Abstract
Heterocysts are terminally differentiated cells devoted to nitrogen fixation in the filamentous cyanobacterium Anabaena sp. strain PCC 7120. We show here that the cell division protein FtsZ is present in vegetative cells but undetectable in heterocysts. These results provide a first rational explanation for the inability of mature heterocysts to undergo cell division.
The relationship between cell division and cell differentiation is a complex problem in biology. The decision of a specific cell to continue proliferation or instead to arrest at a given time during a cell cycle in order to commit itself to differentiate depends on the interaction between the cell and its surrounding environment. Once a cell becomes competent and committed to differentiate, differential gene expression and protein localization must be involved in order to ensure the cell fate determination. Generally in both eukaryotes and prokaryotes, a differentiating cell, once fully committed, stops cell division (6). Forcing ectopic cell division can affect morphogenesis either by interfering with terminal differentiation or through an inappropriate increase in cell number (1, 9). Similarly in Anabaena sp. strain PCC 7120, a cell division arrest is also observed for differentiated cells (24). Anabaena sp. strain PCC 7120 is a filamentous cyanobacterium capable of developing specialized cells, called heterocysts, devoted to nitrogen fixation. Heterocyst differentiation is induced upon the depletion of a combined-nitrogen source in the growth medium, and only 1 in every 10 to 20 cells along each filament can become heterocysts, which are arranged in a semiregular and one-dimensional pattern (4, 25, 26). Developing proheterocysts may regress under certain conditions, but mature heterocysts no longer divide (24). The growth of a filament is thus ensured only by vegetative cells which retain the ability to divide.
It is not known what makes heterocysts lose the competence for cell division, nor is it clear if cell division arrest is a necessary prelude to heterocyst differentiation. Heterocysts have a thick envelope consisting of an inner layer of glycolipids and an outer layer of polysaccharide (25). The structure of heterocyst envelope may eventually provide a physical constraint for cell division. Alternatively, the arrest of cell division accompanying heterocyst differentiation involves differential regulation of gene expression and enzymatic activities. To gain insight into these questions, we started to investigate the regulation of cell division during heterocyst development. One key element in bacterial division is the GTPase FtsZ, the earliest known element acting on bacterial cell cycle. Upon GTP hydrolysis, FtsZ polymerizes to form a ring structure attached to the membrane through ZipA at the midpoint of a dividing cell and recruits other cell division elements to form the septum (for recent reviews, see references 3, 14, 17, and 22). FtsZ is found in almost all bacteria, Chlamydia trachomatis being the only known exception. It is also present in plant chloroplasts. The FtsZ protein from Anabaena sp. strain PCC 7120 (referred to as FtsZAna hereafter) has been shown to be highly similar to those found in other bacteria (5, 29). In this study, we demonstrate that FtsZAna is undetectable in heterocysts, providing a rational explanation for the inability of mature heterocysts to undergo cell division.
Overexpression of FtsZIAna in Escherichia coli disrupts septum formation.
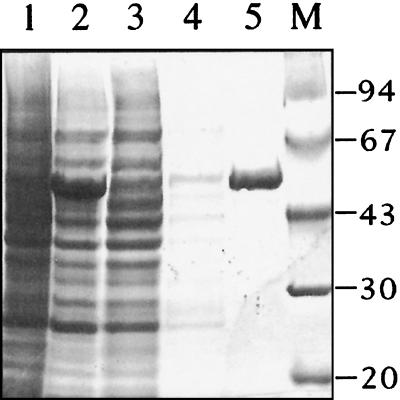
The ftsZAna coding region was amplified by PCR with sense (CCGGAATTCCATATGACACTTGATAATAA) and antisense (GCGGGATCCTTAATTTTTGGGTGGTC) primers. The PCR product was cloned into the pET15b vector (Novagen) and transformed into the E. coli host BL21 for the overproduction of FtsZAna. The expected recombinant FtsZ from this construct would have a His tag attached to its N-terminal end. After induction for 3 h with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG), a major protein band with an estimated molecular mass of about 50 kDa was induced (Fig. 1). The molecular mass of this protein was close to that calculated from the His-FtsZAna fusion (47 kDa).
FIG. 1.
Production and purification of recombinant FtsZAna from E. coli. E. coli BL21 transformed with pET15b overexpressing FtsZAna was grown to an optical density of 0.5 at 600 nm (lane 1) and then induced by 0.4 mM IPTG (lane 2). Soluble proteins from induced cells were loaded onto a His tag affinity column. The flowthrough fraction is shown in lane 3. The column was washed once (lane 4), and then the retained fraction was eluted (lane 5). M, protein molecular weight standard (positions shown in kilodaltons at the right).
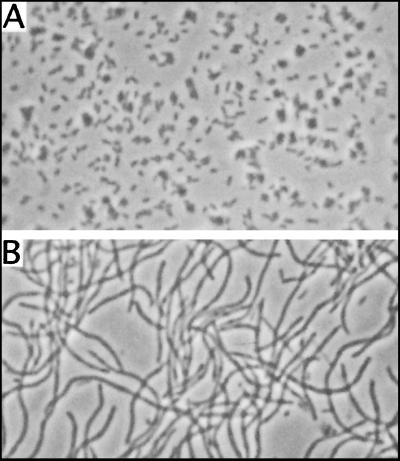
The E. coli host strain became filamentous in the presence of the overexpressed FtsZAna after growth at 37°C for 7 h (Fig. 2). The E. coli filaments had no visible septum. The filamentation phenotype of the E. coli host depended on the concentration of IPTG. Without IPTG as an inducer, cells appeared normal. When incubated for 7 h at 37°C with 0.002 mM IPTG, cells were slightly elongated, with short filaments twice the size of normal cells. When IPTG was added at 0.008 mM or above, cells became fully filamentous. A similar phenotype has been found in E. coli when its endogenous FtsZ protein was overexpressed (23). These results indicated that the presence of FtsZAna at a high concentration interfered with the cell division process in E. coli. A similar observation was made when ftsZ from Rhizobium meliloti was expressed in E. coli (15).
FIG. 2.
Filamentation phenotype of E. coli caused by overexpression of FtsZAna. E. coli BL21 transformed with pET15b overexpressing FtsZAna was grown for 7 h in LB medium in the absence (A) or presence (B) of 0.4 mM IPTG as an inducer.
GTPase activity of recombinant FtsZAna.
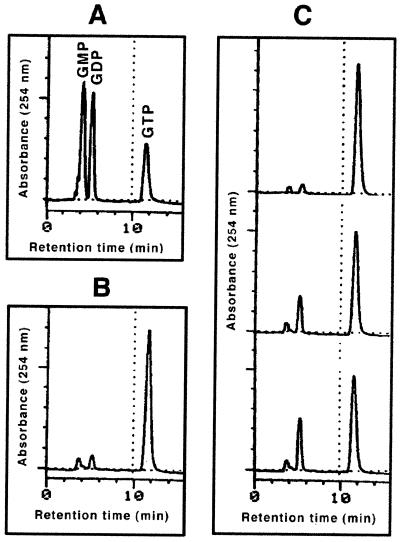
For purification of the recombinant FtsZAna, E. coli host cells were collected by centrifugation, disrupted by sonication, and loaded on a His tag affinity column (Pharmacia) as instructed by the supplier (Fig. 1). The eluted protein, with apparent homogeneity (Fig. 1), was further dialyzed against a buffer consisting of 50 mM HEPES (pH 7.2), 0.1 mM EDTA, and 10% glycerol. For the GTPase assay, recombinant FtsZAna (0.125 mg/ml) was incubated at 37°C in a solution containing 1 mM GTP, 5 mM MgCl2, 50 mM Tris (pH 7.2), 50 mM KCl, and 5% glycerol (16). Samples were withdrawn at different time points and analyzed by high-performance liquid chromatography (HPLC). As shown in Fig. 3, GDP appeared over time at the expense of GTP. Only a basal level of GDP was observed when GTP was incubated without FtsZAna or with FtsZAna inactivated at 100°C for 15 min. This is the first cyanobacterial FtsZ shown to have a GTPase activity.
FIG. 3.
GTPase activity of FtsZAna detected by HPLC on an ion-exchange column (Partisil 10; Waters). Samples were eluted with 0.8 M KH2PO4 (pH 2.6) at a flow rate of 1 ml/min. (A) Identification of nucleotides in a mixture of authentic GMP, GDP, and GTP. (B) GTP was incubated for 2 h with FtsZAna previously heated at 100°C for 15 min. (C) GTP was incubated with FtsZAna for 0 min (top), 60 min (middle), or 120 min (bottom).
It has been reported that 3-methoxybenzamide (3-MBA), an inhibitor of ADP-ribosyltransferase, induces cell filamentation in Bacillus subtilis, and this phenotype could be suppressed by a mutation in the ftsZ gene (18). Since it is conceivable that 3-MBA acts directly on FtsZ, we tested the possibility of this drug as a GTPase inhibitor of FtsZAna. Under our assay conditions, 3-MBA up to a concentration of 5 mM had no significant effect on the GTPase activity of FtsZAna in vitro. 3-MBA at a concentration of 5 mM had an inhibitory effect on the growth ability of Anabaena sp. strain PCC 7120, but septum formation was not affected (data not shown).
Immunodetection of FtsZAna during heterocyst development.
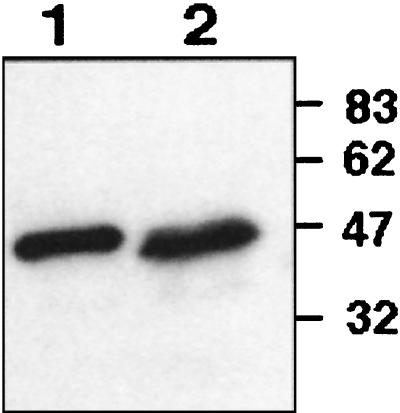
The purified recombinant FtsZAna (Fig. 1) was used as an antigen to produce polyclonal antibodies from a rabbit. The specificity of the antibodies was controlled by immunoblotting with protein lysate from Anabaena sp. strain PCC 7120 prepared as described previously (30), and the recombinant FtsZAna was purified from an E. coli overexpressing strain. The results of this experiment are shown in Fig. 4. A band of 47 kDa was revealed from Anabaena sp. strain PCC 7120 (Fig. 4, lane 2). The size of this signal correlates well with the theoretical molecular weight of FtsZAna, as well as that of the purified recombinant FtsZAna antigen detected on the same blot (lane 1).
FIG. 4.
Detection of FtsZAna by immunoblotting. Purified FtsZAna produced from E. coli (lane 1) and protein lysate from Anabaena sp. strain PCC 7120 (lane 2) were immunodetected with a serum raised against the recombinant FtsZAna. Immunodetection was carried out as described elsewhere (30). Sizes are indicated in kilodaltons.
To determine whether the amount of FtsZ changes during heterocyst development, a 500-ml culture of Anabaena sp. strain PCC 7120 was grown to an optical density of 0.5 at 700 nm in nitrate-containing BG11 medium (21) and then transferred to combined nitrogen-free medium BG110 to induce heterocyst development (28); 60-ml aliquots of cells were collected at different time points from 10 min to 3 days after induction to make protein preparations. After protein separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, similar amounts of FtsZAna were detected in all protein samples by immunoblotting (data not shown). These results suggested that the overall amount of FtsZAna was little changed in filaments induced to differentiate heterocysts. As in E. coli (19), most FtsZAna was found in the soluble fraction in Anabaena sp. strain PCC 7120 (data not shown).
Cell-type-specific localization of FtsZAna.
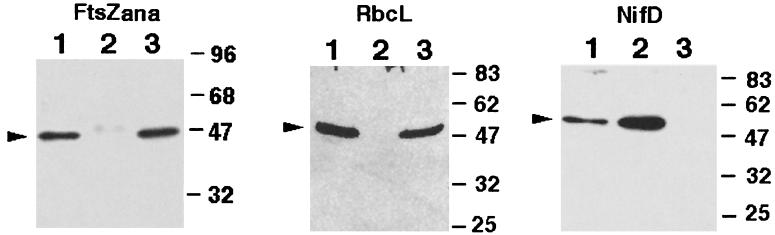
Since heterocysts represent only 5 to 10% of all cells under nitrogen-fixing conditions (4, 25, 26), immunodetection of FtsZAna with proteins prepared from total filaments could not determine whether FtsZAna displayed a differential pattern of regulation in heterocysts compared to vegetative cells. Therefore, a heterocyst preparation was made by treating filaments by lysozyme followed by weak sonication (8). Under such conditions, most vegetative cells were lysed to give vegetative proteins, while heterocysts were resistant due to its thick cell wall. Short and repetitive sonications were performed to eliminate vegetative cells as much as possible. Total proteins from purified heterocysts were extracted. Similar amounts of proteins from total filaments, heterocysts, and vegetative cells were separated by electrophoresis and blotted with anti-FtsZAna antibodies. The results of the immunodetection experiments indicated that FtsZAna was undetectable in protein preparations from heterocyst fractions, while it was detected strongly in total cells (heterocysts plus vegetative cells) in similar amount as in vegetative cells (Fig. 5). This detected protein showed a molecular weight similar to that of the recombinant FtsZAna detected on the same blot (data not shown). A similar pattern of protein localization was found with polyclonal antibodies against RbcL (12), the larger subunit of ribulose-1,5-bisphosphate carboxylase, a well-established element specifically localized in vegetative cells (7, 27). Another polyclonal antibody against NifD, the dinitrogenase α subunit known to be present only in heterocysts (7, 27), was also used in this experiment. Consistent with the fact that NifD is heterocyst specific, the immunodetection experiment revealed a signal of high intensity in heterocyst proteins, a signal of low intensity in total filaments, and no signal in vegetative cells (Fig. 5).
FIG. 5.
Cell-type-specific localization of FtsZAna in Anabaena sp. strain PCC 7120. Proteins were prepared from total filaments (lane 1), heterocysts (lane 2), or vegetative cells (lane 3). Similar amounts of proteins were loaded onto each lane. Immunodetection was carried out as described elsewhere (30) with a polyclonal antibody against FtsZAna, RbcL of Nicotiana sylvestris (12), or NifD of Rhodospirillum rubrum (kindly provided by P. W. Ludden, University of Wisconsin), as indicated. The position of each detected antigen is indicated by an arrow; sizes are indicated in kilodaltons.
From these results, we conclude that FtsZ is a vegetative-cell-specific element in Anabaena sp. strain PCC 7120. Our results correlate with the fact that heterocysts are terminally differentiated cells; they also indicate that the inability of heterocysts to undergo division is not simply due to a physical constraint of the thick heterocyst cell wall but rather is a result of an actively regulated process.
Sporulation in B. subtilis involves asymmetrically localized septum, and a switch of FtsZ location from medial to polar is one of the earliest signs of sporulation. In this organism, FtsZ polar localization is regulated by elements directly involved in sporulation (2, 11, 13). Although no asymmetrical septum formation has been observed at the onset of heterocyst formation in Anabaena sp. strain PCC 7120, cell-type-specific localization of FtsZ observed in the present study is likely to be regulated by some elements involved in heterocyst development. It has been shown in another developmental bacterium, Caulobacter crescentus, that FtsZ is found in the stalk cell and absent in the swarmer cell immediately after division and before the latter differentiates to a stalk cell. This cell-type-specific localization is the result of both transcriptional and proteolytic regulations of FtsZ (10, 20). Similar regulatory mechanisms could account for the cell-type-specific localization of FtsZ in Anabaena sp. strain PCC 7120. FtsZ is thus likely to provide a control point for the coordination between cell division and cell differentiation.
Acknowledgments
We thank P. Ludden (University of Wisconsin) for anti-Nif antibodies and C. Fleck (CNRS, Strasbourg, France) for anti-RuBisCO antibodies.
This work was supported by the French Ministère de l'Education Nationale, de la Recherche et de la Technologie and CNRS.
REFERENCES
- 1.Asano M, Nevins J R, Wharton R P. Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev. 1996;10:1422–1432. doi: 10.1101/gad.10.11.1422. [DOI] [PubMed] [Google Scholar]
- 2.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 3.Bramhill D. Bacterial cell division. Annu Rev Cell Dev Biol. 1997;13:395–424. doi: 10.1146/annurev.cellbio.13.1.395. [DOI] [PubMed] [Google Scholar]
- 4.Buikema W J, Haselkorn R. Molecular genetics of cyanobacterial development. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:33–52. [Google Scholar]
- 5.Doherty H M, Adams D G. Cloning and sequence of ftsZ and flanking regions from the cyanobacterium Anabaena PCC 7120. Gene. 1995;163:93–96. doi: 10.1016/0378-1119(95)00416-4. [DOI] [PubMed] [Google Scholar]
- 6.Edgar B A, Lehner C F. Developmental control of cell cycle regulators: a fly's perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- 7.Elhai J, Wolk C P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990;9:3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden J W, Robinson S J, Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985;314:419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- 9.Karim F D, Rubin G M. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Kelly A J, Sackett M J, Din N, Quardokus E, Brun Y V. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khvorova A, Zhang L, Higgins M L, Piggot P J. The spoIIE in involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J Bacteriol. 1998;180:1256–1260. doi: 10.1128/jb.180.5.1256-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lett M-C, Fleck J, Fritsch C, Durr A, Hirth L. Suitable conditions for characterization, identification, and isolation of the mRNA of the small subunit of ribulose-1,5-biphosphate carboxylase from Nicotiana sylvestris. Planta. 1980;148:211–216. doi: 10.1007/BF00380029. [DOI] [PubMed] [Google Scholar]
- 13.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 14.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Margolin W, Corbo J C, Long S R. Cloning and characterization of a Rhizobium meliloti homolog of the Escherichia coli cell division gene ftsZ. J Bacteriol. 1991;173:5822–5830. doi: 10.1128/jb.173.18.5822-5830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanninga N. Morphogenesis of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohashi Y, Chijiwa Y, Suzuki K, Takahashi K, Nanamiya H, Sato T, Hosoya Y, Ochi K, Kawamura F. The lethal effect of a benzamide derivative, 3-methoxybenzamide, can be suppressed by mutations within a cell division gene, ftsZ, in Bacillus subtilis. J Bacteriol. 1999;181:1348–1351. doi: 10.1128/jb.181.4.1348-1351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pla J, Sanchez P, Palacios P, Vincente M, Aldea M. Preferential cytoplasmic location of FtsZ, a protein essential for Escherichia coli septation. Mol Microbiol. 1991;5:1681–1686. doi: 10.1111/j.1365-2958.1991.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 20.Quardokus E, Din N, Brun Y V. Cell cycle regulation and cell type-specific localization of the FtsZ division initiation protein in Caulobacter. Proc Natl Acad Sci USA. 1996;93:6314–6319. doi: 10.1073/pnas.93.13.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 22.Rothfield L I, Justice S S. Bacterial cell division: the cycle of the ring. Cell. 1997;88:581–584. doi: 10.1016/s0092-8674(00)81899-1. [DOI] [PubMed] [Google Scholar]
- 23.Ward J E, Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox M, Mitchison G J, Smith R J. Pattern formation in the blue-green alga Anabaena. II. Controlled proheterocyst regression. J Cell Sci. 1973;13:637–649. doi: 10.1242/jcs.13.3.637. [DOI] [PubMed] [Google Scholar]
- 25.Wolk C P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D F, editor. The molecular biology of cyanobacteria. Norwell, Mass: Kluwer Academic Publishers; 1994. pp. 769–823. [Google Scholar]
- 26.Wolk C P. Heterocyst formation. Annu Rev Genet. 1996;30:59–78. doi: 10.1146/annurev.genet.30.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Yoon H-S, Golden J W. Heterocyst pattern formation controlled by a diffusible peptide. Science. 1998;282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C-C. A gene encoding a protein related to eukaryotic protein kinases from the filamentous heterocystous cyanobacterium Anabaena PCC 7120. Proc Natl Acad Sci USA. 1993;90:11840–11844. doi: 10.1073/pnas.90.24.11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C-C, Huguenin S, Friry A. Analysis of genes encoding the cell division protein FtsZ and a glutathione synthetase homologue in the cyanobacterium Anabaena PCC 7120. Res Microbiol. 1995;146:445–455. doi: 10.1016/0923-2508(96)80290-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C-C, Libs L. Cloning and characterisation of the pknD gene encoding an eukaryotic type protein kinase in the cyanobacterium Anabaena sp. PCC 7120. Mol Gen Genet. 1998;258:26–33. doi: 10.1007/s004380050703. [DOI] [PubMed] [Google Scholar]