Abstract
Background/Aim: Smaller, earlier-stage breast tumors are being found in breast cancer screening, and neoadjuvant chemotherapy is the gold standard when chemotherapy is indicated. Precise marking and localization of the tumor are thus becoming increasingly important. Wire-free localization techniques are under investigation in order to reduce presurgical radiography, pain, the risk of wire dislocation, and allow scheduling flexibility for patients and surgery departments.
Patients and Methods: This single-center observational study from June 2020 to October 2021 included 15 patients with mammographically or sonographically detected nonpalpable breast lesions. Radiofrequency identification (RFID) tags were placed preoperatively under ultrasound or radiologic guidance to localize lesions for planned surgery. All patients underwent breast conservation surgery, including one bilateral and one targeted axillary dissection.
Results: Histology identified two benign and 13 malignant lesions, including three ductal carcinomas in situ and 11 invasive breast cancers. Placement, control radiography, and handling of the RFID tag were feasible in everyday routine for different radiologists and surgeons and managed cost-effectively. All of the RFID tags were found in the specimen radiographs.
Conclusion: The feasibility and cost-effectiveness of this non-wire localization method were demonstrated in this rather small cohort of patients. Further studies including larger numbers of patients are needed to confirm the method’s accuracy.
Keywords: Breast cancer, localization, surgery, RFID
Breast cancer (BC) is the most common type of cancer found in women and the most common cause of death in women between the ages of 35 and 55 years. In Germany, approximately 70,000 women are diagnosed with BC annually (1). In addition to surgery, every BC patient requires systemic therapy to some extent. Tumor size, histology, and biomarkers are the most important factors that influence the therapy strategy in BC (2,3). The inclusion of mammography in screening procedures in order to achieve earlier detection of BC and thereby reduce the mortality rate has been one of the main strategies in recent years, and screening mammography is now included in recent local guidelines in Germany (4,5).
International BC guidelines recommend preoperative confirmation of suspicious lesions (2). In addition, nonpalpable breast lesions should be marked before surgery (2,6,7).
Most BC guidelines recommend chemotherapy in a neoadjuvant setting, if chemotherapy is indicated (2,8-10), due to its effectiveness and impact on the long-term survival of BC patients (11,12). In addition, the effectiveness of these treatments is improving through new drugs. An increase in the rate of pathological complete remissions (pCR) has been achieved with the help of these novel therapies (13). Along with the increase in pCR rates, problems are arising more frequently with the marking and identification of lesions for radiologists, surgeons, and pathologists. Localizing a lesion that has disappeared from view in order to carry out definitive breast surgery is one of the most challenging problems. Several studies have been published on the use of marker clips, demonstrating the feasibility of this method during neoadjuvant treatment and for preoperative localization of breast lesions (14-17). In addition, less radical approaches to axillary breast surgery are now being adopted. Methods of detecting lymph nodes more easily have been investigated and implemented in everyday clinical routine work — for example, with targeted axillary dissection (TAD). Studies have also validated TAD techniques for reducing the radicality of surgical approaches after neoadjuvant chemotherapy, with higher success rates than sentinel lymph-node biopsy (SLNB). Reports in the literature show that clipped lymph-node biopsy can detect sentinel lymph nodes in 95.65% of cases, in comparison with 80.16% with standard sentinel lymph-node dissection. The use of magnetic seeds for localizing axillary lymph nodes with a wireless localization method identified 37 of 38 magnetic seeds (97%) for definitive surgery (18,19). Local recommendations in Germany also suggest that TAD should be used after neoadjuvant therapy in selected cases (20).
In the breast or axilla, marker clips make it possible to locate lesions using mammography or ultrasound. However, the surgeon requires a second localization process intraoperatively. The most common method is wire-guided localization (WGL). This proven method has some disadvantages, and improvements are therefore needed to reduce the risk of infections, pneumothorax, and pain due to the additional invasive procedure, as well as to reduce the risk of wire dislocation. Another point for improvement is to make the scheduling of the procedure independent of the radiologist’s availability for wire placement. A promising method for overcoming these issues is localization using microchipping with radiofrequency identification (RFID) tags (21-25). For this procedure, an RFID tag that can be located with a special probe during surgery is placed in the lesion.
On the basis of our extensive experience with marker systems (14-17), the aims of this study were to evaluate the clipping method with RFID tags and its feasibility in clinical everyday routine work, and to compare the costs of RFID tagging to the standard wire-based method.
Patients and Methods
Study population. Fifteen consecutive patients with suspected or recurrent breast lesions and one axillary lesion, with an indication for preoperative marking of the nonpalpable lesion, were marked with RFID tags as part of clinical routine work. Histological samples were obtained preoperatively using a core needle biopsy or vacuum-assisted biopsy. The patients were examined, marked, and operated on at the University Breast Center for Franconia between June 2020 and October 2021.
Ultrasound-guided RFID marking. The procedures were carried out between zero and 7 days before surgery. The position of the RFID tag was analyzed and displayed to the surgeon using digital full-field mammography with a standard mediolateral oblique and craniocaudal projections, or via digital breast tomosynthesis (Selenia Dimensions 3D, Hologic) and ultrasound (2-D, Acuson Antares, 13 MHz, Siemens, Erlangen, Germany) in the operating theater.
The same three experienced radiologists performed intramammary RFID tagging. A single-use marking system (LOCalizer, Hologic Medicor GmbH, Kerpen, Germany) was used for RFID tagging.
The LOCalizer system is a wireless system for precise localization of breast lesions. Tag applicators with lengths of 5, 7, and 10 cm are available for marking intramammary lesions. The tag applicator is loaded with a ready-to-use RFID tag. After disinfection and application of local anesthetic, the single-use breast biopsy system (an 11-gauge coaxial cannula) is placed over the focal tumor under ultrasound or radiologic guidance and the clip is placed in the suspicious lesions. The tag is 10.6 mm long and 2 mm in diameter and has a special coating that minimizes dislocation. Each tag has its own identification number, so that each lesion can be separately identified.
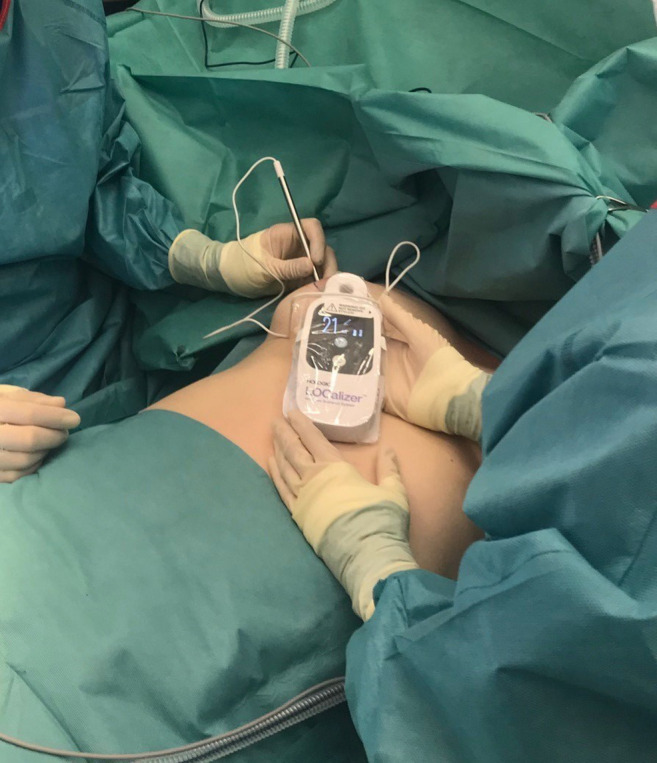
Using a hand-held probe, the tag can be located with its unique identification number. The distance between the probe, which looks like a pencil, and the tag is displayed on the hand-held display (Figure 1).
Figure 1. Intraoperative use of the LOCalizer device.
All of the patients underwent surgery, and a radiograph of the breast specimen was taken during surgery to demonstrate removal of the tag and of the suspicious lesion. After surgery, an expert pathologist with experience in breast pathology analyzed each specimen.
Results
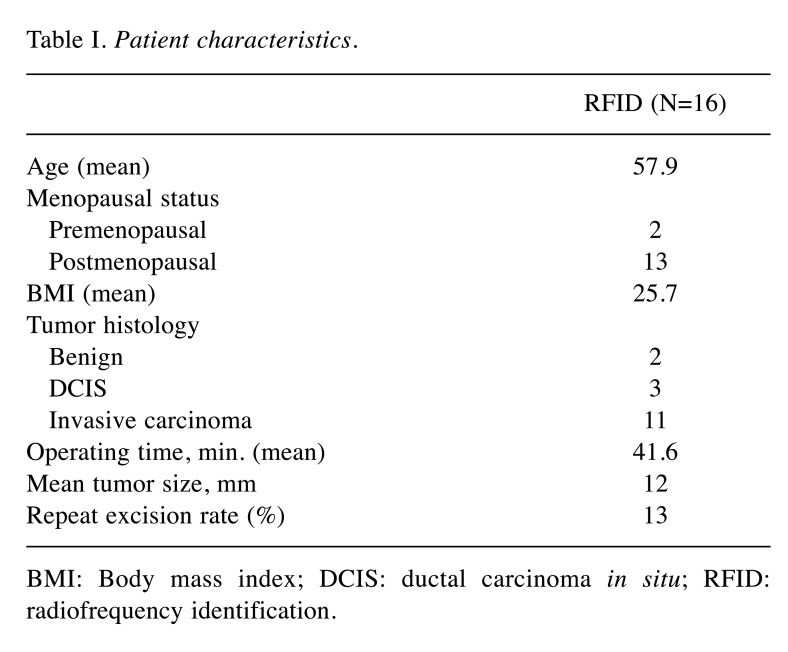
Preoperative deployment of RFID Tag and radiological evaluation of marker position. Sixteen RFID tags were deployed in 15 patients; bilateral tags were placed in one patient. Eleven RFID tags were deployed under ultrasound guidance and five with stereotactic guidance. The patients’ mean age was 57.9 years; two were premenopausal and 13 were postmenopausal. The final pathological histology findings were benign in two cases, carcinoma in situ in three cases, and invasive BC in 11 cases. After the clip had been placed in the breast, its location was verified using ultrasound and digital breast tomosynthesis (DBT) (Figure 2 and Figure 3). No dislocation of the clip was observed after removal of the applicator system. A specimen radiograph is shown in Figure 4, with the RFID tag placed in the middle of the lesion. Patient and tumor characteristics are listed in Table I.
Figure 2. Mammography for clip localization after ultrasound-guided lesion tagging, craniocaudal projection.
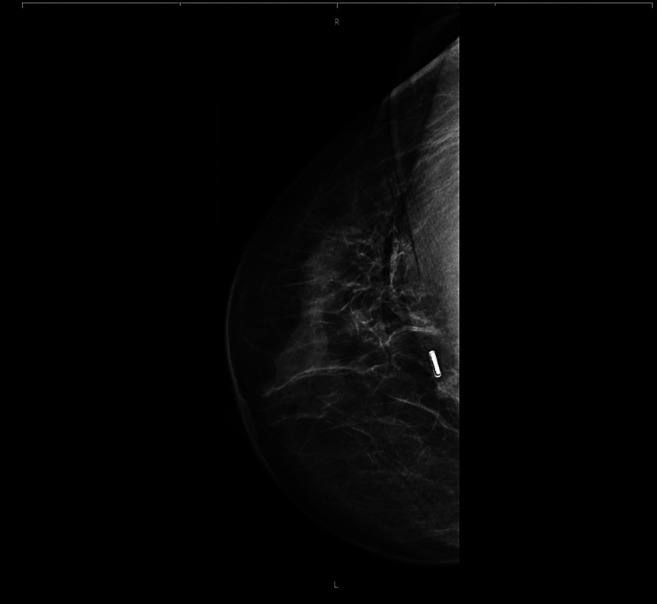
Figure 3. Mammography for clip localization, mediolateral oblique projection.
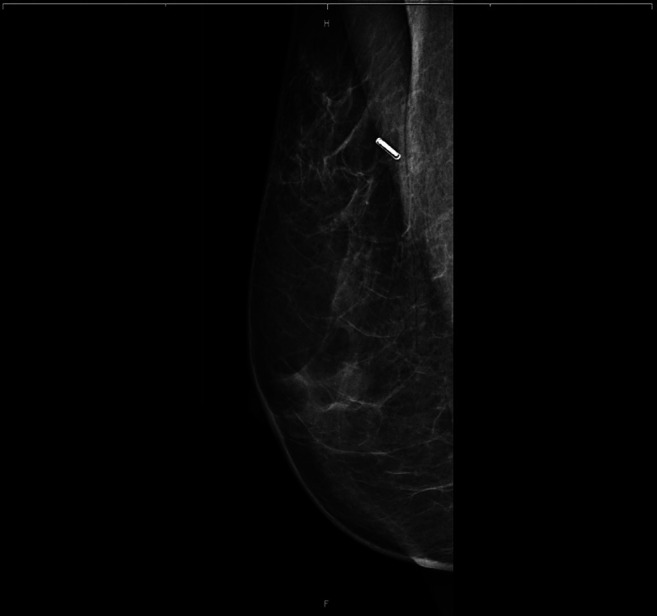
Figure 4. Specimen radiograph.
Table I. Patient characteristics.
BMI: Body mass index; DCIS: ductal carcinoma in situ; RFID: radiofrequency identification.
Postoperative pathological assessment and exclusion of intraoperative loss of clip. Fourteen patients underwent breast-conserving surgery and one patient received targeted axillary dissection. Intraoperative specimen x-rays showed the RFID tag in all of the specimens, and no clip migration was observed. Pathological assessment was carried out for all specimens. The pathologist reported no difficulties in preparing and evaluating the specimens resulting from the RFID tags. No special actions in relation to the specimens had to be taken into account – e.g., in connection with radioactivity.
Implications for clinical routine. As the marking took place on the day of the operation at the latest, there was no need for wire-guided localization on the day of surgery. It was possible to schedule the operations independently of the marking of the lesion. No loss of time in the operating theater was observed, thanks to localization and easy use of the device (Figure 1).
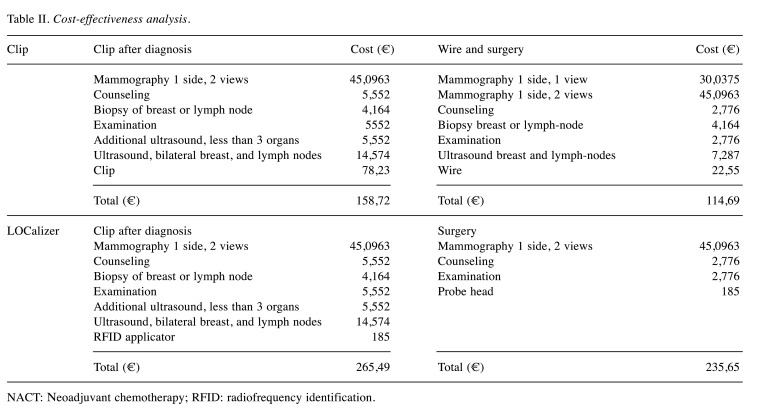
Cost-effectiveness. Costs for patients with placement of radiopaque clips before neoadjuvant chemotherapy were compared with costs for patients with RFID tags (Table II). The cost of RFID tags, including ultrasound and mammography, breast examination, placement of the clip, RFID applicator, and RFID tag is approximately twice as expensive as regular titanium clips. This calculation does not include indirect costs – for example, for scheduling operations only after WGL when this is possible for radiologists. For smaller hospitals, there is more flexibility for planning surgery when a radiologist is on duty on the day of the operation. If the radiology department and the operating theater are in different locations, patients do not have to be taken from one department to the other and back.
Table II. Cost-effectiveness analysis.
NACT: Νeoadjuvant chemotherapy; RFID: radiofrequency identification.
Discussion
The aim of this study was to evaluate the clipping method using RFID tags and its feasibility in everyday clinical routine. Currently, up to 80% of breast-conserving operations for nonpalpable breast lesions are wire-guided (26). Techniques for localizing breast lesions are becoming more important, as neoadjuvant treatment regimens are becoming more frequent and larger numbers of smaller lesions are being detected on screening mammography. Many limitations with the wire-guided approach are known, such as migration and wire breakage, patient anxiety and pain, fixed scheduling of the operation procedure, risk of pneumothorax, and very severe cases of wire dislocation (21).
Observations in the present study showed that wireless localization of nonpalpable breast lesions is feasible and safe in clinical routine work, and this has also been reported in other studies (27-29). Published data on the use of LOCalizer tags in Germany include only four patients with benign lesions, in whom the RFID tags were placed on the day of surgery, whereas in the present study the tags were placed up to 7 days in advance and also in malignant lesions (28). With regard to the time of placement of the RFID tags, there is potential for the LOCalizer tags, which can remain in breast tissue for more than 30 days, with no upper limit, as specified in the U.S. Food and Drug Administration (FDA) approval (24,27,29). In our experience, use of the LOCalizer system is time-efficient, there are no differences in operating times in comparison with breast-conserving surgery in which the lesion is wire-located, and the system does not create any problems for the histopathological preparation and assessment of the specimen. Additional advantages include improved scheduling flexibility and lack of a wire with the disadvantages mentioned above –especially in patients with large breasts and a high body mass index. In lesions located near the pectoral muscle, in which safe insertion of the wire and the risk for dislocation are greatest, the LOCalizer tags also showed promising results. The potential for removing less nontargeted tissue, with the resulting improved cosmetic results, is beneficial for all patients, particularly those with smaller breast tissue. Because of the tags’ size, they can improve access for targeted axillary dissection and be applied in approximately 10 minutes.
The RFID tag can be deployed with ultrasound or stereotactic guidance with a high degree of precision. The dislocation rate is low in comparison with wire-guided marking (30,31). The size of the study was fairly small, but all of the LOCalizer tags in close proximity to the tumor tissue were visible on specimen radiography, and no clip migration occurred. These results are in line with the literature findings (27,29).
With regard to cost aspects, the LOCalizer system is more expensive than standard marking methods, but no costs arise for scheduling of patients or for ensuring that a specialized radiologist is present in the hospital on the day of surgery. Other cost considerations such as psychological effects on the patient and potential complications with the wire-based technique are avoided. The time required for surgery and application of the clip are identical to standard marking, and depending on the organization of the department, the two methods are therefore comparable in terms of cost.
The tags can be placed with the assistance of ultrasound, mammography, and magnetic resonance imaging (MRI), and are also advantageous because there are minimal artifacts on MRI. Each RFID tag has a unique identification number, so that identification of each individual lesion is possible in patients with multiple marked lesions. This allows the surgeon to adjust the resection margins relative to the underlying malignancy.
Several other localization techniques have been examined. A Cochrane analysis published in 2015 compared different methods of radioguided occult lesion localization with wire-guided localization. No statistical differences were observed for successful localization of the clip, positive excision margins, or repeat surgery rates (32).
Migration of surgical clips is one of the major limitations of that method. Increasing pCR rates with neoadjuvant chemotherapy are leading to difficulties for radiologists in marking and localizing tumors. There is a need for localization methods that can be applied at the start of neoadjuvant chemotherapy, with little or no migration of the markers over time. These issues were not addressed in the present group of patients, and further trials are needed in which patients have markers placed before neoadjuvant chemotherapy so that potential migration of the LOCalizer tags can be investigated.
In the literature, the radioactive seed localization technique was assessed in 3,879 breast cancer patients. No significant differences between radioactive seed localization and wire-guided localization were observed in relation to surgical margins and repeat surgery rates (33). With the radiofrequency technique used for the LOCalizer system in the present study, there is no radioactivity with its associated problems, no regulatory issues, no radiation exposure for patients and surgeons, and no potential problems with disposal management. Long-term implantation of the LOCalizer tags might therefore be possible.
Another method was investigated by the iBRA-NET localization study, including 2,300 women with nonpalpable breast lesions. Patients underwent wire-guided vs. magnetic seed localization, with similar rates of lesion identification, at 99.8% versus 99.1% (p=0.048) (34). MAGSEED ferrous-steel surgical tools produce undesirable interference during detection, requiring the use of nonmagnetic instruments, and the results were not convincing for lesions deeper than 4 cm (35). In contrast, the LOCalizer works up to a tissue depth of 6 cm, with standard surgical instruments. The LOCalizer probe is only 8 mm in diameter and can be used for small incisions, and unlike most other technologies, the probe depicts the distance from the tag.
As expected, indocyanine green fluorescence lumpectomy was associated with a significantly higher rate of clear margins (87.5%) in comparison with wire-guided localization (63.3%; p=0.026) (36). In the present study, repeat surgery was necessary in two patients, in one of whom the tumor size had been estimated at 1.5 cm in diameter preoperatively. At the final pathological evaluation, the lesion was 4.6 cm in size, with the LOCalizer tag located inside the tumor on the specimen radiograph. In the other patient, the LOCalizer tag and tumor tissue were also included in the specimen radiograph, but with positive margins, even after subsequent repeat excision. Safe localization of the tumor does not correlate with clear margins and tumor size. The sample of patients was too small, with different surgeons and a lack of a learning curve, for any conclusions to be drawn regarding correlations between marking and the repeat excision rate. Repeat excision rates of 3-17% are reported in the literature, but also with limited numbers of patients (22,27).
Sample size is one of the major limitations of this study, and there was some recruitment bias, with no patients receiving neoadjuvant chemotherapy.
Prospects. The fact that the LOCalizer system displays the distance between the tag and the probe may be helpful in selecting the correct size for the specimen removed. A low repeat excision rate has been reported in other studies (22). The repeat excision rate was 13% in the present study, which is slightly higher than the rate in our institution in recent years, but in view of the small sample size no general conclusions can be drawn regarding higher repeat excision rates with the LOCalizer.
With targeted axillary dissection and a less radical approach in axillary surgery in recent years, the marking of suspicious but nonpalpable axillary lymph nodes is challenging. Marking on the skin is imprecise and marking using wire is painful and associated with discomfort for the patients. Marking of lymph nodes with clips is well established and feasible but does not make it possible to locate the clip intraoperatively without further devices (e.g., intraoperative ultrasound or x-ray). The option of ultrasound-guided placement of RFID tags may be able to solve this problem, and it should be evaluated in further studies.
Conclusion
LOCalizer RFID tagging is a new method that was successfully applied in 15 patients with both malignant and nonmalignant disease, as an effective localization system for nonpalpable breast lesions. It provides advantages for patient scheduling and flexibility and is therefore cost-effective and should be further evaluated in larger trials including patients treated with neoadjuvant chemotherapy.
Conflicts of Interest
R.E. has received honoraria from Roche, Eisai, Pfizer, BioNTech, Veracyte (PROCURE), Diaceutics, and Novartis, and research funding from Roche, NanoString Technologies, Biocartis, ZytoVision, Novartis, Cepheid, and BioNTech. J.E. has received honoraria from Novartis, Pfizer and Eisai. All of the Authors have declared that they have no conflicts of interest in relation to this study.
Authors’ Contributions
F.H.: Conceptualization, formal analysis, investigation, writing – original draft, data curation; R.S-W.: conceptualization, investigation, writing – review & editing; S.J.: conceptualization, investigation, writing – review & editing; R.E.: investigation, writing – review & editing; C.C.H.: investigation, writing – review & editing; C.P.: investigation, writing – review & editing; A.B.: investigation, writing – review & editing; P.P.: conceptualization, formal analysis, investigation, writing – original draft, data curation; J.E.: conceptualization, formal analysis, investigation, writing – original draft, data curation. The publication has been approved by all co-authors.
Acknowledgements
Hologic Medicor had no influence in the preparation of this manuscript. Sole responsibility for the content of the manuscript rests with the Authors.
References
- 1.Krebs in Deutschland für 2017/2018. 13. Ausgabe. Robert KochInstitut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Berlin, 2021. . Available at: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Krebsregisterdaten/krebs_node.html. [Last accessed on July 4, 2022]
- 2.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.4, 2021, AWMF Registernummer: 032-045OL. Available at: http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ [Last accessed on February 23, 2022]
- 3.Schütz F, Fasching PA, Welslau M, Hartkopf AD, Wöckel A, Lux MP, Janni W, Ettl J, Lüftner D, Belleville E, Kolberg HC, Overkamp F, Taran FA, Brucker SY, Wallwiener M, Tesch H, Fehm TN, Schneeweiss A, Müller V. Update Breast Cancer 2019 Part 4 - Diagnostic and therapeutic challenges of new, personalised therapies for patients with early breast cancer. Geburtshilfe Frauenheilkd. 2019;79(10):1079–1089. doi: 10.1055/a-1001-9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742. doi: 10.1007/s00330-009-1588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 6.Wallis M, Tardivon A, Helbich T, Schreer I, European Society of Breast Imaging Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur Radiol. 2007;17(2):581–588. doi: 10.1007/s00330-006-0408-x. [DOI] [PubMed] [Google Scholar]
- 7.Biganzoli L, Cardoso F, Beishon M, Cameron D, Cataliotti L, Coles CE, Delgado Bolton RC, Trill MD, Erdem S, Fjell M, Geiss R, Goossens M, Kuhl C, Marotti L, Naredi P, Oberst S, Palussière J, Ponti A, Rosselli Del Turco M, Rubio IT, Sapino A, Senkus-Konefka E, Skelin M, Sousa B, Saarto T, Costa A, Poortmans P. The requirements of a specialist breast centre. Breast. 2020;51:65–84. doi: 10.1016/j.breast.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ditsch N, Kolberg-Liedtke C, Friedrich M, Jackisch C, Albert US, Banys-Paluchowski M, Bauerfeind I, Blohmer JU, Budach W, Dall P, Fallenberg EM, Fasching PA, Fehm T, Gerber B, Gluz O, Harbeck N, Heil J, Huober J, Kreipe HH, Krug D, Kühn T, Kümmel S, Loibl S, Lüftner D, Lux MP, Maass N, Mundhenke C, Nitz U, Park-Simon TW, Reimer T, Rhiem K, Rody A, Schmidt M, Schneeweiss A, Schütz F, Sinn HP, Solbach C, Solomayer EF, Stickeler E, Thomssen C, Untch M, Witzel I, Wöckel A, Müller V, Janni W, Thill M. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: Update 2021. Breast Care (Basel) 2021;16(3):214–227. doi: 10.1159/000516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stickeler E, Aktas B, Behrens A, Belleville E, Ditsch N, Fasching PA, Fehm TN, Hartkopf AD, Jackisch C, Janni W, Kolberg-Liedtke C, Kolberg HC, Lüftner D, Lux MP, Müller V, Schneeweiss A, Schütz F, Schulmeyer CE, Tesch H, Thomssen C, Uleer C, Untch M, Welslau M, Wöckel A, Wurmthaler LA, Würstlein R, Thill M. Update Breast Cancer 2021 Part 1 - Prevention and early stages. Geburtshilfe Frauenheilkd. 2021;81(5):526–538. doi: 10.1055/a-1464-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomssen C, Fehm TN, Stickeler E, Fasching PA, Janni W, Kolberg-Liedtke C, Kolberg HC, Lüftner D, Müller V, Schütz F, Belleville E, Bader S, Untch M, Welslau M, Thill M, Hartkopf AD, Tesch H, Ditsch N, Lux MP, Wöckel A, Aktas B, Schneeweiss A, Würstlein R. Update Breast Cancer 2021 Part 4 - Prevention and early stages. Geburtshilfe Frauenheilkd. 2022;82(2):206–214. doi: 10.1055/a-1724-9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 12.Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22(5):1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 13.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J, KEYNOTE-522 Investigators Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 14.Schulz-Wendtland R, Heywang-Köbrunner SH, Aichinger U, Krämer S, Wenkel E, Bautz W. [Do tissue marker clips after sonographically or stereotactically guided breast biopsy improve follow-up of small breast lesions and localisation of breast cancer after chemotherapy?] Rofo. 2002;174(5):620–624. doi: 10.1055/s-2002-28278. [DOI] [PubMed] [Google Scholar]
- 15.Schulz-Wendtland R, Dankerl P, Dilbat G, Bani M, Fasching PA, Heusinger K, Lux MP, Loehberg CR, Jud SM, Rauh C, Bayer CM, Beckmann MW, Uder M, Meier-Meitinger M, Brehm B. Evaluation of newly adapted clip marker system in ultrasound-guided core needle biopsy for suspicion of breast cancer. Geburtshilfe Frauenheilkd. 2013;73(11):1135–1138. doi: 10.1055/s-0033-1351086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz-Wendtland R, Dankerl P, Dilbat G, Bani M, Fasching PA, Heusinger K, Lux MP, Loehberg CR, Jud SM, Rauh C, Bayer CM, Beckmann MW, Wachter DL, Uder M, Meier-Meitinger M, Brehm B. Comparison of sonography versus digital breast tomosynthesis to locate intramammary marker clips. Geburtshilfe Frauenheilkd. 2015;75(1):72–76. doi: 10.1055/s-0034-1396164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz-Wendtland R, Dankerl P, Bani MR, Fasching PA, Heusinger K, Lux MP, Jud SM, Rauh C, Bayer CM, Schrauder MG, Beckmann MW, Uder M, Brehm B, Loehberg CR. Evaluation of a marker clip system in sonographically guided core needle biopsy for breast cancer localization before and after neoadjuvant chemotherapy. Geburtshilfe Frauenheilkd. 2017;77(2):169–175. doi: 10.1055/s-0042-124191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores-Funes D, Aguilar-Jiménez J, Martínez-Gálvez M, Ibáñez-Ibáñez MJ, Carrasco-González L, Gil-Izquierdo JI, Chaves-Benito MA, Ayala-De La Peña F, Nieto-Olivares A, Aguayo-Albasini JL. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: Preliminary results. Surg Oncol. 2019;30:52–57. doi: 10.1016/j.suronc.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood HI, Wong JM, Mukhtar RA, Alvarado MD, Price ER. Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol. 2019;213(4):953–957. doi: 10.2214/AJR.19.21378. [DOI] [PubMed] [Google Scholar]
- 20.Ago Breast Committee Diagnosis and treatment of patients with primary and metastatic breast cancer. Recommendations 2021. Available at: www.ago-online.de. [Last accessed on February 17, 2022]
- 21.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol. 2015;204(6):W720–W723. doi: 10.2214/AJR.14.13201. [DOI] [PubMed] [Google Scholar]
- 22.DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC. Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat. 2019;175(1):165–170. doi: 10.1007/s10549-019-05143-w. [DOI] [PubMed] [Google Scholar]
- 23.Wazir U, Tayeh S, Perry N, Michell M, Malhotra A, Mokbel K. Wireless breast localization using radio-frequency identification tags: The first reported European experience in breast cancer. In Vivo. 2020;34(1):233–238. doi: 10.21873/invivo.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGugin C, Spivey T, Coopey S, Smith B, Kelly B, Gadd M, Hughes K, Dontchos B, Specht M. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat. 2019;177(3):735–739. doi: 10.1007/s10549-019-05355-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee MK, Sanaiha Y, Kusske AM, Thompson CK, Attai DJ, Baker JL, Fischer CP, DiNome ML. A comparison of two non-radioactive alternatives to wire for the localization of non-palpable breast cancers. Breast Cancer Res Treat. 2020;182(2):299–303. doi: 10.1007/s10549-020-05707-1. [DOI] [PubMed] [Google Scholar]
- 26.Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;(12):CD009206. doi: 10.1002/14651858.CD009206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullinane CM, Byrne J, Akmenkalne L, O’ Leary DP, Connors AM, Corrigan MA, Redmond HP, Kelly L, O’ Sullivan MJ. The LOCalizer radiofrequency identification system: An effective new technology for localizing non-palpable breast lesions for surgery. Surg Innov. 2021;28(4):473–478. doi: 10.1177/1553350620967853. [DOI] [PubMed] [Google Scholar]
- 28.Malter W, Holtschmidt J, Thangarajah F, Mallmann P, Krug B, Warm M, Eichler C. First reported use of the Faxitron LOCalizer™ radiofrequency identification (RFID) system in Europe - A feasibility trial, surgical guide and review for non-palpable breast lesions. In Vivo. 2019;33(5):1559–1564. doi: 10.21873/invivo.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parisi S, Ruggiero R, Gualtieri G, Volpe ML, Rinaldi S, Nesta G, Bogdanovich L, Lucido FS, Tolone S, Parmeggiani D, Gambardella C, Docimo L. Combined LOCalizer™ and intraoperative ultrasound localization: First experience in localization of non-palpable breast cancer. In Vivo. 2021;35(3):1669–1676. doi: 10.21873/invivo.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberman L, Dershaw DD, Morris EA, Abramson AF, Thornton CM, Rosen PP. Clip placement after stereotactic vacuum-assisted breast biopsy. Radiology. 1997;205(2):417–422. doi: 10.1148/radiology.205.2.9356622. [DOI] [PubMed] [Google Scholar]
- 31.Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology. 1997;205(2):407–415. doi: 10.1148/radiology.205.2.9356621. [DOI] [PubMed] [Google Scholar]
- 32.Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;(12):CD009206. doi: 10.1002/14651858.CD009206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang GL, Tsikouras P, Zuo HQ, Huang MQ, Peng L, Bothou A, Zervoudis S, Tobias Teichmann A. Radioactive seed localization and wire guided localization in breast cancer: A systematic review and meta-analysis. J BUON. 2019;24(1):48–60. [PubMed] [Google Scholar]
- 34.Dave RV, Barrett E, Morgan J, Chandarana M, Elgammal S, Barnes N, Sami A, Masudi T, Down S, Holcombe C, Potter S, Somasundaram SK, Gardiner M, Mylvaganam S, Maxwell A, Harvey J, iBRA-NET Localisation Study collaborative Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br J Surg. 2022;109(3):274–282. doi: 10.1093/bjs/znab443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes MK. Update on preoperative breast localization. Radiol Clin North Am. 2017;55(3):591–603. doi: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Tong M, Guo W. Indocyanine green fluorescence-guided lumpectomy of nonpalpable breast cancer versus wire-guided excision: A randomized clinical trial. Breast J. 2019;25(2):278–281. doi: 10.1111/tbj.13207. [DOI] [PubMed] [Google Scholar]