Abstract
Biopharmaceutical protein production using transgenic plant cell bioreactor processes offers advantages over microbial and mammalian cell culture platforms in its ability to produce complex biologics with simple chemically defined media and reduced biosafety concerns. A disadvantage of plant cells from a traditional batch bioprocessing perspective is their slow growth rate which has motivated us to develop semicontinuous and/or perfusion processes. Although the economic benefits of plant cell culture bioprocesses are often mentioned in the literature, to our knowledge no rigorous technoeconomic models or analyses have been published. Here we present technoeconomic models in SuperPro Designer® for the large-scale production of recombinant butyrylcholinesterase (BChE), a prophylactic/therapeutic bioscavenger against organophosphate nerve agent poisoning, in inducible transgenic rice cell suspension cultures. The base facility designed to produce 25 kg BChE per year utilizing two-stage semicontinuous bioreactor operation manufactures a single 400 mg dose of BChE for $263. Semicontinuous operation scenarios result in 4–11% reduction over traditional two-stage batch operation scenarios. In addition to providing a simulation tool that will be useful to the plant-made pharmaceutical community, the model also provides a computational framework that can be used for other semicontinuous or batch bioreactor-based processes.
Keywords: biopharmaceutical, butyrylcholinesterase, plant cell culture, semicontinuous, technoeconomic analysis
1. |. INTRODUCTION
Growing global demand and public spotlight on the biopharmaceutical industry is driving increased importance on production costs. This spotlight also exacerbates the importance of viral contamination control (Aranha, 2011). These external pressures position the industry to consider alternatives to microbial fermentation and mammalian cell culture production systems.
Plant cell suspension cultures have demonstrated promise as an alternative production system. Plant cells are higher eukaryotes, able to produce a wide array of complex protein products through a versatile set of expression and processing techniques (Huang & McDonald, 2012; Nandi & McDonald, 2014). Plant cell cultures are relatively inexpensive to operate due to their simple, often chemically defined culture medium free from animal-derived components (Häkkinen et al., 2018). They have been used at the commercial manufacturing scale for production of multiple drug products, including the secondary metabolite paclitaxel (Tabata, 2006) and the recombinant human enzyme glucocerebrosidase produced by Protalix Biotherapeutics (Ratner, 2010). Currently, Protalix is the only company with an FDA approved recombinant biologic produced in plant cell suspension culture (Tekoah et al., 2015), and they have several more products in clinical development (Almon et al., 2017; Schiffmann et al., 2019). Protalix’s process, which has paved the way for regulatory approval of this technology, serves as an excellent guide for design of future plant cell culture processes.
We have recently demonstrated the utility of plant cell culture technology for production of a challenging recombinant human therapeutic, the human enzyme butyrylcholinesterase (BChE). BChE is a large (~340 kDa) and heavily glycosylated tetrameric protein that functions as a bioscavenger agent to provide protection against organophosphorus compounds that have been used in chemical warfare and also used as agricultural pesticides. The previously reported cell culture system is able to produce BChE in a metabolically regulated transgenic rice culture (referred to as rice recombinant BChE or rrBChE) over multiple cycles in a stirred tank bioreactor (Corbin et al., 2016) and can operate semicontinuously for >6 months with no decrease in the rrBChE production (unpublished data). Using a combination of scalable, commonly used operations including tangential flow filtration and column chromatography, rrBChE can be purified to >95% with a 41% overall process recovery at laboratory scale. Furthermore, rrBChE has shown comparable structure, activity, and in vitro organophosphate inhibition efficacy to native human BChE (hBChE) (Corbin et al., 2018). These factors indicate that manufacturing-scale implementation of this technology could lead to effective and affordable production of this important drug.
Despite the promise of plant cell cultures for biopharmaceutical production and their demonstrated efficacy and ease of use by Protalix, manufacturing scale use of these cultures has been limited. Due to the high cost of entry into the pharmaceutical manufacturing business, novel processes are often viewed as too risky for development. To mitigate risk associated with adoption of a new process, risk severity and probability must both be considered.
Technoeconomic analysis is one method to reduce economic uncertainty of manufacturing costs and gauge risks. It can also be helpful to assess process operation strategies and predict theoretical costs to identify process and economic parameters with the highest impact on manufacturing costs. This can be done using “back-of-the-envelope” calculations, spreadsheets, computer modeling, and simulation tools such as SuperPro Designer® (Petrides, Carmichael, Siletti, & Koulouris, 2014).
Several traditional biopharmaceutical manufacturing processes have been studied using SuperPro Designer® and other process simulation tools, including tissue plasminogen activator (Rouf, Douglas, Moo-Young, & Scharer, 2001; Rouf, Moo-Young, Scharer, & Douglas, 2000) and monoclonal antibody (Xenopoulos, 2015) production in transgenic mammalian cells. Other studies have focused on whole plant-based biopharmaceutical processes, including lactoferrin (Nandi et al., 2005) and lysozyme production in transgenic rice (Wilken & Nikolov, 2012), and transient expression of monoclonal antibody (Mir-Artigues et al., 2019; Nandi et al., 2016), recombinant BChE (Tusé, Tu, & McDonald, 2014), antimicrobial proteins (McNulty et al., 2019), and Griffithsin (Alam et al., 2018) in Nicotiana benthamiana plants. These studies suggest that plant-based protein expression can produce high quality recombinant proteins with a substantial cost savings, though the magnitude of this savings depends on the specific molecule, as well as the production and processing system.
However, to our knowledge, no such analyses have been performed for a plant cell culture-based biomanufacturing process. In this work, we present a techno-economic model, simulation, and analysis of a large-scale version of the process our group has developed for semicontinuous production of rrBChE in rice cell suspension culture. Our design inputs draw from laboratory-scale process data we have generated and demonstrate the potential cost savings that can be obtained by implementing this process for production of a challenging human biopharmaceutical. The base case facility is designed to produce 25 kg of purified rrBChE/year at >95% purity as bulk drug substance with single-use bioreactors used in the seed train and stainless steel bioreactors used for production. The rrBChE was assumed to be cell-associated, extracted from the rice biomass, and purified using tangential flow filtration and chromatographic operations. An additional goal of this model development is to create a tool that can be easily modified, adapted, and broadly applicable to similar processes. To the best of our knowledge, this work represents the first techno-economic analysis reported for production of recombinant protein in plant cell culture and the first facility simulation model for semicontinuous bioreactor operation over long time frames (~6 months). We believe this analysis can be considered as a general model, and the simulation tool can be used for widespread evaluation of semicontinuously operated cell culture platforms for production of moderate-volume biopharmaceutical products.
2 |. MATERIALS AND METHODS
2.1 |. Target selection
The target product, BChE, was selected based on the suitability of plant cell culture operation for small to moderate drug indications, such as chemical or biological defense stockpiles and rare disease treatment. The target production level for rrBChE is 25 kg per year. With a single dose at 400 mg, this corresponds to production of 62,500 doses of rrBChE annually. The production level was estimated on the basis of stockpile generation and emergency deployment. Many orphan diseases would require similarly small production capabilities, such as α-1-antitrypsin deficiency, which affects ~100,000 people in the US (Stoller & Aboussouan, 2009), or amyotrophic lateral sclerosis, commonly known as Lou Gehrig’s disease, which affects ~30,000 people in the US (Miller & Appel, 2017).
The large-scale biomanufacturing facility designs are based on laboratory-scale data. However, process design inputs assume modest improvements in culture performance and downstream recovery based on anticipated process optimization work to be done as part of scale up to pilot and commercial manufacturing.
2.2 |. Process assumptions
Upstream process performance was assumed to improve from a previous report of laboratory-scale operation (Corbin et al., 2016) in two major categories, cell doubling time (3–4 days) and cell-associated rrBChE expression (20–25 to 200 mg rrBChE/kg fresh weight [FW] rice cell). Table 1 displays recently obtained values at the laboratory-scale along with the projected values at manufacturing scale to be used for this process model. A detailed justification of the projected upstream and downstream values can be found in Supporting Information.
TABLE 1.
Upstream design parameters, current and projected
| Category Parameters | Current laboratory scale values | Base case model values | Units |
|---|---|---|---|
| Cell growth Dry/fresh weight | 0.1 | 0.1 | - |
| Scale-up step | varying | 10 | % WV |
| Inoculation density | 7 | 10 | g FW/L |
| Transfer density | 70 | 100 | g FW/L |
| Doubling time | 4 | 3 | days |
| Step duration | 13 | 10 | days |
| rrBChE expression Expression level | 60 | 200 | mg BChE/kg FW |
| Expression period | 4 | 3 | days |
| Concentration | 1 | 3 | % TSP |
Abbreviations: FW, fresh weight; rrBChE, rice recombinant butyrylcholinesterase; TSP, total soluble protein.
The downstream processing scheme consists of each of the major steps described in the previous report of laboratory-scale downstream process development (Corbin et al., 2018). Current laboratory-scale and projected modeling values of rrBChE recovery are shown in Table 2.
TABLE 2.
Downstream design parameters, actual and projected
| Current laboratory scale values |
Base case model values |
|||||
|---|---|---|---|---|---|---|
| Step | Equipment | rrBChE recovery (Step) | rrBChE recovery (Overall) | Equipment | rrBChE recovery (Step) | rrBChE recovery (Overall) |
| Medium Removal | Buchner funnel | - | - | Decanter | 100% | 100% |
| Cell disruption | Tissue homogenizer | - | - | Bead Mill | 100% | 100% |
| Centrifuge | 100% | 100% | Centrifuge | 94% | 94% | |
| Microfiltration | 0.45 μm | - | - | 0.45 μm | 99% | 93% |
| 0.2 μm | - | - | 0.2 μm | 99% | 92% | |
| UF/DF | TFF | 95% | 95% | TFF | 96% | 88% |
| DEAE | 0.22 μm | - | - | 0.22 μm | 100% | 88% |
| Column | 75% | 70% | Column | 80% | 70% | |
| Hupresin | Column | 60% | 42% | Column | 85% | 60% |
| UF/DF 2 | TFF | - | - | TFF | 95% | 57% |
Abbreviations: DEAE, Diethylethanolamine; rrBChE, rice recombinant butyrylcholinesterase; UF/DF, ultrafiltration/diafiltration.
Clean-in-Place (CIP) assumptions were developed using working process knowledge and literature (Bremer & Seale, 2010; Chisti & Moo-Young, 1994; Lydersen, D’Elia, & Nelson, 1994). Details of the procedures used in the modeling can be found in Table S1.
2.3 |. Process simulation and economics
All process modeling was performed in SuperPro Designer® version 10 build 7 (Intelligen, Inc.), and additional calculations were performed in Microsoft Excel. The process models are publicly available at http://mcdonald-nandi.ech.ucdavis.edu/tools/technoeconomics/. A free trial version of SuperPro Designer® (http://www.intelligen.com/demo.html) can be used to view the model. Process simulation operating expenditure (OPEX) and capital expenditure (CAPEX) assumptions such as startup costs, labor pay rates, and utility rates are based on current Good Manufacturing Practices (cGMP) operation and are listed in Table S2.
3 |. RESULTS
3.1 |. Base case processing
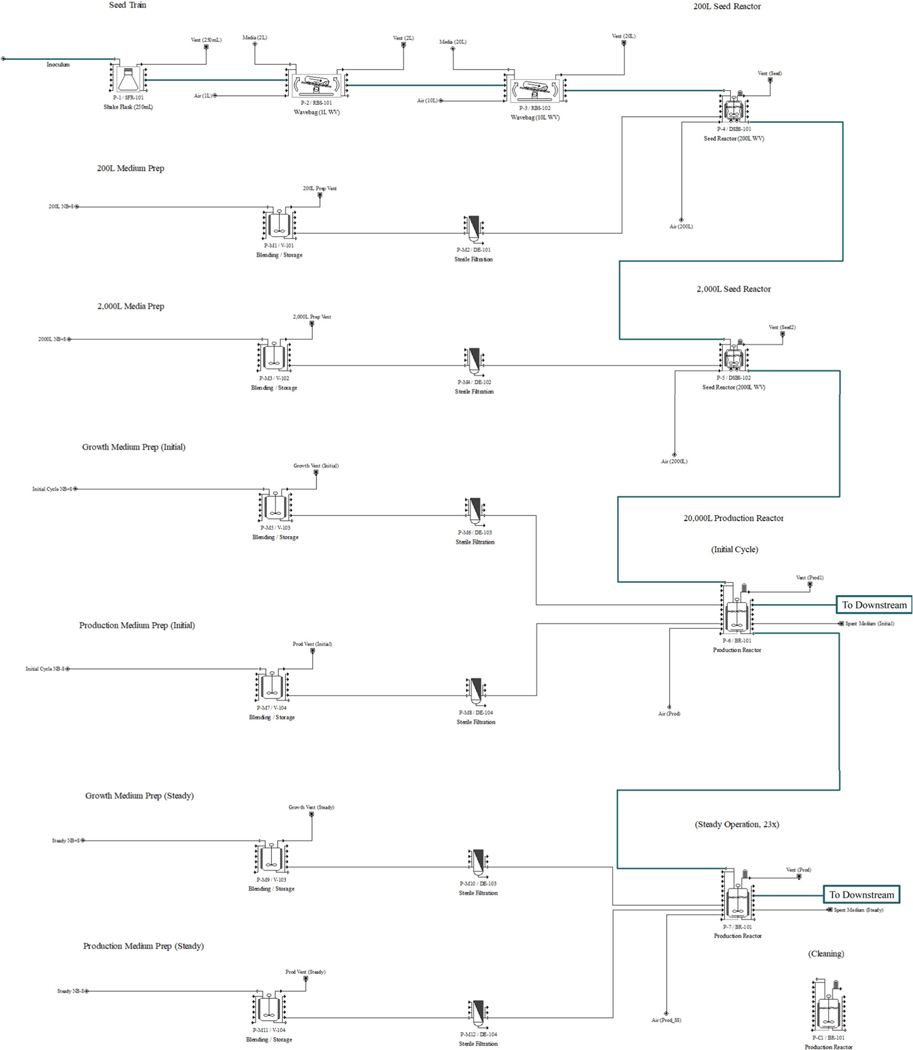
The upstream processing model is shown in Figure 1. It contains five seed train stages before the full-scale production bioreactor. Each seed train step represents a 10-fold increase in working volume over the previous step and is operated in batch mode. The culture is inoculated at 10 g FW/L, then allowed a 10 day growth phase to reach 100 g FW/L. The entire culture is then transferred to the next stage of the seed train to inoculate at 10 g FW/L. At the 20,000 L production bioreactor stage, the culture begins operating semicontinuously in alternating phases of growth and rrBChE expression as shown in Figure 2 for the two-stage semicontinuous operation. The transgenic rice cell culture controls rrBChE expression with an inducible promoter (rice α amylase 3D or RAmy3D promoter) that is triggered by sugar starvation. A more in-depth explanation of the two-stage semicontinuous operation is included in the Supporting Information.
FIGURE 1.
Upstream process flowsheet for the two-stage semicontinuous operation base case scenario in the SuperPro Designer® model
FIGURE 2.
Two-stage semicontinuous operation of transgenic rice cell culture with the RAmy3D expression system
The downstream process flowsheet is shown in Figure 3. The harvested material from the upstream process is composed of rice cell biomass containing 200 mg rrBChE/kg FW and spent expression medium. The medium is separated from the biomass using a decanter, where 95% of the spent medium is removed. The biomass is then mixed in a 1:3 (wt/vol) ratio with extraction buffer and homogenized in a bead mill. After extraction, the resulting supernatant is clarified using a disk-stack centrifuge followed by two dead-end filtration steps (0.45 μm then 0.2 μm pore size). The clarified extract is concentrated 10-fold before diafiltration with four equivalent volumes of buffer in a tangential flow filtration operation and is then passed through a 0.2 μm dead-end filter before the first of two chromatography steps.
FIGURE 3.
Downstream process flowsheet for the two-stage semicontinuous operation base case scenario in the SuperPro Designer® model.DEAE, Diethylethanolamine
An anion exchange resin is first used as a capture chromatography step before being polished using an affinity resin developed specifically for BChE (Hupresin, CHEMFORASE, Rouen, France). The linear flow rate for all chromatography steps for both resin types is 300 cm/hr and they are operated in bind-and-elute mode with the same buffer compositions (20 mM sodium phosphate buffer at pH 7.4) as previously described (Corbin et al., 2018). Each chromatography operation is paired with a holding tank for pooling of elution fractions, which are passed through a 0.2 μm filter before the following unit operation. The pooled, eluted fractions from Hupresin are sent to a final ultrafiltration/diafiltration (UF/DF) operation, where they are concentrated 20-fold, diafiltered into phosphate buffer, and aliquoted in 1 L single-use bioprocess bags stored in totes (plastic storage bins). The overall rrBChE downstream process recovery, from homogenization through storage, is 57%.
This process was modeled as a single recipe, which involves one seed train, 24 harvest cycles from the production bioreactor, and 24 downstream process cycles. The production bioreactor produces 0.2 kg rrBChE per harvest cycle and 4.8 kg rrBChE per entire recipe. After downstream processing, 0.1 kg rrBChE per harvest and 2.7 kg rrBChE per entire recipe are recovered at 99.7% purity. To reach the target production of 25 kg pure rrBChE per year, nine recipes are executed to completion.
3.2 |. Base case process economics
In the base case simulation, we assume the described process will be performed in a contract manufacturing facility (CMO) rather than a new facility to be used exclusively for this process. This can be economically favorable for low to moderate volume drug products, especially those with intermittent demand requirements that can be stockpiled such as BChE. Therefore, all facility-dependent costs, such as equipment maintenance, insurance, local taxes, factory expense, and depreciation, are excluded from determination of the drug price, and an extra 20% is added to the operating costs to account for a fee charged by the CMO.
A summary of production costs for base case process scenarios is shown in Table 3. Given the stated base case design parameters, a single batch produces 2.7 kg of pure rrBChE for total OPEX of $1.5 million, which corresponds to a unit production cost of $656/g or $263 per 400 mg dose. Upstream processing comprises 21% of the OPEX, while downstream processing costs comprise the remaining 79%.
TABLE 3.
A summary of production costs for scenarios within this study
| Case | Adjustments | COGS ($/400 mg rrBChE) | OPEX ($ million/year) | % Upstream (OPEX) | CAPEX ($ million) | % Upstream (CAPEX)a |
|---|---|---|---|---|---|---|
| Base case two-stage semicontinuous, CMO | +20% CMO Fee | 263 | 16.2 | 21 | - | - |
| Two-stage batch, CMO | +20% CMO Fee | 274 | 17.5 | 53 | - | - |
| Single-stage batch, CMO | +20% CMO Fee | 266 | 17.0 | 52 | - | - |
| Two-stage semicontinuous, new facility | With Depreciation | 573 | 35.3 | 62 | 168 | 86 |
| Without Depreciation | 389 | 23.9 | 50 | |||
| Two-stage batch, new facility | With Depreciation | 644 | 41.1 | 77 | 151 | 88 |
| Without Depreciation | 428 | 27.3 | 70 | |||
| Single-stage batch, new facility | With Depreciation | 607 | 38.7 | 75 | 140 | 87 |
| Without Depreciation | 406 | 25.9 | 69 |
Abbreviations: CAPEX, capital expenditures: COGS, cost of goods sold: CMO, contract manufacturing organization: OPEX, operating expenditures.
Excluding contributions of unlisted equipment (designated as 20% total equipment).
3.3 |. Base case scenario analysis
We evaluated the impact of process parameter variation on the model facility production costs, univariately investigating (a) rrBChE expression level in rice biomass, (b) the proportion of culture harvested per cycle of semicontinuous operation, and (c) the dynamic binding capacity of the Hupresin in the affinity chromatography procedure. The facility model was redesigned (e.g. resized) for each parameter variation scenario to maintain the same production level and final product consistency with the base case, while all other parameters were fixed for the analysis. The ranges of the process parameter variation tested in the analyses were determined using working process knowledge. The results of the scenario analyses are shown in Figure 4.
FIGURE 4.
Results of the base case scenario analysis. (a) Tornado chart displaying sensitivity of rrBChE cost of goods sold (COGS) to variation in the tested process parameters over the selected analysis range. Individual scenario analyses of (b) expression level, (c) harvest size, and (d) Hupresin capacity variation on rrBChE COGS. (e) Variation in COGS breakdown in the three scenario analyses. The simulated facility is resized for each scenario analysis result to maintain base case production level and concentration in product formulation. Values corresponding to the base case are circled in black. FW, fresh weight; rrBChE, rice recombinant butyrylcholinesterase
Cost of goods sold (COGS) is most sensitive to expression level variation within the selected parameter ranges, as shown in Figure 4. In each of the analyses there is a clear display of COGS decreasing monotonically with increasing parameter value with diminishing returns. As an illustrative example, the COGS decreases by 46% ($549/g rrBChE reduction) when increasing expression from 100 to 200 mg rrBChE/kg FW, but a larger increase from 200 to 500 mg rrBChE/kg FW is required for a comparable 49% reduction in COGS from that point (at $325/g rrBChE reduction).
3.4 |. Alternate case 1: New facility
To build on the model of our base case, which utilizes CMO production, we have also modeled the case in which a new facility constructed ground-up on an empty lot of land (referred to as a “greenfield” facility) is exclusively devoted to the production of rrBChE using the two-stage semicontinuous operating strategy. To do so, our models are adapted to consider CAPEX associated with purchasing and maintaining the required equipment and facilities. We calculated that the most cost-effective facility would have four complete sets of seed train equipment, four production bioreactors, and one set of downstream processing equipment (data not shown). The equipment and fixed capital costs are all scaled accordingly, along with all the facility-dependent OPEX contributions.
With these modifications, the cost of a 400 mg dose of rrBChE produced in a new facility is $573 when depreciation is included, and $389 when it is omitted, with CAPEX of $168 million (Table 3). The inclusion of facility-dependent costs increases the relative costs of the upstream processing from 21% in the CMO case to 62% with depreciation, which is expected as four full sets of upstream processing equipment are paired with one set of downstream processing equipment.
3.5 |. Alternate case 2: Batch operation
To evaluate the impact of the semicontinuous processing strategy on the rrBChE COGS, we adapted the semicontinuous operation models to examine the process costs associated with the equivalent facility operated in a traditional two-stage batch mode. Here, each production bioreactor operation results in a single cycle of growth, expression, and harvest before CIP, steam-in-place (SIP), and introduction of a fresh inoculum. Five sets of seed train and production bioreactors are required to maintain base case production capacity. Each harvest produces 0.2 kg of pure rrBChE, as the entire 20,000 L culture is collected at the time of harvest. To minimize the size of the decanter, 80% of the spent medium is removed in the bioreactor via gravity sedimentation, and the remaining 15% is removed by the decanter to match the overall 95% medium removal in the semicontinuously operated base case. Otherwise, the performance of all other downstream steps remains unchanged.
COGS and CAPEX of the two-stage batch operation cases are listed in Table 3. Most notably, OPEX contributions are more heavily weighted by the upstream (52%), as compared to the base case. A comparison of the two-stage semicontinuous and two-stage batch mode operation OPEX contributions is shown in Figure 5 and a comparison of CAPEX contributions is shown in Figure 6.
FIGURE 5.
Annual operating expenditures for production of rrBChE in two-stage semicontinuous and two-stage batch mode operation using a contract manufacturing organization and with a greenfield single-product facility broken down by (a) cost items, and (b) manufacturing section. Depreciation costs are not included in the annual operating costs for the new facility scenarios. DEAE, Diethylethanolamine; rrBChE, rice recombinant butyrylcholinesterase; UF/DF, ultrafiltration/diafiltration
FIGURE 6.
Total equipment costs for a greenfield single-product facility producing rrBChE in two-stage semicontinuous and two-stage batch mode operation broken down by manufacturing section. DEAE, Diethylethanolamine; rrBChE, rice recombinant butyrylcholinesterase; UF/DF, ultrafiltration/diafiltration
3.6 |. Alternate case 3: Single-stage batch operation (simple induction)
The expression phase is initiated by sugar starvation, which is achieved using gravity sedimentation-assisted medium exchange. A simple induction method would be to let the sugar deplete naturally—to tune the culture sugar concentration such that the time to depletion is set to coincide with desired final cell concentration. This is referred to as single-stage induction since a medium exchange operation is not required. Preliminary data suggest that this method has the potential to yield comparable growth and expression kinetics, but that the culture is slow to recover in semicontinuous operation (unpublished data). The batch mode operation models were adapted to the simple induction procedure by eliminating the medium exchange operation from sucrose-rich growth medium to sucrose-free medium and reducing sucrose concentration in the production bioreactor growth media by one-half of the base case growth medium.
COGS and CAPEX of the single-stage batch cases are listed in Table 3. These results correspond to a 3–6% reduction in COGS over the two-stage batch mode operation.
To better understand the economic impact of the two-stage (medium exchange) and single-stage (simple) induction methods, we performed a sensitivity analysis of the COGS to the cost of culture medium. The culture medium used in this study is inexpensive ($0.10/L growth medium; $0.09/L growth medium [half-sucrose]; $0.11/L production medium). The costs, while calculated based on bulk price estimates of the raw material components, are comparable to a previously published analysis on cost-optimized plant cell culture media (Häkkinen et al., 2018). Other sources of culture medium for eukaryotic cell culture in batch mode operation cite $5–10/L (Harrison, Todd, Rudge, & Petrides, 2015; Kelley, 2009; Xu, Gavin, Jiang, & Chen, 2017).
The results of the analysis for a new facility including depreciation costs are shown in Figure 7. COGS increases linearly with culture medium cost in the two-stage and single-stage scenarios but at different rates proportional to culture medium requirements. There is a 6% reduction in COGS using single-stage batch operation when media costs are neglected, which increases to a 23% reduction in COGS at a scenario of $10/L culture medium.
FIGURE 7.
(a) Sensitivity analysis of rrBChE cost of goods sold to the cost of culture media for batch mode operation using two-stage (medium exchange) and single-stage (simple) induction strategies for new greenfield single-product facility design including depreciation costs. (b) The variation in operating cost contributions as a function of culture medium cost. The base case scenario price is at $0.10/L. PBR, production bioreactor; rrBChE, rice recombinant butyrylcholinesterase
4 |. DISCUSSION
The technoeconomic process simulation in this work demonstrates the potential cost-savings for production of a moderate volume drug substance in a two-stage semicontinuously operated plant cell suspension culture. It also illustrates viability of batch-mode operation of plant cell suspension culture for commercial manufacturing and highlights significant differences in facility design between these two modes of operation. This simulation uses recombinant BChE as a model product, which has long been a challenging and costly molecule to produce but could represent any complex biologic molecule needed at moderate production levels (10s of kg per year).
In this analysis, two-stage semicontinuous operation yields 4% lower COGS than two-stage batch operation in the CMO scenario, 11% lower in the new facility scenario, and 9% lower in the new facility scenario excluding depreciation costs. Based on the product of interest and the stability of the product in the cell culture environment (e.g., resistance to protease degradation, pH denaturation), semicontinuous operation may provide significant benefits over batch operation which are not captured in this model since the product was assumed to be cell-associated.
We found that semicontinuous operation may be particularly favorable for facilities with high upstream costs; the economic benefits of semicontinuous operation realized in these models are in the 31–63% lower upstream operating costs. As compared to two-stage batch operation, there are 100 fewer executions of the seed train per year. The higher starting biomass density in the “steady state” semicontinuous growth phase results in production reactor cycles every 6 days as opposed to every 13 days in batch. However, raw material costs are 58% higher than in two-stage batch operation. Media requirements are 97% volumetrically higher in semicontinuous operation wherein a full 20,000 L of each growth and expression media are consumed for a return on only 10,000 L of culture harvested in each cycle. Interestingly, the CIP costs of semicontinuous operation are 70% higher than the batch case despite 100 fewer executions of production bioreactor cleaning. This is due to the lower harvest size of semicontinuous (10,000 L) compared to batch (20,000 L) resulting in twice as many annual downstream processing batches.
We demonstrate that a simple induction strategy to let the sugar in the media naturally deplete could provide additional benefits to batch operation, reducing COGS to within 1% of that of two-stage semicontinuous operation. However, there is appreciable uncertainty as to whether the assumptions of comparable growth and expression kinetics between the medium exchange and simple induction strategies are appropriate. Simple induction is a promising avenue for research and development to improve manufacturing of rrBChE, or other recombinant products under the control of the RAmy3D promoter, particularly in case gravity sedimentation and medium exchange in large-scale conventional bioreactors may be difficult to implement. The benefit of simple induction with the Ramy3D promoter would also be expected to increase substantially with the cost of culture media.
The semicontinuous process modeled here has some similarities and differences to the one used by Protalix for production of their product Elelyso®, an orphan drug used for treatment of Gaucher’s disease. Elelyso® is produced intracellularly in carrot root cell culture and uses a semicontinuous process (Grabowski, Golembo, & Shaaltiel, 2014). Thus, Protalix’s process provides an additional reference point to justify the feasibility of the process described in this model. Another major hurdle overcome by Protalix was initial establishment of the regulatory pathway for plant-made recombinant human biologics. The mammalian viral contamination-related shutdown of a competing mammalian cell culture production facility, along with the competing product’s market exclusivity at the time, served to accelerate regulatory evaluation of Protalix’s product and establish a more trusting and favorable view of plant-made pharmaceuticals (Mor, 2015).
Despite this, a few hurdles remain for mainstream adoption of plant cell culture technologies. Pharmaceutical manufacturing processes require stably preserved cell-banking to supply a well-defined starting material and prevent genetic drift in the culture. Cryopreservation techniques have been established for plant cell cultures (Kwon et al., 2013; Mustafa, de Winter, van Iren, & Verpoorte, 2011), but there is no protocol that can be universally applied to all species (Santos, Abranches, Fischer, Sack, & Holland, 2016). There is also an ongoing literature debate as to the potential immunogenicity of plant glycan structures. While some studies indicate a potential for an immune response to plant glycans on human therapeutics (Chung et al., 2008), several other studies of actual in vivo administration indicate that this does not occur in practice (Rup et al., 2017; Shaaltiel & Tekoah, 2016). However, the difficulty in proving that something does not occur will likely continue to challenge regulatory approval and mainstream acceptance of this technology.
For BChE specifically, this study provides manufacturing models which demonstrate a substantial improvement over current production technology in terms of product safety, reliability, and cost. To date, no form of BChE has been approved for therapeutic use in humans. Recombinant hBChE produced in transgenic goats (Protexia®, product by PharmAthene, now Altimmune) reached Phase I clinical trials (ClinicalTrials.gov Identifier: NCT00744146), and results indicated that it was well-tolerated (Jurchison, 2009). However, the project was discontinued after project funding expired in 2010 and the production facilities were sold (PharmAthene, 2015). No production cost analysis was reported. Aside from Protexia®, the most well-developed technology for BChE production involves purification of hBChE from human blood plasma. This product, too, has passed Phase I clinical trials (ClinicalTrials.gov Identifier: NCT00333528). Though many technical aspects of pilot scale purification of hBChE have been documented (Saxena, Tipparaju, Luo, & Doctor, 2010), to our knowledge, no cost analyses have been publicly reported for this process either. However, in February of 2012, the Defense Advanced Research Projects Agency (DARPA, 2012) released a call for research proposals titled “Butyrylcholinesterase Expression in Plants.” In this document, DARPA cites a BChE dose size of 400 mg and estimates a cost per dose of hBChE as ~$10,000 (DARPA, 2012), though no references are given for this value. In addition to the extremely high cost of plasma-derived hBChE, availability is extremely limited: the entire theoretically available blood supply in the US could only produce 1–2 kg of pure hBChE, or 2,500–5,000 doses, per year (Ashani, 2000). Therefore, cost-effective production of recombinant BChE has been a long-standing goal. Our models suggest that plant cell suspension culture manufacturing has the potential to reduce the COGS to <3% of the 2012 DARPA manufacturing estimate.
To that end, we have not only studied rrBChE production in rice cell culture, but have also evaluated production of recombinant BChE using transient expression in N. benthamiana plants through agrionfiltration (Alkanaimsh et al., 2016), and published a techno-economic analysis of this system (Tusé et al., 2014). In this work, a single dose of recombinant BChE is estimated to cost $234 when produced in an existing facility and $474 when a new facility is constructed. Overall, these values are lower than, but comparable to, our findings for rrBChE production in rice cell. However, the two models differ in several important ways. Tusé et al. (2014) assume an expression level of 500 mg BChE/kg FW of plant tissue, which is significantly higher projection than what is assumed in the rice cell culture model. The Tusé et al. (2014) model assumes a low downstream recovery of 20%, which is supported by literature surrounding purification of BChE from N. benthamiana whole plant systems (Geyer et al., 2010). Much of the BChE loss occurs in the initial recovery steps; assumptions regarding the costs and binding capacities of the chromatography steps are comparable to this model.
While these two plant-based systems appear to give similar product costs, the choice of expression host depends on other factors, in addition to cost. Transient expression avoids the long lead times associated with development of a transgenic line, which can be essential in rapid response applications. However, transgenic bioreactor-based systems benefit from increased process controllability, reproducibility, and compatibility with existing infrastructure and regulatory guidelines. For BChE and similar targets, a combination of both these strategies may prove beneficial in meeting global defense needs for both stockpiling and rapid response situations. For other products, such as orphan drugs to treat rare disease, cell culture systems may be preferred for the regulatory process familiarity.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this project was provided by the National Institutes of Health, National Institute of General Medical Sciences (NIGMS-NIH, #T32-GM008799) and the Defense Threat Reduction Agency (DTRA, #HDTRA1-15-0054). This work was also supported by a NASA Space Technology Research Fellowship (NASA grant number 80NSSC18K1157). Its contents are solely the responsibility of the authors and do not necessarily representthe official views of the NIGMS, NIH, DTRA, or the National Aeronautics and Space Administration (NASA).
Funding information
Defense Threat Reduction Agency, Grant/Award Number: HDTRA1-15-0054; National Institute of General Medical Sciences, Grant/Award Number: T32-GM008799; NASA Space Technology Research Fellowship, Grant/Award Number: 80NSSC18K1157
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
Karen A. McDonald is a cofounder of Inserogen, Inc., a plant-based biotechnology company with a focus on the development of orphan drugs for replacement therapy.
REFERENCES
- Alam A, Jiang L, Kittleson GA, Steadman KD, Nandi S, Fuqua JL, & McDonald KA (2018). Technoeconomic modeling of plant-based Griffiths in manufacturing. Frontiers in Bioengineering and Biotechnology, 6, 102. 10.3389/fbioe.2018.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkanaimsh S, Karuppanan K, Guerrero A, Tu A, Hashimoto B, Hwang MS, & McDonald KA (2016). Transient expression of tetrameric recombinant human butyrylcholinesterase in Nicotiana benthamiana. Frontiers in Plant Science, 7, 743. 10.3389/fpls.2016.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon E, Khoury T, Drori A, Gingis-Velitski S, Alon S, Chertkoff R, & Ilan Y. (2017). An oral administration of a recombinant anti-TNF fusion protein is biologically active in the gut promoting regulatory T cells: Results of a phase I clinical trial using a novel oral anti-TNF alpha-based therapy. Journal of Immunological Methods, 446, 21–29. 10.1016/J.JIM.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Aranha H. (2011). Virus safety of biopharmaceuticals. Retrieved from https://www.contractpharma.com/issues/2011-11/view_features/virus-safety-of-biopharmaceuticals
- Ashani Y. (2000). Prospective of human butyrylcholinesterase as a detoxifying antidote and potential regulator of controlled-release drugs. Drug Development Research, 308, 2000–2308. [DOI] [Google Scholar]
- Bremer PJ, & Seale RB (2010). Clean-in-Place (CIP). In Encyclopedia of industrial biotechnology, Hoboken, NJ: John Wiley & Sons, Inc. 10.1002/9780470054581.eib231 [DOI] [Google Scholar]
- Chisti Y, & Moo-Young M. (1994). Clean-in-place systems for industrial bioreactors: Design, validation and operation. Journal of Industrial Microbiology, 3, 201–207. [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le Q-T, Berlin J, Morse M, & Slebos RJ (2008). Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. The New England Journal of Medicine, 11(358), 1109–1117. 10.1126/scisignal.2001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JM, Hashimoto BI, Karuppanan K, Kyser ZR, Wu L, Roberts BA, & Nandi S. (2016). Semicontinuous bioreactor production of recombinant butyrylcholinesterase in transgenic rice cell suspension cultures. Frontiers in Plant Science, 7, 1–9. 10.3389/fpls.2016.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JM, Kailemia MJ, Cadieux CL, Alkanaimsh S, Karuppanan K, Rodriguez RL, & Nandi S. (2018). Purification, characterization, and N-glycosylation of recombinant butyrylcholinesterase from transgenic rice cell suspension cultures. Biotechnology and Bioengineering, 115(5), 1301–1310. 10.1002/bit.26557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARPA (2012). Broad agency announcement butyrylcholinesterase expression in plants DSO DARPA-BAA-12-31.
- Geyer BC, Kannan L, Cherni I, Woods RR, Soreq H, & Mor TS (2010). Transgenic plants as a source for the bioscavenging enzyme, human butyrylcholinesterase. Plant Biotechnology Journal, 8(8), 873–886. 10.1111/j.1467-7652.2010.00515.x [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Golembo M, & Shaaltiel Y. (2014). Taliglucerase alfa: An enzyme replacement therapy using plant cell expression technology. Molecular Genetics and Metabolism, 112(1), 1–8. 10.1016/j.ymgme.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Häkkinen ST, Reuter L, Nuorti N, Joensuu JJ, Rischer H, & Ritala A. (2018). Tobacco BY-2 media component optimization for a cost-efficient recombinant protein production. Frontiers in Plant Science, 9, 45. 10.3389/fpls.2018.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG, Todd PW, Rudge SR, & Petrides DP (2015). In Gubbins KE, Barteau MA, Lauffenburger DA, Morari M, Ray WH & Russel WB (Eds.), Bioseparations Science and Engineering (2nd ed.).Oxford University Press. [Google Scholar]
- Huang TK, & McDonald KA (2012). Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnology Advances, 20, 79. 10.1016/j.biotechadv.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Jurchison S. (2009). PharmAthene, Inc. Presents Phase I Clinical Trial Results and New Therapeutic Animal Model Data for Protexia (R) | BioSpace. Retrieved from https://www.biospace.com/article/releases/pharmathene-inc-presents-phase-i-clinical-trial-results-andnew-therapeutic-animal-model-data-for-protexia-r-/
- Kelley B. (2009). Industrialization of mAb production technology: The bioprocessing industry at a crossroads. mAbs, 1(5), 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Jeong SH, Choi JW, Pak YY, & Kim D, Il. (2013). Assessment of long-term cryopreservation for production of hCTLA4Ig in transgenic rice cell suspension cultures. Enzyme and Microbial Technology, 53(3), 216–222. 10.1016/j.enzmictec.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Lydersen BK, D’Elia N, & Nelson KL (1994). Bioprocess engineering: Systems, equipment and facilities, New York, NY: Wiley. [Google Scholar]
- McNulty MJ, Gleba Y, Tusé D, Hahn-Löbmann S, Giritch A, Nandi S, & McDonald KA (2019). Techno-economic analysis of a plant-based platform for manufacturing antimicrobial proteins for food safety. Biotechnology Progress, 36, 2896. 10.1002/btpr.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, & Appel SH (2017). Introduction to supplement: The current status of treatment for ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18(suppl 1), 1–4. 10.1080/21678421.2017.1361447 [DOI] [PubMed] [Google Scholar]
- Mir-Artigues P, Twyman RM, Alvarez D, Cerda Bennasser P, Balcells M, Christou P, & Capell T. (2019). A simplified technoeconomic model for the molecular pharming of antibodies. Biotechnology and Bioengineering, 116(10), 2526–2539. 10.1002/bit.27093 [DOI] [PubMed] [Google Scholar]
- Mor TS (2015). Molecular pharming’s foot in the FDA’s door: Protalix’s trailblazing story. Biotechnology Letters, 37(11), 2147–2150. 10.1007/s10529-015-1908-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa NR, de Winter W, van Iren F, & Verpoorte R. (2011). Initiation, growth and cryopreservation of plant cell suspension cultures. Nature Protocols, 6(6), 715–742. 10.1038/nprot.2010.144 [DOI] [PubMed] [Google Scholar]
- Nandi S, Kwong AT, Holtz BR, Erwin RL, Marcel S, & McDonald KA (2016). Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs, 8(8), 1456–1466. 10.1080/19420862.2016.1227901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, & McDonald K. (2014). Expression of recombinant proteins in plant cell culture. In Hefferon K. (Ed.), Plant-derived Pharmaceuticals: Principles and applications for developing countries, Wallingford, UK: CAB International. Retrieved from https://www.cabi.org/bookshop/book/9781780643434 [Google Scholar]
- Nandi S, Yalda D, Lu S, Nikolov Z, Misaki R, Fujiyama K, & Huang N. (2005). Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Research, 14(3), 237–249. 10.1007/s11248-004-8120-6 [DOI] [PubMed] [Google Scholar]
- Petrides D, Carmichael D, Siletti C, & Koulouris A. (2014). Biopharmaceutical process optimization with simulation and scheduling tools. Bioengineering, 1, 154–187. 10.3390/bioengineering1040154 [DOI] [PubMed] [Google Scholar]
- PharmAthene. (2015). United States Security and Exchange Commissions Form 10-K for the fiscal year ended December 31, 2014.
- Ratner M. (2010). Pfizer stakes a claim in plant cell-made biopharmaceuticals. Nature Biotechnology, 28(2), 107–108. 10.1038/nbt0210-107 [DOI] [PubMed] [Google Scholar]
- Rouf S, Douglas P, Moo-Young M, & Scharer J. (2001). Computer simulation for large scale bioprocess design. Biochemical Engineering Journal, 8(3), 229–234. 10.1016/S1369-703X(01)00112-7 [DOI] [Google Scholar]
- Rouf S, Moo-Young M, Scharer J, & Douglas P. (2000). Single versus multiple bioreactor scale-up: Economy for high-value products. Biochemical Engineering Journal, 6(1), 25–31. 10.1016/S1369-703X(00)00066-8 [DOI] [PubMed] [Google Scholar]
- Rup B, Alon S, Amit-Cohen B-C, Almon EB, Chertkoff R, Tekoah Y, & Rudd PM (2017). Immunogenicity of glycans on biotherapeutic drugs produced in plant expression systems—The taliglucerase alfa story. PLOS One, 12(10), 0186211. 10.1371/journal.pone.0186211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RB, Abranches R, Fischer R, Sack M, & Holland T. (2016). Putting the spotlight back on plant suspension cultures. Frontiers in Plant Science, 7, 1–12. 10.3389/fpls.2016.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Tipparaju P, Luo C, & Doctor BP (2010). Pilot-scale production of human serum butyrylcholinesterase suitable for use as a bioscavenger against nerve agent toxicity. Process Biochemistry, 45(8), 1313–1318. 10.1016/j.procbio.2010.04.021 [DOI] [Google Scholar]
- Schiffmann R, Goker-Alpan O, Holida M, Giraldo P, Barisoni L, Colvin RB, & Hughes D. (2019). Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. Journal of Inherited Metabolic Disease, 42(3), 12080–12544. 10.1002/jimd.12080 [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y, & Tekoah Y. (2016). Plant specific N-glycans do not have proven adverse effects in humans. Nature Biotechnology, 34(7), 706–708. 10.1038/nbt.3556 [DOI] [PubMed] [Google Scholar]
- Stoller JK, & Aboussouan LS (2009). Myths and misconceptions about α1-antitrypsin deficiency. Archives of Internal Medicine, 169(6), 546–550. [DOI] [PubMed] [Google Scholar]
- Tabata H. (2006). Production of paclitaxel and the related taxanes by cell suspension cultures of taxus species. Current Drug Targets, 7, 453–461. 10.2174/138945006776359368 [DOI] [PubMed] [Google Scholar]
- Tekoah Y, Shulman A, Kizhner T, Ruderfer I, Fux L, Nataf Y, & Shaaltiel Y. (2015). Large-scale production of pharmaceutical proteins in plant cell culture-the protalix experience. Plant Biotechnology Journal, 13(8), 1199–1208. 10.1111/pbi.12428 [DOI] [PubMed] [Google Scholar]
- Tusé D, Tu T, & McDonald KA (2014). Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. BioMed Research International, 2014, 2014. 10.1155/2014/256135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken LR, & Nikolov ZL (2012). Recovery and purification of plant-made recombinant proteins. Biotechnology Advances, 30(2), 419–433. 10.1016/j.biotechadv.2011.07.020 [DOI] [PubMed] [Google Scholar]
- Xenopoulos A. (2015). A new, integrated, continuous purification process template for monoclonal antibodies: Process modeling and cost of goods studies. Journal of Biotechnology, 213, 42–53. 10.1016/J.JBIOTEC.2015.04.020 [DOI] [PubMed] [Google Scholar]
- Xu S, Gavin J, Jiang R, & Chen H. (2017). Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnology Progress, 33(4), 867–878. 10.1002/btpr.2415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.