Abstract
Background/Aim: Vitamin D deficiency and vitamin D receptor (VDR) gene polymorphisms are involved in a variety of biological processes including cell proliferation, apoptosis, and adhesion in malignant tumors. This study investigated whether vitamin D levels and genetic variations of VDR are risk factors for thyroid cancer.
Patients and Methods: Patients who underwent surgery for differentiated thyroid carcinoma (n=113) and those with benign thyroid pathology (n=150) were genotyped for VDR gene polymorphisms (ApaI, TaqI, FokI, and BsmI) and their 25(OH)D levels were simultaneously measured. Demographic data and histopathologic reports were also acquired for all patients.
Results: Vitamin D levels were significantly lower in the thyroid cancer group (p=0.03). FokI and TaqI polymorphisms were more frequent in the thyroid cancer patients (p<0.001). Compared to control, the proportion of the FokI Ff genotype was increased (p<0.0006) and the proportion of the TaqI Tt genotype was also higher among patients with thyroid cancer (p<0.0001). The Ff genotype of FokI was also associated with multifocality, invasive pattern, and risk for local metastasis.
Conclusion: The VDR gene polymorphism FokI may be associated with the risk of thyroid cancer and its more aggressive forms.
Keywords: Thyroid cancer, vitamin D, 25OHvitamin D, VDR polymorphisms, FokI
Thyroid cancer has rapidly become the most frequent endocrine malignancy, accounting for 3.2% of all new cancer diagnoses (1). Its increasing incidence with a constant death rate of 0.5/100,000 persons has been attributed to a more extensive imaging thyroid screening (2). Recent studies focus on finding prognostic factors for the aggressive cases of thyroid cancer and provide evidence indicating that vitamin D, its metabolic pathways, and molecular mechanisms of action play an important role in disease progression (3-5). Multiple studies found a significant correlation between low levels of vitamin D and increased thyroid cancer risk by approximately 30% (6). The prognostic value of vitamin D in differentiated thyroid cancer staging is also extensively studied; vitamin D deficiency has been significantly associated with increased papillary thyroid cancer stage and local and distant metastasis (7-9). The latest factor in the vitamin D pathway involved in thyroid cancer evolution is the vitamin D receptor (VDR); its over-expression has been associated with lymph node metastasis and the knockdown of VDR attenuated the antiproliferative and proapoptotic effects of vitamin D in thyroid cancer tissues (10,11). Vitamin D regulates gene transcription through VDR binding and exerts antitumoral effects by stimulating differentiation, apoptosis and reducing proliferation, angiogenesis, and invasion (12). VDR gene polymorphisms (BsmI, ApaI, TaqI, FokI) have been shown to increase the risk of some types of cancer (breast, prostate, renal cell carcinoma, malignant melanoma, ovarian, colorectal, etc.) (13-15). The correlation between VDR gene polymorphisms and thyroid cancer needs to be further studied. So far, results have shown that ApaI and FokI (AA and FF genotypes, respectively) offer protection against follicular cancer, whereas FokI (TT genotype) is associated with T3/T4 stage, extrathyroid invasion and a tumor size ≥10 mm (16-18).
The aim of this study was to investigate the correlation between vitamin D status, VDR gene polymorphisms, and clinical and histopathologic findings of patients with differentiated thyroid carcinoma.
Patients and Methods
A total of 113 patients with differentiated thyroid carcinoma and 150 control subjects were enrolled in this case-controlled study. All subjects were recruited among patients who were treated in our institution between 2018 and 2020 and their diagnosis was confirmed by pathology. Serum 25(OH)D was measured in our laboratory using electrochemiluminescence on a Cobas E601 C analyzer (Roche Diagnostics, Indianapolis, IN, USA; with a measuring range=3–70 ng/ml, functional sensitivity 4.01 ng/ml, and variation coefficient of 18.5%). Current guidelines recommend a value range of 10 to 20 ng/ml for vitamin D deficiency, 20-30 ng/ml for vitamin D insufficiency, and >30 ng/ml were considered normal. Total DNA was isolated from blood in EDTA vials in maximum seven days after sample collection and verified with Nanoquant spectrophotometry. Genotyping was performed with Restriction Fragment Length Polymorphism (PCR-RFLP) technology in PCR amplified DNA fragments. In our study we determined the 4 commonly studied VDR gene polymorphisms (FokI – rs2228570, BsmI – rs1544410, ApaI – rs7975232, TaqI – rs731236). Ultrasound features (nodule size, number, presence/absence of calcifications and suspicious adenopathy, vascularization, extension) were obtained for every patient.
Histopathologic features of thyroid carcinoma were assessed according to the tumor-node-metastasis (TNM) cancer staging included tumor size (T), multifocality, local and extrathyroid invasion, lymph node metastasis (N), distant metastasis (M) and angiolymphatic invasion.
Each patient was given a written informed consent and the study was approved by the ethics committee of the C.I. Parhon National Institute of Endocrinology.
Statistical analysis was performed using MedCalc Software Ltd. version 20.027, Ostend, Belgium and Microsoft Office 365, Microsoft Excel software version 2206. Data are presented as mean±standard deviation (DS), percentages (%), odds ratios (OR), and 95% confidence intervals (CIs). p-Values were calculated using t-test or χ2 as appropriate. Statistical significance was considered at a p-value<0.05. Correlations were made using Pearson’s correlation coefficient calculator.
Results
Regarding description of demographic data (Table I), mean age value in the cancer group was 50±14.46 years old and in the control group 55.87±11.67 years old. In both groups female sex was more frequent; the cancer group included 91 female patients (80.56%) and 22 male patients (19.44%), and the control group included 137 female cases (91.33%) and 13 males (8.67%). More than half of patients came from the urban environment: 72.56% (82 cases) in the thyroid cancer group and 68.66% (103 cases) in the benign group.
Table I. Statistical data description of patients.
SD: Standard deviation.
Ultrasound features in the thyroid cancer group revealed a hipoechoic echotexture in 91 cases (80.53%) and an isoechoic echotexture in 22 patients (19.47%). Calcifications were present in 54.86% of the cancer patients (n=62) out of which 74.19% (n=46) were described as microcalcifications, 20.96% (n=13) as macrocalcifications and 4.85% (n=3) as both micro- and macrocalcifications. Most of the patients had multinodular goitre (n=83, 73.45%) and the rest of 30 (26.54%) were diagnosed with a single nodule. Mean size of thyroid nodules was 2.9 cm±1.42 DS. Eighty-seven patients (76.99%) had laterocervical lymph nodes out of which 38 (43.67%) were considered suspicious. Mean size of cervical lymph nodes was 1.07 cm±0.61 DS. Regarding localization, fifty-one of cancer sites were in the right thyroid lobe representing 54.86% of cases. The left lobe was affected in thirty-five patients (30.97%) and the isthmus in twenty-seven cases (14.17%). Regarding the control group, the ultrasound echotexture was hipoechoic in 122 cases (81.33%) and isoechoic in twenty-eight of them (18.67%). Out of the total of 150 patients, thirty-two had microcalcifications (21.33%) and 3 (2%) had both micro- and macrocalcifications. Many of the patients were diagnosed previously with multinodular goitre (n=112, 74.66%); 28 of them (18.66%) had a single thyroid nodule and 10 of them (6.68%) had diffuse enlarged goitre. Mean size of the nodules was 2.81 cm±1.69 DS. 41.33% (n=62) of the patients had laterocervical lymph nodes out of which 12 (19.35%) were considered suspicious. Mean size of cervical lymph nodes was 0.75 cm±0.41 DS. Regarding localization, the nodule was localized in the right lobe in 68 patients (45.33%) followed by the left lobe with 56 cases (37.33%) and the isthmus with 16 patients (10.66%) and 10 patients had diffuse goitre (6.68%) (Table I).
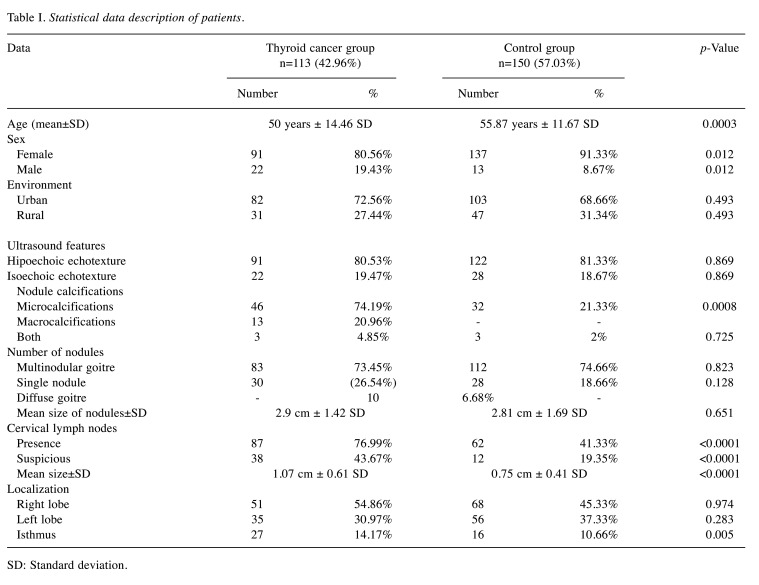
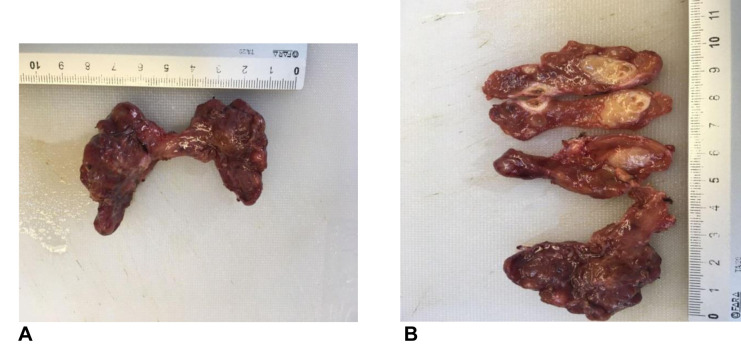
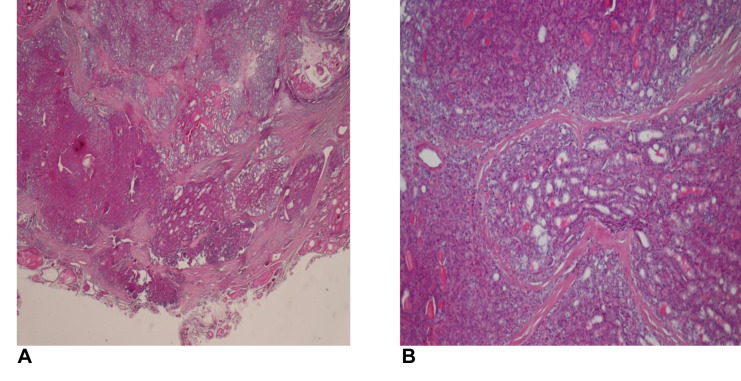
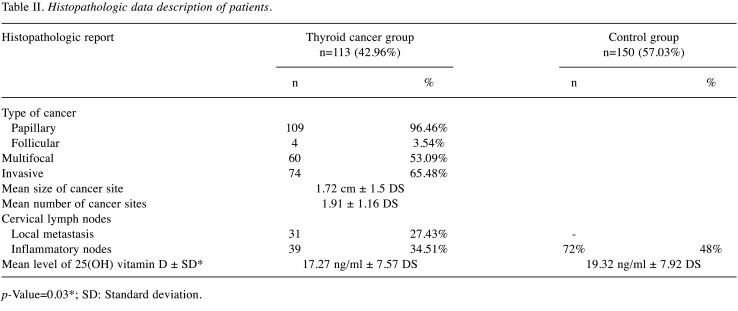
The histopathologic report (Figure 1 and Figure 2) described reactive inflammatory lymph nodes in 72 patients (48%) with benign disease. In the thyroid cancer examinations, histopathology exam revealed 109 cases of papillary thyroid carcinoma (96.46%) and 4 cases of follicular carcinoma (3.54%). Out of all thyroid cancer cases, 60 (53.09%) were multifocal and 74 (65.48%) were invasive or infiltrative in the surrounding tissue. Mean size of the cancer foci site was 1.72 cm±1.5 DS and mean number of cancer sites was 1.91±1.16 DS. The histopathologic report also showed 31 cases of local cervical metastasis (27.43%) and 39 (34.51%) reactive inflammatory lymph nodes.
Figure 1. Papillary thyroid carcinoma. A, B) macroscopic aspect.
Figure 2. Histopathologic findings. A) Papillary thyroid carcinoma with marginal and capsular invasion (HE staining ×20); B) Papillary thyroid carcinoma with solid, sclerosing and follicular variant areas (HE staining ×20).
Mean levels of 25(OH)D in the thyroid cancer group was 17.27 ng/ml±7.57 DS, which was significantly lower compared to the control group with mean levels of 19.32 ng/ml±7.92 DS (p=0.03) (Table II).
Table II. Histopathologic data description of patients.
p-Value=0.03*; SD: Standard deviation.
Although weak, vitamin D levels negatively correlated with the nodule size both in the thyroid cancer group (r=–0.36, p=0.00006) and in the control group (r=–0.33, p=0.00002) suggesting a necessity for larger studies.
After TNM classification, the patients were distributed as follows: 1 patient (0.88%) with T4, 57 (50.44%) with T3, 12 (10.61%) with T2 and 43 (38.07%) with T1. Levels of vitamin D varied by T (tumor size): mean levels of 25(OH)D for T1 were 17.11 ng/ml±8.06 SD, for T2 20.31 ng/ml±6.94 SD and for T3 it was 17.01 ng/ml±7.18 SD. For the only T4 case, the levels of 25(OH)D were 5.13 ng/ml.
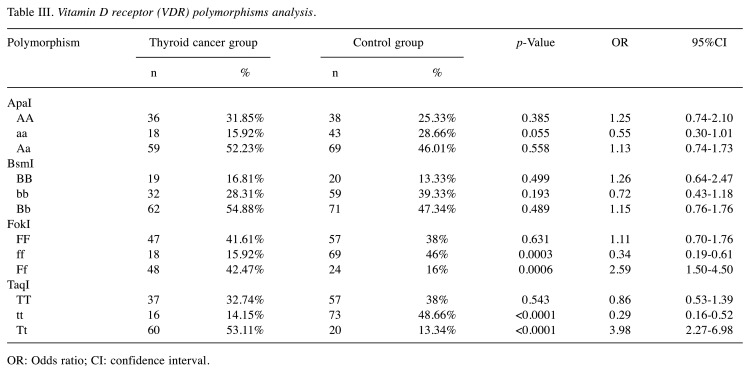
Regarding VDR polymorphism, we studied FokI (rs2228570), BsmI (rs1544410), ApaI (rs7975232), TaqI (rs731236). By comparison, statistically significant differences between the two groups were found for the FokI polymorphism and TaqI polymorphism (p<0.001). Compared to control, the rate of the FokI Ff genotype was higher in patients with thyroid cancer (OR=2.59, 95%CI=1.5-4.5, p=0.0006) and the rate of TaqI Tt genotype was also higher in patients with thyroid cancer (OR=3.98, 95%CI=2.27-6.98, p<0.0001) (Table III).
Table III. Vitamin D receptor (VDR) polymorphisms analysis.
OR: Odds ratio; CI: confidence interval.
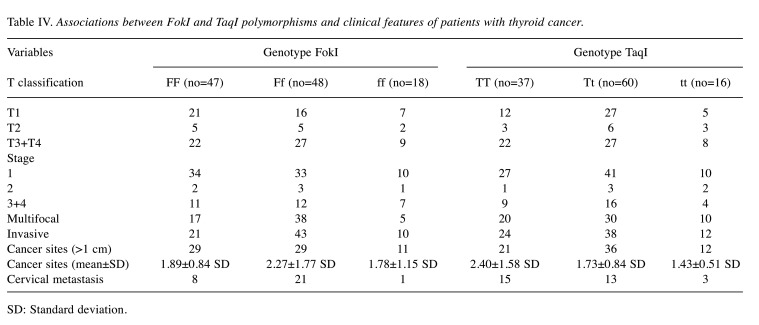
Associations between VDR FokI and TaqI polymorphisms and clinical features of patients with thyroid cancer are shown in Table IV. Patients carrying the FokI Ff genotype had increased risk for cervical metastases compared to FokI FF patients (OR=2.57, 95%CI=1.03-6.37, p=0.004). Mean size of cancer sites was also larger for Ff patients than for FF ones. Multifocality was also significantly higher in patients with Ff genotype than those with the FF genotype (OR=2.18, 95%CI=1.08-4.40, p=0.02). Patients with FokI Ff genotype had increased risk for local invasion compared to those with the FF genotype (OR=2, 95%CI=1.03-3.87, p=0.003). Although it also appears to be correlated with T3/T4, stages 3 and 4, multifocality and invasive types, there were no statistically significant differences in the association with the Tt genotype compared to the TT genotype.
Table IV. Associations between FokI and TaqI polymorphisms and clinical features of patients with thyroid cancer.
SD: Standard deviation.
Discussion
Secondary to the increasing incidence of thyroid cancer and the continuous search for risk and progression predictors, we studied the relevance of vitamin D status and VDR gene polymorphisms in a Romanian population. The study revealed a significantly lower levels of 25(OH) D in differentiated thyroid cancer patients compared to control and a significantly higher rate of FokI and TaqI polymorphisms in thyroid cancer patients compared to control. The Ff genotype of FokI and the Tt genotype of TaqI were more frequent and the Ff genotype increased the risk for cervical metastases, multifocality, invasiveness and a larger cancer site size mean. There were no statistically significant associations with TNM staging although it appears that the Ff genotype was more frequent in stages 3 or 4 of cancer. Additional studies with higher numbers of patients are needed to draw a safe conclusion.
Vitamin D is nowadays considered to be an immunomodulator with antitumoral effects and it has been associated with several types of cancer (colon, breast, prostate, etc.) and a protective effect on thyroid tissue (12). It has been suggested that vitamin D can regulate all steps in the tumorigenesis process including proliferation, differentiation, apoptosis, angiogenesis and inflammation (19). The main mechanisms for the antiproliferative and prodifferentiation effects of calcitriol comprise the regulation of growth factors, cell cycle, and signaling pathways. It increases expression of cyclin-dependent (CDK) inhibitors (p21 and p27) and inhibits expression of CDK2 leading to inhibition of cell-cycle progression (20,21). Other important anticancer pathways include: inhibition of Wnt/β-catenin signaling pathway, activation of transcription factors forkhead box o3/4, inhibition of telomerase activity, down-regulation of anti-apoptotic proteins Bcl-2 and Bcl-XL and upregulation of pro-apoptotic proteins Bax, Bak and Bad (22). These anti-tumoral effects are mediated by VDR. Regarding thyroid cancer, its occurrence and progression depends on impaired 1.25(OH)2D3 signaling, which has been confirmed in thyroid cell lines. Local signaling of the vitamin D-VDR complex has been shown to be decreased in thyroid cancer tissue with local metastasis with a full loss of signaling in metastatic anaplastic thyroid carcinoma (12,13). The anti-tumoral effect of vitamin D and potential use of therapeutic doses of vitamin D in thyroid cancer, requires VDR presence and function.
Many studies reviewed the correlation of vitamin D levels or VDR locus and different diseases (18,19). The VDR gene has been studied for its correlation with several types of cancer, but the results have been inconsistent among different populations indicating ethnic and geographical differences or interactions with multiple genetic or environmental factors. VDR polymorphisms can behave as markers for functional variants that influence expression of VDR. VDR polymorphisms may determine the levels of VDR mRNA, VDR protein and consecutive vitamin-D-mediated effect (18-21). For example, the allele f of the FokI gene polymorphism leads to a longer VDR protein by inserting a start codon, which can affect VDR activity and reduces its effectiveness as a transcriptional activator. Also, the f allele has been correlated with higher 25(OH) vitamin D levels (22-24).
Furthermore, it has been shown that VDR expression is negatively correlated with tumor malignancy, and is considered a biomarker for high-risk patients (23,24). VDR gene polymorphisms (ApaI, BsmI, TaqI, FokI) were also studied in order to establish their role as potential risk factors and predictors for tumor aggressiveness. Relevant associations between VDR polymorphisms and breast (FokI, BsmI, ApaI), prostate (FokI, BsmI, TaqI), colorectal (FokI, BsmI, TaqI) and skin cancer (Fok1, Bsm1, Taq1) have been reported demonstrating that there are certain interactions between VDR and carcinogenesis (25-27).
Data on correlations between thyroid cancer and VDR gene polymorphisms are only in vitro and very controversial. None of the polymorphisms were associated with higher risk of PTC in a German study (25,26). In other studies the genotypes were not associated with the risk of thyroid cancer (25-29) but their correlations with TNM stage and pathology characteristics was not studied. In our study, vitamin D deficiency was confirmed in thyroid cancer patients compared to control and FokI and TaqI were the only two polymorphisms with significant difference between the two groups: VDR gene FokI genotype ff (p=0.0003, OR=0.34, 95%CI=0.19-0.61) and Ff (p=0.0006, OR=2.59, 95%CI=1.5-4.5) and VDR gene TaqI genotype tt (p<0.0001, OR=0.29, 95%CI=0.16-0.52) and Tt (p<0.0001, OR=3.98, 95%CI=2.27-6.98) were more frequent in the thyroid cancer patients. Similar to our study, it has been revealed that FokI might play an important role in the etiology of papillary thyroid cancer and was associated with T3/4, stage 3 or 4, extra-thyroidal invasion, multifocality and tumor size ≥10 mm (12). Our study suggests that VDR gene FokI and TaqI polymorphisms may be associated with the appearance of differentiated thyroid carcinoma and also its prognosis being correlated with aggressiveness factors. VDR gene FokI might be taken into consideration as a prediction factor for poor clinical and pathology features and advanced stage of thyroid cancer. Studies have shown that the FokI polymorphism is the only locus with a potential impact on the VDR protein structure, being found in the coding sequence (in the first ATG starting code of VDR protein) (30-32). The small sample size and seasonal variations in vitamin D levels are limitations of this study.
Conclusion
In conclusion, our study suggests that VDR gene FokI polymorphism, especially the Ff genotype and TaqI polymorphism genotype Tt might contribute to thyroid cancer risk and more aggressive types of thyroid cancer (multifocal, invasive, metastasis) in a Romanian population. To our knowledge, this is the only study performed in Romania addressing this matter. To further investigate this, we will continue to enroll patients in order to determine whether FokI and TakI could be used as prognostic factors for the more severe forms of differentiated thyroid carcinoma.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
A.M.C. designed the study, collected the data and the statistical analysis, A.M. isolated the DNA for VDR gene polymorphisms, A.C. was responsible for vitamin D testing, M.G. was the operating surgeon in all of the cases, D.I. was the pathologist and supplied all histopathology reports and C.P. coordinated the study.
References
- 1.Bains A, Mur T, Wallace N, Noordzij JP. The role of vitamin D as a prognostic marker in papillary thyroid cancer. Cancers (Basel) 2021;13(14):3516. doi: 10.3390/cancers13143516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan KL, Li S, Tseng CH, Kim J, Nguyen DT, Dawood NB, Livhits MJ, Yeh MW, Leung AM. Rising incidence and incidence-based mortality of thyroid cancer in California, 2000-2017. J Clin Endocrinol Metab. 2020;105(6):dgaa121. doi: 10.1210/clinem/dgaa121. [DOI] [PubMed] [Google Scholar]
- 3.Tam S, Boonsripitayanon M, Amit M, Fellman BM, Li Y, Busaidy NL, Cabanillas ME, Dadu R, Sherman S, Waguespack SG, Williams MD, Goepfert RP, Gross ND, Perrier ND, Sturgis EM, Zafereo ME. Survival in differentiated thyroid cancer: Comparing the AJCC cancer staging seventh and eighth editions. Thyroid. 2018;28(10):1301–1310. doi: 10.1089/thy.2017.0572. [DOI] [PubMed] [Google Scholar]
- 4.Niculescu DA, Capatina CAM, Dusceac R, Caragheorgheopol A, Ghemigian A, Poiana C. Seasonal variation of serum vitamin D levels in Romania. Arch Osteoporos. 2017;12(1):113. doi: 10.1007/s11657-017-0407-3. [DOI] [PubMed] [Google Scholar]
- 5.Kim MJ, Kim D, Koo JS, Lee JH, Nam KH. Vitamin D receptor expression and its clinical significance in papillary thyroid cancer. Technol Cancer Res Treat. 2022;21:15330338221089933. doi: 10.1177/15330338221089933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Wang H, Zhang Z, Zhou X, Yao J, Zhang R, Liao L, Dong J. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition. 2019;57:5–11. doi: 10.1016/j.nut.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Sulibhavi A, Rohlfing ML, Jalisi SM, McAneny DB, Doherty GM, Holick MF, Noordzij JP. Vitamin D deficiency and its relationship to cancer stage in patients who underwent thyroidectomy for papillary thyroid carcinoma. Am J Otolaryngol. 2019;40(4):536–541. doi: 10.1016/j.amjoto.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Kim JR, Kim BH, Kim SM, Oh MY, Kim WJ, Jeon YK, Kim SS, Lee BJ, Kim YK, Kim IJ. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid. 2014;24(11):1618–1624. doi: 10.1089/thy.2014.0090. [DOI] [PubMed] [Google Scholar]
- 9.Roskies M, Dolev Y, Caglar D, Hier MP, Mlynarek A, Majdan A, Payne RJ. Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg. 2012;41(3):160–163. [PubMed] [Google Scholar]
- 10.Pang R, Xu Y, Hu X, Liu B, Yu J. Vitamin D receptor knockdown attenuates the antiproliferative, pro apoptotic and anti invasive effect of vitamin D by activating the Wnt/β catenin signaling pathway in papillary thyroid cancer. Mol Med Rep. 2020;22(5):4135–4142. doi: 10.3892/mmr.2020.11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T, Zhang H, He L, Wang Z, Dong W, Sun W, Zhang P. Potential use of 1-25-dihydroxyvitamin D in the diagnosis and treatment of papillary thyroid cancer. Med Sci Monit. 2018;24:1614–1623. doi: 10.12659/msm.909544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beysel S, Eyerci N, Pinarli FA, Apaydin M, Kizilgul M, Caliskan M, Ozcelik O, Kan S, Cakal E. VDR gene FokI polymorphism as a poor prognostic factor for papillary thyroid cancer. Tumour Biol. 2018;40(11):1010428318811766. doi: 10.1177/1010428318811766. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal MUN, Khan TA. Association between Vitamin D receptor (Cdx2, Fok1, Bsm1, Apa1, Bgl1, Taq1, and Poly (A)) gene polymorphism and breast cancer: A systematic review and meta-analysis. Tumour Biol. 2017;39(10):1010428317731280. doi: 10.1177/1010428317731280. [DOI] [PubMed] [Google Scholar]
- 14.Dovnik A, Dovnik NF. Vitamin D and ovarian cancer: systematic review of the literature with a focus on molecular mechanisms. Cells. 2020;9(2):335. doi: 10.3390/cells9020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izreig S, Hajek M, Edwards HA, Mehra S, Sasaki C, Judson BL, Rahmati RW. The role of vitamin D in head and neck cancer. Laryngoscope Investig Otolaryngol. 2020;5(6):1079–1088. doi: 10.1002/lio2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho IS, Gonçalves CI, Almeida JT, Azevedo T, Martins T, Rodrigues FJ, Lemos MC. Association of vitamin D pathway genetic variation and thyroid cancer. Genes (Basel) 2019;10(8):572. doi: 10.3390/genes10080572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma V, Fretwell D, Crees Z, Kerege A, Klopper JP. Thyroid cancer resistance to vitamin D receptor activation is associated with 24-hydroxylase levels but not the ff FokI polymorphism. Thyroid. 2010;20(10):1103–1111. doi: 10.1089/thy.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mele C, Caputo M, Samà MT, Bullara V, Mauri MG, Prodam F, Aimaretti G, Pagano L, Marzullo P. The role of metabolic setting in predicting the risk of early tumour relapse of differentiated thyroid cancer (DTC) Eur J Clin Nutr. 2020;74(7):1038–1046. doi: 10.1038/s41430-020-0671-y. [DOI] [PubMed] [Google Scholar]
- 19.Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50(4):1–14. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larriba MJ, González-Sancho JM, Barbáchano A, Niell N, Ferrer-Mayorga G, Muñoz A. Vitamin D is a multilevel repressor of Wnt/b-catenin signaling in cancer cells. Cancers (Basel) 2013;5(4):1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27(32):4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 22.Díaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60(8):2304–2312. [PubMed] [Google Scholar]
- 23.Al-Azhri J, Zhang Y, Bshara W, Zirpoli G, McCann SE, Khoury T, Morrison CD, Edge SB, Ambrosone CB, Yao S. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin Cancer Res. 2017;23(1):97–103. doi: 10.1158/1078-0432.CCR-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashok T, Palyam V, Azam AT, Odeyinka O, Alhashimi R, Thoota S, Sange I. Relationship between vitamin D and thyroid: an enigma. Cureus. 2022;14(1):e21069. doi: 10.7759/cureus.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu N, Zhang H. CYP24A1 depletion facilitates the antitumor effect of vitamin D3 on thyroid cancer cells. Exp Ther Med. 2018;16(4):2821–2830. doi: 10.3892/etm.2018.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penna-Martinez M, Ramos-Lopez E, Stern J, Hinsch N, Hansmann ML, Selkinski I, Grünwald F, Vorländer C, Wahl RA, Bechstein WO, Zeuzem S, Holzer K, Badenhoop K. Vitamin D receptor polymorphisms in differentiated thyroid carcinoma. Thyroid. 2009;19(6):623–628. doi: 10.1089/thy.2008.0388. [DOI] [PubMed] [Google Scholar]
- 27.Rai V, Abdo J, Agrawal S, Agrawal DK. Vitamin D receptor polymorphism and cancer: an update. Anticancer Res. 2017;37(8):3991–4003. doi: 10.21873/anticanres.11784. [DOI] [PubMed] [Google Scholar]
- 28.Köstner K, Denzer N, Müller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29(9):3511–3536. [PubMed] [Google Scholar]
- 29.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano D, Gnagnarella P, Raimondi S, Gandini S. Meta-analysis on vitamin D receptor and cancer risk: focus on the role of TaqI, ApaI, and Cdx2 polymorphisms. Eur J Cancer Prev. 2016;25(1):85–96. doi: 10.1097/CEJ.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huss L, Butt ST, Borgquist S, Elebro K, Sandsveden M, Rosendahl A, Manjer J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019;21(1):84. doi: 10.1186/s13058-019-1169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balla B, Tobiás B, Kósa JP, Podani J, Horváth P, Nagy Z, Horányi J, Járay B, Székely E, Krenács L, Árvai K, Dank M, Putz Z, Szabó B, Szili B, Valkusz Z, Vasas B, Győri G, Lakatos P, Takács I. Vitamin D-neutralizing CYP24A1 expression, oncogenic mutation states and histological findings of human papillary thyroid cancer. J Endocrinol Invest. 2015;38(3):313–321. doi: 10.1007/s40618-014-0165-7. [DOI] [PubMed] [Google Scholar]