Abstract
Objective
The serum lipidomic profile associated with neuropathy in type 2 diabetes is not well understood. Obesity and dyslipidemia are known neuropathy risk factors, suggesting lipid profiles early during type 2 diabetes may identify individuals who develop neuropathy later in the disease course. This retrospective cohort study examined lipidomic profiles 10 years prior to type 2 diabetic neuropathy assessment.
Methods
Participants comprised members of the Gila River Indian community with type 2 diabetes (n = 69) with available stored serum samples and neuropathy assessment 10 years later using the combined Michigan Neuropathy Screening Instrument (MNSI) examination and questionnaire scores. A combined MNSI index was calculated from examination and questionnaire scores. Serum lipids (435 species from 18 classes) were quantified by mass spectrometry.
Results
The cohort included 17 males and 52 females with a mean age of 45 years (SD = 9 years). Participants were stratified as with (high MNSI index score > 2.5407) versus without neuropathy (low MNSI index score ≤ 2.5407). Significantly decreased medium‐chain acylcarnitines and increased total free fatty acids, independent of chain length and saturation, in serum at baseline associated with incident peripheral neuropathy at follow‐up, that is, participants had high MNSI index scores, independent of covariates. Participants with neuropathy also had decreased phosphatidylcholines and increased lysophosphatidylcholines at baseline, independent of chain length and saturation. The abundance of other lipid classes did not differ significantly by neuropathy status.
Interpretation
Abundance differences in circulating acylcarnitines, free fatty acids, phosphatidylcholines, and lysophosphatidylcholines 10 years prior to neuropathy assessment are associated with neuropathy status in type 2 diabetes.
Introduction
Peripheral neuropathy is a common complication of type 2 diabetes, ranging in prevalence from 10 to over 50% in various cohorts. 1 , 2 , 3 , 4 , 5 , 6 Peripheral neuropathy symptoms manifest as a loss of sensation and pain in a length‐dependent manner. 1 Peripheral neuropathy in type 2 diabetes remains recalcitrant to effective treatment; glucose control only marginally prevents neuropathy onset and development. 7 This has spurred interest in identifying metabolic factors early during type 2 diabetes, including modifiable factors, which identify patients most at risk to implement lifestyle interventions in those patients.
Over the past decade, numerous studies have provided evidence that obesity and dyslipidemia are important neuropathy risk factors, 8 independent, even, of glycemic status. In the Danish ADDITION type 2 diabetes cohort, peripheral neuropathy is associated with decreased high‐density lipoprotein cholesterol. 3 This relationship was also present in older populations, in the Health, Aging and Body Composition study, 9 and in youths, in the SEARCH for Diabetes in Youth study. 5 Elevated triglycerides associated with peripheral neuropathy in the Danish Centre for Strategic Research in Type 2 Diabetes cohort 10 and risk for nontraumatic lower‐extremity amputations in the DISTANCE study. 11 In these investigations, measurements were limited to a basic lipid profile. Recent mass spectrometry advances allow the identification and quantification of a larger array of lipids, termed the lipidome, from biosamples. These lipidome studies can identify relevant disease biomarkers at a more granular level and shed light on pathogenesis to develop rationalized, targeted therapies. 12 , 13 , 14 , 15 , 16 , 17
Previously, we reported serum abundance differences in the lipidome in type 2 diabetes patients, which correlated with diabetic kidney disease (DKD) one decade later. 13 In cross‐sectional studies, we also reported stepwise trends in plasma abundance in free fatty acids, acylcarnitines, diacylglycerols, sphingomyelins, and various additional complex lipids by carbon and double bond number in type 2 diabetes patients with compared to those patients without neuropathy. 18 We similarly found lipidomic signatures differentiated obese patients with versus without peripheral neuropathy, independent of glycemic status, 19 a finding replicated in preclinical animal models. 15 , 20 , 21 Collectively, these observations suggest that free fatty acids, acylcarnitines, and complex lipids may be differentially linked with a known diagnosis of neuropathy. However, they do not address whether the serum lipidome can predict future incident neuropathy.
This present retrospective cohort study profiled free fatty acid, acylcarnitine, and complex lipid abundance in 18 lipid classes by carbon number and saturation level in individuals with type 2 diabetes 10 years prior to their neuropathy assessment. The objective was to evaluate correlations between serum lipidomics profile to future incident peripheral neuropathy development. The peripheral neuropathy stage was stratified by Michigan Neuropathy Screening Instrument (MNSI) index scores (low, score ≤ 2.5407; high, score > 2.5407), an index that combines the MNSI physical examination score and MNSI symptom questionnaire. Study participants are members of the Gila River Indian community and comprise one of the longest‐running studies of type 2 diabetes. 22 Since we found that the lipidome early during type 2 diabetes correlates with the onset and progression of DKD severity a decade later, 13 we anticipated a relationship would also emerge with neuropathy and specific lipid profiles. Indeed, we identified abundance differences in circulating acylcarnitines, free fatty acids, phosphatidylcholines, and lysophosphatidylcholines, which were linked to neuropathy severity 10 years later in this type 2 diabetes cohort. This is, to our knowledge, the first study to demonstrate that serum lipidomic signatures can associate with the presence and severity of future peripheral neuropathy.
Participants and Methods
Participant population and diabetic peripheral neuropathy diagnosis
Study population details and participant recruitment are published elsewhere. 23 Briefly, the population comprised American Indians from the Gila River Indian Community who were participating in a longitudinal study of diabetes and its complications (n = 169). They were recruited between 1996 and 2001 for a randomized, double‐blind, placebo‐controlled clinical trial to assess the efficacy of an angiotensin receptor blocker on the development and progression of DKD in type 2 diabetes (ClinicalTrials.gov, NCT00340678). 23 Of the 169 clinical trial participants, 23 89 were subsequently enrolled in a long‐term observational study, which included a neuropathy evaluation (Fig. 1). Of these, 69 participants met the eligibility criteria for this study, which included the availability of a stored serum sample 10 years prior to the neuropathy evaluation by MNSI examination and MSNI questionnaire. 24 , 25 All participants meeting the eligibility criteria were included in this study.
Figure 1.
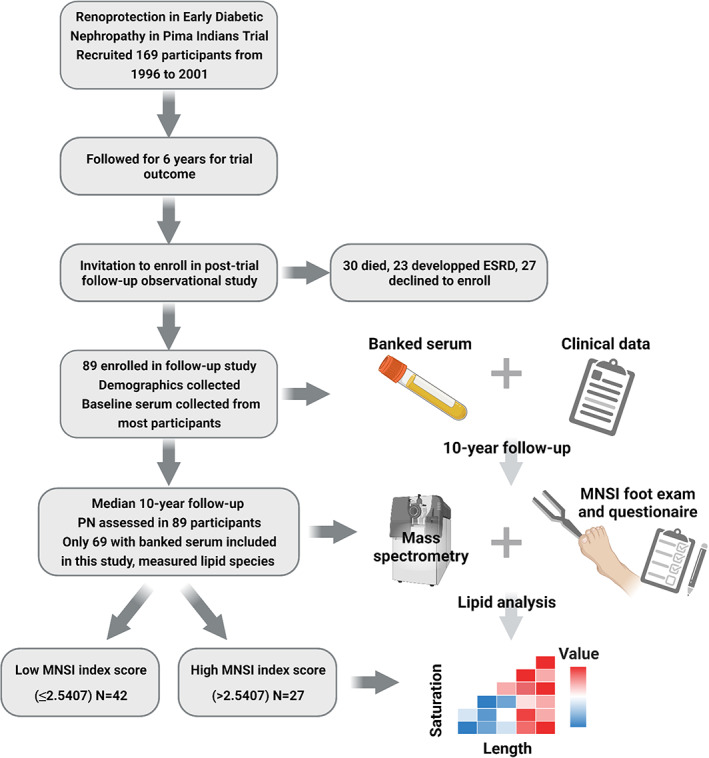
Flow diagram of the study strategy. Participants (n = 169) were originally recruited for the Renoprotection in Early Diabetic Nephropathy in Pima Indians trial from 1996 to 2001. Participants were followed for 6 years to assess the trial outcome. Of the original 169 clinical trial participants, 89 were subsequently enrolled in a long‐term observational study, which banked baseline serum and collected baseline clinical data (age, sex, height, weight, BMI, blood pressure, heart rate, diabetes duration, FPG, HbA1c, total cholesterol, triglycerides, GFR, urine ACR, and medication use). At a mean 10‐year follow‐up, all 89 participants were reexamined, and peripheral neuropathy was assessed by MNSI examination (foot ulceration, vibration, and ankle reflexes) and questionnaire. Of these, 69 participants met the eligibility criteria for this study, which included the availability of a stored serum sample 10 years prior to the neuropathy evaluation. Banked serum from 10 years prior was analyzed by mass spectrometry; 435 lipids from the 18 classes were quantitated and their abundance by chain length and saturation were analyzed. ACR, albumin creatinine ratio; ESRD, end‐stage renal disease; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; MNSI, Michigan Neuropathy Screening Instrument; PN, peripheral neuropathy. Figure created in BioRender.com. [Colour figure can be viewed at wileyonlinelibrary.com]
The MNSI examination consists of a foot exam, which assesses ulceration, vibration, and ankle reflexes, and scores 0 to 8. The MNSI questionnaire consists of 15 self‐administered questions related to symptoms and clinical history and scores of 0–15. The combined MNSI index score (hereafter referred to as MSNI index score) is calculated by a weighted sum of the individual four MSNI examination domains (the eight scores for left and right foot are combined to give four) and 15 MSNI questionnaire components. The weight of components is regression coefficients from a multivariable logistic regression model, which most accurately predicts definite neuropathy. 25 An MNSI index cutoff of >2.5407 indicates the presence of neuropathy.
Participants did not have a baseline MNSI examination or questionnaire. To evaluate baseline status, the risk of baseline of diabetic neuropathy was estimated using a “diabetic neuropathy prediction risk score”. 26 This method leverages an artificial intelligence neural network‐based approach to predict neuropathy status from clinical risk factors, including age, height, weight, diabetes duration, glycated hemoglobin, urine albumin‐to‐creatinine ratio, and total cholesterol. The model achieves over 70% accuracy for predicting neuropathy status, as assessed by continuous‐scale vibration perception threshold using a neurothesiometer. 26 Based on the diabetic neuropathy prediction risk score, the probability of baseline neuropathy was only 5.5% (SD = 1.0%) in patients with low MNSI index and 5.7% (SD = 0.7%) in patients with high MNSI index, which did not differ significantly (p = 0.367). Therefore, participants likely did not have neuropathy at baseline.
The clinical data at the time of serum sample collection were used to describe baseline participant characteristics. The study was approved by the Institutional Review Board #0000006 at the National Institute of Diabetes, Digestive, and Kidney Diseases, Bethesda, Maryland. All participants gave signed informed consent prior to their participation in the study.
Sample preparation and mass spectrometry
Samples were prepared and mass spectrometry quantified lipids from 18 classes per our published protocols. 12 , 14 , 27 Details of sample preparation and lipid analyses are provided in Supplemental Methods.
Measured lipids
Four hundred and thirty‐five lipids were quantitated from the 18 classes (Table S1). Classes that consisted of two or fewer lipid species (monoacylglycerols, plasmenyl‐phosphatidylcholines, phosphatidic acids, phosphatidylglycerols, phosphatidylserines, ceramide phosphates) were eliminated. After combining the different mass spectrometry adducts of the same feature, 236 unique lipids were included in the final analysis, including 16 free fatty acids (6.8%), 76 glycerolipids (32.2%; diacylglycerols and triacylglycerols), 12 cholesteryl‐esters (5.1%), 83 phospholipids (35.1%; phosphatidylcholines, phosphatidylethanolamines, lysophosphatidylcholines, lysophosphatidylethanolamines, plasmenyl‐phosphatidylethanolamines, phosphatidylinositols), 20 sphingomyelins (8.5%), and 29 acylcarnitines (12.3%).
Quality control
A pool of study samples was injected at the beginning and after every 20 mass spectrometry runs in the lipidomic study, and after every 15 mass spectrometry runs in the acylcarnitine study, to assess the stability of measures over time and identify any batch effects.
Statistical analysis
Mean (±SD) or frequency (percentage) was used to describe normally distributed continuous and categorical variables, respectively. The median and interquartile ranges were used to describe nonnormally distributed continuous and categorical variables, respectively. Participant baseline characteristics for normally distributed continuous variables were compared using the t test for two groups, whereas Kolmogorov–Smirnov test compared skewed continuous variables and chi‐square to compare categorical variables. Lipidomics data were prepared for analysis by batching and sum normalizing the raw peak intensities by lipid species within each lipid subclass, which were logit transformed and z‐score standardized. 12 Models were adjusted by age, sex, body mass index (BMI), and systolic and diastolic blood pressure, glycated hemoglobin (HbA1c), statin use, and use of other lipid‐lowering agents due to their established association with neuropathy, and with lipid class carbon number and number of double bond due to test the effect of chain length and saturation status with the outcome, that is, the presence of neuropathy (high MNSI index >2.5407) or the absence of neuropathy (low MNSI index ≤2.5407). We used backward elimination of nonsignificant covariates. We also performed a Pearson's correlation analysis treating the MNSI index as a continuous variable correlated to each lipid within each lipid class and to each component of the metabolic syndrome.
Results
Cohort characteristics
Sixty‐nine participants with type 2 diabetes, including 17 males and 52 females, received a neuropathy assessment. Mean age was 45 ± 9 years (±SD) and mean BMI was 36.0 ± 7.5 kg/m2 in females and 34.8 ± 7.5 kg/m2 in males (p = 0.575). When we assessed neuropathy at the 10‐year follow‐up, there were 27 participants with neuropathy (i.e., with a high MNSI index score of >2.5407) and 42 participants without neuropathy (i.e., with a low MNSI index score of ≤2.5407; Table 1). Systolic (p = 0.010) and diastolic (p = 0.006) blood pressure were significantly greater in participants with versus without neuropathy. 13 , 28 Additionally, the median urine albumin creatinine ratio (ACR) was 15 mg/g in participants without neuropathy, which is within the normal range. This contrasts with a median ACR of 54 mg/g in participants with neuropathy, which indicates microalbuminuria and DKD onset and is significantly elevated versus the group without neuropathy (p = 0.005). The correlation of DKD with peripheral neuropathy is anticipated, based on our earlier study of Pima participants. 6 There were no other significant differences in baseline characteristics by neuropathy status. However, there were several trends of increased fasting plasma glucose (FPG), HbA1c, BMI, total cholesterol, and triglycerides in participants with versus without neuropathy, as anticipated based on uncontrolled diabetes, obesity, and dyslipidemia being known peripheral neuropathy risk factors. 3 , 9 , 29 , 30 , 31 Analysis of MNSI index to components of the metabolic syndrome yielded the same results, with the only significant correlation a positive association between MNSI index and diastolic blood pressure (Table S2).
Table 1.
Participant characteristics by neuropathy status.
| Variables | Without Neuropathy | With Neuropathy | p value |
|---|---|---|---|
| MNSI index ≤ 2.5407 | MNSI index > 2.5407 | ||
| N | 42 | 27 | |
| Age (years) | 46 ± 8 | 45 ± 9 | 0.666 |
| Male sex (%) | 8 (19.0) | 9 (33.3) | 0.179 |
| Height (m) | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.168 |
| Weight (kg) | 92 ± 20 | 100 ± 25 | 0.160 |
| Body mass index (kg/m2) | 35.0 ± 6.3 | 36.8 ± 8.9 | 0.316 |
| Systolic blood pressure (mmHg) | 117 ± 14 | 126 ± 14 | 0.010 |
| Diastolic blood pressure (mmHg) | 74 ± 8 | 79 ± 7 | 0.006 |
| Pulse (/min) | 72 ± 9 | 74 ± 9 | 0.381 |
| Diabetes duration (years) | 15.5 ± 5.4 | 15.6 ± 6.4 | 0.925 |
| Fasting plasma glucose (mg/dL) | 192 ± 78 | 223 ± 96 | 0.147 |
| HbA1c (mmol/mol) | 72.7 ± 18.2 | 81.4 ± 14.9 | 0.102 |
| HbA1c (%) | 8.8 ± 2.2 | 9.6 ± 1.8 | 0.102 |
| Total cholesterol (mg/dL) | 162 ± 41 | 165 ± 37 | 0.788 |
| Triglyceride (mg/dL) | 184 ± 160 | 195 ± 240 | 0.839 |
| GFR (mL/min) | 146 ± 45 | 162 ± 62 | 0.264 |
| Urine albumin creatinine ratio (mg/g)# | 15 [5–53] | 54 [18–155] | 0.005 |
| Intervention arm (%) | 25 (59.5) | 12 (44.4) | 0.220 |
| Medication | |||
| Antihypertensive (%) | 17 (40.5) | 10 (37.0) | 0.775 |
| Metformin (%) | 25 (59.5) | 18 (66.7) | 0.550 |
| Insulin (%) | 17 (40.5) | 11 (40.7) | 0.983 |
| Oral hypoglycemic (%) | 31 (73.8) | 24 (88.9) | 0.128 |
| Statins (%) | 9 (21.4) | 6 (22.2) | 0.938 |
| Other lipid‐lowering (%) | 10 (23.8) | 7 (25.9) | 0.842 |
Significant p values are in bold. Data represented as mean ± SD (for continuous variables) or frequency (percentage; for categorical variables), except nonnormally distributed data, for example, urine albumin creatine ratio#, represented as median [interquartile range]. ANOVA assessed normally distributed continuous variables; Kolmogorov–Smirnov test assessed skewed continuous variables; chi‐square test assessed categorical variables. ANOVA, analysis of variance; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; MNSI, Michigan Neuropathy Screening Instrument.
Baseline acylcarnitine and free fatty acid abundance associated with future neuropathy status
We next conducted lipidomics on serum, which had been banked one decade earlier from these participants to identify early lipidomic changes correlating with neuropathy development. Participants with significantly reduced overall baseline abundance of medium‐chain (C6–C14) acylcarnitines correlated with the development of peripheral neuropathy at a 10‐year follow‐up (p < 0.001; Fig. 2A), that is, had high MNSI index scores. Species‐specific differences by chain length and saturation level were nonsignificant, likely attributable to a lack of statistical significance from limited power of low sample size (Fig. 2B). However, the trend for all individual medium‐chain species with 0, 1, or 2 double bonds followed the aggregate trend and were lower in participants with versus without neuropathy. In short‐chain acylcarnitines, there were nonsignificant trends for reduced L‐carnitine and C4 and elevated C2 and C3 in participants with versus without neuropathy. In long‐chain acylcarnitines, there were also trends for decreased unsaturated species and increased saturated species by participants with versus without neuropathy. Elevated aggregate free fatty acid values correlated with participants that developed neuropathy (p = 0.042; Fig. 3A). When we examined individual free fatty acids by carbon and double bond numbers, there were no significant differential levels in single species (Fig. 3B). However, long‐chain (C20–C24) saturated and unsaturated free fatty acids had nonsignificant trends for being generally elevated in participants with neuropathy compared to participants without neuropathy. Differences in free fatty acids with 16 and 18 carbons were far less pronounced. Finally, correlation analysis of the MNSI index to lipids similarly found significant correlations between acylcarnitines (p = 0.015) and free fatty acids (p = 0.006) (Fig. S2A and B).
Figure 2.
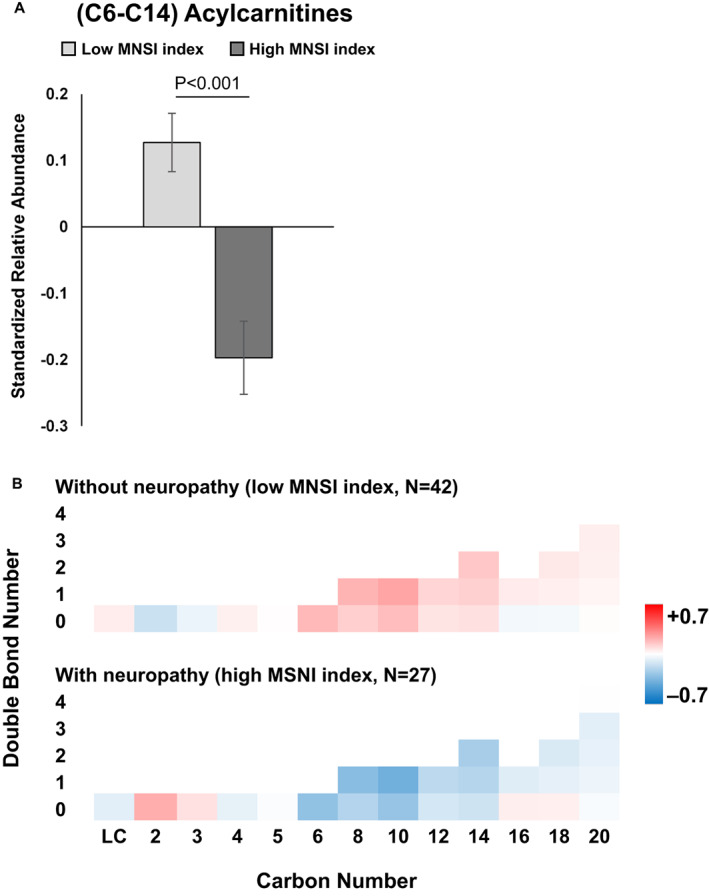
Acylcarnitine abundance by neuropathy status. (A) Overall, mean C6–C14 acylcarnitine abundance was significantly decreased in participants with (dark gray, high MNSI index) versus without neuropathy (light gray, low MNSI index; p < 0.001). (B) Heatmap of acylcarnitine abundance by chain length (carbon number) and saturation (double bond number) for participants with (n = 27, high MNSI index) versus without neuropathy (n = 42, low MNSI index). The scale represents acylcarnitines species that are increased (red) or decreased (blue) in groups. A and B are based on generalized linear mixed models with carbon number, double bond number, and MNSI index groups as main effect variables and the MNSI index group by carbon number interaction, adjusted for other covariates. B, bars represent z‐score standardized mean values ± SEM. LC, L‐carnitine; MNSI, Michigan Neuropathy Screening Instrument. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
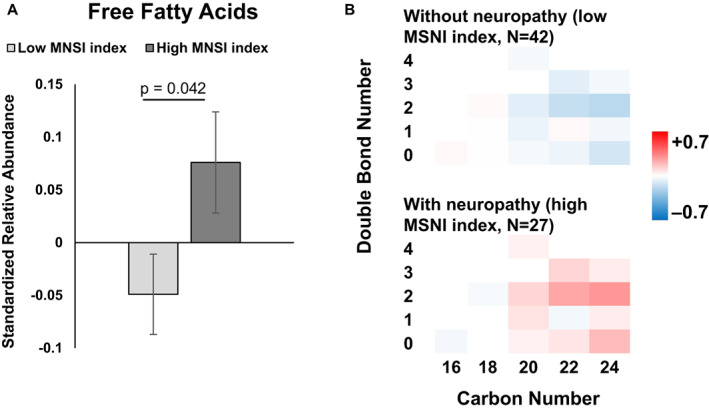
Free fatty acid abundance by neuropathy status. (A) Overall, mean free fatty acid abundance was significantly increased in participants with (dark gray, high MNSI index) versus without neuropathy (light gray, low MNSI index; p = 0.042). (B) Heatmap of free fatty acid abundance by chain length (carbon number) and saturation (double bond number) for participants with (n = 27, high MNSI index) versus without neuropathy (n = 42, low MNSI index). The scale represents acylcarnitines species that are increased (red) or decreased (blue) in groups. (A and B) are based on generalized linear mixed models with a double bond number, carbon number, and MNSI index groups as main effect variables. (B) bars represent z‐score standardized mean values ± SEM. MNSI, Michigan Neuropathy Screening Instrument. [Colour figure can be viewed at wileyonlinelibrary.com]
Overall, at baseline, lower aggregate medium‐chain acylcarnitines and higher aggregate free fatty acids levels in serum were associated with the presence and severity of peripheral neuropathy in participants with type 2 diabetes at the 10‐year follow‐up.
Baseline phosphatidylcholine and lysophosphatidylcholine abundance associated with future neuropathy status
Of the complex lipids, we found that baseline abundance differences in phosphatidylcholines and lysophosphatidylcholines are associated with future peripheral neuropathy status. Overall, decreased phosphatidylcholine aggregate abundance correlated with neuropathy in participants at the 10‐year follow‐up (p = 0.016; Fig. 4A). Although nonsignificant, most mono‐ and polyunsaturated phosphatidylcholines were diminished in banked serum from participants that eventually developed neuropathy (i.e., high MNSI index; Fig. 4B). In contrast, the class level of lysophosphatidylcholine abundance was significantly higher in participants with versus without neuropathy (p = 0.017; Fig. 5A). This observation is aligned with lower phosphatidylcholines in the neuropathy group, since lysophosphatidylcholines are generated from phosphatidylcholines by phospholipase A2‐mediated removal of a fatty acid chain. There were no significant chain length‐ and saturation‐dependent differences by neuropathy status (Fig. 5B). Broadly, individual lysophosphatidylcholine species followed the aggregate trend, with few exceptions, most notably the saturated C18 species. As anticipated, correlation analysis of the MNSI index to lipids identified a significant correlation to lysophosphatidylcholines (p = 0.006) (Fig. S2C).
Figure 4.
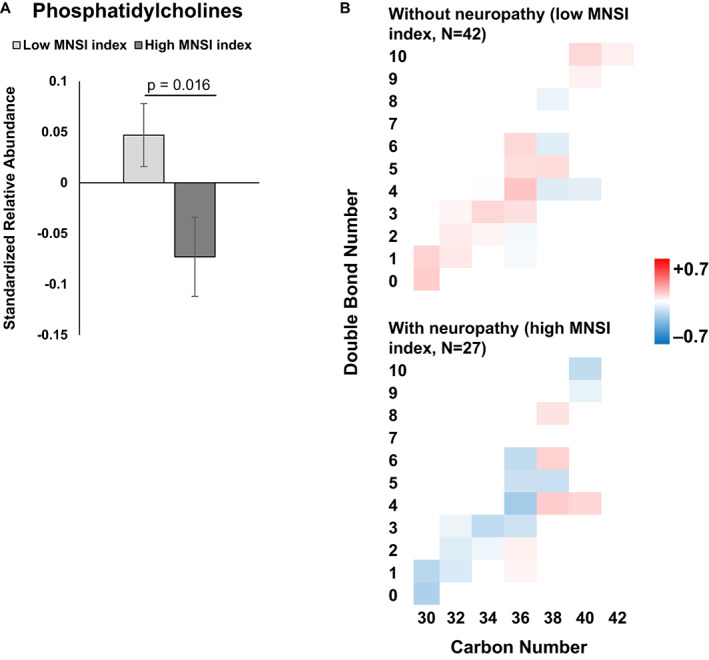
Phosphatidylcholine abundance by neuropathy status. (A) Overall, mean free fatty acid abundance was significantly decreased in participants with (dark gray, high MNSI index) versus without neuropathy (light gray; low MNSI index; p = 0.016). (B) Heatmap of phosphatidylcholine abundance by chain length (carbon number) and saturation (double bond number) for participants with (n = 27, high MNSI index) versus without neuropathy (n = 42, low MNSI index). The scale represents acylcarnitines species that are increased (red) or decreased (blue) in groups. A and B are based on generalized linear mixed models with a double bond number and MNSI index groups as main effect variables, adjusted for other covariates. B, bars represent z‐score standardized mean values ± SEM. MNSI, Michigan Neuropathy Screening Instrument. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.
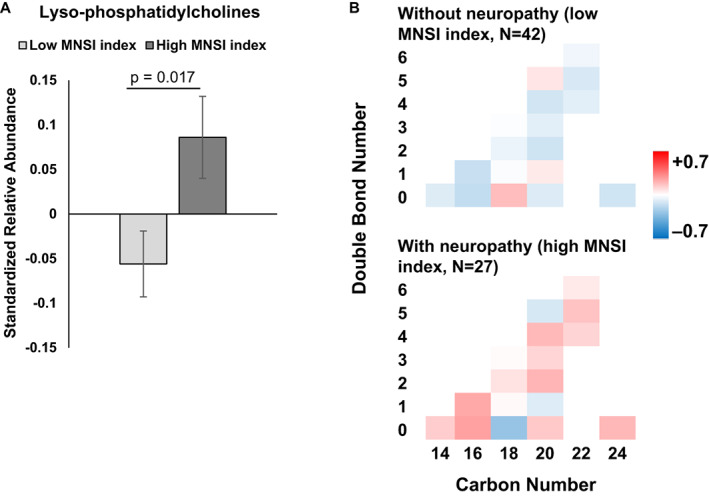
Lysophosphatidylcholines abundance by neuropathy status. (A) Overall, mean free fatty acid abundance was significantly increased in participants with (dark gray, high MNSI index) versus without neuropathy (light gray, low MNSI index; p = 0.017). (B) Heatmap of lysophosphatidylcholine abundance by chain length (carbon number) and saturation (double bond number) for participants with (n = 27; high MNSI index) versus without neuropathy (n = 42, low MNSI index). The scale represents acylcarnitines species that are increased (red) or decreased (blue) in groups. A and B are based on generalized linear mixed models with a double bond number and MNSI index groups as main effect variables, adjusted for other covariates. B, bars represent z‐score standardized mean values ± SEM. MNSI, Michigan Neuropathy Screening Instrument. [Colour figure can be viewed at wileyonlinelibrary.com]
Cumulatively, of complex lipids, differential baseline serum phosphatidylcholine and lysophosphatidylcholine levels correlated with the presence and severity of peripheral neuropathy one decade later in Pima participants with type 2 diabetes. Abundances of other signaling and complex lipids did not significantly affect future neuropathy status (Figs. S1 and S2D–L). Among these lipid classes, none of the covariates were used to adjust models associated with lipid level variation.
Discussion
Herein, for the first time, to our knowledge, we demonstrate that a serum lipidomics signature associates with future incident peripheral neuropathy. Specifically, in Pima participants with type 2 diabetes, decreased baseline serum medium‐chain acylcarnitines and increased free fatty acids associated with peripheral neuropathy, were assessed by the MNSI index, one decade later. Participants that developed neuropathy also had lower phosphatidylcholines and higher lysophosphatidylcholines versus participants that did not develop neuropathy. The abundance of other lipid classes did not significantly vary with neuropathy. These findings indicate lipid changes related to impaired mitochondrial β‐oxidation in participants that develop peripheral neuropathy.
Diabetes is the greatest risk for peripheral neuropathy, 4 , 31 although dyslipidemia, 3 , 5 , 8 , 9 , 10 , 11 obesity, 2 , 3 , 30 and additional components of the metabolic syndrome, such as hypertension, 30 , 32 are additional important metabolic risk factors. Herein, systolic and diastolic blood pressure were elevated in participants with versus without neuropathy. 13 , 28 Moreover, microalbuminuria and DKD at baseline correlated with neuropathy at the follow‐up in Pima participants with type 2 diabetes. 6 However, there were no significant differences in baseline glycemic (FPG, HbA1c) and basic lipid (triglycerides, total cholesterol) profiles or anthropometric measures (BMI). This may be due to the relatively smaller sample size in the group with (n = 27) versus without (n = 42) neuropathy or the presence of additional risk factors beyond glycemic and basic lipid profiles, such as intermediate and complex lipids, which may associate with future incident neuropathy. We previously noted no differences in basic lipid, glycemic, or anthropometric metrics in type 2 diabetes participants with (n = 49) versus without neuropathy (n = 48) in our larger ADDITION study. 18 Since there were no differences in basic lipid profiles, we next investigated the lipidomics profiles from stored serum baseline samples.
We found a specific lipidomics profile of differential aggregate levels of decreased medium‐chain acylcarnitines, increased free fatty acids, decreased phosphatidylcholines, and increased lysophosphatidylcholines associated with future incident neuropathy in Pima participants with type 2 diabetes. This agrees with metabolomics and lipidomics profiles of incident peripheral neuropathy in the Danish ADDITION cohort, 18 which collected plasma at the time of neuropathy assessment. Participants with neuropathy had a trending increase in triacylglycerols in both the ADDITION and Pima studies. We saw abundance variation by carbon and double bonds in diacylglycerols and other sphingo‐ and phospholipids in both the Pima and ADDITION studies, although trends were more uniform in ADDITION, especially for diacylglycerols, sphingomyelins, ceramides, and phosphatidylethanolamines. 18 Differences between the two studies may have arisen from the distinct temporal assessments of lipid signatures relative to peripheral neuropathy development in the two studies, from our smaller sample size in the current study, or from natural variation in the populations.
Few other studies have compared plasma or serum lipidomics of participants with type 2 diabetes to peripheral neuropathy status. We recently assessed cross‐sectional lipidomics in obese participants, independent of glycemic status, and found peripheral neuropathy was characterized by differential diacylglycerols, phosphatidylcholines, sphingomyelins, ceramides, and dihydroceramides. 19 Ziegler et al. analyzed cross‐sectional plasma signatures from participants from the German Diabetes Study with recent‐onset type 2 diabetes (n = 95) to cardiac autonomic neuropathy. 33 Several phosphatidylcholines and sphingomyelins correlated inversely with cardiac autonomic neuropathy in participants with type 2, but not type 1, diabetes, concluding this may arise from dyslipidemia as a major driver of nerve damage secondary to type 2 diabetes. 33
We have also reported lipidomic analyses of other diabetic complications in the Pimas, including nephropathy 13 and retinopathy. 34 The neuropathy lipidomic signatures from the current study were distinct to both the DKD and the retinopathy signatures, although signatures in all three diabetic complications centered around impaired β‐oxidation. This aligns with our mouse data of tissue‐specific metabolic and lipidomic differences in nerve, kidney, and retina in type 2 diabetes. 16 , 35 Overall, these studies underscore the importance of baseline plasma lipidomics profiles on the development, even a decade later, of type 2 diabetes complications, 13 , 34 including peripheral neuropathy.
Lipids are a diverse class of molecules with numerous biological functions, especially in the nervous system. Herein, the lipidomic signatures associated with neuropathy in Pima participants with type 2 diabetes centered on lipids related to mitochondrial function. Indeed, dysfunctional mitochondrial dynamics underpin diabetic neuropathy, 36 including dyslipidemia, which impairs mitochondrial trafficking 20 , 21 , 37 and bioenergetics, leading to energy failure and resulting nerve injury. 37 In the current study, serum linked to neuropathy was characterized by elevated free fatty acids and diminished medium‐chain acylcarnitines. Free fatty acids are an important energy source through mitochondrial β‐oxidation. Fatty acids are shuttled into mitochondria by conjugating with L‐carnitine, forming acylcarnitine intermediates, which are converted back to the fatty acid acyl within mitochondria, where they are metabolized by β‐oxidation. 38 Free fatty acids accumulation and decreased medium‐chain acylcarnitines suggest blockade in fatty acid to acylcarnitine conversion and disrupted mitochondrial β‐oxidation, a scenario likely arising from fatty acid substrate excess, 38 as occurs in dyslipidemia.
Complex phospholipids, such as the ratio of phosphatidylcholines to phosphatidylethanolamines, dictate membrane curvature, regulating mitochondrial biogenesis and bioenergetics. 39 , 40 Herein, reduction phosphatidylcholines signals potential changes to mitochondrial structure and, in turn, mitochondrial function. Moreover, lysophosphatidylcholines, which were elevated in aggregate in participants that developed neuropathy, correlate with insulin resistance 41 and are linked to retinal neurodegeneration in preclinical studies. 42 Lysophosphatidylcholines are also precursors to lysophosphatidic acid, which is related to neuropathic pain, 43 a frequent symptom in type 2 diabetes patients and peripheral neuropathy. Although lipidomics identified differential abundance in these lipid species in participants with type 2 diabetes and neuropathy, preclinical studies are needed to infer causality from these specific lipids and/or elucidate pathomechanisms.
This study has several strengths. The Pima Indian type 2 diabetes cohort is long‐established and very homogenous and has been deeply phenotyped for several diabetic complications. 13 , 28 , 34 The great extent of homogeneity minimizes potential confounders, creating a unique opportunity to explore the biology of neuropathy in a type 2 diabetes cohort. Physical examinations and data collection followed well‐specified research protocols, resulting in high‐quality data. Additionally, the mass spectrometry quality control protocol ensured high‐quality lipidomic data on a large array of lipid classes, with low coefficients of variations and minimal to no batch‐to‐batch variability.
This study also has limitations. Serum lipidomics can identify biomarkers of diabetic peripheral neuropathy, but the relationship between serum versus nerve tissue lipids and damage is uncertain. Our study did not assess nerve conduction velocities as a neuropathy outcome; however, the MNSI index has good diagnostic characteristics for neuropathy (area under the curve of 0.86 for the receiver operating characteristic curve). 44 Moreover, the study did not assess baseline MNSI index; however, diabetic neuropathy prediction risk scores indicate participants likely did not have neuropathy at baseline. 26 As an observational study, we cannot infer causality between lipid species abundances to later development of neuropathy; however, our observations align with our preclinical model studies, which inferred causality. 15 , 20 , 21 The cohort is relatively small and findings will need replication in larger cohorts, although findings in this cohort have broadly been confirmed in other populations, 18 especially for DKD. 13 , 45 Our study also noted multiple interesting nonsignificant trends, which larger studies might be powered to rigorously assess. Our study was too small for analyses by sex, though sex‐dependent differences in the plasma lipidome have been reported. 46 , 47 , 48 Finally, as with other Omic studies, our lipidomics platform generated a relatively large number of lipids; hence, applying traditional statistical methods to individualized lipids is limited by our sample size and hence the potential of false discovery. To overcome these limitations, we continuously applied data reduction strategies. This included applying intralipid class mixed models, which reduced multiplicity and potential for false discovery, and enhanced statistical power. Yet, the approach retained deep pathophysiological insight by accounting for lipid profiles in relation to neuropathy phenotype.
We conclude that aggregate abundance differences in circulating medium‐chain acylcarnitines, free fatty acids, phosphatidylcholines, and lysophosphatidylcholines, early in the course of the disease are linked to the later development of human type 2 diabetic neuropathy. Our findings have important clinical implications. They suggest a potential diagnostic route through biomarker discovery and risk stratification to identify type 2 diabetes patients at the highest risk of peripheral neuropathy, facilitating better management in this patient subset. Additionally, further research validating and delineating the relationship of serum lipid species to neuropathy in preclinical models could enhance our understanding of pathogenesis and open avenues for targeted therapeutic development. Importantly, since lipidomics highlighted impaired mitochondrial β‐oxidation, it suggests that conventional lipid‐lowering medication, such as statins and fenofibrates, which act through cholesterol and apoprotein synthetic pathways, may be ineffective for treating neuropathy. Indeed, statin use does not appear to impact neuropathy onset. 49 As an alternative approach, this study underscores a possible need for therapeutics that optimize fatty acid metabolism and enhance mitochondrial β‐oxidation.
Conflicts of Interest
BCC declares consulting fees from Dynamed. All other authors have nothing to disclose.
Authors' Contribution
FA designed the lipidomic study, prepared serum samples for mass spectrometry, analyzed and interpreted the data, and wrote the first draft. TMR performed serum sample preparation for lipidomic analysis and mass spectrometry runs. TS retrieved mass spectrometry data. JB performed serum sample preparation for lipidomic analysis and mass spectrometry runs. MGS interpreted data and wrote the first draft. HCL and RGN contributed to clinical study design, data collection, and manuscript drafting. GM performed statistical analyses and contributed to manuscript drafting. SP contributed to the lipidomic study design, mass spectrometry, data interpretation, and manuscript drafting. ELF trained clinical research coordinators on MNSI administration and oversaw data collection, contributed to the lipidomic study design, data interpretation, manuscript drafting, and secured study funding. All authors critically evaluated the paper and have approved the final version. ELF is the guarantor.
Supporting information
Table S1 Identified lipids by adduct, mass‐to‐charge ratio (m/z), and retention time (RT) in positive and negative modes. The mass accuracy was ±0.001 Da in positive mode and ±0.005 Da in negative mode, with an overall mass error of <2 ppm. CE, cholesterol ester; CerP, ceramide‐phosphate; CL, cardiolipin; DAG, diacylglycerol; FFA, free fatty acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MAG, monoacylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; pPC, plasmenyl‐phosphatidylcholine; pPE, plasmenyl‐phosphatidylethanolamine; SM, sphingomyelin; TAG, triacylglycerol.
Table S2. Correlation of MNSI index with baseline variables. *p = 0.017; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; MNSI, Michigan Neuropathy Screening Instrument; SBP, systolic blood pressure.
Figure S1. Various lipid class abundance by neuropathy status. Heatmap of lipid abundances reveals no statistically significant differences in participants with (n = 27; high MNSI index) versus without neuropathy (n = 42; low MNSI index) for (A) sphingomyelins (SM), (B) phosphatidylinositols (PI), (C) lysophosphatidylethanolamines (LPE), (D) plasmenyl‐phosphatidylethanolamines (pPE), (E) cholesteryl‐esters (CE), (F) phosphatidylethanolamines (PE), (G) diacylglycerols (DAG), (H) triacylglycerols (TAG).
Figure S2. Correlation between neuropathy severity and lipids. Heatmap of Pearson correlation coefficients of neuropathy severity (MNSI index) with each lipid by lipid class by carbon number (x‐axis) and double bond number (y‐axis) for (A) acylcarnitines (AC), (B) free fatty acids (FFA), (C) lysophosphatidylcholines (LPC), (D) phosphatidylcholines (PC), (E) triacylglycerols (TAG), (F) phosphatidylinositols (PI), (G) cholesteryl‐esters (CE), (H) diacylglycerols (DAG), (I) lysophosphatidylethanolamines (LPE), (J) phosphatidylethanolamines (PE), (K) plasmenyl‐phosphatidylethanolamines (pPE), and (L) sphingomyelins (SM).
Acknowledgments
The authors thank the Pima individuals who participated in this study and Camille and Bernadine Waseta and Lois I. Jones, RN at the Chronic Kidney Disease Section, NIDDK, for collecting data.
Funding Information
This study was supported by the NIH (grant nos. R24DK082841 [ELF, SP], K08DK106523 [FA], R03DK121941 [FA], P30DK089503 [SP], P30DK081943 [SP], P30DK020572 [SP], K99DK129785 [ELR], and T32NS07222 [ELR]), the NeuroNetwork for Emerging Therapies, and the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Funding Statement
This work was funded by Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of Diabetes and Digestive and Kidney Diseases grants K08DK106523, K99DK129785, P30DK020572, P30DK081943, P30DK089503, R03DK121941, and R24DK082841; National Institute of Neurological Disorders and Stroke grant T32NS07222; the NeuroNetwork for Emerging Therapies.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.
References
- 1. Feldman EL, Callaghan BC, Pop‐Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 2. Callaghan BC, Reynolds EL, Banerjee M, et al. The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Diabetes Care. 2020;43(3):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersen ST, Witte DR, Dalsgaard EM, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen‐detected type 2 diabetes followed for 13 years: ADDITION‐Denmark. Diabetes Care. 2018;41(5):1068‐1075. [DOI] [PubMed] [Google Scholar]
- 4. Reynolds EL, Callaghan BC, Banerjee M, Feldman EL, Viswanathan V. The metabolic drivers of neuropathy in India. J Diabetes Complicat. 2020;34(10):107653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care. 2017;40(9):1226‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaiswal M, Fufaa GD, Martin CL, Pop‐Busui R, Nelson RG, Feldman EL. Burden of diabetic peripheral neuropathy in Pima Indians with type 2 diabetes. Diabetes Care. 2016;39(4):e63‐e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;13(6):Cd007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savelieff MG, Callaghan BC, Feldman EL. The emerging role of dyslipidemia in diabetic microvascular complications. Curr Opin Endocrinol Diabetes Obes. 2020;27(2):115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 2016;39(5):801‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christensen DH, Knudsen ST, Gylfadottir SS, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5,249 patients in the Danish centre for strategic research in type 2 diabetes (DD2) cohort. Diabetes Care. 2020;43(6):1266‐1275. [DOI] [PubMed] [Google Scholar]
- 11. Callaghan BC, Feldman E, Liu J, et al. Triglycerides and amputation risk in patients with diabetes: ten‐year follow‐up in the DISTANCE study. Diabetes Care. 2011;34(3):635‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Afshinnia F, Rajendiran TM, Soni T, et al. Impaired b‐oxidation and altered complex lipid fatty acid partitioning with advancing CKD. J Am Soc Nephrol. 2018;29(1):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Afshinnia F, Nair V, Lin J, et al. Increased lipogenesis and impaired beta‐oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight. 2019;4(21):e130317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Afshinnia F, Rajendiran TM, Karnovsky A, et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int Rep. 2016;1(4):256‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Brien PD, Guo K, Eid SA, et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech. 2020;13(2):dmm042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sas KM, Lin J, Rajendiran TM, et al. Shared and distinct lipid‐lipid interactions in plasma and affected tissues in a diabetic mouse model. J Lipid Res. 2018;59(2):173‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121(4):1402‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rumora AE, Guo K, Alakwaa FM, et al. Plasma lipid metabolites associate with diabetic polyneuropathy in a cohort with type 2 diabetes. Ann Clin Transl Neurol. 2021;8(6):1292‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo K, Savelieff MG, Rumora AE, et al. Plasma metabolomics and lipidomics differentiate obese individuals by peripheral neuropathy status. J Clin Endocrinol Metabol. 2021;107(4):1091‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rumora AE, LoGrasso G, Haidar JA, Dolkowski JJ, Lentz SI, Feldman EL. Chain length of saturated fatty acids regulates mitochondrial trafficking and function in sensory neurons. J Lipid Res. 2019;60(1):58‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rumora AE, LoGrasso G, Hayes JM, et al. The divergent roles of dietary saturated and monounsaturated fatty acids on nerve function in murine models of obesity. J Neurosci. 2019;39(19):3770‐3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet. 1971;2(7716):125‐128. [DOI] [PubMed] [Google Scholar]
- 23. Weil EJ, Fufaa G, Jones LI, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes. 2013;62(9):3224‐3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281‐1289. [DOI] [PubMed] [Google Scholar]
- 25. Herman WH, Pop‐Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubey VN, Dave JM, Beavis J, Coppini DV. Predicting diabetic neuropathy risk level using artificial neural network and clinical parameters of subjects with diabetes. J Diabetes Sci Technol. 2022;16(2):275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Afshinnia F, Rajendiran TM, Wernisch S, et al. Lipidomics and biomarker discovery in kidney disease. Semin Nephrol. 2018;38(2):127‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds EL, Akinci G, Banerjee M, et al. The determinants of complication trajectories in American Indians with type 2 diabetes. JCI Insight. 2021;6(10):e146849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73(12):1468‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas‐Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95(7):1342‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu Y, Xing P, Cai X, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: estimates of the INTERPRET‐DD study. Front Public Health. 2020;8:534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ziegler D, Strom A, Straßburger K, et al. Association of cardiac autonomic dysfunction with higher levels of plasma lipid metabolites in recent‐onset type 2 diabetes. Diabetologia. 2021;64(2):458‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fort PE, Rajendiran TM, Soni T, et al. Diminished retinal complex lipid synthesis and impaired fatty acid β‐oxidation associated with human diabetic retinopathy. JCI Insight. 2021;6(19):e152109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sas KM, Kayampilly P, Byun J, et al. Tissue‐specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rumora AE, Savelieff MG, Sakowski SA, Feldman EL. Disorders of mitochondrial dynamics in peripheral neuropathy: clues from hereditary neuropathy and diabetes. Int Rev Neurobiol. 2019;145:127‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sajic M, Rumora AE, Kanhai AA, et al. High dietary fat consumption impairs axonal mitochondrial function in vivo. J Neurosci. 2021;41(19):4321‐4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Eunen K, Simons SM, Gerding A, et al. Biochemical competition makes fatty‐acid β‐oxidation vulnerable to substrate overload. PLoS Comput Biol. 2013;9(8):e1003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basu Ball W, Neff JK, Gohil VM. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018;592(8):1273‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9 Pt B):1558‐1572. [DOI] [PubMed] [Google Scholar]
- 41. Liu P, Zhu W, Chen C, et al. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020;247:117443. [DOI] [PubMed] [Google Scholar]
- 42. Cheng L, Han X, Shi Y. A regulatory role of LPCAT1 in the synthesis of inflammatory lipids, PAF and LPC, in the retina of diabetic mice. Am J Physiol Endocrinol Metab. 2009;297(6):E1276‐E1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Velasco M, O'Sullivan C, Sheridan GK. Lysophosphatidic acid receptors (LPARs): potential targets for the treatment of neuropathic pain. Neuropharmacology. 2017;113(Pt B):608‐617. [DOI] [PubMed] [Google Scholar]
- 44. Callaghan BC, Xia R, Reynolds E, et al. Better diagnostic accuracy of neuropathy in obesity: a new challenge for neurologists. Clin Neurophysiol. 2018;129(3):654‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nelson RG, Knowler WC, Kretzler M, et al. Pima Indian contributions to our understanding of diabetic kidney disease. Diabetes. 2021;70(8):1603‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beyene HB, Olshansky G, AA TS, et al. High‐coverage plasma lipidomics reveals novel sex‐specific lipidomic fingerprints of age and BMI: evidence from two large population cohort studies. PLoS Biol. 2020;18(9):e3000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torretta E, Barbacini P, Al‐Daghri NM, Gelfi C. Sphingolipids in obesity and correlated co‐morbidities: the contribution of gender, age and environment. Int J Mol Sci. 2019;20(23):5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne). 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kristensen FP, Christensen DH, Callaghan BC, et al. Statin therapy and risk of polyneuropathy in type 2 diabetes: a Danish cohort study. Diabetes Care. 2020;43:2945‐2952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Identified lipids by adduct, mass‐to‐charge ratio (m/z), and retention time (RT) in positive and negative modes. The mass accuracy was ±0.001 Da in positive mode and ±0.005 Da in negative mode, with an overall mass error of <2 ppm. CE, cholesterol ester; CerP, ceramide‐phosphate; CL, cardiolipin; DAG, diacylglycerol; FFA, free fatty acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MAG, monoacylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; pPC, plasmenyl‐phosphatidylcholine; pPE, plasmenyl‐phosphatidylethanolamine; SM, sphingomyelin; TAG, triacylglycerol.
Table S2. Correlation of MNSI index with baseline variables. *p = 0.017; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; MNSI, Michigan Neuropathy Screening Instrument; SBP, systolic blood pressure.
Figure S1. Various lipid class abundance by neuropathy status. Heatmap of lipid abundances reveals no statistically significant differences in participants with (n = 27; high MNSI index) versus without neuropathy (n = 42; low MNSI index) for (A) sphingomyelins (SM), (B) phosphatidylinositols (PI), (C) lysophosphatidylethanolamines (LPE), (D) plasmenyl‐phosphatidylethanolamines (pPE), (E) cholesteryl‐esters (CE), (F) phosphatidylethanolamines (PE), (G) diacylglycerols (DAG), (H) triacylglycerols (TAG).
Figure S2. Correlation between neuropathy severity and lipids. Heatmap of Pearson correlation coefficients of neuropathy severity (MNSI index) with each lipid by lipid class by carbon number (x‐axis) and double bond number (y‐axis) for (A) acylcarnitines (AC), (B) free fatty acids (FFA), (C) lysophosphatidylcholines (LPC), (D) phosphatidylcholines (PC), (E) triacylglycerols (TAG), (F) phosphatidylinositols (PI), (G) cholesteryl‐esters (CE), (H) diacylglycerols (DAG), (I) lysophosphatidylethanolamines (LPE), (J) phosphatidylethanolamines (PE), (K) plasmenyl‐phosphatidylethanolamines (pPE), and (L) sphingomyelins (SM).
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.


