Abstract
The flavonoid naringin was found to induce the expression of hrmA, a gene with a symbiotic phenotype in the cyanobacterium Nostoc punctiforme. A comparative analysis of several flavonoids revealed the 7-O-neohesperidoside, 4′-OH, and C-2 C-3 double bond in naringin as structural determinants of its hrmA-inducing activity.
Cyanobacteria of the genus Nostoc can form N2-fixing symbiotic associations with a diverse variety of plants (10). Under the influence of the plant partner, motile units of Nostoc, termed hormogonia, infect the plant tissue. Once inside, hormogonia dedifferentiate into filaments composed of both vegetative cells and N2-fixing cells, called heterocysts. Symbiotically associated Nostoc form heterocysts at frequencies significantly higher than those found in free-living filaments (10). These heterocysts release the majority of their fixed nitrogen to the plant (10). Various extracts and exudates from both symbiotic and nonsymbiotic plants have been shown to influence the development of Nostoc (2, 3, 8, 11). Aqueous ground extract of the symbiotically competent bryophte Anthoceros punctatus increases transcript levels of the hrm locus in wild-type Nostoc punctiforme (E. L. Campbell and J. C. Meeks, personal communication) and in strains containing luxAB fusions within the locus (3). Mutants in hrmU and hrmA have a phenotype of increased infection frequency of A. punctatus which correlates with an increased sensitivity to plant-activated hormogonium formation (3, 4). Although the role of these genes in hormogonium formation has not been fully elucidated, sequence analysis shows that the hrmU gene belongs to a family of NAD(P)H-dependent oxidoreductases, while hrmA has no identifiable sequence motifs (3).
In contrast to our extensive knowledge of the signaling mechanisms in the more specific Rhizobium-legume symbioses, none of the signaling molecules involved in Nostoc symbioses have been identified. Flavonoids secreted by legumes establish communication with Rhizobium by binding the transcriptional activator protein NodD (6), and flavonoids are also likely to have a role in plant symbioses with mycorrhizal fungi (13–15). Seed rinse from Gunnera, a symbiotic angiosperm host of Nostoc, has been shown capable of inducing expression of nod genes in Rhizobium, thus raising the possibility of common chemical signals among host plants (12). For this study, several flavonoids were screened for the ability to increase expression of luciferase from a hrmA-luxAB transcriptional fusion in mutant strain UCD 328, formed by transposition of Tn5-1063 into hrmA of N. punctiforme (3, 4).
Strain UCD 328 was cultivated in a Nostoc basal growth medium at 23°C under light with continuous shaking (5). In test tubes, cells were suspended to a concentration of 0.6 μg of chlorophyll (Chl) a in 2,475 μl of medium and incubated under growth conditions. After 30 min, a 25-μl solution of 95% ethanol with or, for controls, without dissolved flavonoid was added to each cell suspension. To assay for LuxAB luciferase activity at various time intervals, 1 ml of cell suspension was combined with 111 μl of a 3 mM decylaldehyde–1.4% ethanol solution in a 12- by 55-mm test tube, vortexed, and placed in a luminometer (Compactlumi VS500; Microtek Nichion, Shizuoka, Japan). Luminescence (relative light units) from the cells was monitored for 90 s following a 50-s delay. To normalize the values, the Chl a content of each assay tube was determined. Luciferase activities are reported relative to the activity of cells from parallel control experiments.
Flavanones, flavan-3-ols, kaempferol, and myricetin were purchased from Sigma Chemical Co. (St. Louis, Mo.); anthocyanins, rutin, and rhoifolin were from Extrasynthèse (Genay, France); quercitin and flavone were from Nacalai Tesque (Kyoto, Japan).
Induction of hrmA-luxAB expression by naringin.
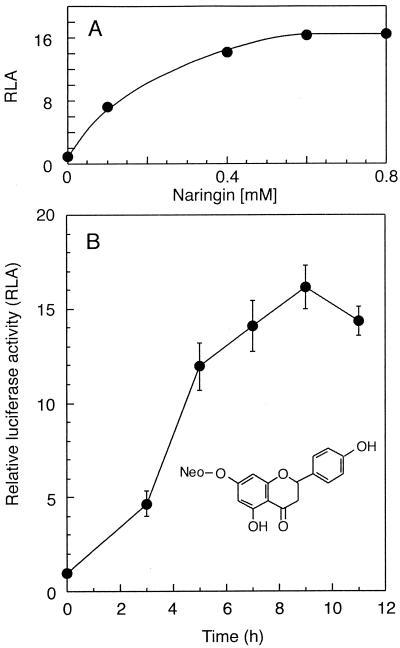
Cells of strain UCD 328 incubated with 0.4 mM naringin showed a peak 16.1 ± 1.1-fold (mean ± standard error; n = 7) increase in luciferase activity 9 h after addition of the flavonoid (Fig. 1). Near-maximal induction was reached with 0.6 mM naringin (Fig. 1). Compared to the reported induction of luciferase activity by A. punctatus extract in strain UCD 328 and other hrmA-luxAB fusion strains (3), the response induced by naringin peaks a few hours earlier at nearly double the intensity.
FIG. 1.
Effect of naringin on hrmA-luxAB in strain UCD 328. (A) Luciferase activity of cells incubated 7 h in various concentrations of naringin relative to the value for control cells incubated without naringin (means from at least three experiments). (B) Cells incubated in 0.4 mM naringin were assayed for luciferase activity at various time intervals; values are the means ± standard errors from at least four experiments. A two-dimensional structure of naringin is presented in the inset. Neo, neohesperidose.
Under the incubation conditions used for the assay, naringin had no discernible effect on the growth of strain UCD 328. Over a 2-day period, cells incubated with 0.4 mM naringin had a growth rate of 0.62 ± 0.03 d−1 (n = 5), identical to that of control cells, 0.62 ± 0.03 d−1 (n = 6).
Structural determinants of hrmA-luxAB-inducing activity.
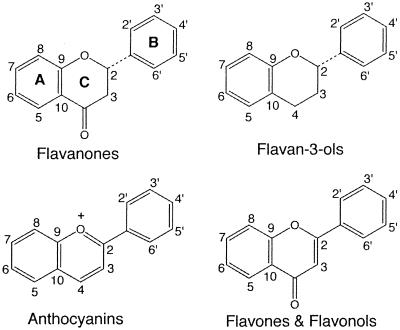
We assayed several other flavonoids in order to ascertain the structural specificity required for induction of hrmA. Flavonoids are characterized by a common three-ring structure whose A, B, and C rings are shown in Fig. 2. Subtle differences in the ring substitution pattern and localization of a flavonoid can have major effects on its function (9, 13, 16). Our survey included representatives from four flavonoid subclasses: the flavanones, the flavan-3-ols, the anthocyanins, and the flavones and flavonols, distinguished by differences in their basic structures (Fig. 2). Cells of strain UCD 328 were incubated for 7 h under growth conditions in 0.1 mM each compound before determination of luciferase activity. Rhoifolin, neohesperidin, and prunin were found to induce (Table 1) but at levels significantly lower than naringin (Fig. 3).
FIG. 2.
The basic three-ring structures of the flavonoids examined in this study. Positions of the A, B, and C rings are indicated.
TABLE 1.
Flavonoids assayed for hrmA-luxAB activity
| Flavonoid | Structurea | Inductionb |
|---|---|---|
| Flavanones | ||
| Narigenin | 5,7,4′-OH | + |
| Prunin | Naringenin-7-OGlc | + |
| Naringin | Naringenin-7-ONeo | + |
| Hesperitin | 5,7,3′-OH, 4′-OMe | − |
| Neohesperidin | Hesperitin-7-ONeo | − |
| Hesperidin | Hesperitin-7-ORut | − |
| Flavan-3-ols | ||
| (+)Catechin | (+)3,5,7,3′,4′-OH | − |
| (−)Epigallocatechingallate | 5,7,3′,4′,5′-(−)3-O-gallate | − |
| Anthocyanins | ||
| Pelargonidin | 3,5,7,4′-OH | − |
| Pelargonin-3-glucoside | Pelargonidin-3-OGlc | − |
| Cyandin | 3,5,7,3′,4′-OH | − |
| Flavones and flavonols | ||
| Flavone | − | |
| Rhoifolin | 5,4′-OH, 7-ONeo | + |
| Kaempferol | 3,5,7,4′-OH | − |
| Quercitin | 3,5,7,3′,4′-OH | − |
| Rutin | Quercitin-3-ORut | − |
| Myricetin | 3,5,7,3′,4′,5′-OH | − |
OGlc, O-glucose; ONeo, O-neohesperidose; ORut, O-rutinose.
See Fig. 3 for relative activities of inducing compounds.
FIG. 3.
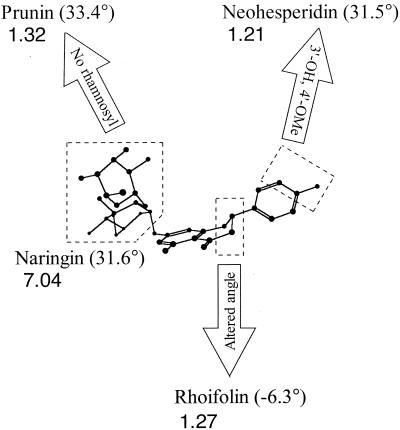
Three-dimensional view of naringin contrasting its structural differences with the flavonoids that displayed low hrmA-luxAB-inducing activity. Relative inducing activity is indicated below the name of each compound. The C-2 C-3 C-4 C-10 tetrahedral bond angles, in parentheses, were determined from the MM2-minimal energy conformations of the structures (Chem3D; Cambridge Software, Cambridge, Mass.). Dashed lines enclose regions of contrast between the flavonoids.
A comparison of naringin to rhoifolin shows the importance the C-ring conformation for hrmA-inducing activity. Naringin is a flavanone and therefore differs from rhoifolin, a flavone, in having a saturated C-2 C-3 bond. This confers a 37.9° difference in the C-2 C-3 C-4 C-10 tetrahedral bond angle of the otherwise identical compounds (Fig. 3). Rhoifolin gave a 1.27 ± 0.07-fold (n = 8) increase in luciferase activity, substantially lower than the 7.04 ± 0.26-fold (n = 4) increase induced by the same concentration of naringin (Fig. 3). Thus, the bent confirmation of the C ring found in naringin appears to favor hrmA-luxAB induction.
The substitution pattern of the B ring also influences the hrmA-inducing activity of naringin. The flavanone neohesperidin differs from naringin only in the B ring; naringin contains a single -OH at C-4′, whereas neohesperidin has a -OCH3 at C-4′ and a -OH at C-3′. Luciferase activity rose only 1.21 ± 0.07-fold in response to neohesperidin (Fig. 3). Therefore, since flavanones are known to interact with proteins (9), hydrogen bond formation between the 4′ -OH of naringin and a putative target protein in N. punctiforme may be involved in the hrmA induction process.
Further comparisons illustrate the importance of the A-ring sugar. Neohesperidose, the 7-O-dissaccharide of naringin, is a rhamnosyl-glucose. Prunin and naringenin differ from naringin only in their 7-O linkage. Interestingly, prunin (having a 7-O-glucose) induced only a 1.32 ± 0.07-fold increase in luciferase activity (Fig. 3), while naringenin (having a 7-OH) did not induce at all. Therefore, the terminal rhamnosyl moiety on the 7-O-glucose is another hrmA-inducing determinant of naringin.
No induction of hrmA-luxAB by any of the other 12 flavonoids listed in Table 1 was observed, nor did we observe induction by 34 additional compounds found in plants, including hydroxycinnamic acids, caffeine, tannic acid, riboflavin, ascorbate, sucrose, and oxalate (data not shown).
Physiological implications.
This report of hrmA-luxAB induction by naringin is the first direct evidence of a flavonoid influencing the gene expression of a cyanobacterium. Naringin is a relatively common flavonoid distributed among several plant families (1). It can accumulate in plants to near millimolar levels (7), concentrations that we have found to maximally induce hrmA. Although we have clearly shown strong hrmA induction by naringin, we do not consider that naringin will be the only molecule found to have this effect on Nostoc. Plants that establish symbioses with Nostoc might fine-tune the substitution pattern of their flavonoids in order to maximally exert control over their symbiont. However, knowledge of the flavonoid content in symbiotic hosts of Nostoc is limited. We have found that extract of Azolla, a symbiotically competent water fern, has a higher hrmA-luxAB-inducing activity than naringin (M. F. Cohen and H. Yamasaki, unpublished data). The identification of naringin as an inducer of hrmA should aid in elucidating the molecular mechanisms regulating Nostoc differentiation and provide important clues for the isolation of potential hrmA inducers from symbiotic plant partners including Azolla and Anthoceros. Such molecules could conceivably function in plant symbiotic cavities to prevent the formation hormogonia and thus permit higher frequencies of N2-fixing heterocysts (3).
Acknowledgments
We are indebted to Jack Meeks and Elsie Campbell for providing Nostoc strains and for critical reading of the manuscript. We thank Yojiro Takagi for assistance with the luciferase assays.
REFERENCES
- 1.Bohm B A. The minor flavonoids. In: Harborne J B, editor. The flavonoids: advances in research since 1986. London, England: Chapman and Hall; 1994. pp. 387–440. [Google Scholar]
- 2.Campbell E L, Meeks J C. Characteristics of hormogonia formation by symbiotic Nostoc spp. in response to the presence of Anthoceros punctatusor its extracellular products. Appl Environ Microbiol. 1989;55:125–131. doi: 10.1128/aem.55.1.125-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen M F, Meeks J C. A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Mol Plant-Microbe Interact. 1997;10:280–289. doi: 10.1094/MPMI.1997.10.2.280. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M F, Wallis J G, Campbell E L, Meeks J C. Transposon mutagenesis of Nostocsp. strain ATCC 29133, a filamentous cyanobacterium with multiple differentiation alternatives. Microbiology. 1994;140:3233–3240. doi: 10.1099/13500872-140-12-3233. [DOI] [PubMed] [Google Scholar]
- 5.Enderlin C S, Meeks J C. Pure culture and reconstitution of the Anthoceros-Nostocsymbiotic association. Planta. 1983;158:157–165. doi: 10.1007/BF00397709. [DOI] [PubMed] [Google Scholar]
- 6.Fisher R F, Long S R. Rhizobium-plant signal exchange. Nature. 1992;357:655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- 7.Fuhr U, Kummert A L. The fate of naringin in humans—a key to grapefruit drug interactions. Clin Pharmacol Ther. 1995;58:365–373. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 8.Gantar M, Kerby N W, Rowell P. Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria: III. The role of a hormogonia-promoting factor. New Phytol. 1993;124:505–513. doi: 10.1111/j.1469-8137.1995.tb04304.x. [DOI] [PubMed] [Google Scholar]
- 9.Grayer R J. Flavanoids. In: Harborne J B, editor. Plant phenolics. Vol. 1. London, England: Academic Press; 1989. pp. 283–323. [Google Scholar]
- 10.Meeks J C. Symbiosis between nitrogen-fixing cyanobacteria and plants. Bioscience. 1998;48:266–276. [Google Scholar]
- 11.Rasmussen U, Johansson C, Bergman B. Early communication in the Gunnera-Nostocsymbiosis: plant-induced cell differentiation and protein synthesis in the cyanobacterium. Mol Plant-Microbe Interact. 1994;6:696–702. [Google Scholar]
- 12.Rasmussen U, Johansson C, Renglin A, Petersson C, Bergman B. A molecular characterization of the Gunnera-Nostoc symbiosis: comparison with the Rhizobium-plant and Agrobacterium-plant interactions. New Phytol. 1996;133:391–398. [Google Scholar]
- 13.Shirley B W. Flavonoind biosynthesis: ‘new’ functions for an ‘old’ pathway. Trends Plant Sci. 1996;1:377–382. [Google Scholar]
- 14.Stafford H A. Roles of flavonoids in symbiotic and defense functions in legume roots. Bot Rev. 1997;63:27–39. [Google Scholar]
- 15.Xie Z-P, Staehelin C, Vierheilig H, Wiemken A, Jabbouri S, Broughton W J, Vögeli-Lange R, Boller T. Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans. Plant Physiol. 1995;108:1519–1525. doi: 10.1104/pp.108.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]