Abstract
Objective
Assess whether propranolol modulates the trigeminovascular system in both men and women.
Methods
We investigated the effect of propranolol (80 mg, 90 min after oral administration, corresponding to T max) on the increase in dermal blood flow of the forehead skin (innervated by the trigeminal nerve) by capsaicin application (0.6 mg/mL) and electrical stimulation (0.2–1.0 mA) before and after placebo (grapefruit juice) or propranolol (oral solution diluted in grapefruit juice) in a randomized, double‐blind, placebo‐controlled cross‐over study, including healthy males (n = 10) and females on contraceptives (n = 11). Additionally, we compared our results with data from the Dutch IADB.nl prescription database by analyzing the change in triptan use after propranolol prescription in a population similar to our dermal blood flow study subjects (males and females, 20–39 years old).
Results
Dermal blood flow responses to capsaicin were significantly attenuated after propranolol, but not after placebo. When stratifying by sex, no significant changes in the capsaicin‐induced dermal blood flow were observed in females after propranolol, whereas they remained significant in males. Dermal blood flow responses to electrical stimulation were not modified in any case. In our prescription database study, after propranolol, a more pronounced decrease in triptan use was observed in male patients than in female patients.
Interpretation
Propranolol (80 mg) inhibits capsaicin‐induced increases in dermal blood flow in a sex‐dependent manner. In patients, a more pronounced decrease in triptan use is observed in males when compared with females, suggesting an interaction between propranolol and sex steroids in the modulation of the trigeminovascular system.
Introduction
Migraine is a highly disabling neurovascular disorder 1 ; it is estimated that around 16% of the world population suffers from migraine, with 70% being women. 2 The pathophysiology of migraine remains largely unknown; however, it is considered to involve a dysfunctional activation of the trigeminovascular system and vasodilation of the trigeminal‐innervated vessels, mainly mediated by the release of calcitonin gene‐related peptide (CGRP), a neuropeptide present in perivascular sensory fibers. 3 , 4
Migraine treatment can be either acute, aimed to reverse the attack once it has begun, or prophylactic, designed to reduce the frequency and severity of migraine attacks. In the former case, drugs have been developed specifically for the treatment of migraine based on the current knowledge of the pathophysiological mechanisms underlying migraine. 5 , 6 , 7 , 8 In contrast, for decades, prophylactic treatment was not developed specifically for this indication, but for other disorders and was later empirically discovered to reduce migraine attack frequency in some patients, as is the case for propranolol. In 1960, Rabkin and colleagues 9 noted that one patient under propranolol treatment for angina pectoris showed a marked relief of his “vascular headaches.” Nowadays, propranolol is widely prescribed for the preventative treatment of migraine, but its mechanism of action is not yet clear, as not all β‐blockers are effective for the prophylactic treatment of migraine and the lack of efficacy does not correlate with their β‐adrenoceptor selectivity (i.e. non‐selective/selective β1‐adrenoceptor). 10 This suggests that their mechanism of action may well not be related to their antagonistic properties on β‐adrenoceptors and/or their antihypertensive properties, but to other unknown mechanism(s).
Currently, one of the main targets for both the acute and prophylactic treatment of migraine is the inhibition of the CGRP pathway, either with antagonists of the CGRP receptor (gepants), 11 monoclonal antibodies against CGRP or its receptor, 12 or, by inhibiting the release of CGRP (i.e., triptans and ditans). 13 , 14 Within this context, our group has developed a non‐invasive, human model that can be used to evaluate trigeminal nerve‐induced vasodilation mediated by CGRP release. For this purpose, capsaicin is topically applied on the forehead (innervated by the trigeminal nerve) and the increases in dermal blood flow (DBF) are measured. 15 We have previously shown with this model that sumatriptan modulates the human trigeminovascular system. 14
An important aspect to consider is that migraine is more prevalent in women than in men. In accordance with this, we have shown that the CGRP‐mediated trigeminovascular responses fluctuate throughout the menstrual cycle, 16 which points to a possible interaction between sex hormones and the trigeminovascular system, and the importance of sex‐specific studies (and treatments) for migraine. 17 Thus, in view of the importance of the trigeminovascular system in the pathophysiology and treatment of migraine, the high prevalence of migraine in female patients and the fact that the mechanism of action of propranolol remains unknown, the aim of this study was to investigate the effect of propranolol on the modulation of the human trigeminovascular system and compare the responses between males and females. Moreover, to analyze whether in a clinical “real life” setting, we could observe sex‐dependent differences in propranolol efficacy, we assessed the change in triptan use after propranolol prescription, a surrogate marker of therapeutic success, as a decrease on migraine days, would result in a decrease on the use of triptans.
Methods
Standard protocol approvals, registrations, and patient consents
The study protocol was reviewed and approved by the independent Ethics Committee of Erasmus MC, Rotterdam, the Netherlands, (MEC 2016–196), and was registered at the Netherlands Trial Register (ID: NTR6007). All participants gave written informed consent after explanation of the study, which was conducted in accordance with local laws, the ethical principles of the Declaration of Helsinki, as well as the principles of Good Clinical Practice.
Design and procedures
This was a randomized, double‐blind, placebo‐controlled, crossover study. Healthy, non‐smoking male and female (age range 18–57) individuals, body mass index (BMI) 20–28 kg/m2, without history of migraine, cardiovascular disease, or use of medication, were eligible. Subjects with systolic blood pressure (SBP) values lower than 110 mmHg or heart rate (HR) lower than 60 beats per min (while sitting) were excluded from the study for safety reasons, in view of the cardiovascular effects of propranolol. Perimenopausal females were excluded of the study, and the women included were using oral contraceptives, with experiments not being performed during the first and the “stop week” to avoid the confounding influence of varying sex steroid levels. 17 , 18
Recruitment of subjects started in April 2017. For allocation of the participants, the Department of Biostatistics of Erasmus University Medical Center (Rotterdam, the Netherlands) created a randomization list, and the preparation of the grapefruit juice (placebo or with propranolol) was performed by a person that was not involved in the experiments. All experiments were performed in a quiet, temperature‐controlled room. Study subjects had two visits, scheduled with a 1‐week washout in between and during the same time of day. Participants were not allowed to use any type of drugs (including nonsteroidal anti‐inflammatory drugs) for >48 h prior to the study visit. Furthermore, they could not consume alcohol, caffeine‐containing beverages and chocolate for >12 h prior to the start of experiments. For experiments in the morning, a light breakfast 3 h before the start of the experiment was allowed; whereas for experiments in the afternoon, a light lunch 3 h before the start of an experiment was allowed.
After arriving, all the subjects underwent a weight and height measurement. Female subjects had to take a pregnancy test. Furthermore, an electrocardiogram was performed to identify and exclude subjects with cardiac disorders, especially conduction disorders. Before each measurement, a standardized light meal was provided. Measurements were performed before and 90 min after either placebo or propranolol (corresponding to the time to propranolol peak plasma concentration 19 , 20 ) during the two research visits. During the experiment, the subjects rested supine on a bed and were not allowed to speak. Measurements before administration of propranolol or placebo were always performed on the right side of the forehead, while measurements after administration of propranolol or placebo were performed on the left side of the forehead. We have previously shown that responses do not differ between sides. 15 , 21 Capsaicin solution (2 mM, corresponding to 0.6 mg/ml, diluted in a mixture of ethanol 100%, tween 20 and distilled water; 3:3:4) and physiological saline (0.9% NaCl, 0.5 ml) were placed in reservoirs specifically designed for this purpose (drug delivery electrodes, Perimed AB, Järfälla, Sweden). For the capsaicin solution, this electrode was merely used as a reservoir, since no iontophoresis was used for capsaicin, which was simply applied to the skin. Furthermore, a ground electrode (Perimed AB, Järfälla, Sweden) was placed in the neck region 15 cm apart from the other electrodes with the negative lead of the iontophoresis device (Periont 382b, Perimed, Sweden) connected to the electrode containing saline (used for electrical stimulation, ES), and the positive lead connected to the electrode in the neck. Capsaicin‐ and ES‐induced dermal forehead vasodilatory responses were measured with a laser Doppler perfusion imager as previously described. 14 , 15 , 16 Briefly, after 15 min of supine rest, DBF at the site of the capsaicin electrode was continuously measured with the PIM 3 laser Doppler flow device for 40 min. After baseline measurement for 2 min, iontophoresis of saline was applied at 0.2 mA for 1 min. DBF was subsequently measured for 6 min. This process was repeated with increasing currents 0.4, 0.6, 0.8, and 1.0 mA.
After the first measurement, subjects were given propranolol (80 mg, Syprol® 50 mg/5 ml, Rosemont Pharmaceuticals Ltd. Leeds, UK) dissolved in 150 ml of grapefruit juice, as recommendation of the product leaflet, or placebo (grapefruit juice only). Grapefruit juice was used in this study because its bitter taste masked the taste of the propranolol solution, which had a tangerine taste. Grapefruit juice does not affect the metabolism of propranolol. 22 After the DBF measurements two vials (6 ml each) of blood were collected to determine the plasma levels of propranolol. A questionnaire was given to all subjects to ask about the side effects they experienced during the experiments.
To verify whether changes in DBF were due to the hemodynamic changes induced by propranolol, HR, SBP, and diastolic blood pressure (DBP) values were measured at the beginning and at the end of each measurement in triplicate, and later correlated with the changes in DBF. As changes in HR, SBP, and DBP could unblind the researcher, experiments were performed always by two researchers: one that always performed the blood pressure measurements and a second one that performed the DBF measurements and was blinded for the blood pressure values.
Propranolol levels
Blood was collected via the cubital vein. Propranolol levels were determined by the laboratory of the hospital pharmacy of Erasmus Medical Center (ISO 15189 accredited) with a Thermo Vantage LC–MS/MS (Thermo Fisher Scientific, Massachussetts, USA) by means of a EMA/FDA validated method. The range of quantification was 10–2500 μg/L. Samples were measured in two blinded batches.
Retrospective prescription database study
For our descriptive, retrospective study on the use of drugs, we used data from the Dutch prescription database (http://www.IADB.nl) from Groningen University. The database contains information on delivery date, anatomical therapeutic chemical (ATC)‐code, delivered quantity, dose per day and the number of delivered defined daily doses (DDD, a statistical measure of drug consumption, defined by the World Health Organization) of 60 public pharmacies in the north‐east of The Netherlands, including about 600,000 patients. 23 Sex and date of birth were also registered.
Patients enter the database once a drug is delivered to them via one of the pharmacies that are registered at IADB.nl. The database contains prescriptions disregarding the prescriber or the medical insurance. Information about medication that was used during hospital admission or ‘over‐the‐counter’ medication is not included in the database. The database does not contain information on the indication of a drug, nor does it contain information on ethnicity, socioeconomic status or lifestyle factors. The database has been validated and is representative for the Dutch population. 23
Research population and inclusion period of the retrospective prescription database study
The research population included all men and women (20–39 years old, roughly representative for the population in our DBF study) that started propranolol treatment in the period between 1995 and 2017. For inclusion in this study, the patients should have been registered in the database for at least 12 months before until 12 months after the start of propranolol and should have received at least two prescriptions for triptans in the 12 months before the start of propranolol prescription. Further, the use of hormonal contraceptives was registered.
Statistical analysis
For the DBF measurements, the sample size was based on previous studies from our group, 14 at 5% significance (two‐tailed) with 80% power. Baseline and maximal DBF responses (expressed in arbitrary units, a.u.) to capsaicin and ES were calculated before and after propranolol or placebo. Differences in DBF responses to capsaicin (our primary endpoint) and ES during propranolol and placebo were calculated for each participant in a blinded manner. Responses to propranolol and placebo were compared within participants by using Student's paired t‐test. Current‐response curves of the ES sequence (0.2–1.0 mA) were analyzed with repeated‐measures analysis of variance (ANOVA). The Mann–Whitney U test was used to compare propranolol plasma levels between males and females.
For the retrospective prescription database study, triptan use, before and after the start of propranolol was determined based on the number of DDD and the number of prescriptions during 12 months, both mean and median were calculated. The change in triptan use was defined as the difference in delivered DDD's in the 12 months after the start of propranolol use, relative to the 12 months before. The results were stratified by sex, age, and the use of hormonal contraceptives. A log transformation was performed to correct for the skewness of the data. Change in triptan use was then compared within subjects by using a paired t‐test. To compare the absolute change in DDD's (i.e., DDD's after propanolol minus DDD's before propranolol) between males and females, an unpaired t‐test was used. A p < 0.05 was considered to indicate significance. Group values are provided as mean values and SEM.
Results
Subjects
Twenty‐one healthy volunteers (10 males), aged 27 ± 2 years (range 18–57) participated (Fig. 1, Table 1). Demographics are described in Table 1.
Figure 1.
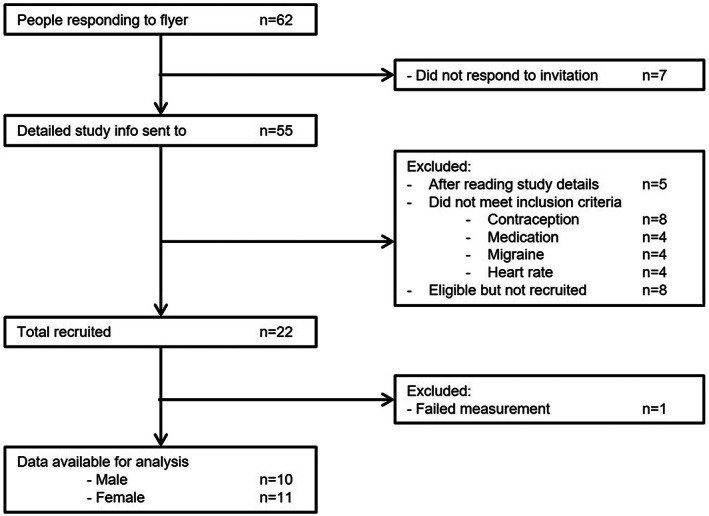
Recruitment flow diagram.
Table 1.
Demographics of the DBF study population.
| Male | Female | Both | |
|---|---|---|---|
| Population (n) | 10 | 11 | 21 |
| Age, years | 29 ± 3 | 25 ± 1 | 27 ± 2 |
| BMI, kg/m2 | 24.10 ± 0.92 | 23.38 ± 0.69 | 23.74 ± 0.56 |
| Height | 1.80 ± 0.02 | 1.64 ± 0.02 | 1.72 ± 0.02 |
| Weight | 78.4 ± 3.3 | 62.9 ± 1.5 | 70.3 ± 2.4 |
| BP, mmHg | |||
| Systolic | 123 ± 3 | 122 ± 4 | 122 ± 2 |
| Diastolic | 74 ± 3 | 74 ± 2 | 74 ± 2 |
| HR, bpm | 67 ± 2 | 79 ± 3 | 73 ± 2 |
Data are counts or mean ± SEM. BMI, body mass index; BP, blood pressure; HR, heart rate.
Hemodynamic changes
After propranolol, as expected, SBP, DBP, and HR were significantly decreased (Table 2).
Table 2.
Hemodynamic changes after placebo and propranolol.
| Males | Females | Both | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p value | Before | After | p value | Before | After | p value | |
| Placebo | |||||||||
| BP, mmHg | |||||||||
| Systolic | 109 ± 1 | 111 ± 2 | 0.237 | 109 ± 2 | 109 ± 3 | 0.891 | 109 ± 1 | 110 ± 2 | 0.369 |
| Diastolic | 64 ± 1 | 65 ± 2 | 0.470 | 63 ± 1 | 64 ± 2 | 0.570 | 64 ± 1 | 65 ± 1 | 0.301 |
| HR, bpm | 64 ± 3 | 62 ± 3 | 0.015 | 69 ± 2 | 70 ± 2 | 0.360 | 66 ± 2 | 66 ± 2 | 0.692 |
| Propranolol | |||||||||
| BP, mmHg | |||||||||
| Systolic | 111 ± 2 | 108 ± 2 | 0.009 | 107 ± 2 | 103 ± 2 | 0.004 | 109 ± 1 | 105 ± 2 | <0.0001 |
| Diastolic | 65 ± 1 | 64 ± 2 | 0.152 | 62 ± 1 | 60 ± 2 | 0.120 | 64 ± 1 | 62 ± 1 | 0.029 |
| HR, bpm | 61 ± 2 | 54 ± 3 | <0.0001 | 69 ± 2 | 61 ± 2 | <0.0001 | 65 ± 2 | 58 ± 2 | <0.0001 |
Data are mean ± SEM. Bold text indicates significant difference in blood pressure parameters before versus after placebo or propranolol, p < 0.05. BP, blood pressure; HR, heart rate.
Forehead DBF responses to capsaicin or electrical stimulation
Changes in forehead DBF to capsaicin were not significantly correlated with the changes in SBP (p = 0.403, r 2 = 0.037), DBP (p = 0.678, r 2 = 0.009), or HR (p = 0.963, r 2 = 0.0001) after propranolol (Fig. 2). Changes in DBF to capsaicin were not significantly different before and after placebo (before: 504 ± 31 a.u. vs. after: 505 ± 41 a.u., respectively; p = 0.966). In contrast, DBF responses to capsaicin were significantly decreased after propranolol (before: 513 ± 33 a.u. vs. after: 466 ± 35 a.u.; p = 0.042; Fig. 3).
Figure 2.
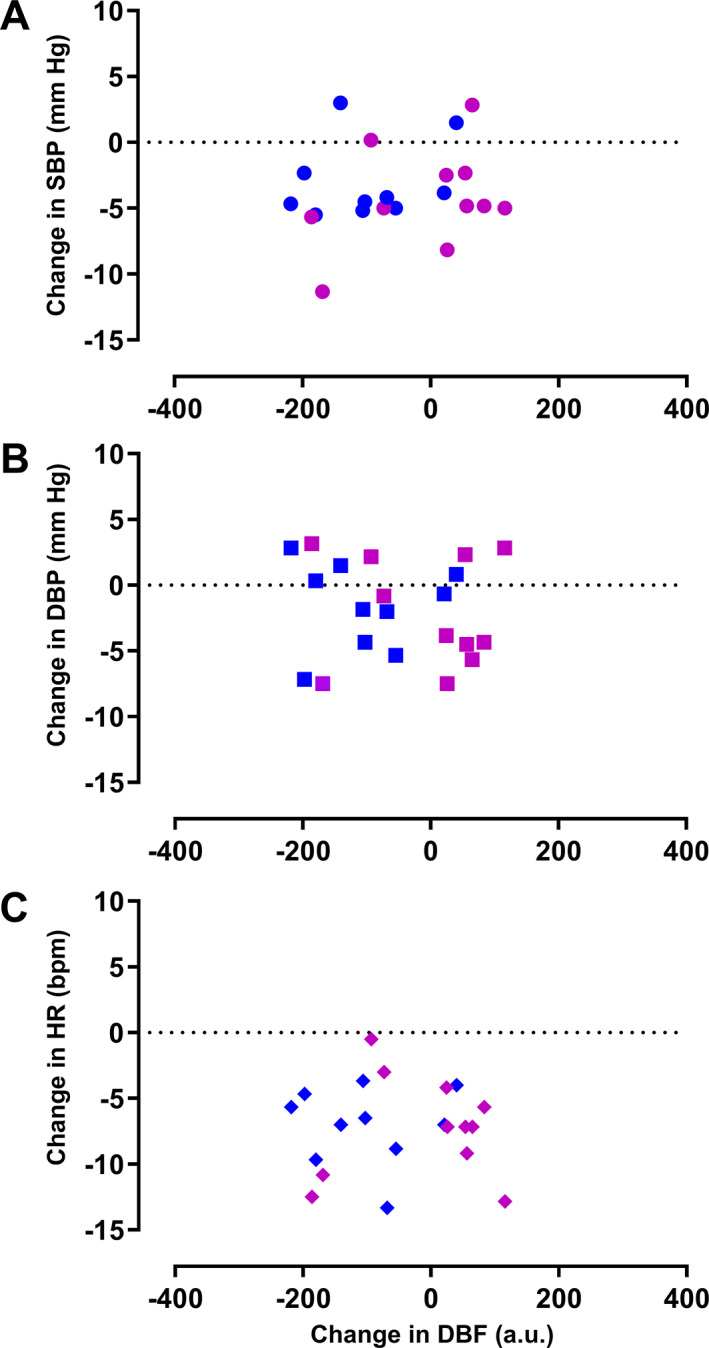
Correlation of the changes in DBF and the hemodynamic variables after propranolol intake. No correlation was observed between the changes in DBF and SBP (A), DBP (B), nor HR (C), n = 21. Female subjects are shown in purple (n = 11) and male subjects in dark blue (n = 10). DBF, dermal blood flow; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
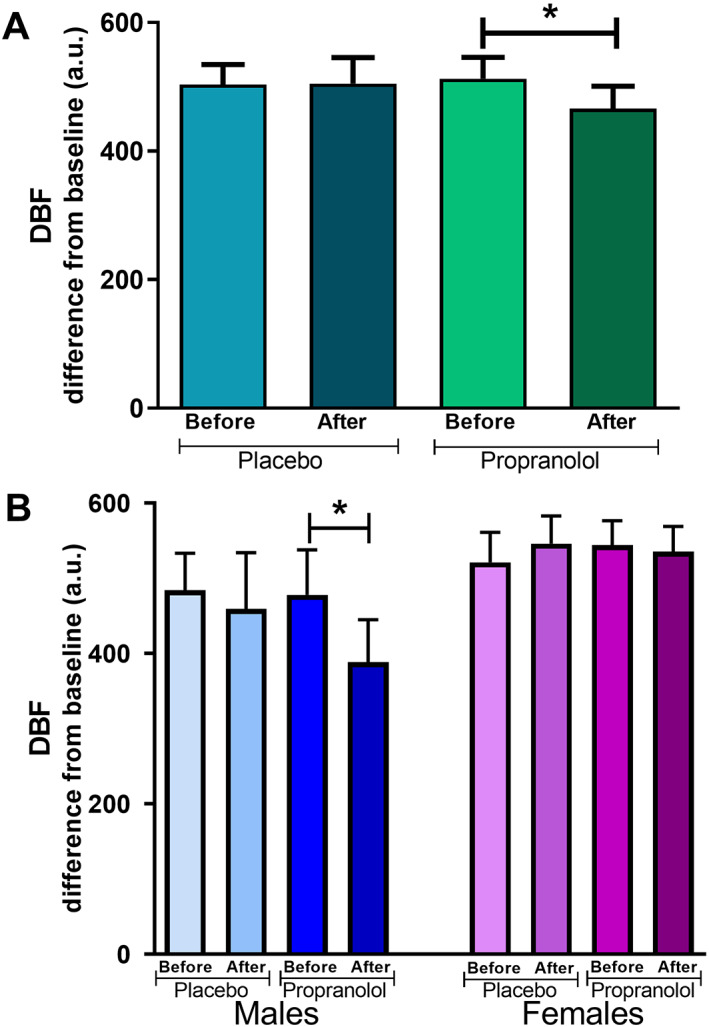
Forehead DBF responses to capsaicin. DBF response to capsaicin before and after placebo or propranolol as change from baseline (in a.u.) in (A) both sexes (n = 21) and (B) when divided by sex (males, lower left, n = 10; females, lower right, n = 11). Data are presented as mean ± SEM. *Significant decrease in DBF response to capsaicin after propranolol versus DBF response to capsaicin after placebo, p < 0.05. DBF, dermal blood flow. [Colour figure can be viewed at wileyonlinelibrary.com]
When stratifying the data by sex, the DBF responses to capsaicin were significantly decreased after propranolol in males (478 ± 60 a.u. vs. 389 ± 56 a.u.; p = 0.005), but not in female subjects (544 ± 32 a.u. vs. 536 ± 33 a.u.; p = 0.788) (Fig. 3). No significant difference was observed after placebo in males (484 ± 49 a.u. vs. 459 ± 75 a.u.; p = 0.662), or in females (521 ± 40 a.u. vs. 546 ± 37 a.u.).
The DBF responses to ES were not affected by either propranolol (p = 0.744) or placebo (p = 0.400, Fig. 4). When sub‐grouping by sex, DBF responses in males were also not significantly modified after placebo (p = 0.514), or propranolol (p = 0.404); similarly, in female subjects, no significant difference was observed after placebo (p = 0.372) or propranolol (p = 0.556; data not shown).
Figure 4.
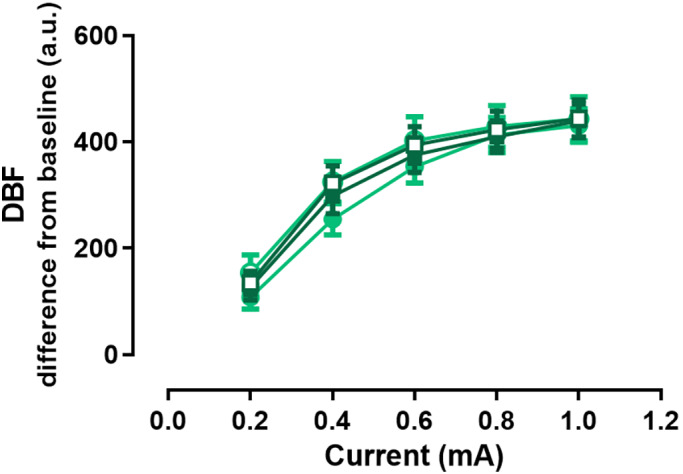
Forehead DBF responses to ES. Maximal DBF response to ES with an increasing stimulation current before placebo (full circle) or propranolol (full square) and after placebo (empty circle) or propranolol (empty square) as change from baseline (in a.u.). Data are presented as mean ± SEM, n = 21. DBF, dermal blood flow; ES, electrical stimulation. [Colour figure can be viewed at wileyonlinelibrary.com]
Pain in (neck) electrode site was the most reported side effect (5/21), followed by tingling in electrode site (2/21), weird sensation (1/21 after placebo vs. 1/21 after propranolol), and drowsiness (1/21 after placebo vs. 1/21 after propranolol). Fatigue and weakness were reported after propranolol (1/21, each), but not after placebo. One subject reported neck stiffness due to uncomfortable pillow and two subjects reported nausea and flickering lights after placebo.
Propranolol levels
Mean plasma levels of propranolol were 63.4 ± 12 μg/L (Fig. 5, therapeutic levels between 20 and 300, NVZA toxicologie.org info). When stratifying by sex, propranolol levels were significantly higher in women when compared with men (90.5 ± 21 μg/L vs. 36.3 ± 7 μg/L, respectively; p = 0.023), also when adjusted for body weight (data not shown).
Figure 5.
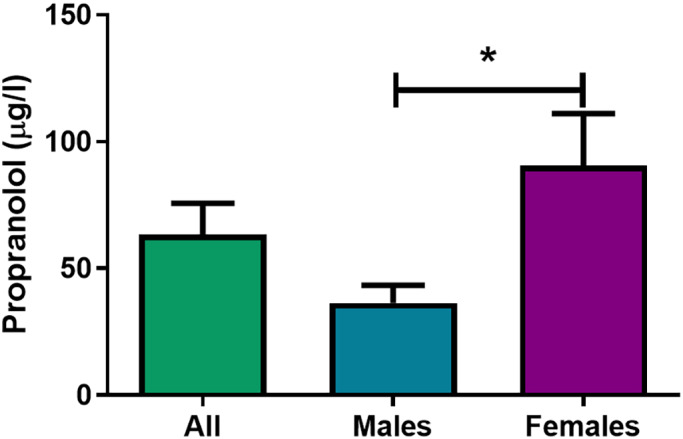
Propranolol plasma levels. Propranolol plasma levels (in μg/L) pooled (n = 21), and divided by sex (males, n = 10; females, n = 11). *Significant difference in propranolol levels of male versus female subjects, p < 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
Retrospective prescription database study
A total of 291 subjects were included in our analysis (31 ± 0.3 years old, range 20–39). The average DDD of triptans in the 12 months before and after propranolol prescription is described (Table 3). The average DDD of triptans in the 12 months before propranolol prescription was slightly decreased in the 12 months after propranolol prescription. When stratifying by sex, 47 males (16%, 31 ± 0.9 years old, range 20–39) and 244 females (84%, 31 ± 0.4 years old, range 20–39) were part of the study. When further stratifying female subjects by use of contraceptives, 151 women on contraceptives were included in our analysis (52%, 30 ± 0.5 years old, range 20–39).
Table 3.
Change in triptan DDD after propranolol prescription.
| N | % of total | Age Years | Triptan DDD before propranolol | Triptan DDD after propranolol | Absolute change | % of change | p value | |
|---|---|---|---|---|---|---|---|---|
| Total | 291 | 100 | 31 ± 0.3 | 61.16 ± 3.6 | 59.06 ± 4.3 | −2.10 | −3% | <0.0001 |
| Males | 47 | 16 | 31 ± 0.9 | 42.15 ± 6.0 | 35.79 ± 5.8 | −6.36 | −15% | 0.002 |
| Females | 244 | 84 | 31 ± 0.4 | 64.82 ± 4.1 | 63.55 ± 4.9 | −1.27 | −2%* | <0.0001 |
| Females on contraceptives | 151 | 52 | 30 ± 0.5 | 63.04 ± 4.7 | 58.43 ± 5.4 | −4.61 | −7%** | <0.0001 |
Absolute and percentage of change of triptan use after propranolol prescription in all the subjects included in the retrospective study, and stratified by sex and contraceptive use. Data are counts or mean ± SEM. DDD, defined daily doses.
Significant difference in the reduction of triptan DDD between female and male patients (p < 0.05).
Significant difference in the reduction of triptan DDD between females on contraceptives and males (p < 0.05).
Male subjects had a lower DDD of triptans in the 12 months before propranolol than females. Furthermore, in males, the average DDD of triptans in 12 months after propranolol, was decreased, representing a reduction of 15%, whereas in females, there was a modest decrease of 2%. When further stratifying to women on contraceptives, a reduction of 7% was observed. The percentage of change between males and females was significantly different (−15% vs. −2%; p = 0.0218), as well as the percentage of change between females on contraceptives and males (−7% vs. −15%; p = 0.0464).
Discussion
Since Rabkin and colleagues 9 reported that propranolol could be effective for the treatment of “vascular headaches,” it has become one of the most widely prescribed drugs for the prophylactic treatment of migraine, however, its exact mechanism of action is not yet known. On this basis, in this study, we investigated the effect of propranolol on the modulation of the human trigeminovascular system, currently one of the main targets for migraine treatment.
Our results show that a single dose of propranolol (80 mg) significantly inhibits the trigeminal nerve‐mediated vasodilation, more specifically, the capsaicin‐dependent vasodilation, as the DBF responses to ES were not significantly modified (Figs. 3 and 5). Our group has previously shown that, sumatriptan, an acutely acting antimigraine drug, inhibits the DBF responses to capsaicin to approximately 75% of the control response. 14 In the present study, we also observed a significant decrease in the CGRP‐mediated DBF responses; however, the decrease was not as pronounced as with sumatriptan (to ~90%). This may explain why propranolol is not effective as an acutely acting antimigraine drug, but rather as a prophylactic antimigraine agent. Also, the study took place at T max after a single dose, while in clinical practice, after continuous administration, the elimination of propranolol becomes saturated and it accumulates to a greater extent than predicted by its half‐life, due to a decrease in presystemic extraction (from 78% after the first dose, to 66% following 80 mg 20 ). In fact, after chronic administration of the same oral dose to different patients, a 20‐fold variation in plasma levels has been described, 24 which could also explain the differences in therapeutic efficacy among patients. Another aspect to consider, is that in the present study, we chose the lowest effective dose of propranolol that has been used in the clinical trials for migraine treatment (80 mg) 10 as acute intake of higher doses was not ethically feasible. Thus, it is reasonable to assume that (in male patients), higher propranolol doses might exert a more pronounced inhibition of the capsaicin‐induced increases in DBF.
Previously, studies have investigated the effect of propranolol in preclinical models of migraine. In murine models of cortical spreading depression (CSD) (considered the underlying cause of migraine with aura), it has been shown that propranolol prevents the changes in behavior (e.g., freezing) and cerebral blood flow induced by CSD, 25 with studies also showing prevention of CSD onset and migration. 26 , 27 Additionally, it has been shown that chronic administration of l‐propranolol, but not of d‐propranolol, inhibits the CSD migration and/or onset. 28 Nevertheless, the role of CSD in the pathophysiology of migraine is still debated, 28 and both enantiomers have been proven to be effective for the prophylactic treatment of migraine. 29 Therefore, inhibition of the CSD‐related changes seems unlikely to be the only mechanism behind the efficacy of propranolol. Moreover, in a murine model of central trigeminovascular activation, it has been shown that microiontophoretic ejection of propranolol inhibits thalamocortical activity in response to superior sagittal sinus stimulation and L‐glutamate‐evoked neuronal activation 30 . However, not all the β‐blockers that are effective for the treatment of migraine are able to cross the blood–brain barrier (e.g., atenolol); therefore, their efficacy cannot be mediated purely via central mechanisms. In the present study, we showed that a single dose of propranolol modulates the human peripheral trigeminovascular system, which broadens the therapeutic implications of our results.
A question that remains open in this study is the exact nature of the receptor(s)/mechanism(s) involved in the modulation of the trigeminovascular system by propranolol. Indeed, (presynaptic/prejunctional) β‐adrenoceptors would seem to be the most likely candidates, as it has been shown that propranolol inhibits glutamate release via presynaptic receptors. 31 , 32 Moreover, the inhibition by propranolol of thalamocortical activity in response to dural stimulation was shown to be mediated via β1‐adrenoceptors. 30 However, not all β‐blockers are effective for the prophylactic treatment of migraine, and their efficacy does not depend on their selectivity, as the β1‐adrenoceptor antagonists atenolol and metoprolol are effective for the prophylactic treatment of migraine, whereas acebutolol is not. 10 Interestingly, while all the effective β‐blockers (e.g., propranolol, metoprolol, atenolol) are β‐adrenoceptor antagonists, all the non‐effective ones (e.g., pindolol, acebutolol, labetalol) have intrinsic sympathomimetic activity, which means that those “β‐blockers” behave, in fact, as partial agonists of the β‐adrenoceptors, 31 which could suggest that total blockade of β‐adrenoceptors is needed for an effective therapeutic response in migraine treatment. Additionally, propranolol has been shown to not only act as a β‐adrenoceptor antagonist, but also as a mixed agonist/antagonist of 5‐HT receptors in a tissue‐dependent manner. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 In relation to this, it has been shown that a single point mutation increases the affinity of the β‐adrenoceptor antagonists for the 5‐HT1B, 5‐HT1D, and 5‐HT1F receptors, 45 and activation of prejunctional 5‐HT1D and 5‐HT1F receptors is thought to inhibit the release of CGRP from the trigeminal fibers that innervate the dura (and the forehead skin), 13 , 46 which is in accordance with our previous results with sumatriptan. 14 Moreover, as high concentrations of propranolol are needed in order to observe an effective therapeutic response in migraine patients, 10 it would seem reasonable that propranolol is not only acting as a β‐adrenoceptor antagonist, but also via other prejunctional receptors present on trigeminal fibers, possibly 5‐HT1D and/or 5‐HT1F receptors. However, if the inhibition of the capsaicin‐induced DBF responses after propranolol was mediated only via prejunctional 5‐HT1D/1F receptors, then the inhibition of the capsaicin‐induced DBF responses by sumatriptan (a 5‐HT1B/1D/1F receptor agonist 47 ) previously reported by our group, 14 would have presented the same sex‐dependent profile, and we did not find significant differences between sexes.
As mentioned above, in the present study we observed that when stratifying our results by sex, the inhibition of the DBF responses to capsaicin was sex‐dependent, as a pronounced (and significant) inhibition remained present in men, but not in women (Fig. 3). Interestingly, the plasma levels of propranolol in females were significantly higher than in males (Fig. 5), even when correcting for weight, thus excluding a pharmacokinetic cause for the sex‐dependent effects. This may point to the desensitization of the receptors involved in the modulation of the trigeminovascular system, which would suggest that lower doses of propranolol are needed for migraine prophylaxis in women; and/or that at this dose, there is activation of other receptors that counteract this response. Interestingly, our group has previously shown in the same forehead model that the trigeminal nerve‐induced vasodilatory responses and hormone levels are altered in female migraine patients. 19 Moreover, a recent study in rodents showed that application of CGRP to the cranial meninges results in behavioral responses consistent with headache in preclinical models in a female‐specific manner, 48 reinforcing the interaction between sex hormones and CGRPergic fibers in the modulation of the trigeminovascular system. 20 Additionally, sexually dimorphic responses to propranolol have also been reported in a murine model of addiction, where in males the mechanism of action of propranolol involved 5‐HT signaling, whereas in females it involved both β‐adrenergic and 5‐HT signaling. 49
Considering our abovementioned results and that women represent around 70% of migraine patients, 2 it is important to address whether our results can be translated into the clinical practice. With this goal in mind, we performed a retrospective study where we correlated our results with data from the Dutch IADB.nl prescription database. For this, we analyzed the change in triptan use after prescription of propranolol in a population similar to our DBF study subjects. We analyzed the change in triptan use as a surrogate marker of therapeutic efficacy of propranolol, as a decrease in migraine days would result in a decrease in triptan use. In agreement with our forehead study, our results show that even though both sexes reduced their use of triptans after propranolol, a significantly more pronounced decrease was observed in male subjects when compared with females on contraceptives (15% in males vs. 7% in females on contraceptives, Table 3). Moreover, when female patients were not stratified by contraceptive use, a decrease of only 1% in the use of triptans was observed, which, although significant, cannot be considered clinically meaningful. Certainly, these results must be interpreted with caution, as it was a retrospective study and the exact indication for propranolol was not stated. More studies that fall beyond the scope of our current study, should assess whether other antimigraine drugs show sex‐dependent efficacy (e.g., triptans), and the possible receptors/mechanisms involved.
In conclusion, our study shows that an acute dose of propranolol (80 mg) inhibits the capsaicin‐induced increases in DBF in a sex‐dependent manner. Additionally, in a retrospective study, a more pronounced decrease in triptan use was observed in male patients when compared with females on contraceptives. These results, taken together, suggest an interaction between propranolol and sex steroids in the modulation of the trigeminovascular system and support the usefulness of our human model to evaluate trigeminovascular modulation by current and prospective antimigraine drugs, as well as the need for sex‐specific migraine treatments.
Conflict of Interest
Nothing to report.
Authors' Contributions
ERB, CMV, JV, AHJD, AHvdM, KI, and AMvdB contributed to conception and design of the study. ERB, RMS, JvdB, CCMSV, and BCPK contributed to the acquisition and analysis of data. ERB and AMvdB drafted the manuscript. All authors revised and approved the manuscript.
Acknowledgments
We thank all of the participants of the study. We also thank Dr Mariam Kavousi for her invaluable help with the statistical analysis. Finally, we thank Birgitte Breemerkamp and Anouk Buurman for their unconditional and vital support throughout the study. This study was funded by the Dutch Research Council (Vidi grant 917.113.349, Antoinette MaassenVanDenBrink). Eloísa Rubio‐Beltrán was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT; fellowship No. 409865; Mexico City).
Funding Information
This study was funded by the Dutch Research Council (Vidi grant 917.113.349, Antoinette MaassenVanDenBrink). Eloísa Rubio‐Beltrán was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT; fellowship No. 409865; Mexico City).
Funding Statement
This work was funded by Consejo Nacional de Ciencia y Tecnología grant 409865; Dutch Research Council grant Vidi 917.113.349.
References
- 1. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension‐type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):954‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) study. Headache. 2013;53(8):1278‐1299. [DOI] [PubMed] [Google Scholar]
- 3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine — current understanding and treatment. N Engl J Med. 2002;346(4):257‐270. [DOI] [PubMed] [Google Scholar]
- 4. Edvinsson L. The Trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache. 2017;57(Suppl 2):47‐55. [DOI] [PubMed] [Google Scholar]
- 5. Feniuk W, Humphrey PPA. The development of a highly selective 5‐HT1 receptor agonist, sumatriptan, for the treatment of migraine. Drug Dev Res. 1992;26(3):235‐240. [Google Scholar]
- 6. Rubio‐Beltran E, Labastida‐Ramirez A, Villalon CM, MaassenVanDenBrink A. Is selective 5‐HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88‐97. [DOI] [PubMed] [Google Scholar]
- 7. Hong P, Tan T, Liu Y, Xiao J. Gepants for abortive treatment of migraine: a network meta‐analysis. Brain Behav. 2020;10(8):e01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs. 2019;28(6):555‐567. [DOI] [PubMed] [Google Scholar]
- 9. Rabkin R, Stables DP, Levin NW, Suzman MM. The prophylactic value of propranolol in angina pectoris. Am J Cardiol. 1966;18(3):370‐380. [DOI] [PubMed] [Google Scholar]
- 10. Jackson JL, Cogbill E, Santana‐Davila R, et al. A comparative effectiveness meta‐analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10(7):e0130733‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Negro A, Lionetto L, Simmaco M, Martelletti P. CGRP receptor antagonists: an expanding drug class for acute migraine? Expert Opin Investig Drugs. 2012;21(6):807‐818. [DOI] [PubMed] [Google Scholar]
- 12. Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol. 2015;80(2):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labastida‐Ramirez A, Rubio‐Beltran E, Haanes KA, et al. Lasmiditan inhibits calcitonin gene‐related peptide release in the rodent trigeminovascular system. Pain. 2020;161:1092‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahimi K, Danser AHJ, Terwindt GM, Van Den Meiracker AH, MaassenVanDenBrink A. A human trigeminovascular biomarker for antimigraine drugs: a randomised, double‐blind, placebo‐controlled, crossover trial with sumatriptan. Cephalalgia. 2016;37(1):94‐98. [DOI] [PubMed] [Google Scholar]
- 15. Ibrahimi K, Vermeersch S, Danser A, et al. Development of an experimental model to study trigeminal nerve‐mediated vasodilation on the human forehead. Cephalalgia. 2014;34(7):514‐522. [DOI] [PubMed] [Google Scholar]
- 16. Ibrahimi K, van Oosterhout WPJ, van Dorp W, et al. Reduced trigeminovascular cyclicity in patients with menstrually related migraine. Neurology. 2015;84(2):125‐131. [DOI] [PubMed] [Google Scholar]
- 17. Labastida‐Ramirez A, Rubio‐Beltran E, Villalon CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia. 2019;39:435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibrahimi K, Vermeersch S, Frederiks P, et al. The influence of migraine and female hormones on capsaicin‐induced dermal blood flow. Cephalalgia. 2017;37(12):1164‐1172. [DOI] [PubMed] [Google Scholar]
- 19. Eldon MA, Kinkel AW, Daniel JE, Latts JR. Bioavailability of propranolol hydrochloride tablet formulations: application of multiple dose crossover studies. Biopharm Drug Dispos. 1989;10(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 20. Wood AJ, Carr K, Vestal RE, Belcher S, Wilkinson GR, Shand DG. Direct measurement of propranolol bioavailability during accumulation to steady‐state. Br J Clin Pharmacol. 1978;6(4):345‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linstra KM, Ibrahimi K, Terwindt GM, Wermer MJH, MaassenVanDenBrink A. Migraine and cardiovascular disease in women. Maturitas. 2017;97:28‐31. [DOI] [PubMed] [Google Scholar]
- 22. Flockhart DA, Tanus‐Santos JE. Implications of cytochrome P450 interactions when prescribing medication for hypertension. Arch Intern Med. 2002;162(4):405‐412. [DOI] [PubMed] [Google Scholar]
- 23. Visser ST, Schuiling‐Veninga CCM, Bos JHJ, de Jong‐van den Berg LTW, Postma MJ. The population‐based prescription database IADB.Nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res. 2013;1(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 24. Shand DG. Pharmacokinetics of propranolol: a review. Postgrad Med J. 1976;52(Suppl 4):22‐25. [PubMed] [Google Scholar]
- 25. Kurauchi Y, Haruta M, Tanaka R, et al. Propranolol prevents cerebral blood flow changes and pain‐related behaviors in migraine model mice. Biochem Biophys Res Commun. 2019;508(2):445‐450. [DOI] [PubMed] [Google Scholar]
- 26. Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59(4):652‐661. [DOI] [PubMed] [Google Scholar]
- 27. Richter F, Mikulik O, Ebersberger A, Schaible HG. Noradrenergic agonists and antagonists influence migration of cortical spreading depression in rat—a possible mechanism of migraine prophylaxis and prevention of Postischemic neuronal damage. J Cereb Blood Flow Metab. 2005;25(9):1225‐1235. [DOI] [PubMed] [Google Scholar]
- 28. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9:637‐644. [DOI] [PubMed] [Google Scholar]
- 29. Stensrud P, Sjaastad O. Short‐term clinical trial of propranolol in racemic form (Inderal), D‐propranolol and placebo in migraine. Acta Neurol Scand. 1976;53(3):229‐232. [DOI] [PubMed] [Google Scholar]
- 30. Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain. 2004;128(1):86‐97. [DOI] [PubMed] [Google Scholar]
- 31. Okuda N, Kohara K, Mikami H, et al. Effect of propranolol on central neurotransmitter release in Wistar rats analysed by brain microdialysis. Clin Exp Pharmacol Physiol. 1999;26(3):220‐224. [DOI] [PubMed] [Google Scholar]
- 32. Wang S‐J, Coutinho V, Sihra TS. Presynaptic cross‐talk of β‐adrenoreceptor and 5‐hydroxytryptamine receptor signalling in the modulation of glutamate release from cerebrocortical nerve terminals. Br J Pharmacol. 2002;137(8):1371‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olsson JE, Behring HC, Forssman B, et al. Metoprolol and propranolol in migraine prophylaxis: a double‐blind multicentre study. Acta Neurol Scand. 1984;70(3):160‐168. [DOI] [PubMed] [Google Scholar]
- 34. Schoeffter P, Pfeilschifter J, Bobirnac I. 5‐Hydroxytryptamine 5‐HT1B receptors inhibiting cyclic AMP accumulation in rat renal mesangial cells. Naunyn Schmiedebergs Arch Pharmacol. 1995;351(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 35. Pauwels PJ, Palmier C. Inhibition by 5‐HT of forskolin‐induced cAMP formation in the renal opossum epithelial cell line OK: mediation by a 5‐HT1B like receptor and antagonism by methiothepin. Neuropharmacology. 1994;33(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 36. Glennon RA, Pierson ME, McKenney JD. Stimulus generalization of 1‐(3‐trifluoromethylphenyl)piperazine (TFMPP) to propranolol, pindolol and mesulergine. Pharmacol Biochem Behav. 1988;29(1):197‐199. [DOI] [PubMed] [Google Scholar]
- 37. Hjorth S, Carlsson A. Is pindolol a mixed agonist‐antagonist at central serotonin (5‐HT) receptors? Eur J Pharmacol. 1986;129(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 38. Maura G, Ulivi M, Raiteri M. (−)‐Propranolol and (±)‐cyanopindolol are mixed agonists‐antagonists at serotonin autoreceptors in the hippocampus of the rat brain. Neuropharmacology. 1987;26(7):713‐717. [DOI] [PubMed] [Google Scholar]
- 39. Tsuchihashi H, Nakashima Y, Kinami J, Nagatomo T. Characteristics of 125I‐lodocyanopindolol binding to β‐adrenergic and serotonin‐1B receptors of rat brain: selectivity of β‐adrenergic agents. JPN J Pharmacol. 1990;52(2):195‐200. [DOI] [PubMed] [Google Scholar]
- 40. Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5‐hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46(2):157‐203. [PubMed] [Google Scholar]
- 41. Murphy TJ, Bylund DB. Characterization of serotonin‐1B receptors negatively coupled to adenylate cyclase in OK cells, a renal epithelial cell line from the opossum. J Pharmacol Exp Ther. 1989;249(2):535‐543. [PubMed] [Google Scholar]
- 42. Nishimura Y. Characterization of 5‐hydroxytryptamine receptors mediating contractions in basilar arteries from stroke‐prone spontaneously hypertensive rats. Br J Pharmacol. 1996;117(6):1325‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schoeffter P, Hoyer D. 5‐Hydroxytryptamine 5‐HT1B and 5‐HT1D receptors mediating inhibition of adenylate cyclase activity. Naunyn Schmiedebergs Arch Pharmacol. 1989;340(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 44. Adham N, Ellerbrock B, Hartig P, Weinshank RL, Branchek T. Receptor reserve masks partial agonist activity of drugs in a cloned rat 5‐hydroxytryptamine1B receptor expression system. Mol Pharmacol. 1993;43(3):427‐433. [PubMed] [Google Scholar]
- 45. Adham N, Tamm JA, Salon JA, Vaysse PJ, Weinshank RL, Branchek TA. A single point mutation increases the affinity of serotonin 5‐HT1Dα, 5‐HT1Dβ, 5‐HT1E and 5‐HT1F receptors for β‐adrenergic antagonists. Neuropharmacology. 1994;33(3):387‐391. [DOI] [PubMed] [Google Scholar]
- 46. Eltorp CT, Jansen‐Olesen I, Hansen AJ. Release of calcitonin gene‐related peptide (CGRP) from Guinea pig dura mater in vitro is inhibited by sumatriptan but unaffected by nitric oxide. Cephalalgia. 2000;20(9):838‐844. [DOI] [PubMed] [Google Scholar]
- 47. Rubio‐Beltran E, Labastida‐Ramirez A, Haanes KA, et al. Characterization of binding, functional activity, and contractile responses of the selective 5‐HT1F receptor agonist lasmiditan. Br J Pharmacol. 2019;176(24):4681‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Avona A, Burgos‐Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural calcitonin gene‐related peptide produces female‐specific responses in rodent migraine models. J Neurosci. 2019;39(22):4323‐4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kohtz AS, Aston‐Jones G. Cocaine seeking during initial abstinence is driven by noradrenergic and serotonergic signaling in hippocampus in a sex‐dependent manner. Neuropsychopharmacology. 2016;42:408‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]


