Abstract
Background/Aim: This study aimed to investigate the prognostic significance of preoperative anemia in gastric cancer patients.
Patients and Methods: The medical records of 801 patients with gastric cancer who underwent gastrectomy at the Nara Medical University hospital, were reviewed. Anemia was defined as a hemoglobin (Hb) level of <10 g/dl. Multivariate analysis was performed to identify prognostic factors.
Results: The mean Hb level was 13.1 (SD=2.0). Sixty-four (8.0%) patients were classified into the anemic group. Anemic patients were significantly older than nonanemic patients (p=0.007). Anemia was significantly associated with cardiovascular disease (p=0.041), chronic renal failure (p<0.001), tumor depth (p<0.001), and lymph node metastasis (p=0.001). The overall survival (OS) and cause-specific survival (CSS) rates of anemic patients were significantly lower in comparison to the nonanemic patients (p<0.001). In a subgroup analysis, the OS rate of anemic patients was significantly lower than that of nonanemic patients among patients with stage I and stage II disease. According to a multivariate analysis, preoperative anemia was an independent prognostic factor for OS (p<0.001), but not CSS (p=0.555). The rate of non-cancer deaths among anemic patients was significantly higher than that among nonanemic patients (p<0.001).
Conclusion: Preoperative anemia is a simple and reliable predictor of poor prognosis, and it is associated with a higher risk of non-cancer death.
Keywords: Anemia, gastric cancer, prognosis, cause of death
Gastric cancer is the second most frequent cause of cancer-related deaths worldwide, in spite of recent improvements in early detection, progress in surgical techniques, and the development of chemotherapeutic regimens (1,2). Surgical resection remains the mainstream treatment for gastric cancer. However, recurrence is often observed in gastric cancer patients, even after curative resection. Thus, it is important to identify the factors that predict occurrence and determine their relevance in predicting the outcome.
There is a higher prevalence of anemia in patients with malignancies. Previous reports have shown that anemia is observed in 30-90% of cancer patients, depending on the type of cancer and definition of anemia (3). A number of studies have investigated the prognostic significance of anemia, and have demonstrated that anemia is associated with a worse prognosis in cancer patients (3-10). With regard to gastric cancer, however, there are little data on the clinical significance or prognostic value of preoperative anemia (11-14). We, therefore, retrospectively investigated the association between preoperative anemia and clinicopathological factors, and further attempted to evaluate its prognostic impact of anemia in gastric cancer patients.
Patients and Methods
Patients. A total of 924 patients with histologically confirmed gastric cancer underwent gastrectomy between January 2001 and December 2012 in the Nara Medical University hospital. We excluded 35 patients who underwent R2 resection, 11 patients who underwent preoperative chemotherapy, 36 patients with stage IV disease, 24 patients who simultaneously underwent an operation for another type of cancer, and 17 patients whose perioperative data were unavailable. Thus, 801 patients were analyzed in this study. Written informed consent was obtained from all of the patients. This study was approved by the Local Ethics Committee on Clinical Investigation of Nara Medical University (no. 1341).
Data. In the present study, we retrospectively obtained clinicopathological and surgical findings from the patients’ medical records. The clinicopathological findings included age, sex, preoperative comorbidities (including cerebrovascular disease, cardiovascular disease, and chronic renal failure), tumor depth, and lymph node metastasis. The stage of gastric cancer was classified according to the 7th edition of the American Joint Committee on Cancer TNM classification system (15). The surgical findings included the duration of the operation, amount of blood loss, surgical procedure, use of perioperative red blood cell (RBC) transfusion, incidence of postoperative complications, and length of postoperative hospital stay. The severity of complications was defined according to the Clavien-Dindo classification (16). In addition, we collected data from blood tests that were performed just before the operation, including the preoperative hemoglobin (Hb), albumin (Alb), lymphocyte count, and the carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels in the peripheral blood. The prognostic nutritional index (PNI) was calculated as 10×serum Alb (g/dl)+0.005×total lymphocyte count (per mm3). The body mass index (BMI) was calculated as the patient’s weight (in kilograms) immediately before surgery divided by the square of the height (in meters).
Statistical analysis. All statistical analyses were performed using the SPSS software program (version 22.0 for windows, SPSS, Chicago, IL, USA). Categorical variables were presented as numbers and percentages, groups were compared using the Chi-squared test or Fisher’s exact test. Continuous variables were expressed as the mean and standard deviation, and mean values were compared using the t-test. At the time of final follow-up (April 2016), the median follow-up period was 61.2 months. Overall survival (OS) was defined as the duration from operation to death, and cause-specific survival (CSS) was defined as the duration from the operation to death from gastric cancer. The survival curves were estimated using the Kaplan-Meier method, and the differences between the curves were analyzed by the log-rank test. The univariate and multivariate hazard ratios (HR) were calculated using a Cox proportional hazards model. All the variables that showed statistical significance in a univariate analysis were entered into a multivariate analysis. The Hb cutoff value that was selected gave the optimal separation in the OS of the low-risk and high-risk cases. p-Values of <0.05 were considered to reflect statistical significance, and confidence intervals (CI) were calculated at the 95% level.
Results
Clinicopathological characteristics and perioperative data. The mean preoperative Hb level was 13.1 (SD=2.0). The hazard ratio of a low Hb level (for OS) was highest when the Hb cutoff value was 10 g/dl (HR=3.306). Thus, the Hb cutoff value was set at 10 g/dl. As a result, 64 (8.0%) patients with an Hb level of <10 g/dl were classified into the anemic group, and 737 (92.0%) patients with an Hb level of ≥10 g/dl were classified into the nonanemic group. The relationships between the Hb level and clinicopathological characteristics are shown in Table I. The anemic patients were much older than the nonanemic patients (p=0.007). The incidence of cardiovascular disease (p=0.041) and chronic renal failure (p<0.001) in the anemic patients was higher than that of the nonanemic patients. The mean PNI and BMI values of the anemic patients were significantly lower than those of the nonanemic patients (p<0.001 and p=0.027, respectively). There was also a significant difference between the anemic and nonanemic patients in terms of tumor depth (p<0.001), lymph node metastasis (p=0.001), and baseline CEA (p<0.001) and CA 19-9 levels (p=0.002). The duration of the operation in the anemic patients was significantly shorter in comparison to the nonanemic patients (p=0.007). The anemic patients received perioperative RBC transfusion more frequently than the nonanemic patients (p<0.001). Overall, postoperative complications developed in 208 (26.0%) patients. The rate of postoperative complications in the groups did not differ to a statistically significant extent.
Table I. Clinicopathological characteristics and perioperative data according to the patient hemoglobin status.
PNI: Prognostic nutritional index; BMI: body mass index; CEA: carcinoembryonic antigen; CA: carbohydrate antigen; TG: total gastrectomy; PG: proximal gastrectomy; PPNTG: pylorus preserving nearly total gastrectomy; DG: distal gastrectomy; PPG: pylorus preserving gastrectomy; RBC: red blood cells. Age, PNI, BMI, duration the operation, blood loss, and length of postoperative hospital stay are expressed as the mean and standard deviation; the values in parentheses are percentages. aData were not available for 31 patients. bData were not available for 17 patients. cData were not available for 23 patients. dThe grade of postoperative complications was assessed according to the Clavien-Dindo classification. eIndicates the value obtained using Student’s t-test. fIndicates the value obtained using the chi-squared test. gIndicates the value obtained using Fisher’s exact test.
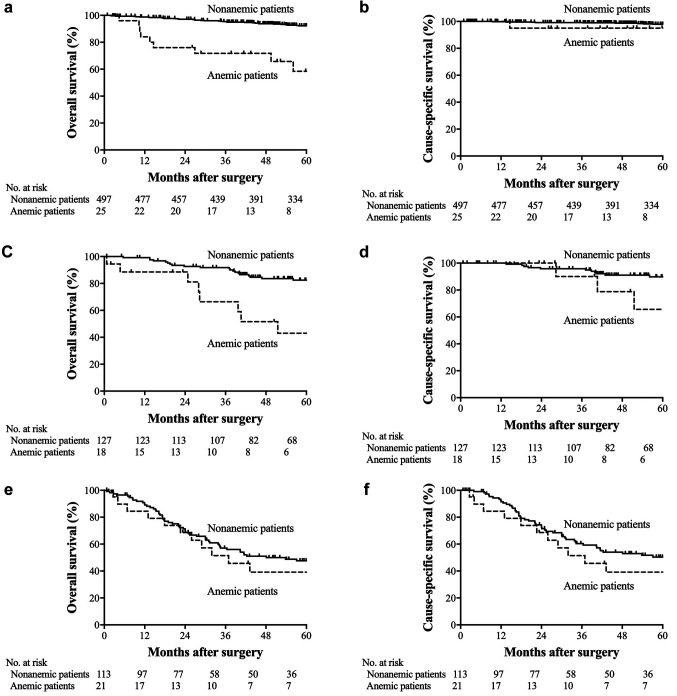
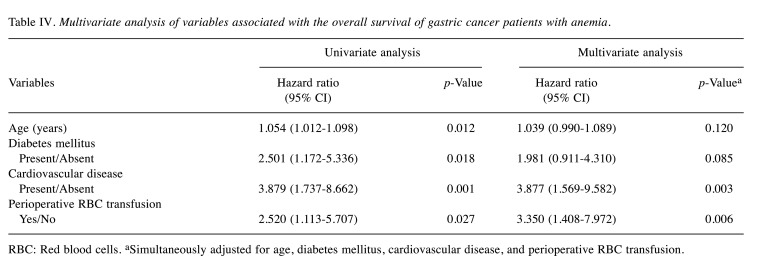
OS and CSS. The 5-year OS rate was 48.7% in the anemic patients and 83.6% in nonanemic patients (p<0.001; Figure 1a). The 5-year CSS rate was 67.6% in the anemic patients and 89.3% in the nonanemic patients (p<0.001; Figure 1b). Among the patients with stage I disease, the 5-year OS and CSS rates in the anemic patients were 58.5 and 95.0%, respectively, while those in the nonanemic patients were 92.2 and 97.8%, respectively (OS, p<0.001; CSS, p=0.321; Figures 2a and b). In stage II, the 5-year OS and CSS rates of the anemic patients were 43.0 and 65.6%, while those of the nonanemic patients were 82.5 and 89.8%, respectively (OS, p=0.001; CSS, p=0.146; Figure 2c and d). In stage III, the 5-year OS and CSS rates of the anemic patients were 39.2 and 39.2%, and those of the nonanemic patients were 47.6 and 50.4%, respectively (OS, p=0.766; CSS, p=0.573; Figure 2e and f).
Figure 1. Kaplan-Meier estimates of overall survival and cause-specific survival according to the preoperative Hb level. (a) Overall survival (p<0.001); (b) Cause-specific survival (p<0.001).
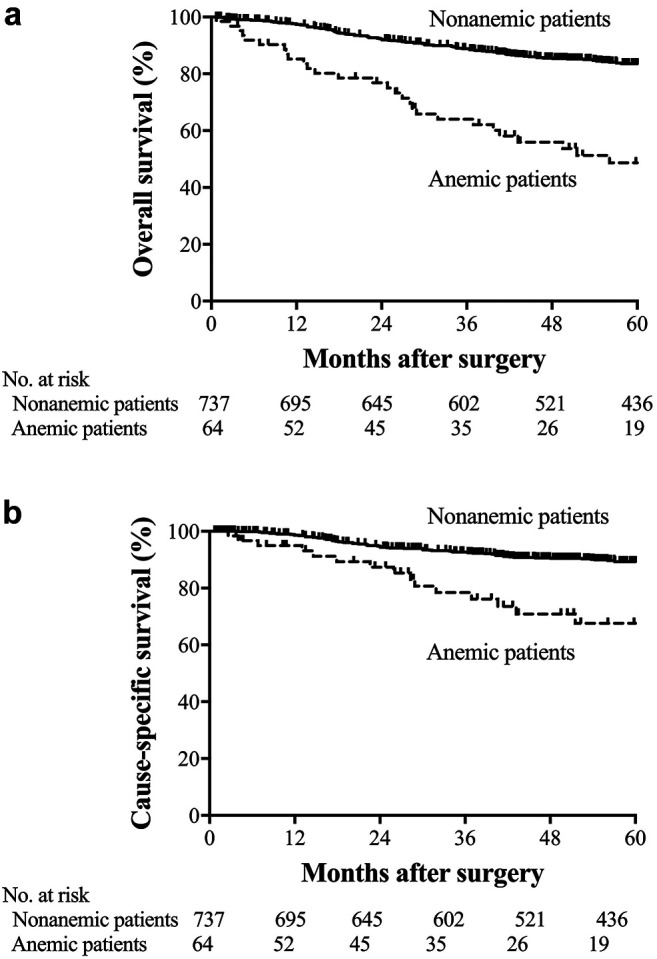
Figure 2. Kaplan-Meier estimates of overall survival and cause-specific survival according to preoperative Hb levels. (a) Overall survival among patients with stage I disease (p<0.001). (b) Cause-specific survival among patients with stage I disease (p=0.321). (c) Overall survival among patients with stage II disease (p=0.001). (d) Cause-specific survival among the patients with stage II disease (p=0.146). (e) Overall survival among patients with stage III disease (p=0.766). (f) Cause-specific survival among patients with stage III disease (p=0.573).
Causes of death. At the final follow-up, 29 of the 64 (45.3%) anemic patients, and 155 of the 737 (21.0%) nonanemic patients had died (p<0.001). The detailed causes of death are shown in Figure 3. The cause of death was tumor relapse in 15 (23.4%) anemic patients and 81 (11.0%) nonanemic patients (p=0.003). The other causes of death in the anemic and nonanemic patients were other types of cancer in 0 (0%) and 25 (3.4%) patients (p=0.134), non-cancer in 14 (21.9%) and 49 (6.6%) patients (p<0.001), respectively. The causes of non-cancer death in the anemic and nonanemic patients were as follows: pneumonia in 6 (9.4%) and 17 (2.3%) (p=0.007), cardiac disease in 1 (1.6%) and 12 (1.6%; p>0.999), cerebral disease in 2 (3.1%) and 1 (0.1%; p=0.018), sepsis in 1 (1.6%) and 4 (0.5%; p=0.341), others in 1 (1.6%) and 7 (0.9%; p=0.488), and unknown in 3 (4.7%) and 8 (1.1%; p=0.051), respectively.
Figure 3. The detailed causes of death.
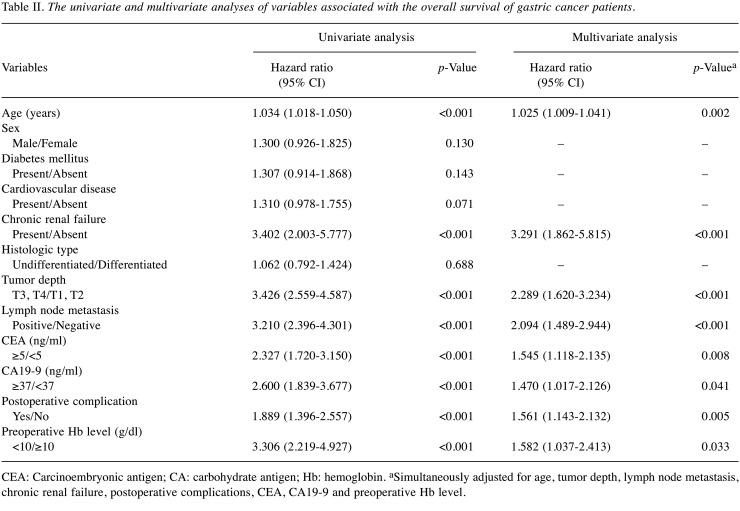
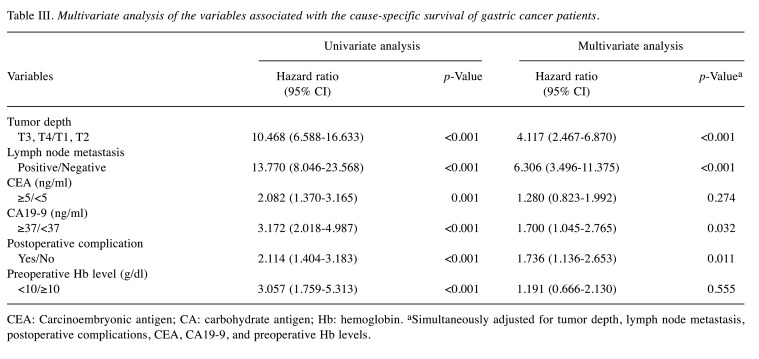
Predictive factors for overall and cause-specific survival. In univariate analysis of factors associated with OS, the HR for anemic patients was 3.306 (95% CI=2.219-4.927, p<0.001). The multivariate analysis demonstrated that the Hb level (p=0.033), as well as age (p=0.002), chronic renal failure (p<0.001), tumor depth (p<0.001), lymph node metastasis (p<0.001), CEA (p=0.008), CA 19-9 (p=0.041), postoperative complications (p=0.005) were independent prognostic factors for OS (Table II). The multivariate analysis did not identify preoperative anemia as an independent prognostic factor for CSS (95% CI=0.666-2.130, p=0.555; Table III).
Table II. The univariate and multivariate analyses of variables associated with the overall survival of gastric cancer patients.
CEA: Carcinoembryonic antigen; CA: carbohydrate antigen; Hb: hemoglobin. aSimultaneously adjusted for age, tumor depth, lymph node metastasis, chronic renal failure, postoperative complications, CEA, CA19-9 and preoperative Hb level.
Table III. Multivariate analysis of the variables associated with the cause-specific survival of gastric cancer patients.
CEA: Carcinoembryonic antigen; CA: carbohydrate antigen; Hb: hemoglobin. aSimultaneously adjusted for tumor depth, lymph node metastasis, postoperative complications, CEA, CA19-9, and preoperative Hb levels.
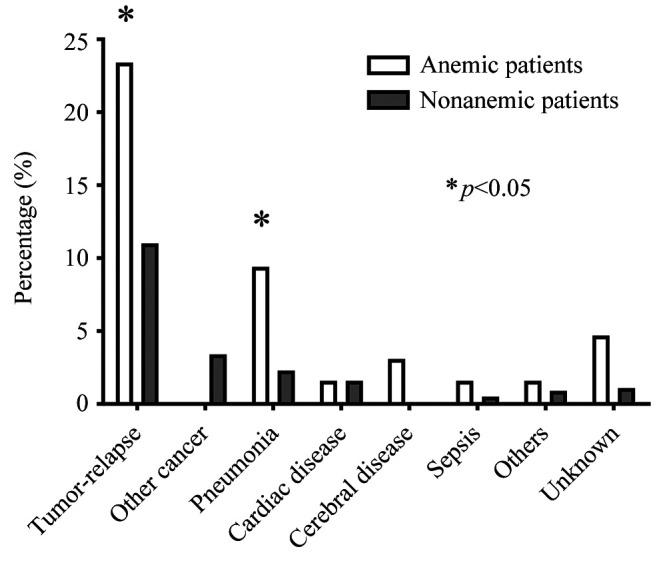
The prognostic impact of RBC transfusion. We finally investigated the prognostic impact of RBC transfusion. Among the whole study population, RBC transfusion was not an independent predictor of OS in multivariate analysis (95% CI=0.903-1.934, p=0.152). The 5-year OS rate was 36.2% in the anemic patients who received RBC transfusion and 66.4% in the anemic patients who did not (p=0.022; Figure 4a). Among anemic patients with stage I disease, the 5-year OS rate of patients who received RBC transfusion was significantly lower in comparison to patients who did not (p<0.001; Figure 4b). Among the patients with stage II and III disease, the 5-year OS rates of the patients who were treated with and without RBC transfusion were similar (p=0.871; Figure 4c). The multivariate analysis identified RBC transfusion as an independent prognostic factor for OS among anemic patients (95% CI=1.408-7.972, p=0.006; Table IV).
Figure 4. Kaplan-Meier estimates of overall survival among the anemic patients according to the performance of RBC transfusion. (a) Overall survival (p=0.002). (b) Overall survival among the anemic patients with stage I disease (p<0.001). (c) Overall survival among anemic patients with stage II and III disease (p=0.871).
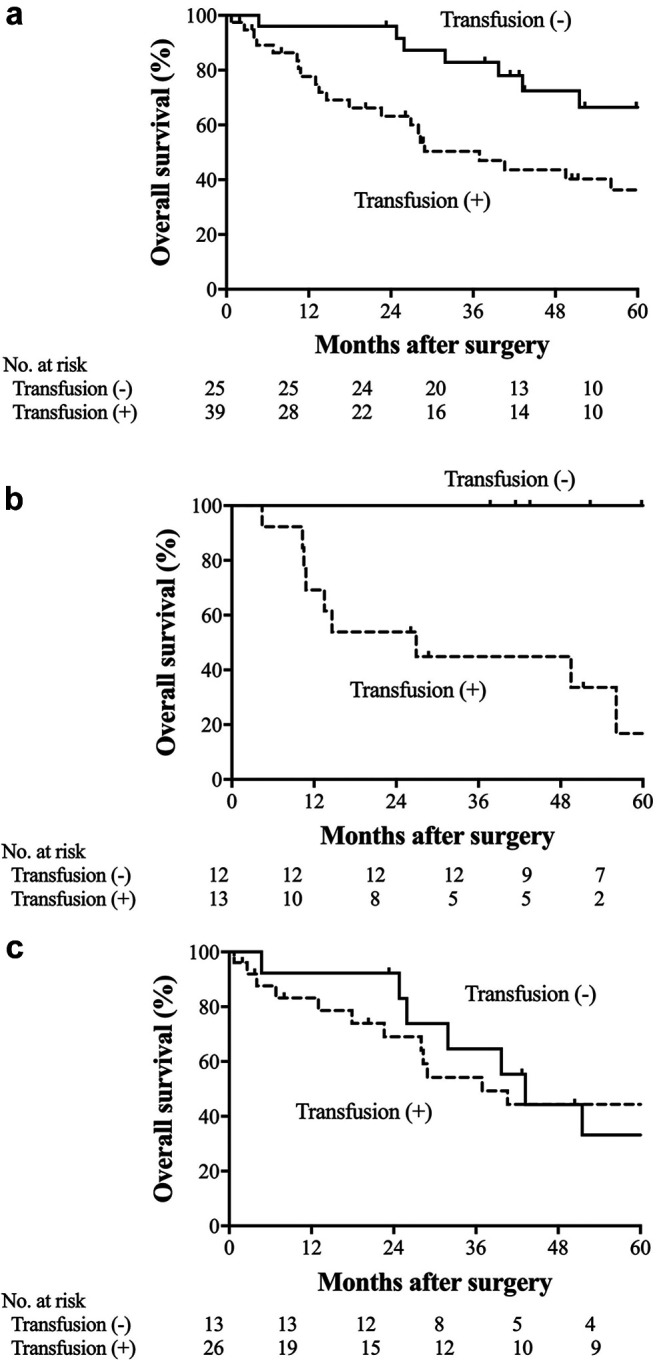
Table IV. Multivariate analysis of variables associated with the overall survival of gastric cancer patients with anemia.
RBC: Red blood cells. aSimultaneously adjusted for age, diabetes mellitus, cardiovascular disease, and perioperative RBC transfusion.
Discussion
In the present study, we evaluated the clinical significance and prognostic impact of preoperative anemia in gastric cancer patients. Previous studies showed that the anemic patients had a significantly lower postoperative survival rate in comparison to nonanemic patients in various cancers, including breast cancer, ovarian cancer, and colon cancer (4,5,10,17). In gastric cancer, one study reported that anemia was an independent prognostic factor for OS after curative resection (11,12), while others did not identify anemia as an independent prognostic factor for OS (13,14). In the present study, the 5-year OS rate of the anemic patients was significantly lower than that of the nonanemic patients. Importantly, the multivariate analysis identified preoperative anemia as an independent prognostic factor for OS (HR=1.582, 95% CI=1.037-2.413; p=0.033). These results indicate that preoperative anemia could be a simple and reliable predictor of postoperative survival in gastric cancer patients.
Previous studies on anemia in gastric cancer patients have used various Hb cutoff values, which have ranged from 10 to 13 g/dl (8,11,14,18). Thus, the prevalence of anemia reported in these studies varies. However, the optimal Hb cutoff value for predicting the long-term outcomes remains uncertain. In the present study, the Hb cutoff value was set at 10 g/dl because the highest hazard ratio for OS was observed at this level.
Some studies evaluated the association between anemia and postoperative survival in gastric cancer patients (11,12). However, the mechanism that leads to a poor prognosis in anemic patients remains uncertain. In the present study, we investigated the detailed causes of death, and found several important findings. First, anemia was associated with a high risk of non-cancer death. Shen et al. reported that anemia was independently associated with postoperative survival in patients with stage I and II gastric cancer (11). In the present study, the OS rate of anemic patients with stage I disease was significantly lower than that of nonanemic patients with stage I disease, but the CSS rates of the two groups were similar. Furthermore, 6.6% of nonanemic patients and 21.9% of the anemic patients died of causes other than cancer (p<0.001). In particular, the rate of pneumonia in the anemic patients was significantly higher than that in the nonanemic patients. These findings suggest that preoperative anemia may be associated with an increased risk of non-cancer death, in particular death from infection. In addition, the mean PNI and BMI values of anemic patients were significantly lower than those of nonanemic patients. Thus, anemia may be associated with the patient’s nutritional status.
Second, anemia does not increase the risk of death from gastric cancer. Previous studies showed that preoperative anemia was an independent prognostic factor for disease-free survival in colon and breast cancer patients (4-6). Moreover, anemia has been reported to influence the response rate to radiotherapy and/or chemotherapy (3,7-10). With regard to the reason for impaired survival and the reduced effect of therapy, it was hypothesized that hypoxia was related to tumor aggressiveness in patients with anemia (10,19-22). In the whole population of the present study, the anemic patients showed a lower CSS rate than the nonanemic patients and a significantly higher rate of gastric cancer death. These findings suggest that anemia may increase the risk of gastric cancer death. However, when the sub-population of patients with stage III disease was analyzed, the OS and CSS rates of the anemic and nonanemic patients did not differ to a statistically significant extent. Moreover, anemia was not identified as an independent prognostic factor for CSS. Our findings indicate that anemia itself may not increase the risk of gastric cancer death. Taken together, the data suggest that patients with preoperative anemia are at greater risk of non-cancer death but not gastric cancer death.
In addition, the present study demonstrated that anemia was significantly associated with factors reflecting a more advanced tumor status, such as a deeper depth of invasion and lymph node metastasis. Shen et al. reported similar results (11). Furthermore, we found a significant correlation between Hb status and elevated tumor marker levels. Thus, the preoperative Hb level may reflect the extent of tumor progression.
The influence of perioperative blood transfusion on the long-term outcomes of gastric cancer patients has been previously reported, with conflicting results. Perioperative transfusion has been demonstrated to be associated with higher rates of recurrence and worse prognoses in gastric cancer patients (23-26). In contrast, some studies have reported no association between perioperative transfusion and long-term outcomes (27,28). In the present study, anemic patients received perioperative transfusions more frequently than nonanemic patients. RBC transfusion was not independently associated with OS in multivariate analysis. These results indicate that transfusion may have little impact on the postoperative survival of gastric cancer patients. However, the 5-year OS rate of anemic patients who received RBC transfusions was significantly lower than that of the patients who did not receive RBC transfusion, despite the fact that two groups displayed similar mean Hb values (8.77±0.92 vs. 8.98±0.75; p=0.335). In addition, multivariate analysis identified perioperative transfusion as an independent prognostic factor for OS among anemic patients. These results suggest that the RBC transfusion may worsen the prognosis of gastric cancer patients with anemia. However, we could not draw the definite conclusions because the number of patients, particularly those with stage II and III disease, was relatively small, and because the indication for transfusion was determined by each surgeon without clear criteria.
Our study has several limitations. First, this study is a single-center retrospective study with a more significant surgical and selective bias than multicenter studies. Second, subgroup analyses do not have sufficient statistical power due to the relatively small sample size. Third, although we set the Hb cutoff value at 10 g/dl according to the hazard ratio for OS, the value is not validated in other populations. A larger multicenter clinical trial is warranted to address these issues.
In conclusion, the present study demonstrated that preoperative anemia was a simple and reliable predictor of a poor prognosis in gastric cancer patients. Preoperative anemia may be at a higher risk of non-cancer death.
Conflicts of Interest
The Authors declare no conflicts of interest in association with the present study.
Authors’ Contributions
T. K. and K. M. made substantial contributions to conception and design. All Authors participated in data analysis, discussed the results, and contributed to the writing of the final manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.An MS, Yoo JH, Kim KH, Bae KB, Choi CS, Hwang JW, Kim JH, Kim BM, Kang MS, Oh MK, Hong KH. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J Surg Oncol. 2015;13:64. doi: 10.1186/s12957-015-0488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Chen Y, Chen D, Jiang Y, Huang W, Ouyang H, Xing W, Zeng M, Xie X, Zeng W. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer. 2014;14:844. doi: 10.1186/1471-2407-14-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Xu B. Association of pretreatment anemia with pathological response and survival of breast cancer patients treated with neoadjuvant chemotherapy: a population-based study. PLoS One. 2015;10(8):e0136268. doi: 10.1371/journal.pone.0136268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SD, Park JW, Park KS, Lim SB, Chang HJ, Kim DY, Jeong SY, Choi HS, Oh JH. Influence of anemia on tumor response to preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis. 2009;24(12):1451–1458. doi: 10.1007/s00384-009-0762-7. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, Park YS, Kang WK, Park K, Kim S, Bang SM, Cho EK, Shin DB, Lee JH. Anemia is the strongest prognostic factor for outcomes of 5-fluorouracil-based first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2006;57(1):91–96. doi: 10.1007/s00280-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 9.Voelter V, Schuhmacher C, Busch R, Peschel C, Siewert JR, Lordick F. Incidence of anemia in patients receiving neoadjuvant chemotherapy for locally advanced esophagogastric cancer. Ann Thorac Surg. 2004;78(3):1037–1041. doi: 10.1016/j.athoracsur.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Obermair A, Handisurya A, Kaider A, Sevelda P, Kölbl H, Gitsch G. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review. Cancer. 1998;83(4):726–731. [PubMed] [Google Scholar]
- 11.Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J Surg Oncol. 2005;91(2):126–130. doi: 10.1002/jso.20272. [DOI] [PubMed] [Google Scholar]
- 12.Jatzko GR, Lisborg PH, Denk H, Klimpfinger M, Stettner HM. A 10-year experience with Japanese-type radical lymph node dissection for gastric cancer outside of Japan. Cancer. 1995;76(8):1302–1312. doi: 10.1002/1097-0142(19951015)76:8<1302::aid-cncr2820760803>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1(2):125–133. doi: 10.1007/s101200050006. [DOI] [PubMed] [Google Scholar]
- 14.Wu CW, Hsieh MC, Lo SS, Tsay SH, Li AF, Lui WY, P’eng FK. Prognostic indicators for survival after curative resection for patients with carcinoma of the stomach. Dig Dis Sci. 1997;42(6):1265–1269. doi: 10.1023/a:1018814426278. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116(22):5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91(12):2214–2221. [PubMed] [Google Scholar]
- 18.Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Satomi T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher DK, Mattheinsen U, Thews O, Vaupel P. Blood flow, oxygenation, and bioenergetic status of tumors after erythropoietin treatment in normal and anemic rats. Cancer Res. 1996;56(20):4728–4734. [PubMed] [Google Scholar]
- 20.Vaupel P, Mayer A, Briest S, Höckel M. Oxygenation gain factor: a novel parameter characterizing the association between hemoglobin level and the oxygenation status of breast cancers. Cancer Res. 2003;63(22):7634–7637. [PubMed] [Google Scholar]
- 21.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 22.Rockwell S. Oxygen delivery: implications for the biology and therapy of solid tumors. Oncol Res. 1997;9(6-7):383–390. [PubMed] [Google Scholar]
- 23.Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, Iida T, Hayata K, Yamaue H. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. 2009;13(10):1821–1830. doi: 10.1007/s11605-009-0973-9. [DOI] [PubMed] [Google Scholar]
- 24.Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Levine EA, Jin LX, Cho CS, Winslow ER, Russell MC, Staley CA, Maithel SK. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US Gastric Cancer collaborative. J Am Coll Surg. 2015;221(3):767–777. doi: 10.1016/j.jamcollsurg.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102–110. doi: 10.1016/j.ijsu.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 26.Yang K, Chen XZ, Zhang WH, Hu JK. Effect of perioperative transfusion on survival and morbidity for gastric cancer patients with gastrectomy. J Am Coll Surg. 2015;221(5):995–996. doi: 10.1016/j.jamcollsurg.2015.07.449. [DOI] [PubMed] [Google Scholar]
- 27.Rausei S, Ruspi L, Galli F, Tirotta F, Inversini D, Frattini F, Chiappa C, Rovera F, Boni L, Dionigi G, Dionigi R. Peri-operative blood transfusion in gastric cancer surgery: prognostic or confounding factor. Int J Surg. 2013;11 Suppl 1:S100–S103. doi: 10.1016/S1743-9191(13)60027-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhou HY, Yi W, Wang J, Zhang J, Wang WJ, Hu ZQ. Association of perioperative allogeneic blood transfusions and prognosis of patients with gastric cancer after curative gastrectomy. Am J Surg. 2014;208(1):80–87. doi: 10.1016/j.amjsurg.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Takayama T, Matsumoto S, Wakatsuki K, Tanaka T, Migita K, Ito M, Nakajima Y. Novel laparoscopic procedure for treating proximal early gastric cancer: laparoscopy-assisted pylorus-preserving nearly total gastrectomy. Surg Today. 2014;44(12):2332–2338. doi: 10.1007/s00595-014-0928-y. [DOI] [PubMed] [Google Scholar]