinase (PI3K), and Src tyrosine kinase, have been conducted, they have not led to substantial improvements in TNBC treatment (5,7-10). Therefore, it is important to identify effective therapeutic targets that may improve disease outcome and prognosis of patients with TNBC, and thus, improve our understanding of TNBC development, progression, and metastasis at the molecular level.
To meet the energy requirements for uncontrolled proliferation, tumor cells increase their glucose uptake and switch their energy source from mitochondrial oxidative phosphorylation to glycolysis, which is known as the Warburg effect (11,12). During glycolysis, each glucose molecule is eventually converted into two molecules each of pyruvate and ATP in the cytoplasm. Pyruvate kinase M2 (PKM2) is a key rate-limiting enzyme in glycolysis and contributes to the genesis and proliferation of breast cancer cells (11-13). The overexpression of PKM2 results in increased glucose uptake, lactate production, and autophagy inhibition, thereby accelerating oncogenic growth (13-17).
The induction of glycolytic dysfunction has emerged as a promising therapeutic strategy for several cancer types, and PKM2 has not been considered as a potential target molecule. Using different strategies that involve regulation of PKM2 expression, some studies have determined the tumorigenic role of PKM2 in breast cancer and the therapeutic potential of PKM2-targeting strategies (6,13-17). However, the therapeutic role of PKM2 in TNBC remains to be discovered. In this study, we identified a correlation between TNBC and PKM2 overexpression. Furthermore, we assessed the anticancer effects of PKM2 inhibitors (shikonin and compound 3K) on human MDA-MB-231 cells (18-21). In particular, we tested a newly identified PKM2 inhibitor gliotoxin (isolated from marine environments), which directly bound to PKM2 and inhibited its glycolytic activity in a U87MG glioma study (22). Previously, gliotoxins were demonstrated to have anticancer activity in various solid cancer models (23-26). Although gliotoxins isolated from soil showed anticancer activity in breast cancer cells (27), their mechanism of action has not been elucidated. In our study, we determined that combination treatment with PKM2 inhibitors (gliotoxin from marine sources, shikonin, or compound 3K) highly increased cytotoxicity in MDA-MB-231 cells. Our results may facilitate the development of PKM2 inhibitor-based therapies for TNBC.
Materials and Methods
Reagents and cell culture. Gliotoxin, compound 3K and shikonin were obtained from SelleckChem (Houston, TX, USA). Vincristine (VIC) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). The human TNBC cell line, MDA-MB-231, was obtained from Dr. Keun Wook Kang (Department of Pharmacy, Seoul University, Seoul, Republic of Korea) and has been previously used as a cell model (28). The human oral squamous carcinoma cell line KB has been previously used as cell models (29). All cell lines were cultured in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (WelGENE, Daegu, Republic of Korea).
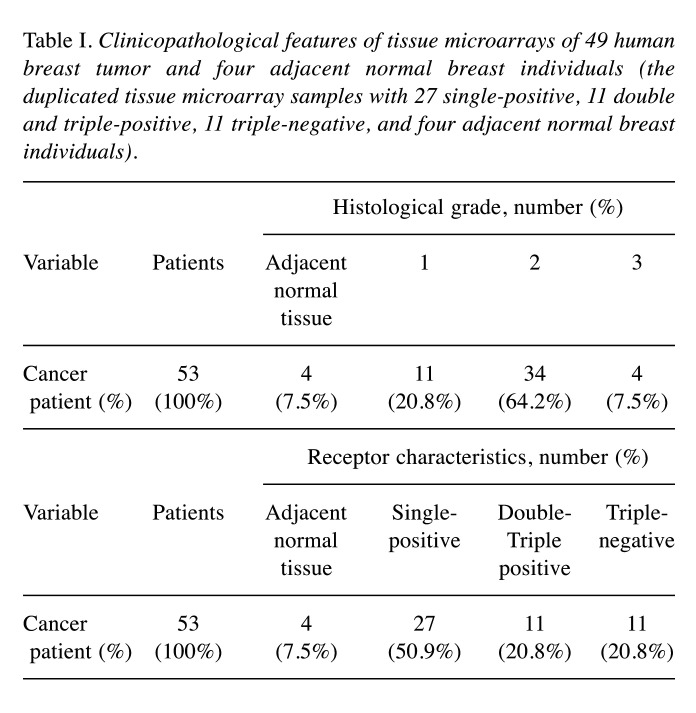
Breast tissue microarray and immunohistochemistry (IHC). The tissue microarray slides (duplicate cores per case) of 4 adjacent normal breasts and 49 breast tumors were obtained from US Biomax Inc. (Rockville, MD, USA). Detailed information on the tissue microarray samples is presented in Table I. Immunohistochemical analysis was performed to investigate PKM2 expression in adjacent normal and breast tumor tissue samples as previously described (18,19). The tissue microarray slides were treated with xylene and ethanol, followed by boiling in sodium citrate buffer for 20 min. Afterward, the slides were treated with 5% H2O2 for 15 min to inactivate endogenous peroxidases and incubated with the PKM2 antibody (1:1,000) at 4˚C for 24 h. The secondary anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) was added and allowed to react at room temperature for 30 min. Subsequently, HRP-streptavidin reagent (Vector Laboratories) was added and allowed to react at room temperature for 30 min. After staining with DAB (Dako, Agilent, Santa Clara, CA, USA) and hematoxylin (Dako), the slides were fixed on a cover glass with mounting solution following ethanol and xylene treatment. The slides were observed at 200× magnification using a confocal K1-Fluo microscope (Nanoscope Systems, Daejeon, Republic of Korea). For assessing immunoreactivity and histological appearance, all tissue microarray slides were examined and scored. The intensity of PKM2 immunostaining in individual cells (average of duplicates) was scored according to the following scale as previously described (18,19): 0, no staining; 1, weak intensity; 2, moderate intensity; and 3, strong intensity. The percentage of cells with positive PKM2 immunostaining at each intensity level was estimated (0 to 100%). The absolute value of the proportion of cells at each intensity level was multiplied by the corresponding intensity value, and the resulting values were added to obtain an immunostaining score ranging from 0 to 300.
Table I. Clinicopathological features of tissue microarrays of 49 human breast tumor and four adjacent normal breast individuals (the duplicated tissue microarray samples with 27 single-positive, 11 double and triple-positive, 11 triple-negative, and four adjacent normal breast individuals).
Microscopic observation. Microscopic observations were performed as previously described (18,30-33), in order to examine the effect of gliotoxin, shikonin, compound 3K, or VIC on cellular growth.
Briefly, MDA-MB-231 or KB cells were grown at low density (1.5×105 cells), and were treated with 5 nM VIC, 1-1.5 μM gliotoxin, 1-1.5 μM shikonin, or 10 μM compound 3K for 1 day. For co-treatment experiments, MDA-MB-231 cells with 50-60% confluence were treated with 5 nM VIC, 1-1.5 μM gliotoxin, 1-1.5 μM shikonin, 10 μM compound, 1-1.5 μM gliotoxin with 1-1.5 μM shikonin, 5 nM VIC with 1-1.5 μM gliotoxin, 5 nM VIC with 1-1.5 μM shikonin, 5 nM VIC with 10 μM compound 3K, 10 μM compound 3K with 1-1.5 μM gliotoxin, 10 μM compound 3K with 1-1.5 μM shikonin, or 0.1% DMSO for 1 day. All cells were observed using an inverted microscope at ×40 magnification.
The effect of these drugs on cell density (cellular confluence) were also examined. Briefly, MDA-MB-231 cells were grown on 60 mm-diameter dishes either at low density (1.5×105 cells) or high density (4×105 cells). After one day, they were treated with a single drug or co-treatments, as indicated above. Then the effect of these drugs on cell growth after one day was examined. Cells were observed using an inverted microscope at ×40 magnification. Results were qualitatively confirmed at least in two independent experiments using an ECLIPSETs2 inverted microscope (Nikon, Tokyo, Japan) with a 40 or 100× objective lens (Nikon Microscopy U).
Fluorescence-activated cell sorting (FACS) analysis. FACS analysis was performed as previously described (18,30-33). Briefly, MDA-MB-231 cells were grown in 60 mm diameter dishes with high density (4×105 cells), and were treated with 5 nM VIC, 1-1.5 μM gliotoxin, 1-1.5 μM shikonin, 10 μM compound, 1-1.5 μM gliotoxin with 1-1.5 μM shikonin, 5 nM VIC with 1-1.5 μM shikonin, 5 nM VIC with 1-1.5 μM gliotoxin, 5 nM VIC with 10 μM compound 3K, 10 μM compound 3K with 1-1.5 μM gliotoxin, 10 μM compound 3K with 1-1.5 μM shikonin, or 0.1% DMSO for 24 h. The detached by trypsin were washed, and suspended in 75% ethanol for 24 h at –20˚C. The cells were then incubated with propidium iodide (PI) staining solution for 30 min at 37˚C. The cell-cycle distribution of stained cells was qualitatively analyzed, and the results confirmed in at least two independent experiments using a Novocyte Flow Cytometer (ACEA Biosciences, San Diego, CA, USA).
Annexin V analysis. Analysis was conducted using an annexin V-fluorescein isothiocyanate (FITC) staining kit (BD Biosciences, Franklin, NJ, USA) as previously reported (18,30-33). Briefly, MDA-MB-231 cells were grown in 60 mm diameter dishes either with low density (1.5×105 cells) or high density (4×105 cells), and were treated with 5 nM VIC, 1-1.5 μM gliotoxin, 1-1.5 μM shikonin, 10 μM compound, 1-1.5 μM gliotoxin with 1-1.5 μM shikonin, 5 nM VIC with 1-1.5 μM shikonin, 5 nM VIC with 1-1.5 μM gliotoxin, 5 nM VIC with 10 μM compound 3K, 10 μM compound 3K with 1-1.5 μM gliotoxin, 10 μM compound 3K with 1-1.5 μM shikonin, or 0.1% DMSO for 24 h. The cells were then detached, pelleted, and washed with PBS. Annexin V-FITC and PI solution was added into the cells and incubated for 30 min at 20˚C. The stained cells were qualitatively analyzed, and the results confirmed in at least two independent experiments using a Novocyte Flow Cytometer (ACEA Biosciences, San Diego, CA, USA).
Cell viability assay. As previously described (18,30-33), cell proliferation was evaluated by colorimetric assays using the EZ-CyTox cell viability assay kit (Daeillab, Seoul, South Korea). Briefly, MDA-MB-231 cells were plated on 96-well plates and grown to either low density (10-20% confluence) or high density (30-40%). The cells were then treated for 48 h with 5 nM VIC, 1-2 μM gliotoxin, 1-2 μM shikonin, 1.5 μM gliotoxin with 1.5 μM shikonin, or 0.1% DMSO. They were then incubated with 10 ml EZ-CyTox solution for 1 h at 37˚C. The absorbance at 450 nm was measured using the VERSA MAX Microplate Reader (Molecular Devices Corp., Sunnyvale, CA, USA). All experiments were performed at least in triplicate and repeated twice. The quantitative analysis was performed in at least two independent experiments in triplicate.
Statistical analysis. Data are presented as the mean±S.D. from at least three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA), analysis of variance followed by Bonferroni’s test. Analysis was performed using GraphPad Prism software (version 5.0; GraphPad Software, CA, USA). Statistical significance was set at p<0.05.
Results
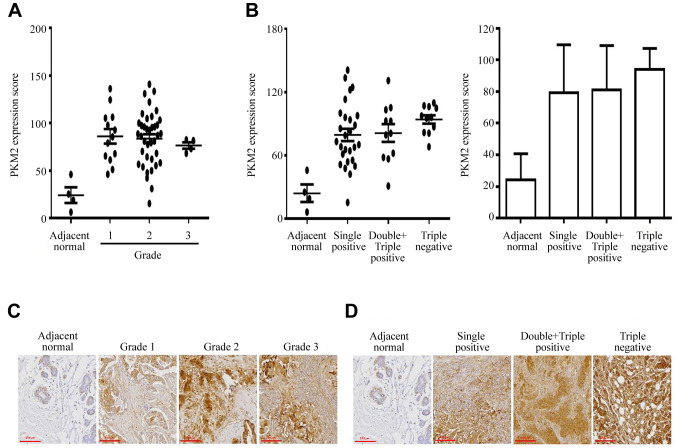
PKM2 overexpression is observed only in TNBC tissues. Herein, PKM2 expression was immunohistochemically examined in 49 human breast cancer samples and compared to that of four adjacent normal breast tissue samples obtained from patients. Each patient was analyzed in duplicate tissue samples. PKM2 expression was higher in most breast cancer tissues than in adjacent normal breast tissues (Figure 1A-D), indicating that the up-regulated expression of PKM2 was highly correlated with tumor progression in the breast. Most samples showed increased expression of PKM2 compared to that in the controls, but certain exceptions were observed. Although we did not identify a correlation between the extent of PKM2 overexpression and breast tumor grade (Figure 1A), we assumed that PKM2 overexpression was required at all stages of breast cancer development. This suggests that PKM2 overexpression is crucial for the development of breast cancer, and PKM2 could be a biomarker for breast cancer that can help distinguish it from other cancers. Although there were no significant differences between TNBC and other receptor breast cancer types, the average PKM2 overexpression level was higher in TNBC than in other receptor-positive types (Figure 1B). A summary of clinicopathological features of all tissues is presented in Table I. Therefore, most breast cancer tissues show increased PKM2 expression levels compared to those of adjacent normal tissues.
Figure 1. PKM2 overexpression is observed in TNBC tissues but not in adjacent normal tissues. (A-B) Breast tumors and adjacent normal breast tissue samples were analyzed for changes in PKM2 expression using immunohistochemistry. The intensity of PKM2 immunostaining in individual cells (average of duplicates) was scored in the breast cancer and adjacent normal breast tissue samples associated with the three different tumor grades and stages. Scale bar, 200 µm. The values represent the mean±S.D. *p<0.05. (C-D) Photographs are shown for representative tissues of adjacent normal, breast grades, or receptors-types with PKM2 expression using immunohistochemistry.
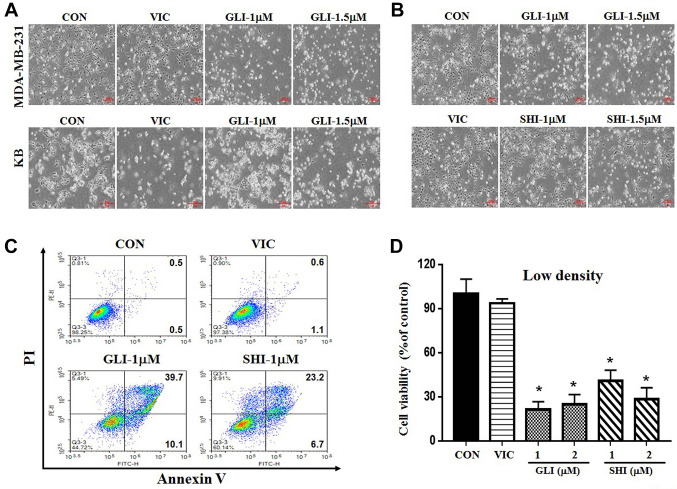
PKM2 inhibitor (gliotoxin or shikonin) sensitizes MDA-MB-231 cells at low dose. Further, we aimed to identify novel sensitization mechanisms for PKM2 inhibition in TNBC cells. We tested the mechanisms of PKM2 inhibition in vitro using the TNBC cell line MDA-MB-231. To inhibit PKM2 function, we tested a recently developed PKM2 inhibitor gliotoxin (22) and assessed whether it can be applied to TNBC. Vincristine (VIC) treatment was used as a positive control to compare the effectiveness of gliotoxin (18,33). We also compared the effectiveness of gliotoxin to that of the known PKM2 inhibitor shikonin (18-21). We performed both microscopic observations and viability assays to observe cytotoxicity in MDA-MB-231 cells. As shown in Figure 2A-C, 1 μM gliotoxin or shikonin was more toxic to MDA-MB-231 cells than 5 nM VIC; thus, even a low dose of PKM2 inhibitors could induce high sensitization. A comparison of cytotoxicity of the above-mentioned agents (VIC, shikonin, and gliotoxin) in other cancer cell lines revealed that KB cells were highly sensitive to VIC but less sensitive to PKM2 inhibitor (Figure 2A), suggesting that PKM2 inhibitors could be exclusively effective for TNBC treatment. Collectively, these results indicate that gliotoxin at relatively low doses has sensitization effects similar to those of the PKM2 inhibitor shikonin in MDA-MB-231 cells.
Figure 2. PKM2 inhibitors gliotoxin or shikonin sensitize MDA-MB-231 cells at low doses. (A) MDA-MB-231 and KB cells were grown on 60 mmdiameter dishes and treated with 5 nM vincristine (VIC), 1 μM gliotoxin (GLI-1), 1.5 μM gliotoxin (GLI-1.5), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification. (B) MDA-MB-231 cells were grown on 60 mm-diameter dishes and treated with 5 nM vincristine, 1 μM gliotoxin (GLI-1), 1.5 μM gliotoxin (GLI-1.5), 1 μM shikonin (SHI-1), 1.5 μM shikonin (SHI-1.5), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification. (C) MDA-MB-231 cells were grown on 60 mm-diameter dishes with low density (1.5×105 cells). They were then treated with 5 nM vincristine (VIC), 1 μM gliotoxin (GLI-1), 1 μM shikonin (SHI-1), or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (D) MDA-MB-231 cells were plated on 96-well plates and grown to 10-20% confluence with low density. The cells were then treated for 48 h with 5 nM vincristine (VIC), 1 μM gliotoxin (GLI-1), 2 μM gliotoxin (GLI-2), 1 μM shikonin (SHI-1), 2 μM shikonin (SHI-2), or 0.1% DMSO (CON). Cell viability assay was performed as described in “Materials and methods”. The data are presented as the mean±S.D. of at least two experiments repeated in triplicate experiments. Data are presented as mean±S.D. *p<0.05 was considered be statistically significant.
Gliotoxin or shikonin highly increases late-apoptosis in cells. To further clarify the mechanism of action of gliotoxin or shikonin, we performed a quantitative apoptosis analysis using annexin V staining. As shown in Figure 2C, apoptotic cell death greatly increased after gliotoxin or shikonin treatment, but not after VIC treatment. Detailed analysis revealed that the number of late-apoptotic cells increased by approximately 4-fold compared to that of early-apoptotic cells after gliotoxin or shikonin treatment. This suggests that gliotoxin or shikonin is a strong and rapid inducer of late apoptosis in MDA-MB-231 cells. Thus, gliotoxin or shikonin can induce rapid apoptosis in TNBC cells.
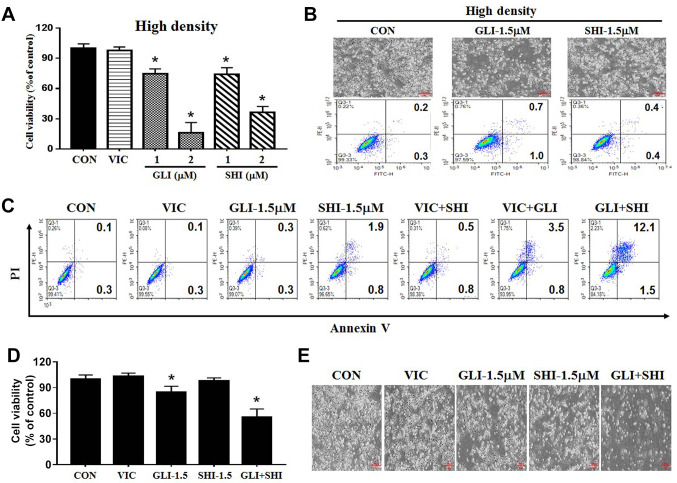
Sensitization by gliotoxin or shikonin is majorly dependent upon cellular density. The characteristics of drug sensitization in cancer cells can be determined by their dependence on cellular density, as high cellular density inhibits drug effects (18). Therefore, we tested the extent to which gliotoxin or shikonin sensitization depends on cellular density. As shown in Figure 2D, we observed that the low-density culture of MDA-MB-231 cells exhibited higher sensitization by either 1 μM gliotoxin or shikonin than by VIC. However, when cellular density was increased, the toxic effect of 1 μM of gliotoxin or shikonin on MDA-MB-231 cells was reduced, whereas VIC showed a similar sensitization effect (Figure 3A). Hence, gliotoxin or shikonin is a low density-specific drug. We confirmed our results using both microscopy and annexin V staining (Figure 3B). Thus, the effectiveness of PKM2 inhibition depends on TNBC cell density.
Figure 3. Combination of two different PKM2 inhibitors synergistically sensitizes high-density MDA-MB-231 cells. (A) MDA-MB-231 cells were plated on 96-well plates and grown to 40%-50% confluence with high density. The cells were then treated for 48 h with 5 nM vincristine (VIC), 1 μM gliotoxin (GLI-1), 2 μM gliotoxin (GLI-2), 1 μM shikonin (SHI-1), 2 μM shikonin (SHI-2), or 0.1% DMSO (CON). Cell viability assay was performed as described in Materials and Methods. Data are presented as the mean±S.D. of at least two experiments repeated in triplicate experiments. *p<0.05 was considered statistically significant. (B) MDA-MB-231 cells were grown on 60 mm-diameter dishes at high density (4×105 cells). They were then treated with 1.5 μM gliotoxin (GLI-1.5), 1.5 μM shikonin (SHI-1.5), or 0.1% DMSO (CON). After 24 h, either microscopic observation or annexin V analyses were performed as described in Materials and Methods. (C) MDA-MB-231 cells were grown on 60 mm-diameter dishes at high density (4×105 cells). They were then treated with 5 nM vincristine (VIC), 1.5 μM gliotoxin (GLI-1.5), 1.5 μM shikonin (SHI-1.5), 5 nM VIC with 1.5 μM gliotoxin (VIC+GLI), 5 nM VIC with 1.5 μM shikonin (VIC+SHI), 1.5 μM gliotoxin with 1.5 μM shikonin (GLI+SHI), or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (D) MDA-MB-231 cells were plated on 96-well plates and grown to 30-40% confluence at high density. The cells were then treated for 48 h with 5 nM vincristine (VIC), 1.5 μM gliotoxin (GLI-1.5), 1.5 μM shikonin (SHI-1.5), 1.5 μM gliotoxin with 1.5 μM shikonin (GLI+SHI), or 0.1% DMSO (CON). Cell viability assay was performed as described in Materials and Methods”. Data are presented as the mean±S.D. of at least two experiments repeated in triplicate experiments. *p<0.05 was considered be statistically significant. (E) MDA-MB-231 cells were grown on 60 mm-diameter dishes with high density (4×105 cells). They were then treated with 5 nM vincristine (VIC), 1.5 μM gliotoxin (GLI-1.5), 1.5 μM shikonin (SHI-1.5), 1.5 μM gliotoxin with 1.5 μM shikonin (GLI+SHI), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification.
Combination of two different PKM2 inhibitors synergistically sensitizes high-density MDA-MB-231 cells. Furthermore, we investigated ways to overcome the reduced sensitization-effect of PKM2 inhibitors in high-density TNBC. We assumed that co-treatment with an anticancer drug (VIC) and a PKM2 inhibitor (gliotoxin or shikonin) could increase sensitization due to the additive effects of both drugs in MDA-MB-231 cells because they have different sensitization mechanisms. As seen in the apoptosis analysis in Figure 3C, we did not observe any enhanced sensitization effects by treatments with VIC+gliotoxin or VIC+shikonin compared to those by single treatments in high-density cultures. Interestingly, co-treatment with gliotoxin and shikonin significantly increased late apoptosis compared to single treatment (Figure 3C). As seen in Figure 3D and E, both viability assay and microscopic observations revealed that gliotoxin+shikonin showed enhanced toxicity in high-density MDA-MB-231 cells compared to single treatments, confirming that the combination of two different PKM2 inhibitors increased the sensitization of high-density MDA-MB-231 cells. These results suggest that different PKM2 inhibitors can be used in combination therapies for TNBC.
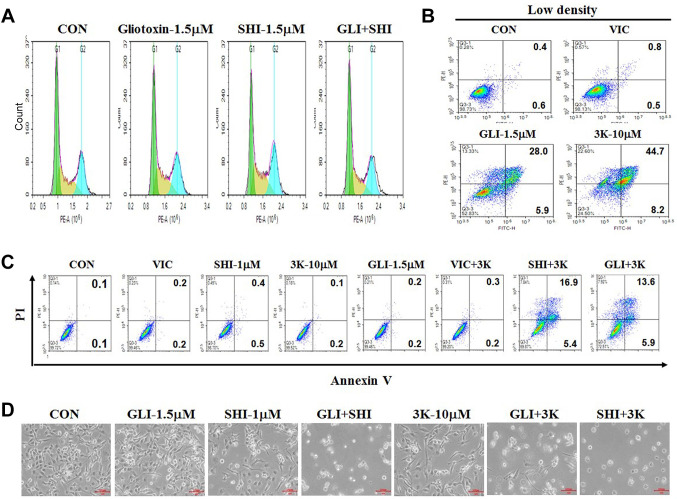
Combination of two different PKM2 inhibitors does not increase S or G2 arrest for the sensitization of high-density MDA-MB-231 cells. Next, we performed FACS analyses to determine whether cell-cycle arrest occurred along with apoptosis in gliotoxin+shikonin treated high-density cultures. As shown in Figure 4A, we did not detect a significant increase in G2 or S phase arrest in MDA-MB-231 cells co-treated with gliotoxin+shikonin (Figure 4A). Considering that G2 or S arrest is one of the survival mechanisms of cancer cells against toxic agents, we concluded that gliotoxin+shikonin co-treatment is highly effective for faster apoptotic death. We concluded that gliotoxin+shikonin co-treatment induces high levels of late-apoptotic death and non-G2 or non-S arrest in high-density MDA-MB-231 cells.
Figure 4. PKM2 inhibitor, compound 3K in combination with gliotoxin exhibits sensitization mechanisms similar to those of gliotoxin+shikonin cotreatment. (A) MDA-MB-231 cells were grown on 60 mm-diameter dishes either at high density (4×105 cells). They were then treated with 1.5 μM gliotoxin (GLI-1.5), 1.5 μM shikonin (SHI-1.5), 1.5 μM gliotoxin with 1.5 μM shikonin (GLI+SHI), or 0.1% DMSO (CON). After 24 h, FACS analyses were performed as described in Materials and Methods. (B) MDA-MB-231cells were grown on 60 mm-diameter dishes at low density (1.5×105 cells). They were then treated with 5 nM vincristine (VIC), 1.5 μM gliotoxin (GLI-1.5), 10 μM compound 3K (3K-10), or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (C) MDA-MB-231 cells were grown on 60 mm-diameter dishes with high density (4×105 cells). They were then treated with 5 nM vincristine (VIC), 1.5 μM gliotoxin (GLI-1.5), 1 μM shikonin (SHI-1), 10 μM compound 3K (3K-10), 5 nM VIC with 10 μM compound 3K (VIC+3K), 1.5 μM shikonin with 10 μM compound 3K (SHI+3K), 1.5 μM gliotoxin with 10 μM compound 3K (GLI+3K), or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (D) MDA-MB-231 cells were grown on 60 mm-diameter dishes at high density (4×105 cells). They were then treated with 1.5 μM gliotoxin (GLI-1.5), 1 μM shikonin (SHI-1), 10 μM compound 3K (3K-10), 1.5 μM shikonin with 10 μM compound 3K (SHI+3K), 1.5 μM gliotoxin with 10 μM compound 3K (GLI+3K), 1.5 μM gliotoxin with 1.5 μM shikonin (GLI+SHI), or 0.1% DMSO (CON). After 1 day, all cells were observed at an inverted microscope at ×100 magnification.
PKM2 inhibitor—compound 3K in combination with gliotoxin exhibits sensitization mechanism similar to that of gliotoxin and shikonin co-treatment. We also tested whether other PKM2 inhibitors could sensitize MDA-MB-231 cells similar to that by gliotoxin or shikonin. Compound 3K has been recently developed and is considered to be a specific PKM2 inhibitor (18,19,34-36). As shown in Figure 4B, compound 3K increased late apoptosis in low-density MDA-MB-231 cells, as observed with gliotoxin treatment. Both, compound 3K and gliotoxin showed an approximately 5-fold increase in late apoptosis in low-density MDA-MB-231 cells compared to that in early apoptosis (Figure 4B), suggesting that sensitization mechanisms of compound 3K are similar to those of shikonin or gliotoxin.
Furthermore, we tested whether co-treatment with compound 3K also increases the toxicity of other PKM2 inhibitors (gliotoxin and shikonin) in high-density MDA-MB-231 cells. As seen in Figure 4C, both gliotoxin+compound 3K and compound 3K+shikonin co-treatments highly increased late apoptosis in high-density cell cultures, as seen in the case of gliotoxin+shikonin co-treatment (Figure 3C-E). However, treatment with VIC+compound 3K did not increase toxicity in high-density MDA-MB-231 cells. This indicates that co-treatment with various PKM2 inhibitors is effective in increasing toxicity in high-density TNBC cells. We confirmed our results by microscopy (Figure 4D). We conclude that co-treatment with PKM2 inhibitors can induce sensitization of high-density MDA-MB-231 cells.
Discussion
The role of PKM2 in tumor initiation and progression in various cancer types has been studied previously (14-16,37). It has been established that inhibition of PKM2 reduces tumor growth and causes cancer cell death (15,16). Studies have revealed overexpression of PKM2 mRNA in various cancers, including liver, bladder, breast, lung, esophagus, gastric, and colorectal cancers (14-16,19,38). Furthermore, overexpression of the PKM2 protein is associated with different types of cancers in humans. As there are only few studies on the association between PKM2 and its role in TNBC (6,39-41), we examined this relationship in the present study. Although, previous studies have shown that PKM2 inhibition can increase the sensitization of breast cancer cells, we attempted to identify a more detailed and novel PKM2 inhibition mechanism in TNBC. We believe that our novel findings on the oncogenic role of PKM2 in TNBC will facilitate its application in TNBC treatment.
Immunohistochemical analysis revealed that PKM2 levels were higher in most patients with breast cancer than in normal adjacent controls. This suggests that the up-regulated expression of PKM2 is required for tumor progression in breast cancer. However, we did not observe any correlation between the extent of PKM2 overexpression and grade dependence or poor prognosis in these patients. Differences in race or age of patients with breast cancer could be responsible for the fact that PKM2 overexpression is not dependent on grade. A greater number of breast cancer samples should be investigated to demonstrate the role of PKM2 in disease prognosis. Further studies are also required to determine the genetic characteristics of patients with high overexpression of PKM2.
Previous studies have reported an elevated PKM2 expression in TNBC (39). In a detailed analysis of other receptor-positive cancer types, we found that most TNBC samples showed an increase in PKM2 levels compared to those of other breast cancer tissues. This indicates that PKM2 overexpression is a common feature of TNBC tissues. We hypothesized that PKM2 overexpression is required for the development of TNBC. We also suggest that PKM2 overexpression could be an indicator of TNBC. Further investigations should be conducted to determine whether PKM2 overexpression can be used as a biomarker for TNBC.
Furthermore, as only a few studies have investigated PKM2 inhibition in TNBC types, it remains unclear whether PKM2 inhibitors are potential TNBC-targeting drugs. Several PKM2 inhibitors such as shikonin, metformin, vitamin K, and temozolomide have been studied (18,19,34,42). Compounds such as compound 3K have shown significant antiproliferative activity in various cancer cell lines with high expression of PKM2 (18-21). Recently, gliotoxin was reported as a PKM2 inhibitor in glioma cells (22). Although gliotoxins have been studied in various cancer models, their role in TNBC has not been studied in detail.
Herein, we aimed to identify the activity of a novel PKM2 inhibitor gliotoxin regarding its anticancer effects in the TNBC cell line MDA-MB-231. We found that they sensitized the human TNBC cell line MDA-MB-231 at a relatively low dose, similar to shikonin. During sensitization analysis of gliotoxin and shikonin, we found that sensitization was maximum in low confluent cultures, suggesting that PKM2 inhibitors sensitize MDA-MB-231 cells with low density. Previously, the PKM2 inhibitors shikonin and compound 3K showed low specificity for glioma cells (18,19). We concluded that PKM2 inhibitors are not effective in high-density cellular states. It is possible that cancer cells do not utilize PKM2 activity for growth in high-density conditions. These results may help in further clinical application of PKM2 inhibitors in TNBC. Previously, gliotoxins were demonstrated to have anticancer activity in various solid cancer models (23-27), including MDA-MB-231 breast cancer cells. Our current results seem to be the first to demonstrate that gliotoxin has cytotoxicity as a PKM2 inhibitor in MDA-MB-231 cells with low-density specificity. Since we did not observe any significant reduction of PKM2 protein levels by PKM2 inhibitors (shikonin, compound 3K, and gliotoxin) in MDA-MB-231 (data not shown), we hypothesize that those inhibitors affect the activity of PKM2, including posttranslational modification, cellular localization, or tetramer formations, etc. In further studies, it should be determined how PKM2 inhibitors regulate PKM2 activity in MDA-MB-231 cells.
Furthermore, to demonstrate the sensitizing efficiency of PKM2 inhibitors in MDA-MB-231 cells, we compared their effects on the KB cancer cell line, which has been shown to be highly sensitive to the anticancer drug, VIC. Interestingly, during sensitization analysis of gliotoxin or shikonin with other organ-generated KB cancer cells (32,33), we found that TNBC showed high sensitization effects at low doses. However, KB cells are highly sensitive to VIC, whereas MDA-MB-231 cells require higher doses of VIC for sensitization, suggesting that PKM2 inhibitors are specific to TNBC. This also suggests that VIC-sensitive cells are poor candidates for PKM2 inhibitor treatment. These results also indicated that PKM2 inhibitors specifically sensitize MDA-MB-231 cells. We also concluded that PKM2 inhibitors generally have similar sensitization mechanisms with respect to specificity in TNBC. A detailed analysis of the sensitization mechanisms of gliotoxin and shikonin was performed. They induced increased late apoptosis levels in MDA-MB-231 cells, as determined by annexin V analysis. We hypothesized that PKM2 inhibitors could be used as targeted drugs for TNBC.
Furthermore, we investigated how PKM2 inhibitors could overcome non-sensitization in high-density cultures. It has been previously reported that co-treatment with two PKM2 inhibitors (shikonin+compound 3K) increased cytotoxicity in U87MG glioma, but VIC+shikonin or VIC+compound 3K treatments did not exhibit this effect (18). We tested whether gliotoxin also increased cytotoxicity when used with shikonin or compound 3K. We demonstrated that co-treatment with two PKM2 inhibitors, gliotoxin+shikonin and gliotoxin+compound 3K, resulted in increased sensitization compared to single treatments. However, we did not detect an increase in toxicity upon co-treatment with VIC and gliotoxin or shikonin in MDA-MB-231 cells. Thus, PKM2 inhibitors may be used as substitutes for anticancer drugs in TNBC treatment and two different PKM2 inhibitor combinations could be used in clinical settings.
We did not detect an increase in G2 or S arrest after PKM2 inhibitor co-treatment. This suggested that co-treatment with PKM2 inhibitors increased late-apoptosis levels faster than non-G2 or non-S arrest. We assumed that there was insufficient time to recover from toxic PKM2 inhibitors in TNBC patients. We concluded that the rapid increase in apoptosis and late-apoptosis levels in a relatively short time by PKM2 inhibitors resulted from non-G2 or non-S arrest and induced late apoptosis.
Herein, we demonstrated that PKM2 overexpression was correlated with TNBC, and combination treatments with its inhibitors (gliotoxin, shikonin, or compound 3K) could highly sensitize high-density MDA-MB-231 cells via increased rate of late apoptosis. Our findings identified PKM2 inhibitors with antitumor activity in TNBC, laying the foundation for the development of PKM2 inhibitors as promising antitumor drugs for TNBC treatment in the future.
Conflicts of Interest
The Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
Ji Sun Lee, Yunmoon Oh, and Jin-Sol Lee: Collected the data, contributed data or analysis tools, wrote the article. Ji Sun Lee, Yunmoon Oh, and Jin-Sol Lee, Jae Hyeon Park, Joo-Kyung Shin, Joo-Hee Han, and Hyung Sik Kim: Contributed data and analysis. Sungpil Yoon: Contributed data and analysis, conceived, and designed the analysis, collected the data, wrote the article.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1A2C2002923).
References
- 1.Lee YM, Oh MH, Go JH, Han K, Choi SY. Molecular subtypes of triple-negative breast cancer: understanding of subtype categories and clinical implication. Genes Genomics. 2020;42(12):1381–1387. doi: 10.1007/s13258-020-01014-7. [DOI] [PubMed] [Google Scholar]
- 2.Borri F, Granaglia A. Pathology of triple negative breast cancer. Semin Cancer Biol. 2021;72:136–145. doi: 10.1016/j.semcancer.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Hossain F, Majumder S, David J, Miele L. Precision medicine and triple-negative breast cancer: current landscape and future directions. Cancers (Basel) 2021;13(15):3739. doi: 10.3390/cancers13153739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmé F, Schneeweiss A. Targeted therapies in triple-negative breast cancer. Breast Care (Basel) 2015;10(3):159–166. doi: 10.1159/000433622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCann KE, Hurvitz SA, McAndrew N. Advances in targeted therapies for triple-negative breast cancer. Drugs. 2019;79(11):1217–1230. doi: 10.1007/s40265-019-01155-4. [DOI] [PubMed] [Google Scholar]
- 6.Agostinetto E, Eiger D, Punie K, de Azambuja E. Emerging therapeutics for patients with triple-negative breast cancer. Curr Oncol Rep. 2021;23(5):57. doi: 10.1007/s11912-021-01038-6. [DOI] [PubMed] [Google Scholar]
- 7.Alquraishi M, Puckett DL, Alani DS, Humidat AS, Frankel VD, Donohoe DR, Whelan J, Bettaieb A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic Biol Med. 2019;143:176–192. doi: 10.1016/j.freeradbiomed.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes-Xavier CE, Martín-Pérez J, Elson A, Pulido R. Protein tyrosine phosphatases as novel targets in breast cancer therapy. Biochim Biophys Acta. 2013;1836(2):211–226. doi: 10.1016/j.bbcan.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Mir MA, Qayoom H, Mehraj U, Nisar S, Bhat B, Wani NA. Targeting different pathways using novel combination therapy in triple negative breast cancer. Curr Cancer Drug Targets. 2020;20(8):586–602. doi: 10.2174/1570163817666200518081955. [DOI] [PubMed] [Google Scholar]
- 10.Sahu R, Pattanayak SP. Strategic developments & future perspective on gene therapy for breast cancer: role of mTOR and Brk/PTK6 as molecular targets. Curr Gene Ther. 2020;20(4):237–258. doi: 10.2174/1566523220999200731002408. [DOI] [PubMed] [Google Scholar]
- 11.Almouhanna F, Blagojevic B, Can S, Ghanem A, Wölfl S. Pharmacological activation of pyruvate kinase M2 reprograms glycolysis leading to TXNIP depletion and AMPK activation in breast cancer cells. Cancer Metab. 2021;9(1):5. doi: 10.1186/s40170-021-00239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arundhathi JRD, Mathur SR, Gogia A, Deo SVS, Mohapatra P, Prasad CP. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol Biol Rep. 2021;48(5):4733–4745. doi: 10.1007/s11033-021-06414-w. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Su CM, Chen HA, Du S, Li CW, Wu H, Tsai SH, Yeh YT. Cryptanshinone inhibits the glycolysis and inhibits cell migration through PKM2/β-catenin axis in breast cancer. Onco Targets Ther. 2020;13:8629–8639. doi: 10.2147/OTT.S239134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013–1019. doi: 10.1038/aps.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey P, Kundu A, Sachan R, Park JH, Ahn MY, Yoon K, Lee J, Kim ND, Kim IS, Lee BM, Kim HS. PKM2 knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells. Cell Physiol Biochem. 2019;52(6):1535–1552. doi: 10.33594/000000107. [DOI] [PubMed] [Google Scholar]
- 16.Dey P, Son JY, Kundu A, Kim KS, Lee Y, Yoon K, Yoon S, Lee BM, Nam KT, Kim HS. Knockdown of pyruvate kinase M2 inhibits cell proliferation, metabolism, and migration in renal cell carcinoma. Int J Mol Sci. 2019;20(22):5622. doi: 10.3390/ijms20225622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni W, Xia Y, Luo L, Wen F, Hu D, Bi Y, Qi J. High expression of ALDH1A3 might independently influence poor progression-free and overall survival in patients with glioma via maintaining glucose uptake and lactate production. Cell Biol Int. 2020;44(2):569–582. doi: 10.1002/cbin.11257. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Lee JS, Oh Y, Lee JS, Park HE, Lee H, Park YS, Kyung SY, Kim HS, Yoon S. PKM2 is overexpressed in glioma tissues, and its inhibition highly increases late apoptosis in U87MG cells with low-density specificity. In Vivo. 2022;36(2):694–703. doi: 10.21873/invivo.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JH, Kundu A, Lee SH, Jiang C, Lee SH, Kim YS, Kyung SY, Park SH, Kim HS. Specific pyruvate kinase M2 inhibitor, compound 3K, induces autophagic cell death through disruption of the glycolysis pathway in ovarian cancer cells. Int J Biol Sci. 2021;17(8):1895–1908. doi: 10.7150/ijbs.59855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B, Wang Z, Ding Y, Wang X, Lu S, Wang C, He C, Piao M, Chi G, Luo Y, Ge P. RIP1 and RIP3 contribute to shikonin-induced glycolysis suppression in glioma cells via increase of intracellular hydrogen peroxide. Cancer Lett. 2018;425:31–42. doi: 10.1016/j.canlet.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149:104463. doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- 22.Tang W, Liu ZL, Mai XY, Qi X, Li DH, Gu QQ, Li J. Identification of Gliotoxin isolated from marine fungus as a new pyruvate kinase M2 inhibitor. Biochem Biophys Res Commun. 2020;528(3):594–600. doi: 10.1016/j.bbrc.2020.05.139. [DOI] [PubMed] [Google Scholar]
- 23.Hubmann R, Sieghart W, Schnabl S, Araghi M, Hilgarth M, Reiter M, Demirtas D, Valent P, Zielinski C, Jäger U, Shehata M. Gliotoxin targets nuclear NOTCH2 in human solid tumor derived cell lines in vitro and inhibits melanoma growth in xenograft mouse model. Front Pharmacol. 2017;8:319. doi: 10.3389/fphar.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Lou Q, He L, Wen C, Lin M, Zhu Z, Wang F, Huang L, Lan W, Iwamoto A, Yang X, Liu H. Reduced-gliotoxin induces ROS-mediated anoikis in human colorectal cancer cells. Int J Oncol. 2018;52(3):1023–1032. doi: 10.3892/ijo.2018.4264. [DOI] [PubMed] [Google Scholar]
- 25.Manh Hung LV, Song YW, Cho SK. Effects of the combination of gliotoxin and adriamycin on the adriamycin-resistant non-small-cell lung cancer A549 cell line. Mar Drugs. 2018;16(4):105. doi: 10.3390/md16040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park GB, Jeong JY, Kim D. Gliotoxin enhances autophagic cell death via the DAPK1-TAp63 signaling pathway in paclitaxel-resistant ovarian cancer cells. Mar Drugs. 2019;17(7):412. doi: 10.3390/md17070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen T, Li LM, Xu ZY, Wang YD, Xie WD. Julichrome derivatives and gliotoxin from a soil derived Streptomyces sp. Nat Prod Res. 2021;35(1):34–40. doi: 10.1080/14786419.2019.1611814. [DOI] [PubMed] [Google Scholar]
- 28.Im JH, Kang KW, Kim SY, Kim YG, An YJ, Park S, Jeong BH, Choi SY, Lee JS, Kang KW. GPR119 agonist enhances gefitinib responsiveness through lactate-mediated inhibition of autophagy. J Exp Clin Cancer Res. 2018;37(1):295. doi: 10.1186/s13046-018-0949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KS, Jiang C, Kim JY, Park JH, Kim HR, Lee SH, Kim HS, Yoon S. Low-dose crizotinib, a tyrosine kinase inhibitor, highly and specifically sensitizes P-Glycoprotein-overexpressing chemoresistant cancer cells through induction of late apoptosis in vivo and in vitro. Front Oncol. 2020;10:696. doi: 10.3389/fonc.2020.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C, Lee SH, Park JH, Lee JS, Park JW, Kim JR, Lee SH, Kim HS, Yoon S. A low dose of aripiprazole has the strongest sensitization effect among 19 repositioned bipolar drugs in P-gp-overexpressing drug-resistant cancer cells. Anticancer Res. 2021;41(2):687–697. doi: 10.21873/anticanres.14820. [DOI] [PubMed] [Google Scholar]
- 31.Jiang C, Zheng T, Park JH, Lee JS, Oh Y, Kundu A, Kim HS, Yoon S. Sensitization effects of repurposed blood pressure-regulating drugs on drug-resistant cancer cells. Anticancer Res. 2021;41(12):6179–6190. doi: 10.21873/anticanres.15437. [DOI] [PubMed] [Google Scholar]
- 32.Oh Y, Lee JS, Lee JS, Park JH, Kim HS, Yoon S. Co-treatment of low dose pacritinib, a Phase III Jak2 inhibitor, greatly increases apoptosis of P-gp over-expressing cancer cells with multidrug resistance. Anticancer Res. 2022;42(5):2433–2442. doi: 10.21873/anticanres.15722. [DOI] [PubMed] [Google Scholar]
- 33.Oh Y, Lee JS, Lee JS, Park JH, Kim HS, Yoon S. JAK2 inhibitor, fedratinib, inhibits P-gp activity and co-treatment induces cytotoxicity in antimitotic drug-treated P-gp overexpressing resistant KBV20C cancer cells. Int J Mol Sci. 2022;23(9):4597. doi: 10.3390/ijms23094597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning X, Qi H, Li R, Jin Y, McNutt MA, Yin Y. Synthesis and antitumor activity of novel 2, 3-didithiocarbamate substituted naphthoquinones as inhibitors of pyruvate kinase M2 isoform. J Enzyme Inhib Med Chem. 2018;33(1):126–129. doi: 10.1080/14756366.2017.1404591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu F, Guo M, Huang W, Feng L, Zhu J, Luo K, Gao J, Zheng B, Kong LD, Pang T, Wu X, Xu Q. Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 2020;36:101634. doi: 10.1016/j.redox.2020.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Z, Lan J, Lei S, Yang Y, He Z, Xue Y, Chen T. Simultaneous inhibition of ornithine decarboxylase 1 and pyruvate kinase M2 exerts synergistic effects against hepatocellular carcinoma cells. Onco Targets Ther. 2020;13:11697–11709. doi: 10.2147/OTT.S240535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One. 2013;8(2):e57610. doi: 10.1371/journal.pone.0057610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu H, Luo H, Zhu X, Hu X, Zheng L, Zhu X. Pyruvate kinase M2 (PKM2) expression correlates with prognosis in solid cancers: a meta-analysis. Oncotarget. 2017;8(1):1628–1640. doi: 10.18632/oncotarget.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma C, Zu X, Liu K, Bode AM, Dong Z, Liu Z, Kim DJ. Knockdown of pyruvate kinase M inhibits cell growth and migration by reducing NF-kB activity in triple-negative breast cancer cells. Mol Cells. 2019;42(9):628–636. doi: 10.14348/molcells.2019.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasul A, Riaz A, Wei W, Sarfraz I, Hassan M, Li J, Asif F, Adem Ş, Bukhari SA, Asrar M, Li X. Mangifera indica extracts as novel PKM2 inhibitors for treatment of triple negative breast cancer. Biomed Res Int. 2021;2021:5514669. doi: 10.1155/2021/5514669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Yu P, Zhu X, Zhu JL, Han YB, Zhang H, Zhou X, Yang L, Xia YZ, Zhang C, Kong LY. The Chk2-PKM2 axis promotes metabolic control of vasculogenic mimicry formation in p53-mutated triple-negative breast cancer. Oncogene. 2021;40(34):5262–5274. doi: 10.1038/s41388-021-01933-z. [DOI] [PubMed] [Google Scholar]
- 42.Ning X, Qi H, Li R, Li Y, Jin Y, McNutt MA, Liu J, Yin Y. Discovery of novel naphthoquinone derivatives as inhibitors of the tumor cell specific M2 isoform of pyruvate kinase. Eur J Med Chem. 2017;138:343–352. doi: 10.1016/j.ejmech.2017.06.064. [DOI] [PubMed] [Google Scholar]