Abstract
In severely affected stroke survivors, cortico-muscular control is disturbed and volitional upper limb movements often absent. Mental rehearsal of the impaired movement in conjunction with sensory feedback provision are suggested as promising rehabilitation exercises. Knowledge about the underlying neural processes, however, remains vague. In male and female chronic stroke patients with hand paralysis, a brain-computer interface controlled a robotic orthosis and turned sensorimotor β-band desynchronization during motor imagery (MI) of finger extension into contingent hand opening. Healthy control subjects performed the same task and received the same proprioceptive feedback with a robotic orthosis or visual feedback only. Only when proprioceptive feedback was provided, cortico-muscular coherence (CMC) increased with a predominant information flow from the sensorimotor cortex to the finger extensors. This effect (1) was specific to the β frequency band, (2) transferred to a motor task (MT), (3) was proportional to subsequent corticospinal excitability (CSE) and correlated with behavioral changes in the (4) healthy and (5) poststroke condition; notably, MI-related enhancement of β-band CMC in the ipsilesional premotor cortex correlated with motor improvements after the intervention. In the healthy and injured human nervous system, synchronized activation of motor-related cortical and spinal neural pools facilitates, in accordance with the communication-through-coherence hypothesis, cortico-spinal communication and may, thereby, be therapeutically relevant for functional restoration after stroke, when voluntary movements are no longer possible.
SIGNIFICANCE STATEMENT This study provides insights into the neural processes that transfer effects of brain-computer interface neurofeedback to subsequent motor behavior. Specifically, volitional control of cortical oscillations and proprioceptive feedback enhances both cortical activity and behaviorally relevant connectivity to the periphery in a topographically circumscribed and frequency-specific way. This enhanced cortico-muscular control can be induced in the healthy and poststroke brain. Thereby, activating the motor cortex with mental rehearsal of the impaired movement and closing the loop by robot-assisted feedback synchronizes ipsilesional premotor cortex and spinal neural pools in the β frequency band. This facilitates, in accordance with the communication-through-coherence hypothesis, cortico-spinal communication and may, thereby, be therapeutically relevant for functional restoration after stroke, when voluntary movements are no longer possible.
Keywords: β-oscillations, cortico-muscular coherence, cortico-spinal excitability, motor recovery, neurofeedback, stroke
Introduction
After stroke, active movements are particularly relevant for motor rehabilitation to be effective in improving outcome beyond spontaneous neurobiological processes (Murphy and Corbett, 2009; Langhorne et al., 2011). When active physical practice, e.g., of the upper extremities is no longer possible, the re-learning of movements remains restricted (Doyon and Benali, 2005; Halsband and Lange, 2006). Motor recovery in severely impaired stroke patients with a long-lasting hand paralysis is therefore limited (Dobkin et al., 2004; Feigin et al., 2008; Jørgensen et al., 1999).
In these patients, motor imagery (MI) might be an alternative to physical practice (Boe et al., 2014; Halsband and Lange, 2006) since it activates the sensorimotor system without any overt movement, particularly when reinforced by feedback (Gao et al., 2011; Szameitat et al., 2012; Vukelić and Gharabaghi, 2015a; Bauer et al., 2015; Naros et al., 2016). Neurofeedback of motor intention or MI-related brain states with brain-computer/brain-machine interfaces (BCI/BMI) or peripheral electrical stimulation is therefore being explored as an experimental training to improve the motor outcome of stroke rehabilitation (Biasiucci et al., 2018). Controlled trials demonstrated that MI with contingent feedback resulted in greater improvement than interventions that provided no or only random feedback (Cervera et al., 2018). However, these studies revealed also considerable heterogeneity of the intervention effects (Cervera et al., 2018). Moreover, these BCI interventions have often not achieved clinical benefits beyond those of dose-matched robot-assisted therapy (Ang et al., 2010, 2014), which has, in turn, provided only little additional benefit over dose-matched classical physiotherapy up to now (Kwakkel et al., 2008; Lo et al., 2010; Klamroth-Marganska et al., 2014). In this context, current BCI interventions resemble (if applied contingently to MI) other priming interventions such as transcranial electrical (Allman et al., 2016) or magnetic brain stimulation techniques (Volz et al., 2016). These approaches increase, unlike sham stimulation, the general responsiveness of the brain for subsequent active practice.
However, to provide the rationale for translating BCI interventions into clinical settings for severely impaired stroke patients, who lack upper limb function and are unable to engage their hand in useful physical training, more specific effects beyond the practice of primed physiotherapy are required (Naros and Gharabaghi, 2015; Brauchle et al., 2015). Such a specificity requires the demonstrating of brain-behavior relationships (Gharabaghi, 2016): we need to demonstrate (1) an evolution of the oscillatory dynamics trained by the neurofeedback task, (2) correlated modulations of functionally relevant networks related to the therapeutic goal, and (3) a correlation between the modulated neural dynamics and the task-specific behavioral changes.
Previous findings serve as building blocks toward this goal: movement-related β-oscillations were shown to be compromised in stroke patients and correlated with the impairment level (Rossiter et al., 2013; Shiner et al., 2015). In the early poststroke period, these oscillations may reflect impairment only and not recovery (Bönstrup et al., 2019). Less is known about the role of β-oscillations in chronic stroke patients. In healthy subjects, however, volitional modulation of these sensorimotor β-oscillations could be learned via BCI feedback and correlated with corticospinal excitability (CSE) increases (Kraus et al., 2016a) and improved motor performance (Naros et al., 2016); β-oscillations in the healthy brain predicted, moreover, a power-dependent and phase-dependent intrinsic response modulation of cortico-spinal signal propagation at rest (Khademi et al., 2018, 2019) and in the active motor system (Naros et al., 2020).
More specific knowledge about the underlying neural processes that transfer effects of the BCI intervention to the following behavioral motor task (MT), particularly in the poststroke condition, remains, however, vague. In this context, we conjectured in accordance with the communication-through-coherence hypothesis (Fries, 2005), synchronized activation of motor-related cortical and spinal neural pools, e.g., captured by cortico-muscular coherence (CMC), as the functionally relevant substrate to mediate the above-mentioned effects. We, furthermore, hypothesized a frequency-specificity to the β-band and the relevance of proprioceptive feedback to volitional modulation of sensorimotor oscillations for this coherence to ensue. We also expected β-CMC as the functionally relevant substrate to mediate BCI training-related network dynamics to subsequent motor improvements in the poststroke condition, thereby, being potentially relevant for functional restoration when voluntary hand opening is no longer possible.
Materials and Methods
Subjects
The research was approved by the ethics committee of the University of Tubingen Faculty of Medicine and conformed to the standards set by the latest version of the Declaration of Helsinki. All participants gave their written informed consent before participation.
In study 1, we recruited twenty-seven, right-handed healthy human subjects (mean age, 27.30 ± 4.38 years, range 19–37 years, 22 male) with no history of psychiatric or neurologic disorders. Fifteen subjects participated in one session with proprioceptive feedback, and twelve subjects participated in one session with visual feedback. The Edinburgh handedness inventory (Oldfield, 1971) was used to confirm right-handedness. In this healthy subject population, we have already reported (Kraus et al., 2016a) an increase of CSE, indexed by motor-evoked potential (MEP) changes in electromyographic (EMG) recordings in response to transcranial magnetic stimulation (TMS). This MEP increase occurred in the steep part of the stimulus-response curve (SRC). In this previous work (Kraus et al., 2016a), we investigated CSE of the stimulated sensorimotor cortex (indexed by MEPs) at rest, after the BCI intervention.
In the present study, we analyzed the electroencephalographic (EEG) data acquired during the intervention, and afterward in the course of a MT that was performed before and after the intervention (Fig. 1). This allowed capturing on a moment-to-moment basis corticocortical (ciCOH) and cortico-muscular oscillatory phase coherence (CMC) of both hemispheres (beyond the right sensorimotor cortex that was previously studied with MEPs) in the course of the BCI intervention and the behavioral MT (Schnitzler and Gross, 2005; Mehrkanoon et al., 2014).
Figure 1.
Experimental design and example data (healthy subjects). A, Schematic illustration of the experimental design and timeline. B, Healthy subjects underwent a neurofeedback intervention of modulating β activity (16–22 Hz) in circumscribed premotor and sensorimotor regions (marked by + on the topography; L and R stand for the left and right cortical hemispheres) of the right hemisphere by kinesthetic MI. Healthy subjects participated in an intervention session (∼40 min), during which they received feedback which was contingent to their MI-associated brain activity in a parallel-group design with one of two different modalities: (1) visual feedback with a brain–computer interface (n = 12) or (2) proprioceptive feedback with a brain–computer interface (n = 15) orthosis attached to the left hand. C, Healthy subjects (both visual and proprioceptive groups) performed a MT (∼5 min) before (pre-MT) and after (post-MT) the intervention. An oscillating target (0.1 Hz) was presented to the subjects on a screen. The subjects were instructed to follow the target using a cursor. This was controlled by isometric flexion and extension of the left hand that was attached to a hand orthosis. D, Motor performance was defined as the difference between the targeted and the actual force applied to the hand orthosis which was paralleled by a deviation between the target and the actual oscillation on the screen, i.e., the closer the target and the actual oscillation, the better the performance.
The data acquired during the intervention and the MT were analyzed separately. Thereby, the task-related network-level connectivity, a potential generalization to the MT, and the underlying neurophysiological mechanisms could be analyzed and compared with each other, and to the reported MEP changes and behavioral effects.
In study 2, eight right-handed human stroke subjects (mean age, 57 ± 11 years, range 34–68 years, seven male) participated in the same BCI intervention with proprioceptive feedback like the healthy subjects. As part of a larger study on the underlying neurophysiological mechanisms of BCI feedback in the lesioned brain, these severely affected chronic stroke subjects performed 20 sessions of this intervention within a four-week training period, i.e., one session per day. Clinical details of this group of stroke subjects (Table 1; P8 of the present study is P9 of Belardinelli et al., 2017; where the original P8 was excluded from further analysis because of left-handedness) have already been reported (Belardinelli et al., 2017; Grimm et al., 2016) and are cited here accordingly. Here, we included right-handed subjects only, since recent work of our group suggests that right and left handers show different oscillatory entrainment of cortical alpha band networks during β-band neurofeedback (interhemispheric vs intrahemispheric interactions), when using the very same approach applied in this study (Vukelić et al., 2019). The stroke subjects were in the chronic phase after stroke (70 ± 34 months) and presented with a severe and persistent hemiparesis of the left side because of a right hemispheric lesion with an upper extremity Fugl–Meyer Assessment (UE-FMA) score of 16.23 ± 6.79 [range 6.80–28.60] (Belardinelli et al., 2017). They had a left-hand paralysis and were unable to extend their fingers. After the four-week intervention, the UE-FMA score of the stroke subjects showed a small but significant improvement by 3.15 ± 2.06 (p = 0.003; paired t test; Cohen's d = 0.42), with a distributed improvement pattern across arm (UE-FMA part A) by 1.63 ± 0.80 (p < 0.001; Cohen's d = 0.37), wrist (UE FMA part B) by 0.80 ± 0.77 (p = 0.022 Cohen's d = 0.33), and hand (UE-FMA part C) by 0.71 ± 1 (p = 0.083, Cohen's d = 0.47).
Table 1.
Clinical characteristics of the stroke subjects (modified from Belardinelli et al., 2017)
| Pat.nr. | Age (year) | FMA pre | FMA post | FMA Change | Sex | Hand dominance | Lesion hemisphere | Lesion type | Lesion volume (voxel) | Disease duration (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 56 | 28.60 | 35.25 | 6.65 | Male | Right | Right | Hemorrhage | 79,013 | 78 |
| P2 | 63 | 16.10 | 18.35 | 2.25 | Female | Right | Right | Ischemic | 95,476 | 78 |
| P3 | 52 | 22.40 | 26.45 | 4.05 | Male | Right | Right | Ischemic | 80,144 | 156 |
| P4 | 67 | 6.80 | 10.85 | 4.05 | Male | Right | Right | Hemorrhage | 87,923 | 75 |
| P5 | 68 | 16.40 | 20.20 | 3.80 | Male | Right | Right | Hemorrhage | 2879 | 34 |
| P6 | 34 | 13.40 | 13.40 | 0.00 | Male | Right | Right | Hemorrhage | 44,957 | 45 |
| P7 | 63 | 15.80 | 16.80 | 1.00 | Male | Right | Right | Ischemic | 111,162 | 58 |
| P8 | 55 | 10.35 | 13.73 | 3.38 | Male | Right | Right | Hemorrhage | 39,073 | 80 |
The clinically important difference (CID) for the UE-FMA score in chronic stroke (i.e., 5.25) Page et al. (2012) has been estimated for stroke subjects with minimal to moderate impairment (UE-FMA score of ≥28 to ≤50 and active extension in the affected wrist of at least 5°; 109), and not for severely impaired stroke subjects with a hand paralysis such as those of this study. Therefore, UE-FM changes of ∼3 might be still clinically important in the severely affected stroke subject population studied here; however, a formal CID estimation in this patient population needs to be done in the future.
However, the underlying neural processes during the BCI intervention with proprioceptive feedback and their impact on actual motor improvements remained unclear and necessitated a secondary analysis on the brain-behavior relationship. The present study therefore evaluated the motor-network changes in the course of the intervention in relation to subsequent behavioral changes. Importantly, the same data analysis pipeline was applied for both studies to allow for comparisons between healthy and stroke subjects. It should be noted that the current study design cannot disentangle the impact of simple tactile as compared with the proprioceptive feedback.
Data acquisition
The data acquisition methods applied in this study have been described in detail in our previous work (Kraus et al., 2016a) and are cited here where appropriate: EMG/EEG data were recorded at a sampling rate of 5 kHz using an antialiasing bandpass filter with cutoff frequencies at 0.16 Hz and 1 kHz. In a next step, data were downsampled to 1 kHz by the BrainAmp Amplifier (Kraus et al., 2016a). Since the estimation of CMC might be particularly sensitive to aliasing effects, the preprocessing needs to be done cautiously. Specifically, the low-pass filter needs to apply a cutoff frequency (LPF = 1KHz) based on the ratio between sampling rate (SR = 5 kHz) and down-sampling factor (d = 5), before down-sampling by this factor d. Furthermore, the data needs to be analyzed with adapted filter boundaries by using finite impulse response (FIR) filters that do not lead to phase or delay distortion.
We used Ag/AgCIAmbuNeuroline 720 wet gel surface electrodes (Ambu GmbH) to record EMG activity from the left extensor digitorum communis (EDC) muscle (Kraus et al., 2016a). Two electrodes were placed 2 cm apart from each other on the muscle belly. To record EEG signals, we used Ag/AgCl electrodes (BrainCap for TMS, Brain Products GmbH) and BrainVision software with DC amplifiers and an antialiasing filter (BrainAmp, Brainproducts GmbH). A 32-channel EEG setup, which complied with the international 10–20 system, was used (FP1, FP2, F3, Fz, F4, FT7, FC5, FC3, FC1, FC2, FC4, FC6, FT8, C5, C3, C1, Cz, C2, C4, C6, TP7, CP5, CP3, CP1, CPz, CP2, CP4, CP6, TP9, P3, P4, and POz with FCz as reference). We kept the impedances at all electrodes below 10 kΩ. The EEG signals were later transferred to the BCI2000 software (Schalk et al., 2004) for online analysis and offline storage (Kraus et al., 2016a).
Experimental conditions
Figures 1 and 2 represent the schematic illustration of the experimental design for the healthy (Fig. 1) and stroke subjects (Fig. 2), respectively. In healthy subjects, the experiment commenced with an isometric MT (5 min), which was followed by the intervention (40 min) and a further isometric MT (5 min). The intervention consisted of a visual or proprioceptive feedback task. The proprioceptive feedback task (intervention) and the pre/post MT differed, entailing dynamic movement and isometric movement, respectively.
Figure 2.
Experimental design and example data (stroke subjects). A, Schematic illustration of the experimental design and timeline. Proprioceptive BCI feedback was applied to the paralyzed left hand in 20 sessions in the course of four weeks. Each session consisted of 15 runs. B, Stroke subjects underwent a neurofeedback intervention of modulating β activity (16–22 Hz) in circumscribed premotor and sensorimotor regions (marked by + on the topography; L and R stand for the left and right cortical hemispheres) of the right, ipsilesional hemisphere by kinesthetic MI.
The proprioceptive feedback task was identical for healthy and stroke subjects. A brain-computer interface controlled a robotic orthosis and turned sensorimotor β-band desynchronization (ERD) during MI of finger extension into contingent hand opening. A full and continuous opening of the hand orthosis was achieved when an individual desynchronization threshold was reached and when ERD was maintained for the MI period. Movement stopped as soon as ERD was absent, but could be reinitiated in the same trial if the threshold was met again.
More, specifically, the intervention consisted of 15 runs. Each run lasted ∼2.5 min and included 11 trials. Each trial began with a 6-s rest phase which was followed by a 2-s preparation phase and a 6-s move phase, i.e., kinesthetic MI (Fig. 1B). The audio taped cues of a female voice: “relax,” “left hand,” and “go” were used to initiate the rest, preparation, and move phases, respectively. All subjects were instructed to keep their muscles relaxed throughout the intervention. During the move phase, the subjects were instructed to perform kinesthetic MI, i.e., to imagine the feeling of opening their left hand, i.e., finger extension, from a first-person perspective. During the move phase, the passive opening of the left hand (proprioceptive group) and changing the color of the cross on a screen (visual group) was initiated by the BCI2000 software after detection of MI-related event-related desynchronization (ERD) in the β-band (16–22 Hz; Gharabaghi et al., 2014). In future, a richer visual feedback (i.e., visualized hand opening) may be considered to explore the full potential of this feedback modality. Details with regard to the online ERD evaluation are specified below. This feedback was contingent, i.e., the participants were rewarded with robotic opening of the hand (proprioceptive group) or color change on a screen (visual group), when the predefined brain state (i.e., β-ERD) was achieved and sustained (Bauer et al., 2016a,b; Bauer and Gharabaghi, 2017). Before the experiment, a desynchronization task, consisting of three MI training runs, was performed for offline calibration to account for each subject's ability for desynchronization. Following this calibration session, an individual desynchronization threshold, described in detail elsewhere (Bauer and Gharabaghi, 2015a,b; Gharabaghi, 2016; Naros and Gharabaghi, 2015, 2017), was implemented for the intervention. Whenever the respective ERD was insufficient during the intervention, the robotic movement ceased but could be restarted and continued when the ERD threshold was reached again. Importantly, the robotic hand opening was synchronized to the respective MI-related brain activation, whereas the robotic hand closing occurred after the relax command automatically and independent of the respective brain state (Kraus et al., 2016a). The frequency band, i.e., 16–22 Hz, to extract the ERD feature was the same for healthy and stroke subjects, and was selected on the basis of previous work by our group in healthy subjects indicating that volitional modulation of these sensorimotor β-oscillations could be learned via BCI and proprioceptive feedback and correlated with CSE increases (Kraus et al., 2016a) and improved motor performance (Naros et al., 2016).
ERD was analyzed over the right surface EEG channels FC4, C4, and CP4 (Fig. 1B) during the move phase (McFarland et al., 2000). ERD detection was performed with an adaptive linear classifier (Gharabaghi et al., 2014; Kraus et al., 2016a). The spectral power was computed with an autoregressive model of order 16 (McFarland and Wolpaw, 2008), fitted to the last 500 ms of the signal and updated every 40 ms. To rule out a noisy control signal for the orthosis, five consecutive 40-ms time periods (i.e., 200 ms) had to be classified as ERD positive (negative) to start (stop) feedback (Bauer and Gharabaghi, 2015a, 2017; Vukelić and Gharabaghi, 2015a,b).
The MT was an isometric one; the fingers of the healthy subject were connected to the robotic orthosis via small magnets which were attached to the finger tips. This ensured that the fingers could not be moved and so the applied forces were instead translated into cursor movements on a screen. Before and after the intervention, the isometric MT was performed (only by healthy subjects). This task has been described in detail elsewhere (Naros et al., 2016) and is cited here accordingly. During the isometric MT, the fingers of the healthy subject were connected to the robotic orthosis via small magnets which were attached to the finger tips. The orthosis was blocked in the middle position between maximum extension and flexion. This ensured that the fingers could not be moved and so the applied forces were instead translated into cursor movements on a screen, where a horizontal target bar, which oscillated vertically with a frequency of 0.1 Hz, was presented ∼150 cm in front of the subject. The healthy subjects were asked to control the vertical position of a simultaneously presented cursor via the force of the fingers (Digits II–V) to follow a vertically oscillating target (Fig. 1C). This task consisted of 15 trials (10 s each) per run (two runs). Each trial had one flexion (5 s) and extension (5 s) phase, i.e., downward and upward direction of the cursor, respectively (Fig. 1C).
Online data analysis
To estimate the task-related brain activity, ERD of the right sensorimotor area (i.e., at the electrodes FC4, C4, and CP4) was analyzed during the MI phase (McFarland et al., 2000). Our approach has been described in detail elsewhere (Kraus et al., 2016a) and is cited here accordingly. A full opening of the hand orthosis was achieved when ERD was maintained for the MI period. Movement stopped as soon as ERD was absent. The power was estimated by an autoregressive model with a model order of 16 based on the Burg Algorithm (McFarland and Wolpaw, 2008). A linear classifier with nine features consisting of three channels (FC4, C4, and CP4) and three independent 2-Hz frequency bins for estimation of spectral power from 16 to 22 Hz were used to detect decreases in sensorimotor rhythm power in the β-band. To ensure robotic movements only during sufficiently long periods of ERD, five consecutive 40-ms time periods had to be classified as ERD to initiate hand opening by the orthosis. Three calibration runs were performed before the experiment to implement an individual desynchronization threshold and to consider each subject's ability for desynchronization. This assured that each subject was facing the same task-related demand. During the intervention feedback was given only when subjects reached 20% of their strongest β-ERD modulation in the motor-imagery epoch (Gharabaghi et al., 2014). Whenever the threshold was not met because of insufficient ERD either no robotic opening of the hand occurred or ongoing robotic opening stopped, but could be reinitiated in the same trial if the threshold was met again. The orthosis moved back to the “hand closed” position after the “relax” command within the first 1.5 s of the rest phase (Kraus et al., 2016a).
Offline data analysis
Apart from the online analysis indicated above, all other data were analyzed offline using MATLAB (The MathWorks) and the FieldTrip open-source MATLAB toolbox (http://fieldtrip.fcdonders.nl/; The MathWorks). This included bandpass filtering (FIR) between 2 and 46 Hz (for both EEG and EMG signals), linear detrending, and visual inspection for ocular/muscular artifact rejection.
Assessing intervention-related cortical and cortico-muscular modulation
We analyzed EEG spectral modulation and EEG-EMG CMC during the BCI interventions with proprioceptive and visual feedback. We studied time periods (with 1000-ms length) of the move phase before feedback onset (in comparison to the rest phase) with at least 500-ms continuous MI-related ERD below the predefined threshold which resulted on average in 93 ± 27 time periods per subject. To capture the evolution of EEG oscillations in the course of the intervention, time periods were divided into 10 subgroups, yielding an average of 9 ± 3 time periods per subgroup. A Hann window was applied on each time period to attenuate edge effects (Nuttall, 1981). The power spectral density (PSD) of each epoch was calculated frequency-wise from 2 to 46 Hz in steps of 1 Hz using fast Fourier transform (FFT). Later, the average of estimated PSD for each subgroup was estimated. For coherence estimation, we applied the FIR filter, and not the infinite impulse responses (IIRs) filter, to avoid interference with the phase of the signal.
Assessing MT-related cortico-muscular modulation
We studied the synchronous oscillatory activity between EEG and rectified EMG of the EDC muscle by analyzing CMC (Myers et al., 2003). To estimate CMC, each trial of the MT (10 s) was divided into ten nonoverlapping time-windows (1-s intervals) denoted by F1–F5 and E1–E5 during flexion and extension, respectively. Data from each interval were visually inspected for ocular/muscular artifacts, yielding an average of 26 ± 4 time periods (each 1000-ms length) per interval and subject.
CMC was estimated as the cross-spectral density matrix frequency between EEG and EMG channels (Schulz et al., 2014). This was calculated frequency-wise using the multi-taper method (nine tapers) over the frequency range from 2 to 46 Hz in steps of 1 Hz. We then obtained the magnitude of the coherence values by normalizing the magnitudes of the summed cross-spectral density matrix for each frequency to the corresponding power values at that frequency (Schulz et al., 2014). Furthermore, we found no changes in EEG or EMG spectral power between conditions (BCI with proprioceptive vs visual feedback), nor did we detect a correlation between EEG or EMG spectral power with the difference in CMC strength values in the course of the intervention in healthy and stroke subjects. This indicates that CMC modulation was not confounded by amplitude changes of neuronal oscillations (von Carlowitz-Ghori et al., 2015), which were shown to affect the estimation of CMC (Bayraktaroglu et al., 2013), since they relate to the signal-to-noise ratio (Nikulin et al., 2011).
Assessing MEP modulation and the corresponding relation with the MT-related cortico-muscular modulation
To estimate the modulation of MEP amplitude we followed the procedure described previously (Kraus et al., 2016a). After peak-to-peak MEP amplitude estimation, we fitted a three parameter Boltzman sigmodal function per healthy subject resulting in a SRC. We have previously reported that the proprioceptive condition resulted in an increase of CSE in the steep part of SRC (Kraus et al., 2016a). Hence, MEPs of the steep part of the SRC were chosen for further analysis (i.e., 120% resting motor threshold). Then, Spearman's rank correlation was used to evaluate the relationships between the CMC magnitude during the MT (CMC [motor task]) and the corresponding MEP amplitudes in the subsequent CSE evaluation. Because of technical reasons, the SRCs from two of the 15 subjects could not be included in this analysis.
Assessing behavioral changes
The difference between the cursor movements on a screen controlled by the healthy subject and the oscillating horizontal target bar resulted in the error rate during the MT, i.e., area under the curve (AUC; summing all the differences). Reduction of this error rate was defined as a changed behavior in motor performance (Fig. 1D).
Assessing the relationships between intervention-related and MT-related cortico-muscular modulation and behavioral changes
Assessing the relationships between these different measures will reveal brain-behavior relationships even for single-session interventions (as in the healthy subject study) that are not intended to reveal behavioral group or condition effects. We used Spearman's rank correlation to evaluate the relationship between CMC magnitude during the intervention (CMC [intervention]) and the MT (CMC [motor task]), as well as between CMC [motor task] and behavioral change across subjects. For each subject, we had a set of channels that showed significant modulation of the CMC magnitude. To provide a correlation analysis between the measures (i.e., CMC [intervention], CMC [motor task], behavioral changes) we needed to decrease the number of channels to one per subject. The statistically significant clusters of the previous analyses were chosen for this estimation. However, neither the average of all channels in this cluster nor the channel with maximum change could be used for this purpose. The former approach would miss the strongest change, the latter would not be applicable as well, since the channel with maximum change was not the same for all the subjects, e.g., C4 in one and C2 in another participant. We, therefore, implemented an approach that considered both the maximum CMC value and the individual variability of the spatial distribution. Specifically, the maximum CMC value from each cluster was subtracted from the median value of the respective cluster for each subject. Then, one pair of CMC [intervention]/CMC [motor task] per healthy subject was used for further analysis.
Assessing the phase-frequency relationships of cortico-muscular modulation
The phase-frequency relationships of CMC were estimated frequency-wise (every 1 Hz). This estimation allows to determine the dominant directionality between cortex and periphery. Specifically, the phase was estimated by taking the argument from the estimated EEG-EMG cross-spectrum (Witham et al., 2011; see below, Statistical analysis).
Assessing directed CMC
The directed CMC was estimated frequency-wise (every 1 Hz) for the descending (from EEG to EMG) and ascending pathways (from EMG to EEG), separately, using the partial directed coherence (PDC) implemented in Fieldtrip (Oostenveld et al., 2011). PDC estimates the direction of connectivity through two time series and decomposes the partial coherence using a multivariate autoregressive model (Witham et al., 2011).
Stroke subject data
Data analysis for the group of stroke subjects was identical to the approaches described above for the group of healthy subjects. Since the stroke subject group performed 20 intervention sessions (compared with the one session performed by each healthy subject), the sessions were evaluated individually, yielding 20 different datasets for ERD and CMC modulation per stroke subject. For each session (i.e., training day), the ERD and CMC averages across subjects are reported in the results (section: ERD and CMC changes in stroke subjects). The findings indicate an evolution of these measures from the start until the third week, while showing a large session-by-session variability. This ERD and CMC variability in the course of the whole four-week period would not be sufficiently captured by comparing the last to the first session only. However, to allow for comparisons between the ERD and CMC changes in the course of the training period on the one side, and the pre/post behavioral changes on the other side, we needed to operationalize the training-related ERD and CMC changes. For this purpose, we defined the average of the first two sessions as baseline and the rest of the sessions as training related changes, since the first relevant change of both ERD and CMC ensued from the third session on (see Fig. 8H,I). The resulting physiological changes were then compared with the motor improvement assessed by the UE-FMA score, i.e., before and after the four-week intervention.
Figure 8.

Modulation of CMC correlates with motor improvement in stroke subjects. A, Axial MRI for each stroke subject. B, Modulation of MI-related ERD and CMC. Group data of stroke subjects with proprioceptive BCI feedback. Black circles indicate clusters with statistically significant modulation (randomization test) during the training in comparison to baseline. Cortical topographies are presented for the feedback frequency band (16–22 Hz) and the neighboring bands (9–15, 23–29, and 30–36 Hz). C, Changes of CMC magnitude (group analysis; channel F4) for the start (light-gray) versus rest (dark-gray) of training sessions. D, The CMC magnitude differences (start vs rest of training sessions) converted from coherence values to z-values. The solid and dashed lines represent the α level of 5%, uncorrected and corrected for the number of frequency bins, respectively. E, The distribution of the unconverted CMC magnitudes (coherence; 16–22 Hz) after bootstrapping (10,000 times) for the start (dark-gray) versus rest (light-gray) of training sessions. The vertical red and blue lines represent the mean and 95% of the confidence intervals for the corresponding groups. F, The relationship between improvement in the FMA score and the CMC modulation (16–22 Hz; F4, FC2, and FC4 channels). Each circle represents one stroke subject. The regression line is represented in gray (r = 0.88 p = 0.007 Spearman's rank correlation, randomization test, 1000 repetitions). G, Source level analysis of CMC magnitude (16–22 Hz) using the DICS method. H, ERD modulation during the training. Each square represents the average of one session (group analysis). The yellow and red squares represent the first and the last sessions of the training. I, CMC modulation during the training. Each square represents the average of one session (group analysis). The yellow and red squares represent the first and the last session of the training. J, Phase-frequency plots (mean and SE) for the EEG channels that were used to calculate the cortical power for providing feedback (FC4, C4, and CP4), and the frequencies with significant CMC modulation at the onset (gray) and end (black) of the intervention, respectively. The regression slopes for the β frequency band are represented by lines and indicated by magenta when significantly different from zero. K, Topographies of the respective regression slopes. Magenta and yellow colors indicate the directionality of information flow from the cortex to the periphery and from the periphery to the cortex, respectively. Black circles represent the EEG channels which have a regression slope that is significantly different from zero. L, The topography of the modulated directional coherence (postintervention vs preintervention MT; 16–22 Hz) indicates an information flow from cortex to periphery.
Localization of CMC using dynamic imaging of coherent sources (DICS)
We used the DICS method (Gross et al., 2001) to illustrate the cortical map of CMC magnitude at source level. DICS uses the beamformer (Van Veen et al., 1997) to leverage the linear projection of the sensor level activity through a spatial filter. The spatial filter was estimated from the lead field of the source of interest and the estimated cross-spectral density (frequency domain). We employed DICS to estimate the similarity between EEG and EMG oscillatory activities and their linear dependencies (Belardinelli et al., 2017). DICS was applied for each of the sessions of every stroke subject following cross-spectral estimation. As for the sensor level analysis, data of sessions 3–20 of each stroke subject were averaged and compared with baseline (sessions 1 and 2).
Statistical analysis
Testing significance of ERD and CMC modulation
We used a nonparametric randomization test with 1000 repetitions to test the modulation of ERD/CMC magnitude and directed coherence. The null hypothesis was that observed increases in ERD/CMC magnitude were not related to the intervention and were therefore exchangeable within the intervention and for the premotor and post-MT values.
Since the artifact rejection led to an unequal degree of freedom (df; a maximum of 540 for healthy subjects) for each group of time periods (with 1000-ms length), we converted the coherence differences (Fig. 3A,B) to Z-statistic (Fig. 3C,D) before the nonparametric statistical test (Maris et al., 2007). The distribution of the nonconverted coherence is estimated using the bootstrapping method (Fig. 3E,F). Following the same procedure, we converted the coherence difference of study 2 (df, a maximum of 900 for stroke subjects) to Z-statistic. We performed the cluster-based randomization test for each frequency band of interest. The frequency bands were selected to cover the feedback frequency band of the intervention (16–22 Hz) and the neighboring frequency bands with the same bandwidth (9–15, 23–29, and 30–36 Hz) for balanced statistical comparisons. When the maximum of t-statistic (paired t test; ERD) or Z-statistic (CMC) exceeded the threshold α level of 5%, adjacent EEG channels were clustered in the same set (Maris et al., 2007). Cluster-level statistics were then conducted by taking an average of the t-statistics. In cases where multiple clusters were observed, the maximum of the cluster-level statistics was used for later comparisons. The p-value to reject the null hypothesis was the proportion of cluster-based randomizations that resulted in larger test statistics than were observed here (without randomization).
Figure 3.
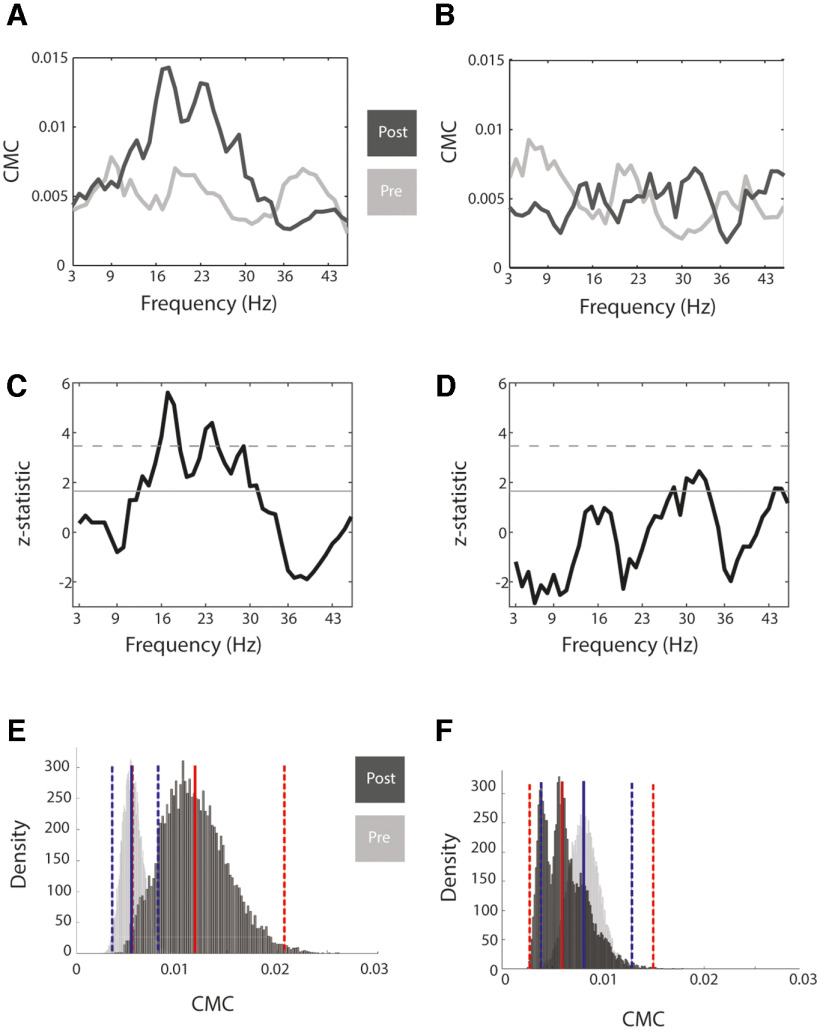
Changes of CMC. A, Preintervention (light-gray) versus postintervention (dark-gray) CMC magnitude during the MT (channel C4; group analysis for the transition from flexion to extension, i.e., E1 in Fig. 4) for the proprioceptive feedback group. B, Same as A but for visual group. C, The CMC magnitude differences (post vs pre) converted from coherence values to z-values. The solid and dashed line represents the α level of 5%, uncorrected and corrected for the number of frequency bins, respectively. D, Same as C but for visual group. E, The distribution of the unconverted CMC magnitudes (coherence; 16–22 Hz) after bootstrapping (10,000 times) for the proprioceptive group, postintervention (dark-gray) versus preintervention (light-gray). The vertical red and blue lines represent the mean and 95% of the confidence intervals for the corresponding groups. F, Same as E but for visual group.
To address the multiple comparison issue, we applied nonparametric cluster-based test statistics that controlled for the family-wise error rate across EEG channels (Maris and Oostenveld, 2007). This cluster-based approach uses one test for the spatio-spectral grid instead of multiple tests.
For the stroke subjects, the null hypothesis was that the observed cortical and corticospinal modulations were not caused by the intervention so that the baseline sessions could be exchanged with the following sessions.
Testing significance of the relationships between intervention-related and MT-related cortico-muscular modulation and behavioral changes
We tested the significance of the relationship between intervention-related and MT-related CMCs as well as between the MT-related CMCs and motor behavioral changes across subjects. Additionally, we tested the significance of the relationship between MT-related CMCs and MEP changes. Our null hypothesis was that they were not correlated and that independent/dependent variables were exchangeable, i.e., intervention-related CMC/MT-related CMC/behavioral change/MEPs. We began by shuffling the independent versus dependent variables with 1000 repetitions. At each repetition, we calculated Spearman's rank correlation. The p-value to reject the null hypothesis was the proportion of randomizations that resulted in larger test statistics than we observed (without randomization).
Testing significance of phase-frequency relationships
We calculated the mean of the estimated CMC phase for each frequency across subjects, yielding one phase-frequency spectrum. We then fitted a line to the phase-frequency plot using a linear regression of phase at the healthy subject level and subsequently averaging estimates of time delay (i.e., over a range of frequencies with significant CMC). The regression slope differed significantly from zero when p < 0.05.
Testing significance of cortico-muscular connectivity using DICS
To test the significance of the changes, the randomization test was employed. For this, the estimated coherent preintervention and postintervention sources were randomly shuffled among subjects. Similar to the sensor level analysis, the threshold of Z > 1.65 (α level of 5%; Maris et al., 2007) was used at each stage of randomization. As we assumed that the observed increase of the coherent source was not because of the intervention, the p-value to reject the null hypothesis was the proportion of cluster-based randomizations that resulted in a larger test statistic than the one observed (with no randomization). The p-value to reject the null hypothesis was the proportion of randomizations that resulted in larger test statistics than the observed one (without randomization).
Results
ERD and CMC modulation
Healthy subjects learned to modulate MI-related sensorimotor ERD in the feedback frequency range (16–22 Hz) independent of the feedback modality; however, significant CMC changes ensued only when proprioceptive feedback was provided; since CMC changes are tiny by nature, their robustness was verified by applying the respective statistical tests (Fig. 3). The ERD and CMC changes in the course of the intervention, i.e., the changes during each training session, were frequency-specific, and were restricted to the right hemisphere, i.e., contralateral to the left hand that was moved by the robotic orthosis, and overlapped in the primary sensorimotor and premotor cortex (Fig. 4). Specifically, a cluster-based randomization test (1000 repetitions) revealed significant ERD modulation in the frequency range of 16–22 Hz for the proprioceptive (EEG channels FC2, C2, C4, Cp2, and CP4; p = 0.017, cluster-based randomization test, 1000 repetitions; Cohen's d = 1.6528; Fig. 4A) and visual (EEG channels C2, C4, C6, CPz, CP2, CP4, CP6, and P4; p = 0.004, cluster-based randomization test, 1000 repetitions; Cohen's d = 1.2044; Fig. 4B) groups. The CMC changes were frequency-specific (16–22 Hz) as well, but occurred in the proprioceptive group only (EEG channels: FC2, FC6, C2, C4, C6, CP2, CP4, CP6, and P4; p = 0.014, cluster-based randomization test, 1000 repetitions; Cohen's d = 0.9474; Fig. 4A).
Figure 4.
Cortical topography of MI-related ERD and CMC. A, Group data of the proprioceptive group in healthy subjects. Black circles indicate clusters with statistically significant modulation (nonparametric randomization test) in the course of the intervention (the contrast between end and start, see methods). Cortical topographies are presented for the feedback frequency band (16–22 Hz) and the neighboring bands (9–15, 23–29, and 30–36 Hz). L and R stand for the left and right cortical hemispheres. B, Same as A except for the visual group.
CMC, MEP, and behavioral changes
This increase in CMC magnitude (16–22 Hz) in the course of the MI intervention was also observed in the MT after the intervention (cluster-based randomization test, 1000 repetitions; Fig. 5). The topography of the MT-related CMC modulation was restricted to the right hemisphere, i.e., contralateral to the left hand that was moved by the robotic orthosis, and projected to the primary sensorimotor and premotor cortex. In the proprioceptive group, the MT-related increase in CMC magnitude occurred at movement intervals reflecting transitions between finger flexion (16–22 Hz, p = 0.002; and 23–29 Hz, p = 0.002, cluster-based randomization test, 1000 repetitions; respective Cohen's d = 0.3373 and 0.3956) and extension (16–22 Hz, p = 0.007; and 23–29 Hz, p = 0.006, cluster-based randomization test, 1000 repetitions; respective Cohen's d = 0.5567 and 0.5370). In the visual group, this increase occurred during finger extension independent of the transition intervals (23–29 Hz, p = 0.007; and 30–36 Hz, p < 0.001, cluster-based randomization test, 1000 repetitions; respective Cohen's d = 0.9492 and 1.5004).
Figure 5.
Modulation of MT-related CMC. A, Group data of the proprioceptive group in healthy subjects. Black circles indicate clusters with statistically significant modulation (nonparametric cluster-based randomization test) in the course of the experiment (the contrast between post-MT vs pre-MT). Cortical topographies are presented across the MT in 1-s intervals, i.e., during flexion (F1–F5) and extension (E1–E5), for the feedback frequency band (16–22 Hz) and the neighboring bands (9–15, 23–29, and 30–36 Hz). L and R stand for the left and right cortical hemispheres. Transitions of the MT are marked with arrows (blue: flexion to extension; purple: extension to flexion). The grand-average of the EMG of the EDC muscle across all subjects is plotted below the topographies. B, Same as A except for the visual group.
In the proprioceptive group, the increase of CMC magnitude correlated significantly (r = 0.56 p = 0.015; Spearman's rank correlation, randomization test, 1000 repetitions; Fig. 6A) between the MI-related finger extension (Fig. 4A, 16–22 Hz) and the MT-related finger extension (Fig. 5A, 16–22 Hz). Additionally, there was a positive correlation between the CMC magnitude during MT-related finger extension and the subsequent increase of the MEP amplitude (r = 0.70 p = 0.004; Spearman's rank correlation, randomization test, 1000 repetitions; Fig. 6B). The increase in CMC magnitude during the transitions between finger flexion and extension in the proprioceptive group correlated (Fig. 6C) with the decrease in error rate in the same movement interval (E1) for 16–22 Hz (r = 0.74, p < 0.01, Spearman's rank correlation, randomization test, 1000 repetitions), but not 23–29 Hz (r = 0.11 p = 0.70, Spearman's rank correlation).
Figure 6.

Modulation of CMC correlates with behavioral changes. A, Group data of the proprioceptive group of healthy subjects. The increase of intervention-related CMC (16–22 Hz) from baseline correlates with the (postintervention vs preintervention) increase of the motor-task-related CMC (16–22 Hz) during the flexion to extension transition of the MT (r = 0.56, p = 0.015, Spearman's rank correlation, randomization test, 1000 repetitions); each triangle represents one subject. The regression line is represented in gray. B, The increase of CMC (16–22 Hz) during the flexion to extension transition of the MT correlates with the postchanges versus prechanges in of the MEP amplitude (r = 0.70, p = 0.004, Spearman's rank correlation, randomization test, 1000 repetitions); each triangle represents one subject. The regression line is represented in gray. C, The behavioral change correlated significantly with the increase in CMC magnitude of the MT (16–22 Hz; r = 0.74 p < 0.001, Spearman's rank correlation, randomization test, 1000 repetitions). Each triangle represents one subject. The regression line is represented in gray.
Cortico-muscular control
The directionality of CMC coupling following the intervention was captured with two complementary measures, i.e., estimation of phase-frequency relationships and of directed transfer functions. Specifically, the intervention led to a significant change in the phase-frequency relationships (in the EEG channels used to estimate the cortical power during the feedback task) in the course of the intervention (Fig. 7A, middle column), which persisted in the MT after the intervention (Fig. 7A, right column). This phase-frequency relationship showed a consistently negative slope that differed significantly from zero, indicating an enhanced direction of interaction from cortex to muscle. The respective cortical topography included extended sensorimotor and premotor areas of the right hemisphere, i.e., contralateral to the left hand that was moved by the robotic orthosis (EEG channels: F4, FC2, FC4, FC6, Cz, C2, C4, C6, CPz, CP2, CP4, and CP6; Fig. 7B), which persisted during the postintervention MT (EEG channels: FC2, FC4, Cz, C2, C4, C6, CPz, CP2, CP4, CP6, P4, CP1, P3, and POz; Fig. 7B). The average phase delays of the phase-frequency relationships were 33.5 ± 10.12 and 25.16 ± 7.45 ms for the MI-intervention and post-MT, respectively. Moreover, the directed CMC revealed a significant modulation for the descending but not the ascending pathways, which was frequency-specific (i.e., 16–22 Hz). The topography of this information flow from cortex to periphery overlapped with the estimated phase-frequency relationships (EEG channels: FC2, FC4, C1, Cz, C2, C4, C6, CPz, CP2, CP4, and P4; p < 0.01; cluster-based randomization test, 1000 repetitions; Fig. 7C).
Figure 7.

Directionality of CMC. A, Phase-frequency plots of healthy subjects in the proprioceptive group (mean and SE) for the EEG channels that were used to calculate the cortical power for providing feedback (FC4, C4, and CP4), and the frequencies with significant CMC modulation. Left and right columns represent the MT-related findings before (gray) and after (black) the intervention, respectively. The middle column represents the intervention-related findings at the onset (gray) and end (black) of the intervention, respectively. The regression slopes for the β frequency band are represented by lines and indicated by magenta when significantly different from zero. B, Topographies of the respective regression slopes. Magenta and yellow colors indicate the directionality of information flow from the cortex to the periphery and from the periphery to the cortex, respectively. Black circles represent the EEG channels, the regression slopes of which differ significantly from zero. C, The topography of the modulated directional coherence (postintervention vs preintervention MT; 16–22 Hz) indicates an information flow from cortex to periphery.
ERD and CMC changes in stroke subjects
Stroke subjects [respective axial magnetic resonance images (MRIs) shown in Fig. 8A] learned modulating MI-related sensorimotor ERD over the feedback frequency range (16–22 Hz) and channels (FC4, C4, and CP4; p < 0.01, randomization test, 1000 repetitions; Cohen's d = 1.1074; Fig. 8B, left column). An increase in ERD magnitude was also observed over the frequency range of 9–15 Hz (p < 0.01 randomization test, 1000 repetitions; Cohen's d = 0.8409). These ERD changes were restricted to the ipsilesional right hemisphere, i.e., contralateral to the paralyzed left hand that was moved by the robotic orthosis. The changes in CMC magnitude, however, were bilateral (Fig. 8B, right column). Specifically, ipsilesional premotor CMC increased in the β-band (16–22 Hz), whereas contralesional CMC of extended sensorimotor, premotor and parietal areas increased in the α-band and β-band (9–15 and 16–22 Hz; p < 0.01 randomization test, 1000 repetitions with the respective Cohen's d = 2.5938 and 2.7518). The average phase delays of the phase-frequency relationships during the intervention were 23.75 ± 12.32 and 26.55 ± 7.09 ms for the ipsilesional and contralesional hemisphere, respectively. The statistical analysis of CMC changes (Fig. 8C–E) is performed in the same way as for the healthy subjects (Fig. 3). The session-wise, average ERD (β = −10.26, p = 0.047 randomization test, 1000 repetitions; Fig. 8H) and CMC (β = 6.64, p = 0.001 randomization test, 1000 repetitions; Fig. 8I) measures showed significant changes until the third training week.
Cortico-muscular control and behavioral changes in stroke subjects
In the stroke subject group, the ipsilesional increase (F4, FC2, and FC4) in CMC magnitude (16–22 Hz) in the right hemisphere (Fig. 8B) correlated significantly with the improvement of the UE-FMA score after the intervention period (r = 0.88 p = 0.007; Spearman's rank correlation; randomization test, 1000 repetitions; Fig. 8F). The source level analysis confirmed the topography of increased CMC demonstrated in the sensor level analysis (Fig. 8G). Furthermore, the intervention also led to a significant change in the phase-frequency relationships in the stroke subject group (Fig. 8J). The phase-frequency relationships at the end of the intervention showed a negative slope that differed significantly from zero, indicating an enhanced direction of interaction from cortex to muscle. The respective cortical topography included premotor (FC4), somatosensory (CP4), and parietal (P4) channels in the ipsilesional right hemisphere, i.e., contralateral to the paralyzed left hand that was moved by the robotic orthosis (Fig. 8K). Finally, the directed CMC revealed a significant modulation for the descending (but not the ascending) pathways in the ipsilesional hemisphere (EEG channels: F2, FC6, C2, C6, CPz, CP2, p < 0.01; randomization test, 1000 repetitions; Fig. 8L) in the β-band (16–22 Hz) only.
Discussion
This work shows that MI and sensory feedback may lead to re-organization of cortico-spinal pathways in the healthy and poststroke brain with an associated improvement in motor function. We provide novel findings about potential neural processes involved in the transfer of effects related to the BCI intervention to the following motor behavior:
Synchronizing MI of hand opening with proprioceptive feedback (and not visual feedback only) to sensorimotor β-band desynchronization increased CMC in healthy subjects and chronic stroke subjects with hand paralysis.
This effect (1) was specific to the β frequency band, (2) transferred to a MT, (3) was proportional to subsequent CSE and correlated with behavioral changes in the (4) healthy, and (5) poststroke condition; specifically, MI-related enhancement of β-band CMC in the ipsilesional premotor cortex correlated with motor improvements after the intervention.
Neural processes related to brain-computer interface feedback
Healthy subjects learned to enhance ERD in the targeted sensorimotor area in a frequency-specific way, i.e., in the feedback frequency band (16–22 Hz), regardless of the feedback modality provided (proprioceptive or visual). This was not surprising, given that sensorimotor oscillations are modulated by thalamo-cortical and corticocortical interactions (Thut and Miniussi, 2009; Jensen and Mazaheri, 2010) and do not necessarily depend on proprioceptive input. However, only when proprioceptive feedback was provided, was this specific activation pattern paralleled by CMC increases as well. These occurred in the same cortical area and frequency band as the ERD modulation, thereby suggesting that interacting processes are at work (Fig. 4A). Since MI-related ERD has been shown to reduce intracortical inhibition (Takemi et al., 2013), it may serve as the presynaptic input for an excitatory drive via proprioceptive input (Kraus et al., 2016a). This is in accordance with previous studies which concluded that pairing specific brain states with peripheral (Mrachacz-Kersting et al., 2012, 2016), cortical (Kraus et al., 2016a,b), or combined stimulation (Gharabaghi et al., 2014; Royter and Gharabaghi, 2016; Kraus et al., 2018) increase CSE. We speculate that the kinesthetic MI applied in this study modulated the susceptibility of an extended cortical motor network to the proprioceptive input provided and thus fulfilled the requirements of associative stimulation (Hebb, 1949; Harel and Carmel, 2016).
The same BCI intervention with proprioceptive feedback was recently studied with TMS and MEPs by applying refined TMS protocols (Kraus and Gharabaghi, 2015, 2016; Mathew et al., 2016). This evaluation provided a link between the ERD modulation and the changed connectivity to the periphery (Kraus et al., 2016a). Specifically, the largest MEP gains were found in those cortical areas that were most strongly modulated by the intervention (Kraus et al., 2016a). Furthermore, this topographic specificity was paralleled by a correlation between the ERD changes and the increased connectivity to the periphery, i.e., the largest MEP gains were observed in the subjects with the strongest ERD modulation range (Kraus et al., 2016a).
β-Band CMC and behavioral changes
The present study complements and augments these earlier findings; it proposes, consistent with the communication through coherence hypothesis (Fries, 2005), that synchronized neural activity at cortical and spinal level in the feedback frequency range may contribute to the effects induced by BCI with proprioceptive feedback, since the control intervention (with visual feedback only) did not lead to such connectivity changes (Fig. 4B). However, since behavioral changes occurred in the BCI condition with visual feedback as well, other factors than changes in CMC, e.g., the cognitive demands of the feedback task, have likely contributed to the behavioral changes.
Importantly however, the enhanced β-band CMC during the BCI task with proprioceptive, i.e., dynamic movement, feedback transferred to the subsequent isometric MT. It occurred during the transition intervals between flexion and extension and in a broader β frequency band (16–22, 23–29 Hz), which may be related to increased attentional demands (Murthy and Fetz, 1992; Kristeva-Feige et al., 2002). The magnitude of β-band CMC during the BCI and the MTs correlated with each other (Fig. 6A) and with the behavioral changes (Fig. 6E). The task-related correlation between β-band CMC and motor behavior is a known phenomenon and reflects its proposed role in effective corticospinal interactions (Baker et al., 1999; Kristeva et al., 2007). Moreover, motor learning has been shown to both increase preexisting (Houweling et al., 2010) and develop new β-band CMC (Mendez-Balbuena et al., 2012).
In this study, the brain-behavior relationship in the proprioceptive group revealed task-specificity by occurring during the initiation of finger extension only, i.e., the very movement that was reinforced during the preceding BCI task (E1 interval; Fig. 5A). Moreover, this brain-behavior relationship was specific for the feedback frequency (16–22 Hz) but not for the higher β-band (23–29 Hz) which changed as well. Together, these observations suggest a link between sensorimotor processing during the BCI task and the following motor-task related β-band CMC; this relationship implies the generation of the later dependent on memory traces induced by the former (Omlor et al., 2011). A similar mechanism has already been described for the primary motor cortex in association with the preceding motor experience (Chouinard et al., 2005; Nowak et al., 2005; Berner et al., 2007; Loh et al., 2010) and for β-band CMC during steady force following a dynamic force task (Omlor et al., 2011). Along these lines, β-band CMC has been proposed to serve as the major functional corticospinal gateway for efficient integration and transmission of sensorimotor information (Omlor et al., 2011; Aumann and Prut, 2015). In our study, the magnitude of β-band CMC during the initiation of finger extension (E1 interval; Fig. 5D) correlated also with the MEP increase after the intervention (Fig. 6B). Since this MEP increase was captured at rest (Kraus et al., 2016a), this observation indicates the memory traces to persist in different brain states. The present findings suggest that BCI with proprioceptive feedback is a means of enhancing this process.
Shaping directionality of CMC
The estimation of the phase–frequency relationships and directional coherence indicated, moreover, an enhancement of the predominant information flow from the cortex to the finger extensors (Halliday et al., 1998; Mima et al., 2000; Witham et al., 2011) in the course of the intervention and thereafter (Fig. 6A). To the best of our knowledge, this is the first observation of CMC directionality being shaped by an intervention. Importantly, this phenomenon occurred in an extended sensorimotor area beyond the primary motor cortex (Fig. 6B). This indicates the functionally relevant engagement of an extended motor network for the behavioral task, thus providing the rationale for the application of this technique in subjects with a lesioned primary corticospinal tract. These observations are in line with earlier studies (He et al., 1993; Kombos et al., 1999; Teitti et al., 2008; Schmidt et al., 2013; Kraus and Gharabaghi, 2016), which had indicated that corticospinal connections are not limited to the primary motor cortex but may originate from different regions of the sensorimotor system. The phase delays estimated in this study are consistent with a range of conduction times and may thereby reflect the involvement of both direct and indirect (e.g., cortico-rubro-spinal, cortico-reticulo-spinal) pathways.
Poststroke reorganization related to brain-computer interface feedback
The BCI intervention with proprioceptive feedback resembled in stroke subjects the neurophysiological processes observed in healthy subjects: enhancement of ERD in the cortical area targeted by the feedback; increase of the CMC magnitude in the course of the intervention; enhancement of the predominant information flow from the cortex to the finger extensors.
However, stroke subjects differ from healthy subjects with regard to their cortical topography and frequency spectrum of the cortico-motor connectivity pattern. Both ERD and CMC changes were not restricted to the feedback frequency in the β-band, but also included the α frequency band. Based on the topographic overlap of these to frequencies it remains an open question whether the observed α and β changes are truly independent. Consistent with this observation, cortical networks in the oscillatory α and β-band have been related to both BCI control (Buch et al., 2012; Vukelić et al., 2014, Bauer et al., 2015; Vukelić and Gharabaghi, 2015a,b) and poststroke recovery (Dubovik et al., 2012; Westlake et al., 2012; Nicolo et al., 2015). One might speculate that the stroke subjects in our study relied on a different mode of sensorimotor processing than healthy subjects during integration of sensory reafference into the motor command to ensure maintenance of a stable output (Witham et al., 2011). This observation complements earlier work on the relevance of lower frequency bands for motor restoration (Hall et al., 2014; Ramanathan et al., 2018; Bönstrup et al., 2019; Khanna et al., 2021). Specifically, invasive recordings of units and field potentials suggest that movement execution and restoration after stroke may be linked to low frequency oscillations and correlated local spiking. We may, therefore, assume that activity in different frequencies may have complementary roles for movement control and proprioceptive feedback during poststroke motor recovery. Furthermore, different frequencies may play a role during early and late phases of recovery (Bönstrup et al., 2019).
Nonprimary motor areas and cortical reorganization after stroke
The cortical CMC topography of the stroke subjects group was, moreover, characterized by the extended bi-hemispheric involvement during the intervention (Fig. 8B,G). Bearing in mind that not all poststroke neuronal reorganization relates to functional restoration, CMC may nonetheless serve as a measure to detect functionally relevant efferent drive (Gerloff et al., 2006a,b; Braun et al., 2007; Rossiter et al., 2013) and neural plasticity (von Carlowitz-Ghori et al., 2014; Belardinelli et al., 2017). Therefore, our observation of increased influence of nonprimary motor areas over the finger extensors in the course of the intervention complements earlier reports of widespread changes of brain activity in stroke subjects with more severe impairment (Serrien et al., 2004; Gerloff et al., 2006b; Ward et al., 2006; J.A. Brown, 2008; Volz et al., 2015; Belardinelli et al., 2017; Diekhoff-Krebs et al., 2017).
Furthermore, experimental work indicates that the motor control system maintains a variability of representations so that it can adapt to unpredictable changes (Peters et al., 2017); similarly, the descending corticospinal connectivity is known to be malleable (Brus-Ramer et al., 2007; Mosberger et al., 2017). In this context, our findings indicate that the amount of synchronization between cortical and spinal cord activity (P Brown et al., 1998; Mima and Hallett, 1999; Salenius and Hari, 2003) during the execution of a (robot-assisted) movement will represent the task-relevant recruitment of the available corticospinal output after stroke and the corresponding motor network representation. This view is supported by cross-sectional and longitudinal structural (Koch et al., 2015) and functional MRI (Grefkes and Fink, 2011, 2014) studies in stroke patients. Specifically, in chronic stroke damage to the descending output fibers from one region of the cortical motor system was compensated by activity in areas that retain corticofugal outputs, e.g., from secondary motor areas such as the dorsal premotor cortex (Newton et al., 2006; Riley et al., 2011; Schulz et al., 2012, 2015a,b; Potter-Baker et al., 2016).
Our findings support the observation that the contralesional hemisphere can act as a source of cortical coherence with functionally relevant muscles after stroke (Rossiter et al., 2013). Moreover, we provide empirical support that BCI with proprioceptive feedback serves as a technique to enhance this connectivity. The predominant information flow from cortex to periphery increased, however, in a restricted perilesional area of the affected hemisphere only. Specifically, training-related enhancement of β-band CMC in the ipsilesional premotor cortex correlated with clinical improvements after the intervention. Future approaches may directly address this biomarker by applying CMC feedback (von Carlowitz-Ghori et al., 2015) between this specific area and the targeted muscles. A shift of CMC anteriorly and medially from the ipsilesional primary motor cortex has already been described in chronic stroke patients (Mima et al., 2001). This has led to the development of interventions that specifically target premotor areas with brain stimulation (Cunningham et al., 2015; Sankarasubramanian et al., 2017). Furthermore, inhibition of the dorsal premotor cortices in either hemisphere with magnetic stimulation disrupted motor performance in chronic stroke patients, but not in control subjects (Johansen-Berg et al., 2002; Fridman et al., 2004; Lotze et al., 2006), thereby implicating that these regions contribute to poststroke motor recovery (Belardinelli et al., 2017).
Limitations and future perspectives
Direct comparisons between the healthy subject and stroke subject groups have to be drawn with caution because of apparent differences with regard to age, extent of intervention and motor evaluation. Future work may apply a dose-matched intervention in age-matched healthy subjects to better delineate physiological patterns related specifically to poststroke reorganization. Direct comparisons with MRI metrics may, furthermore, enable us to gain a better understanding as to how structural determinants of connections interrelate with task-related functional connectivity measures (Koch et al., 2015).
The present study aimed to elucidate neurophysiological mechanisms related to BCI feedback and not its impact on motor outcome improvement after stroke. This would have necessitated a controlled study design. The observed correlation of ipsilesional premotor β-band CMC with clinical improvement might have been influenced by nonspecific factors in the course of the training program. This intervention requires therefore replication in controlled studies with larger cohorts and longer follow-up periods.
In conclusion, volitional control of cortical oscillations with proprioceptive feedback enhances both cortical activity and behaviorally relevant connectivity to the periphery. This enhanced cortico-muscular control can be induced in the healthy and poststroke brain. Thereby, activating the motor cortex with MI and closing the loop by robot-assisted feedback may facilitate neurorehabilitation in the absence of volitional muscle control.
Footnotes
This work was supported by the German Federal Ministry of Education and Research (BMBF) Grant INERLINC 16SV8174. F.K. and D.K. were supported by the Graduate Training Center of Neuroscience & International Max Planck Research School, Graduate School of Neural Information Processing (F.K.) and Graduate School of Neural and Behavioral Sciences (D.K.), Tuebingen, Germany. F.K. was affiliated with the Werner Reichardt Center for Integrative Neuroscience, and the Hertie Institute for Clinical Brain Research, University of Tuebingen, Tuebingen, Germany. A.G. was supported by grants from the Baden-Wuerttemberg Foundation and the German Federal Ministry of Education and Research. We thank Bettina Hanna Trunk for her support with the clinical data. We thank support by the Open Access Publishing Fund of the University of Tuebingen.
The authors declare no competing financial interests.
References
- Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, Stagg CJ, Johansen-Berg H (2016) Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med 8:330re1. 10.1126/scitranslmed.aad5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Guan C, Chua KS, Ang BT, Kuah C, Wang C, Phua KS, Chin ZY, Zhang H (2010) Clinical study of neurorehabilitation in stroke using EEG-based motor imagery brain-computer interface with robotic feedback. Conf Proc IEEE Eng Med Biol Soc 2010:5549–5552. [DOI] [PubMed] [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhou L, Tang KY, Ephraim Joseph GJ, Kuah CW, Chua KS (2014) Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Front Neuroeng 7:30. 10.3389/fneng.2014.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann TD, Prut Y (2015) Do sensorimotor β-oscillations maintain muscle synergy representations in primary motor cortex? Trends Neurosci 38:77–85. 10.1016/j.tins.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Baker SN, Philbin N, Spinks R, Pinches EM, Wolpert DM, MacManus DG, Pauluis Q, Lemon RN (1999) Multiple single unit recording in the cortex of monkeys using independently moveable microelectrodes. J Neurosci Methods 94:5–17. 10.1016/s0165-0270(99)00121-1 [DOI] [PubMed] [Google Scholar]
- Bauer R, Gharabaghi A (2015a) Reinforcment learning for adaptive threshold control of restorative brain-computer interfaces: a Bayesian simulation. Front Neurosci 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Gharabaghi A (2015b) Estimating cognitive load during self-regulation of brain activity and neurofeedback with therapeutic brain-computer interfaces. Front Behav Neurosci 9:21. 10.3389/fnbeh.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Gharabaghi A (2017) Constraints and adaptation of closed-loop neuroprosthetics for functional restoration. Front Neurosci 11:111. 10.3389/fnins.2017.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Fels M, Vukelić M, Ziemann U, Gharabaghi A (2015) Bridging the gap between motor imagery and motor execution with a brain-robot interface. Neuroimage 108:319–327. 10.1016/j.neuroimage.2014.12.026 [DOI] [PubMed] [Google Scholar]
- Bauer R, Fels M, Royter V, Raco V, Gharabaghi A (2016a) Closed-loop adaptation of neurofeedback based on mental effort facilitates reinforcement learning of brain self-regulation. Clin Neurophysiol 127:3156–3164. 10.1016/j.clinph.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Bauer R, Vukelić M, Gharabaghi A (2016b) What is the optimal task difficulty for reinforcement learning of brain self-regulation? Clin Neurophysiol 127:3033–3041. 10.1016/j.clinph.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Bayraktaroglu Z, von Carlowitz-Ghori K, Curio G, Nikulin VV (2013) It is not all about phase: amplitude dynamics in corticomuscular interactions. Neuroimage 64:496–504. 10.1016/j.neuroimage.2012.08.069 [DOI] [PubMed] [Google Scholar]
- Belardinelli P, Laer L, Ortiz E, Braun C, Gharabaghi A (2017) Plasticity of premotor cortico-muscular coherence in severely impaired stroke patients with hand paralysis. Neuroimage Clin 14:726–733. 10.1016/j.nicl.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner J, Schönfeldt-Lecuona C, Nowak DA (2007) Sensorimotor memory for fingertip forces during object lifting: the role of the primary motor cortex. Neuropsychologia 45:1931–1938. 10.1016/j.neuropsychologia.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, Schnider A, Schmidlin T, Zhang H, Bassolino M, Viceic D, Vuadens P, Guggisberg AG, Millán JDR (2018) Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun 9:2421. 10.1038/s41467-018-04673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe S, Gionfriddo A, Kraeutner S, Tremblay A, Little G, Bardouille T (2014) Laterality of brain activity during motor imagery is modulated by the provision of source level neurofeedback. Neuroimage 101:159–167. 10.1016/j.neuroimage.2014.06.066 [DOI] [PubMed] [Google Scholar]
- Bönstrup M, Krawinkel L, Schulz R, Cheng B, Feldheim J, Thomalla G, Cohen LG, Gerloff C (2019) Low-frequency brain oscillations track motor recovery in human stroke. Ann Neurol 86:853–865. 10.1002/ana.25615 [DOI] [PubMed] [Google Scholar]
- Brauchle D, Vukelić M, Bauer R, Gharabaghi A (2015) Brain state-dependent robotic reaching movement with a multi-joint arm exoskeleton: combining brain-machine interfacing and robotic rehabilitation. Front Hum Neurosci 9:564. 10.3389/fnhum.2015.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Staudt M, Schmitt C, Preissl H, Birbaumer N, Gerloff C (2007) Crossed cortico-spinal motor control after capsular stroke. Eur J Neurosci 25:2935–2945. 10.1111/j.1460-9568.2007.05526.x [DOI] [PubMed] [Google Scholar]
- Brown JA, Lutsep HL, Weinand M, Cramer SC (2008) Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery 62:853–862. 10.1227/01.neu.0000316287.37618.78 [DOI] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R (1998) Cortical correlate of the piper rhythm in humans. J Neurophysiol 80:2911–2917. 10.1152/jn.1998.80.6.2911 [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH (2007) Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci 27:13793–13801. 10.1523/JNEUROSCI.3489-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Modir Shanechi A, Fourkas AD, Weber C, Birbaumer N, Cohen LG (2012) Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain 135:596–614. 10.1093/brain/awr331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera MA, Soekadar SR, Ushiba J, Millán JDR, Liu M, Birbaumer N, Garipelli G (2018) Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann Clin Transl Neurol 5:651–663. 10.1002/acn3.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T (2005) Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25:2277–2784. 10.1523/JNEUROSCI.4649-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham DA, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, Jones S, Lowe M, Beall E, Sakaie K, Plow EB (2015) Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil 96:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhoff-Krebs S, Pool EM, Sarfeld AS, Rehme AK, Eickhoff SB, Fink GR, Grefkes C (2017) Interindividual differences in motor network connectivity and behavioral response to iTBS in stroke patients. Neuroimage Clin 15:559–571. 10.1016/j.nicl.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Firestine A, West M, Saremi K, Woods R (2004) Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 23:370–381. 10.1016/j.neuroimage.2004.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. 10.1016/j.conb.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Dubovik S, Pignat JM, Ptak R, Aboulafia T, Allet L, Gillabert N, Magnin C, Albert F, Momjian-Mayor I, Nahum L, Lascano AM, Michel CM, Schnider A, Guggisberg AG (2012) The behavioral significance of coherent resting-state oscillations after stroke. Neuroimage 61:249–257. 10.1016/j.neuroimage.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Feigin VL, Barker-Collo S, McNaughton H, Brown P, Kerse N (2008) Long-term neuropsychological and functional outcomes in stroke survivors: current evidence and perspectives for new research. Int J Stroke 3:33–40. 10.1111/j.1747-4949.2008.00177.x [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG (2004) Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127:747–758. 10.1093/brain/awh082 [DOI] [PubMed] [Google Scholar]
- Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9:474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Gao Q, Duan X, Chen H (2011) Evaluation of effective connectivity of motor areas during motor imagery and execution using conditional Granger causality. Neuroimage 54:1280–1288. 10.1016/j.neuroimage.2010.08.071 [DOI] [PubMed] [Google Scholar]
- Gerloff C, Braun C, Staudt M, Hegner YL, Dichgans J, Krägeloh-Mann I (2006a) Coherent corticomuscular oscillations originate from primary motor cortex: evidence from patients with early brain lesions. Hum Brain Mapp 27:789–798. 10.1002/hbm.20220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M (2006b) Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 129:791–808. 10.1093/brain/awh713 [DOI] [PubMed] [Google Scholar]
- Gharabaghi A (2016) What turns assistive into restorative brain-machine interfaces? Front Neurosci 10:456. 10.3389/fnins.2016.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharabaghi A, Kraus D, Leão MT, Spüler M, Walter A, Bogdan M, Rosenstiel W, Naros G, Ziemann U (2014) Coupling brain–machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci 8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR (2011) Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134:1264–1276. 10.1093/brain/awr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR (2014) Connectivity-based approaches in stroke and recovery of function. Lancet Neurol 13:206–216. 10.1016/S1474-4422(13)70264-3 [DOI] [PubMed] [Google Scholar]
- Grimm F, Naros G, Gharabaghi A (2016) Compensation or restoration: closed-loop feedback of movement quality for assisted reach-to-grasp exercises with a multi joint arm exoskeleton. Front Neurosci 10:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001) Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98:694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TM, de Carvalho F, Jackson A (2014) A common structure underlies low-frequency cortical dynamics in movement, sleep, and sedation. Neuron 83:1185–1199. 10.1016/j.neuron.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR (1998) Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett 241:5–8. 10.1016/s0304-3940(97)00964-6 [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK (2006) Motor learning in man: a review of functional and clinical studies. J Physiol Paris 99:414–424. 10.1016/j.jphysparis.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Harel NY, Carmel JB (2016) Paired stimulation to promote lasting augmentation of corticospinal circuits. Neural Plast 2016:7043767. 10.1155/2016/7043767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL (1993) Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13:952–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949) The organization of behavior: a neuropsychological theory. New York: Wiley. [Google Scholar]
- Houweling S, van Dijk BW, Beek PJ, Daffertshofer A (2010) Cortico-spinal synchronization reflects changes in performance when learning a complex bimanual task. Neuroimage 49:3269–3275. 10.1016/j.neuroimage.2009.11.017 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4:186. 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM (2002) Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125:2731–2742. 10.1093/brain/awf282 [DOI] [PubMed] [Google Scholar]
- Jørgensen HS, Reith J, Nakayama H, Kammersgaard LP, Raaschou HO, Olsen TS (1999) What determines good recovery in patients with the most severe strokes? The Copenhagen Stroke Study. Stroke 30:2008–2012. 10.1161/01.STR.30.10.2008 [DOI] [PubMed] [Google Scholar]
- Khademi F, Royter V, Gharabaghi A (2018) Distinct beta-band oscillatory circuits underlie corticospinal gain modulation. Cereb Cortex 28:1502–1515. 10.1093/cercor/bhy016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademi F, Royter V, Gharabaghi A (2019) State-dependent brain stimulation: power or phase? Brain Stimul 12:296–299. 10.1016/j.brs.2018.10.015 [DOI] [PubMed] [Google Scholar]
- Khanna P, Totten D, Novik L, Roberts J, Morecraft RJ, Ganguly K (2021) Low-frequency stimulation enhances ensemble co-firing and dexterity after stroke. Cell 184:912–930. 10.1016/j.cell.2021.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, Felder M, Fellinghauer B, Guidali M, Kollmar A, Luft A, Nef T, Schuster-Amft C, Stahel W, Riener R (2014) Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol 13:159–166. 10.1016/S1474-4422(13)70305-3 [DOI] [PubMed] [Google Scholar]
- Koch MW, Greenfield J, Javizian O, Deighton S, Wall W, Metz LM (2015) The natural history of early versus late disability accumulation in primary progressive MS. J Neurol Neurosurg Psychiatry 86:615–621. 10.1136/jnnp-2014-307948 [DOI] [PubMed] [Google Scholar]
- Kombos T, Suess O, Kern BC, Funk T, Hoell T, Kopetsch O, Brock M (1999) Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir (Wien) 141:1295–1301. 10.1007/s007010050433 [DOI] [PubMed] [Google Scholar]
- Kraus D, Gharabaghi A (2015) Projecting navigated TMS sites on the gyral anatomy decreases inter-subject variability of cortical motor maps. Brain Stimul 8:831–837. 10.1016/j.brs.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Kraus D, Gharabaghi A (2016) Neuromuscular plasticity: disentangling stable and variable motor maps in the human sensorimotor cortex. Neural Plast 2016:7365609. 10.1155/2016/7365609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D, Naros G, Bauer R, Leão MT, Ziemann U, Gharabaghi A (2016a) Brain-robot interface driven plasticity: distributed modulation of corticospinal excitability. Neuroimage 125:522–532. 10.1016/j.neuroimage.2015.09.074 [DOI] [PubMed] [Google Scholar]
- Kraus D, Naros G, Bauer R, Khademi F, Leão MT, Ziemann U, Gharabaghi A (2016b) Brain state-dependent transcranial magnetic closed-loop stimulation controlled by sensorimotor desynchronization induces robust increase of corticospinal excitability. Brain Stimul 9:415–424. 10.1016/j.brs.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Kraus D, Naros G, Guggenberger R, Leão MT, Ziemann U, Gharabaghi A (2018) Recruitment of additional corticospinal pathways in the human brain with state-dependent paired associative stimulation. J Neurosci 38:1396–1407. 10.1523/JNEUROSCI.2893-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W (2007) Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 36:785–792. 10.1016/j.neuroimage.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lücking CH (2002) Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol 113:124–131. 10.1016/s1388-2457(01)00722-2 [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Meskers CG, van Wegen EE, Lankhorst GJ, Geurts AC, van Kuijk AA, Lindeman E, Visser-Meily A, de Vlugt E, Arendzen JH (2008) Impact of early applied upper limb stimulation: the EXPLICIT-stroke programme design. BMC Neurol 8:49. 10.1186/1471-2377-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J, Kwakkel G (2011) Stroke rehabilitation. Lancet 377:1693–1702. 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P (2010) Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 362:1772–1783. 10.1056/NEJMoa0911341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh MN, Kirsch L, Rothwell JC, Lemon RN, Davare M (2010) Information about the weight of grasped objects from vision and internal models interacts within the primary motor cortex. J Neurosci 30:6984–6990. 10.1523/JNEUROSCI.6207-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C (2006) The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26:6096–6102. 10.1523/JNEUROSCI.4564-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P (2007) Nonparametric statistical testing of coherence differences. J Neurosci Methods 163:161–175. 10.1016/j.jneumeth.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Mathew J, Kübler A, Bauer R, Gharabaghi A (2016) Probing corticospinal recruitment patterns and functional synergies with transcranial magnetic stimulation. Front Cell Neurosci 10:175. 10.3389/fncel.2016.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR (2000) Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr 12:177–186. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR (2008) Sensorimotor rhythm-based brain–computer interface (BCI): model order selection for autoregressive spectral analysis. J Neural Eng 5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrkanoon S, Breakspear M, Boonstra TW (2014) The reorganization of corticomuscular coherence during a transition between sensorimotor states. Neuroimage 100:692–702. [DOI] [PubMed] [Google Scholar]
- Mendez-Balbuena I, Huethe F, Schulte-Mönting J, Leonhart R, Manjarrez E, Kristeva R (2012) Corticomuscular coherence reflects interindividual differences in the state of the corticomuscular network during low-level static and dynamic forces. Cereb Cortex 22:628–638. 10.1093/cercor/bhr147 [DOI] [PubMed] [Google Scholar]
- Mima T, Hallett M (1999) Electroencephalographic analysis of cortico-muscular coherence: reference effect, volume conduction and generator mechanism. Clin Neurophysiol 110:1892–1899. 10.1016/s1388-2457(99)00238-2 [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M (2000) Functional coupling of human right and left cortical motor areas demonstrated with partial coherence analysis. Neurosci Lett 287:93–96. 10.1016/s0304-3940(00)01165-4 [DOI] [PubMed] [Google Scholar]
- Mima T, Toma K, Koshy B, Hallett M (2001) Coherence between cortical and muscular activities after subcortical stroke. Stroke 32:2597–2601. 10.1161/hs1101.098764 [DOI] [PubMed] [Google Scholar]
- Mosberger AC, Miehlbradt JC, Bjelopoljak N, Schneider MP, Wahl AS, Ineichen BV, Gullo M, Schwab ME (2017) Axotomized corticospinal neurons increase supra-lesional innervation and remain crucial for skilled reaching after bilateral pyramidotomy. Cereb Cortex 28:625–643. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Jiang N, Stevenson AJ, Niazi IK, Kostic V, Pavlovic A, Radovanovic S, Djuric-Jovicic M, Agosta F, Dremstrup K, Farina D (2016) Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J Neurophysiol 115:1410–1421. 10.1152/jn.00918.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Kristensen SR, Niazi IK, Farina D (2012) Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J Physiol 590:1669–1682. 10.1113/jphysiol.2011.222851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10:861–872. 10.1038/nrn2735 [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE (1992) Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A 89:5670–5674. 10.1073/pnas.89.12.5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, Malley MO, Vaughan CL, Heneghan C, Gibson ASC, Harley YXR, Sreenivasan R (2003) Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods 124:157–165. 10.1016/S0165-0270(03)00004-9 [DOI] [PubMed] [Google Scholar]
- Naros G, Gharabaghi A (2015) Reinforcement learning of self-regulated β-oscillations for motor restoration in chronic stroke. Front Hum Neurosci 9:391. 10.3389/fnhum.2015.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naros G, Gharabaghi A (2017) Physiological and behavioral effects of β-tACS on brain self-regulation in chronic stroke. Brain Stimul 10:251–259. 10.1016/j.brs.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Naros G, Naros I, Grimm F, Ziemann U, Gharabaghi A (2016) Reinforcement learning of self-regulated sensorimotor β-oscillations improves motor performance. Neuroimage 134:142–152. 10.1016/j.neuroimage.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Naros G, Lehnertz T, Leão MT, Ziemann U, Gharabaghi A (2020) Brain state-dependent gain modulation of corticospinal output in the active motor system. Cereb Cortex 30:371–381. 10.1093/cercor/bhz093 [DOI] [PubMed] [Google Scholar]
- Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS (2006) Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain 129:1844–1858. 10.1093/brain/awl106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo P, Rizk S, Magnin C, Pietro MD, Schnider A, Guggisberg AG (2015) Coherent neural oscillations predict future motor and language improvement after stroke. Brain 138:3048–3060. 10.1093/brain/awv200 [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Nolte G, Curio G (2011) A novel method for reliable and fast extraction of neuronal EEG/MEG oscillations on the basis of spatio-spectral decomposition. Neuroimage 55:1528–1535. 10.1016/j.neuroimage.2011.01.057 [DOI] [PubMed] [Google Scholar]
- Nowak DA, Voss M, Huang YZ, Wolpert DM, Rothwell JC (2005) High-frequency repetitive transcranial magnetic stimulation over the hand area of the primary motor cortex disturbs predictive grip force scaling. Eur J Neurosci 22:2392–2396. 10.1111/j.1460-9568.2005.04425.x [DOI] [PubMed] [Google Scholar]
- Nuttall A (1981) Some windows with very good sidelobe behavior. IEEE Trans Acoust Speech Signal Process 29:84–91. 10.1109/TASSP.1981.1163506 [DOI] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Mendez-Balbuena I, Schulte-Mönting J, Kristeva R (2011) Corticospinal beta-range coherence is highly dependent on the pre-stationary motor state. J Neurosci 31:8037–8045. 10.1523/JNEUROSCI.4153-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011) FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Fulk GD, Boyne P (2012) Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 92:791–798. 10.2522/ptj.20110009 [DOI] [PubMed] [Google Scholar]
- Peters S, Wadden KP, Hayward KS, Neva JL, Auriat AM, Boyd LA (2017) A structural motor network correlates with motor function and not impairment post stroke. Neurosci Lett 658:155–160. 10.1016/j.neulet.2017.08.036 [DOI] [PubMed] [Google Scholar]
- Potter-Baker KA, Varnerin NM, Cunningham DA, Roelle SM, Sankarasubramanian V, Bonnett CE, Machado AG, Conforto AB, Sakaie K, Plow EB (2016) Influence of corticospinal tracts from higher order motor cortices on recruitment curve properties in stroke. Front Neurosci 10:79. 10.3389/fnins.2016.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Guo L, Gulati T, Davidson G, Hishinuma AK, Won SJ, Knight RT, Chang EF, Swanson RA, Ganguly K (2018) Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nat Med 24:1257–1267. 10.1038/s41591-018-0058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC (2011) Anatomy of stroke injury predicts gains from therapy. Stroke 42:421–426. 10.1161/STROKEAHA.110.599340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Worthen SF, Witton C, Hall SD, Furlong PL (2013) Gamma oscillatory amplitude encodes stimulus intensity in primary somatosensory cortex. Front Hum Neurosci 7:362. 10.3389/fnhum.2013.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royter V, Gharabaghi A (2016) Brain state-dependent closed-loop modulation of paired associative stimulation controlled by sensorimotor desynchronization. Front Cell Neurosci 10:115. 10.3389/fncel.2016.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S, Hari R (2003) Synchronous cortical oscillatory activity during motor action. Curr Opin Neurobiol 13:678–684. 10.1016/j.conb.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Sankarasubramanian V, Machado AG, Conforto AB, Potter-Baker KA, Cunningham DA, Varnerin NM, Wang X, Sakaie K, Plow EB (2017) Inhibition versus facilitation of contralesional motor cortices in stroke: deriving a model to tailor brain stimulation. Clin Neurophysiol 128:892–902. 10.1016/j.clinph.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR (2004) BCI2000: a general-purpose brain–computer interface (BCI) system. IEEE Trans Biomed Eng 51:1034–1043. 10.1109/TBME.2004.827072 [DOI] [PubMed] [Google Scholar]
- Schmidt S, Fleischmann R, Bathe-Peters R, Irlbacher K, Brandt SA (2013) Evolution of premotor cortical excitability after cathodal inhibition of the primary motor cortex: a sham-controlled serial navigated TMS study. PLoS One 8:e57425. 10.1371/journal.pone.0057425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6:285–296. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS (2012) Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke 43:2248–2251. 10.1161/STROKEAHA.112.662619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Ubelacker T, Keil J, Müller N, Weisz N (2014) Now I am ready-now I am not: the influence of pre-TMS oscillations and corticomuscular coherence on motor-evoked potentials. Cereb Cortex 24:1708–1719. 10.1093/cercor/bht024 [DOI] [PubMed] [Google Scholar]
- Schulz R, Koch P, Zimerman M, Wessel M, Bönstrup M, Thomalla G, Cheng B, Gerloff C, Hummel FC (2015a) Parietofrontal motor pathways and their association with motor function after stroke. Brain 138:1949–1960. 10.1093/brain/awv100 [DOI] [PubMed] [Google Scholar]
- Schulz R, Braass H, Liuzzi G, Hoerniss V, Lechner P, Gerloff C, Hummel FC (2015b) White matter integrity of premotor-motor connections is associated with motor output in chronic stroke patients. Neuroimage Clin 7:82–86. 10.1016/j.nicl.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P (2004) Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol 190:425–432. 10.1016/j.expneurol.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Shiner CT, Tang H, Johnson BW, McNulty PA (2015) Cortical beta oscillations and motor thresholds differ across the spectrum of post-stroke motor impairment, a preliminary MEG and TMS study. Brain Res 1629:26–37. 10.1016/j.brainres.2015.09.037 [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, McNamara A, Shen S, Sterr A (2012) Neural activation and functional connectivity during motor imagery of bimanual everyday actions. PLoS One 7:e38506. 10.1371/journal.pone.0038506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemi M, Masakado Y, Liu M, Ushiba J (2013) Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J Neurophysiol 110:1158–1166. 10.1152/jn.01092.2012 [DOI] [PubMed] [Google Scholar]
- Teitti S, Määttä S, Säisänen L, Könönen M, Vanninen R, Hannula H, Mervaala E, Karhu J (2008) Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage 40:1243–1250. 10.1016/j.neuroimage.2007.12.065 [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C (2009) New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci 13:182–189. 10.1016/j.tics.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997) Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Volz LJ, Eickhoff SB, Pool EM, Fink GR, Grefkes C (2015) Differential modulation of motor network connectivity during movements of the upper and lower limbs. Neuroimage 119:44–53. 10.1016/j.neuroimage.2015.05.101 [DOI] [PubMed] [Google Scholar]
- Volz LJ, Rehme AK, Michely J, Nettekoven C, Eickhoff SB, Fink GR, Grefkes C (2016) Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex 26:2882–2894. 10.1093/cercor/bhw034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Carlowitz-Ghori K, Bayraktaroglu Z, Hohlefeld FU, Losch F, Curio G, Nikulin VV (2014) Corticomuscular coherence in acute and chronic stroke. Clin Neurophysiol 125:1182–1191. 10.1016/j.clinph.2013.11.006 [DOI] [PubMed] [Google Scholar]
- von Carlowitz-Ghori K, Bayraktaroglu Z, Waterstraat G, Curio G, Nikulin VV (2015) Voluntary control of corticomuscular coherence through neurofeedback: a proof-of-principle study in healthy subjects. Neuroscience 290:243–254. 10.1016/j.neuroscience.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Vukelić M, Gharabaghi A (2015a) Oscillatory entrainment of the motor cortical network during motor imagery is modulated by the feedback modality. Neuroimage 111:1–11. 10.1016/j.neuroimage.2015.01.058 [DOI] [PubMed] [Google Scholar]
- Vukelić M, Gharabaghi A (2015b) Self-regulation of circumscribed brain activity modulates spatially selective and frequency specific connectivity of distributed resting state networks. Front Behav Neurosci 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelić M, Bauer R, Naros G, Naros I, Braun C, Gharabaghi A (2014) Lateralized alpha-band cortical networks regulate volitional modulation of beta-band sensorimotor oscillations. Neuroimage 87:147–153. 10.1016/j.neuroimage.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Vukelić M, Belardinelli P, Guggenberger R, Royter V, Gharabaghi A (2019) Different oscillatory entrainment of cortical networks during motor imagery and neurofeedback in right and left handers. Neuroimage 195:190–202. 10.1016/j.neuroimage.2019.03.067 [DOI] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS (2006) Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129:809–819. 10.1093/brain/awl002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake KP, Hinkley LB, Bucci M, Guggisberg AG, Byl N, Findlay AM, Henry RG, Nagarajan SS (2012) Resting state α-band functional connectivity and recovery after stroke. Exp Neurol 237:160–169. 10.1016/j.expneurol.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham CL, Riddle CN, Baker MR, Baker SN (2011) Contributions of descending and ascending pathways to corticomuscular coherence in humans. J Physiol 589:3789–3800. 10.1113/jphysiol.2011.211045 [DOI] [PMC free article] [PubMed] [Google Scholar]