Abstract
An integrated approach involving vermicompost, chromate reducing bacteria and AMF was tested to manage the toxic impacts of Cr(VI) on Ocimum basilicum as a model plant. Pot experiments were conducted on O. basilicum plants in an artificially Cr(VI)-contaminated soil in two phases of experiment as bioinoculants experiment and vermicompost experiment. In the first phase of the bioinoculants experiment the series of gradient concentrations of Cr(VI) (0, 25, 50 and 100 mg kg–1 in soil) were evaluated with previously isolated four efficient Cr(VI)-reducing rhizo-bacterial strains (Bacillus Cereus strain SUCR 44, BC; Microbacterium sp. strain SUCR 140, MB; Bacillus thuringiensis strain SUCR186, BT; and Bacillus subtilis strain SUCR188; BS) along with Arbuscular Mycorrhizal Fungus—Glomus fasciculatum (GF) in alone and in co-inoculation form. In the second experiment (vermicompost) the best performing strain (MB) was tested alone or in combination with GF along with different doses of vermicompost. It was observed that vermicompost by itself could be useful in decreasing the bioavailable Cr(VI), uptake of Cr besides improving the nutritional status of plants. The vermicompost also played an important and indirect role and improved herb yield by supporting the multiplication of MB (Microbacterium sp.), an efficient chromate reducing rhizobacteria, that further decreased the bioavailable and toxic form of Cr and improved population and colonization of GF too. The translocation of Cr(VI) was averted through improved colonization of GF, also prevented higher accumulation of Cr in aerial parts (leafy herb) of O. basilicum.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00203-022-03230-z.
Keywords: Vermicompost, Glomus fasciculatum, Cr(VI), Cr(VI) reducing bacteria, Bioavailability
Introduction
The growing population and their demands for products are ever-increasing. To have a sustainable supply chain, countries are running for rapid industrialization. Various industries involves chromium Cr(VI) [Cr(VI)] uses in several industrial processes such as—in tanning, electroplating, ceramics, dyes, paints and pigments manufacturing, textile processing, metal finishing, wood processing and photographic sensitizer manufacturing units. The byproducts and effluents from these industries are serious threat to the environment and living being. In aqueous phase Cr(VI) bears structural similarity with SO42−, entered into cells via sulfate transport pathways (Patra et al. 2010), rapidly reduced generates free radical (Mabbett and Macaskie 2001). Due to this, it is noxious (Wise et al. 2004), mutagenic (Puzon et al. 2002), carcinogenic (Codd et al. 2003), and teratogenic (Asmatullah et al. 1998) besides causing DNA break (Mabbett and Macaskie 2001) and altered gene expression (Bagchi et al. 2002). Due to this, it is renowned as a precedence pollutant (Cheung and Gu 2007). The adverse health effects associated with Cr(VI) exposure includes respiratory cancer, asthma, skin, nose and eye irritation, chrome ulcers, perforated eardrums, kidney and liver damage, pulmonary congestion, erosion and discoloration of the teeth (USEPA 1998). The median lethal dose of Cr(VI) is 50–150 mg/kg (Katz and Salem 1992). The World Health Organization recommends a maximum allowable concentration of 50 μg/L of Cr(VI) in drinking water (WHO 1996). Although Cr(VI) is highly toxic but its trivalent form, i.e., Cr(III) is an essential micronutrient for animal and human being, involved in protein and glucose metabolism (Vincent 2000), enzyme stimulation (Karuppanapandian et al. 2009), nucleic acid stabilization (Snow and Xu 1991) and is relatively inert and much less toxic (Krishna and Philip 2005).
In underdeveloped and developing countries, the lenient regulations on effluents and discharges from these industries are of great concern (Patra et al. 2010) and even posing serious threats to agricultural soils (Gupta et al. 2019) if discharged in surrounding to it. The continuous discharge and uses of contaminated water or source for irrigation effected by it (Jiménez 2006; Abaidoo et al. 2010) further deteriorates the situation leading to heavy crop yield losses. Once the Cr(VI) entered to the plant system, it gets magnified inside the plant and its products remains unfit to be used due to the concentrations crossing the permissive limits fit to be used (Vernay et al. 2007; Soni et al. 2014a, b; Rai et al. 2002; Oliveira 2012). Excessive Cr(VI) accumulation itself, causes severe toxicity to plants leading to altered metabolic processes, impaired photosynthesis, hampered nutrients uptake, inactivation of mitochondrial electron transport, chlorosis, and membrane damage resulting in reduced root growth, stunting and finally leading to plants death (Soni et al. 2014a, b; Shanker et al. 2005).
Globally a large population (80%) still relay on herbal products, supplements for their primary healthcare and immune boosting (Ekor 2014; Soni et al. 2022). In the time of COVID-19 pandemic, demands and usage of herbal supplements and drugs are ever-increasing (Soni et al 2021, 2022). Particularly in Indian subcontinent, masses still relay on traditional system of medicine obtained directly from medicinal plants for maintenance of their day-to-day life and immune boosting (Soni et al. 2021). The medicinal plants contain high economical value secondary metabolites which either directly or indirectly used as raw materials for pharmaceutical, cosmetic and perfumery industries (Tiwari et al. 2017, 2021). Therefore, there is a continuous surge in the demands of raw materials and herbs and herbal products. It is evident that global warming and climate change have impacted on land and water availability for humans and agriculture. In the present scenario safe water management is almost challenging issue, and it is highly recommended to reuse water for agricultural purposes (Abaidoo et al. 2010). Farmers with no option left, when uses such water for irrigation (Jiménez 2006) or either contaminated sites for cultivation severely impacts plant growth/yield and economy. Not only this if the plants or products from affected areas are used they raise safety issues due to magnified accumulated metals (Zheljazkov and Nielsen 1996a, b). The safety concerns of medicinal herbs-based drugs adulterated with metals beyond permissible limits are not new (Rai et al. 2001; Saper et al. 2008; Dargan et al. 2008; Breeher et al. 2013) and attention has been raised from time to time. Looking from the point of remediation many researchers tried aromatic plants as a remedial solution which has some positive aspects, because of their by-products basically essential oils, remains safe from adulteration of Cr(VI) (Gupta et al. 2013). Therefore, they can be a wise option to look into as a part of integrated management system for Cr(VI) remediation in contaminated sites with usable products obtained without adverse losses (Gupta et al. 2013).
This is well established that plant rhizospheric zone has enriched beneficial microbes which can positively interact with root system and forms first line of defences against both biotic (phytopathogens) and abiotic stresses (Tiwari et al 2017). They not act antagonist microbes but also help in mitigating abiotic stresses such as detoxifying metal stress or hazardous compounds present in the vicinity (Burd et al. 2000; Rajkumar and Freitas 2008). The Cr(VI) stress management thus can be achieved via enriching Cr(VI) tolerant microbes (Soni et al. 2014a, b; Wani et al. 2009) which might have other plant beneficial properties such as—plant growth promoting (PGP) potentials, such as nitrogen fixation, phosphate solubilization, siderophore and growth hormones production (Gupta et al. 2019; Glick et al. 1999; Khan and Zaidi 2007). These microbes adapt either enzymatic strategy or manage metal stress via production of metabolites (Losi and Frankenberger 1994), or even may be via accumulation and sequestration (Mamaril et al. 1997). Therefore, isolation and exploration of such microbes have coupled with broad range of potentials, i.e., PGP, or soil remediation will be a better safe management strategy for sustainable agro-technologies under heavy metal stress (Rajkumar et al. 2005, 2006).
The beneficial role of mycorrhiza spans around enhancing plant health, nutrition, quality by stimulating the synthesis of secondary metabolites/bioactive compounds (Singh et al. 2013a, b, c; Tiwari et al. 2017). They also play important role in tolerance to abiotic (Vivas et al. 2006) and biotic stresses (Saikia et al. 2013; Gupta et al. 2014), or even enhance antioxidant activities (Gianinazzi 2008, 2010). Their co-inoculation with beneficial bacterium further improves plant growth, establishment, stress tolerance and mitigation of heavy metals under metal-contaminated conditions (Vivas et al. 2003, 2005, 2006) including Cr stress mitigation too (Soni et al. 2014a). Mycorrhiza has been regarded to help in mitigating metal stress by immobilizing the metals or sorting the metals and thus lowering the toxicity of metal toxicity in plants (Gother and Paszkowski 2006).
Vermicompost is a finely divided peat-like material mainly originates from organic wastes. They when mixed with soil provides better porosity, aeration, drainage and have provides good moisture content because of its high capacity of water holding (Edwards and Burrows 1988). They are highly recommended for the growers and farmers as they stimulate plant growth and development and provides better coping with abiotic stresses to the plants. (Pande et al. 2007; Jadia and Fulekar 2008; Koka et al. 2019). Even it is evident from the research (Kozˇuh et al. 2000) that humic acid and fulvic acid present in vermicompost have potentials to reduce Cr(VI) stress in plants (Chaab et al. 2016). Experimental evidence from our previous studies also revealed that vermicompost acts as a proliferating substrate for the applied microbes and enhances growth and development (Kalra et al. 2010; Singh et al. 2012a, 2013b).
Sweet basil (Ocimum basilicum L. (family Lamiaceae), is an annual herb and considered as an important medicinal and aromatic plant due to its marvelous beneficial potentials (Grayer et al.1996; Tiwari et al. 2017).They have been traditionally used in many kinds of herbal drug preparations, supplementary treatments for stresses, aches, inflammations, asthma and diabetes (Telci et al. 2006). Their leaves contain an essential oil used as flavorings agents in foods and beverages, as fragrances, as fungicides, or insecticides and in various pharmaceutical and industrial products (Singh et al. 2013b; Tiwari et al. 2017, 2021). Because of its high economical value and industrial importance, sweet basil is highly acclaimed commercial crop in many countries, including Indian subcontinent (Padalia et al. 2017). With the multifaceted potentials and aromatic nature of the crop we selected this as a model plant for Cr (VI) stress studies. We designed the experiments to evaluate them under Cr (VI) stress and their impacts on yield attributes. Furthermore, we looked for an integrated method of management towards minimizing economical losses due to Cr (VI) stress. Considering the Cr reducing and chelating properties of vermicompost, we undertook this study to establish the integrated potentials of vermicompost along with MB and GF in synergizing the chromate reduction activity and Cr toxicity in plants. We also were interested to look into, if GF could participate in improved mycorrhization and nutrient uptake. We hypothesize that microbe based remediation of metal stress will provide safe and wiser stress management strategies which can be employed for future agro-technologies. This study further will pave new avenues for search of efficient microorganisms that can be employed for metal stress mitigation in different crops and plants without much impacting the product. The outcomes from this study will generate a consortium for better management of Cr (VI) stress in in-planta, as well as in soils too. Furthermore, it will contribute to higher yields thus lowering the economical losses in O. basilicum when cultivated under Cr (VI) stress.
Materials and methods
Preparation of soil samples with artificial Cr(VI) contamination
A set of experiments were conducted which were divided into two phases—phase 1—bioinoculants experiment, where we screened different microbial treatments in single and in combination for their potentials of Cr(VI) stress management and plant growth promotion. While the second phase of experiment (vermicompost experiment) dealt with integrated approach involving vermicompost as a substrate having self-potentials for Cr(VI) stress reduction, as well as supporting proliferation of microbes and indirectly contributing in the Cr(VI) management and toxicity reduction. The artificial contamination of soil with Cr(VI) was performed by following the previously described method (Papassiopi et al. 2009). In the bioinoculants experiment, respective concentrations of artificially Cr(VI)- (25 mg kg–1, 50 mg kg–1, and 100 mg kg–1) contaminated soil (autoclaved) were prepared for the pot experiment. In the second experiment (trial with vermicompost), the most effective known concentration of Cr(VI) (100 mg kg–1 soil) was mixed in soil containing different doses of sterilized vermicompost (0 t ha–1, 2.5 t ha–1, 5 t ha–1, and 10 t ha–1).
The left over de-oiled herb distillation waste from aromatic plants named such as Mentha arvensis, Cymbopogon flexuosus and C. winterianus were used for production of vermicompost. The vermicompost was produced at the vermicomposting unit housed at Microbial Technology Department of CSIR–CIMAP, Lucknow, U.P India, using earthworm, Eudrilius eugineae. The leftover plant herb mixture was finely chopped and fed to the vermicomposting unit. The unit was regularly monitored for proper moisture content for 90 days to achieve proper degradation and conversion into vermicompost by Eudrilius eugineae (Kalra et al. 2010; Singh et al. 2012c, 2013b). The vermicompost thus produced was analyzed for constituents and contained 1.10% N, 0.62% P and 0.76% K.
Plant material and growth conditions
The experiments were performed under the controlled glasshouse conditions with optimum temperature range (30–40℃), moisture content (60–70%), and photoperiod [Light (14 h)/Dark (10 h)]. The sandy loam soil (Ustifluvent) was used for this experiment having following characteristics [pH 7.26, EC 0.42 dSm–1, organic carbon 3.37 g kg–1, available N (alkaline permanganate extractable) 183 kg ha–1, available P (0.50 M NaHCO3 extractable) 16.3 kg ha–1, and available K (1 N NH4OAc extractable) 94 kg ha–1].
Preparation of bio-inoculums
Cr(VI) reducing bacterial inoculums
The bacterial culture and their vermicompost based inoculum were produced as per the protocols described earlier (Soni et al. 2014a, b). At the time of application, the population of bacterial strains was maintained at 2.0–2.4 × 109 CFU g–1/vermicompost. A five gram of such vermicompost based inoculum was applied to each pot by placing near to the seedlings rhizospheric vicinity.
Glomus fasciculatum (GF) inoculum
The inoculum of Glomus fasciculatum (GF) species was obtained from repository of Microbial Culture Collection housed at CSIR–CIMAP, Lucknow, India. The propagation and application of GF was done by following the methods as described by Singh et al. (2012b, 2013b). At a time of application to the pot experiments, the inoculum concentration was adjusted to 7.0 ± 0.5 spores g–1.
Seed treatment
Seeds of O. basilicum cv. CIM-Saumya were procured form CSIR–CIMAP farm center (Lucknow, India), were sorted for healthy looking ones. They were surface sterilized with application of sodium hypo-chloride (1%) for 5 min followed by 3 times washing with sterilized deionized water. The seeds were transferred to the earthen pan containing soil—vermicompost mixture (both autoclaved, 10:1 v/v) and placed under glasshouse conditions as described previously under dark conditions till germination. Furthermore, the germinated seeds were allowed to raise to 2 to 3 leaf staged seedlings. The seedlings were used for pot experiments under glasshouse conditions as mentioned before.
Experimental design
The experiment was set up in a completely randomized design (CRD) with three replications per treatment (Table 1). Two seedlings were transplanted in each pot (10 inch diameter containing 8.5 kg sterilized potting mixture) and allowed to get established. After 1 week, pots were homogenized by removing the extra one plant and leaving behind one plant per pot. The optimum moisture level in soil was maintained by application of sterilized water at regular intervals. The experiments were repeated twice at different time intervals under similar set of glasshouse conditions as described previously with 3 biological replications per treatment.
Table 1.
Experimental design and microbial treatments of the pot experiments conducted in controlled conditions
| S. no. | Treatments | Abbreviation used | Description | Bioinoculants experiment | Vermicompost experiment |
|---|---|---|---|---|---|
| 1 | *Control | CN | Uninoculated | Gradient concentration of Cr(VI) (0, 25, 50 and 100 mg kg–1 in soil) were taken | Gradient doses of vermicompost (0 t ha–1, 2.5 t ha–1, 5 t ha–1, 10 t ha–1) were taken at 100 mg kg–1 Cr(VI) in potting mixture |
| 2 | *Glomus fasciculatum | Gf | Gf inoculated | ||
| 3 | Bacillus cereus strain SUCR44) | BC | BC inoculated | ||
| 4 | *Microbacterium sp. strain SUCR140 | MB | MB inoculated | ||
| 5 | Bacillus thuringiensis strain SUCR186 | BT | BT inoculated | ||
| 6 | Bacillus subtilis strain SUCR188 | BS | BS inoculated | ||
| 7 | Bacillus cereus strain SUCR44 + Glomus fasciculatum | BC + GF | BC + GF inoculated | ||
| 8 | *Microbacterium sp. strain SUCR140 + Glomus fasciculatum | MB + GF | MB + GF inoculated | ||
| 9 | Bacillus thuringiensis strain SUCR186 + Glomus fasciculatum | BT + GF | BT + GF inoculated | ||
| 10 | Bacillus subtilis strain SUCR188 + Glomus fasciculatum | BS + GF | BS + GF inoculated |
*Treatments were selected for Experiment-II
Harvesting and biomass measurements
After completion of 75 days from sowing of seeds the harvesting of the plants were manually performed using sickle. The plants were separated from root and scaled using measuring balance. Th roots were properly washed to remove adherent Cr(VI) on surface of the roots. The length of both root and shoot were measured using meter scale. Biomass (dry weight) was estimated separately for root and shoots from oven dried (at 70 °C till a constant weight was obtained) samples. At a time of harvesting, the soil samples were also collected and preserved at 4 °C till the analysis of microbial populations was conducted.
Determination of bioavailable Cr(VI) [Soluble Cr(VI)]in soil
Bioavailable Cr(VI) in the soil was measured by following the methods of Rtidel and Terytze (1999). A cocktail of soil (10 g), phosphate buffer (48 ml, 0.1 M, pH 8.0), Al2(SO4)3 (1 ml, 0.4 M) and Na2SO3 (1 ml, 1 M) was prepared and shaked (30 min at 250 rpm). Afterwards, 10 mL filtrate (using 0.45 µm filter) was taken and mixed with DDW (20 ml) and NaOCl (1 ml) followed by NaCl (5 g) and H3PO4 (1 ml, 7 M) were added. The whole solution was then transferred in a 50 ml volumetric flask. Furthermore, 1 mL of diphenylcarbazide (DPCZ) solution was added and the flask was filled up to the mark with water. After 10 min of incubation the absorbance was recorded at 540 nm. A separate 10 ml of soil filtrate treated in the same manner with only 1 ml of acetone instead of DPCZ solution was used as blank.
Estimation of Cr in plants
The total Cr concentration in root and shoot samples was determined using atomic absorption spectroscopy (Perkin Elmer), using a method previously described by Soni et al. (2014a, b). The pulverized plant samples (200 mg) from each treatment were taken in Teflon tubes containing 10 ml of digestion mixture [Concentrated HNO3 and HF (2:1, v/v)]. The samples were digested using microdigester (Analytik Jena, Germany) at 200 °C and 200 bar pressure for 75 min (Soni et al. 2014a, b). After digestion, the samples were allowed to cool and filtered through Whatmann (no. 1) filter in a 25 ml of measuring conical flask and the volume of filtrate was made to 25 ml using deionized water. Total Cr content of the digested samples was determined by AAS (Perkin Elmer).
Cr uptake in each plant parts (root and shoot) was calculated by bioaccumulation factor (BAF) formula (Ghosh and Singh 2005).
The translocation efficiency, i.e., ratio of Cr in shoot and root tissue was calculated by translocation factor (TF) formula (Tappero et al. 2007).
Microbial population and NPK estimation
The abundance of Gf population in Ocimum roots were recorded by following a method described by McGonigle et al. (1990). Wet sieving and a decanting method were used for isolation and estimation of AM fungal spores from soil (Bharti et al. 2013). The populations of bacterial strains were determined using a previously described method by Soni et al. (2014a, b). The serial dilution method was used for estimation of microbial population of the soil samples. The samples were serially diluted in 0.85% saline. 100 µl of such diluted sample were taken and spread on nutrient agar culture plates [supplemented with 100 mg l–1 of Cr(VI)]. Thereafter, plates were incubated at 28 °C. For population estimation, the bacterial colonies developed on culture plates were counted using colony counter (Himedia India).The analysis of uptake of NPK in plant samples was performed according to the procedure of Jackson (1973).
Statistical analysis
The statistical analysis of collected data was performed by analysis of variance (ANOVA), assess through β-version of software ASSISTAT 7.6 (http://www.assistat.com). The significant differences among treatments were based on the F test in ANOVA, and means were calculated using Duncan’s multiple range test (DMRT) with a significance level of P ≤ 0.05 and P ≤ 0.01. The standard error mean (SEM) in vertical bar chart was computed using Sigma plot 10 (systat software, Inc.).
Results
Effects on plant growth and yield
Bioinoculants experiment
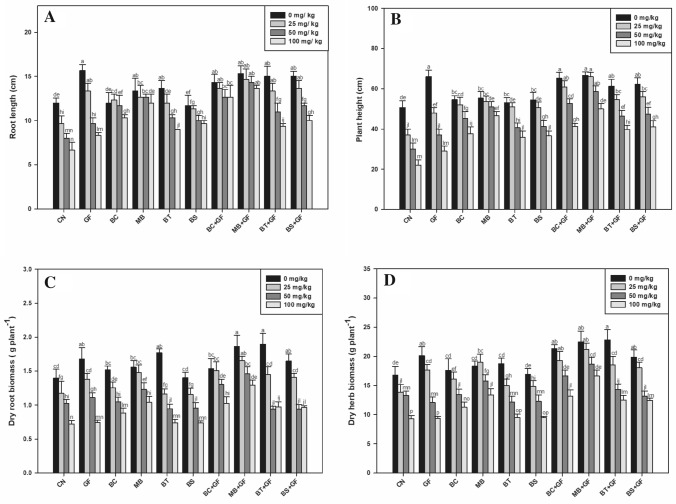
The plants treated with a gradient concentration of Cr(VI) showed an inverse relationship with plant growth parameters. The toxic impacts of Cr(VI) were observed among all the treatments; however, in comparison to control, the bioinoculants treated showed improvements in growth yield attributes (Tables S1–S9, supplementary files). The maximum reduction in plant growth was observed in plants grown in the soil supplemented with 100 mg kg–1of Cr(VI) concentration. This concentration of Cr(VI) (100 mg kg–1 of soil) was found to be highly toxic to the plants as the length of the roots, plant height, dry root and shoot biomass were significantly reduced (44.44%, 56.57%, 48.21% and 44.71%, respectively) as compared to the plants not treated with Cr (Fig. 1). However, the application of chromate resistant and reducing strains showed a significant (at P ≤ 0.05) improvement in plant growth parameters; maximum beneficial impacts were found with MB. These effects were more pronounced when these strains were co-inoculated with GF. The co-inoculation of MB + GF showed maximum increase in plant growth and yields. As compared to control, co-inoculation of MB + GF showed 105%, 127%, 79% and 78% increase in root length, plant height, and dry root and shoot biomass, respectively, at 100 mg kg–1 concentration of Cr(VI). In this regard the potentials of MB (Microbacterium sp.) alone and in combination with GF showed a promising response in reducing chromate induced damage although even under severe Cr(VI) stress (100 mg kg–1). Thus, we selected these combinations of treatments for further evaluation under integrated approach of Cr(VI) stress management with vermicompost supplementation (vermicompost experiment—Phase 2).
Fig. 1.
Effect of bioinoculants on (A) root length, (B) plant height, (C) dry root biomass, and (D) dry herb biomass of Ocimum basilicum in graded level of Cr(VI) amended soil (0, 25, 50, 100 mg/kg). Error bars shown as standard error of mean (SE), different letters above the error bars show significant difference at P ≤ 0.05
Vermicompost experiment
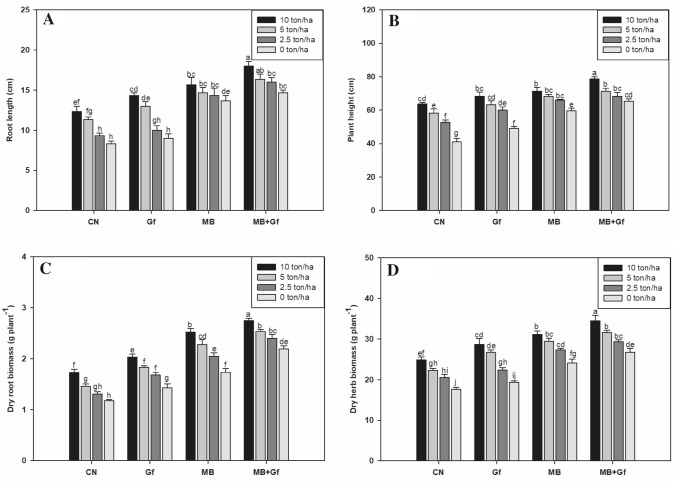
The effect of MB based on its performance in the phase 1-bioinoculants experiment (detailed results are included in Tables S1–S9, supplementary files), was selected and evaluated alone and in combination with GF in the pots supplemented with gradient concentration of vermicompost. It was found that with increase the dose of vermicompost the toxicity of Cr(VI) was mitigated thus supported growth and yield of plants (Tables S10–S18, supplementary files). The maximal increment was observed when supplemented with 10 t ha–1 vermicompost. The treatment improved length of the roots, plant height, dry root and shoot biomass increased by 48%, 52%, 47%, and 41%, respectively, as compared to the plants not treated with vermicompost. The co-inoculation of MB + GF in presence of 10 t ha–1 of vermicompost have much better mitigation of toxic effect of Cr(VI) than compared to vermicompost alone. The co-inoculation along with vermicompost improved the root length (116%), plant height (92%), dry root biomass (133%) and dry shoot biomass (96%) of O. basilicum as compared to the plants not treated with either microbes as well as vermicompost alone (Fig. 2).
Fig. 2.
Effect of bioinoculants on (A) root length, (B) plant height, (C) dry root biomass, and (D) dry herb biomass of Ocimum basilicum in Cr(VI) amended soil (100 mg kg–1) with graded level of vermicompost doses (0, 2.5, 5.0 and 10 ton/ha). Error bars shown as standard error of mean (SE), different letters above the error bars show significant difference at P ≤ 0.05
Effect on Cr bioavailability in soil
Bioinoculants experiment
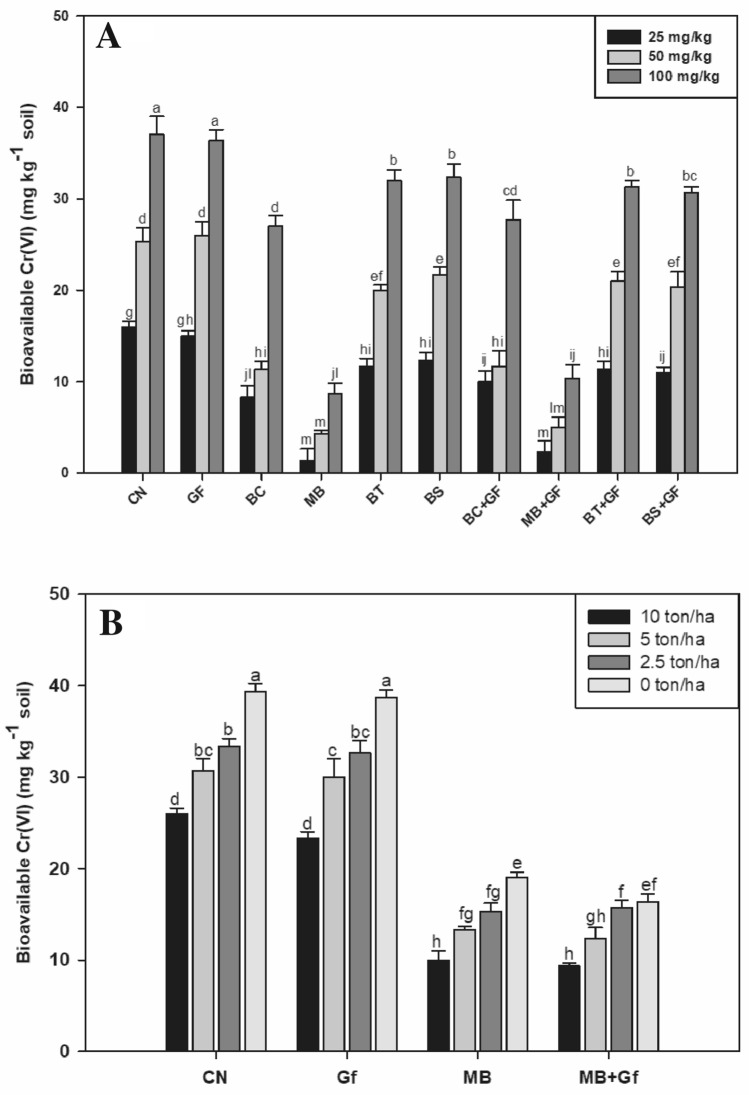
At time of harvesting the bioavailable Cr(VI) was about 37 mg kg–1 (at 100 mg kg−1) in the control pots of both phases of experiments (bioinoculants and vermicompost) (Fig. 3a, b). We found that the inoculation of bacterial strains sufficiently reduced bioavailable Cr(VI) in soil, whereas no such effects were noticed in pots inoculated with GF only. It was also found that no further reduction was observed in bioavailable Cr when bacterial strains and GF were co-inoculated. Considering the control value as 100%, the bioavailability of Cr was reduced by 24% and 28% with the treatments of MB and MB + GF co-inoculations, respectively; although statistically at par with each other.
Fig. 3.
A Effect of bioinoculants on Cr(VI) bioavailability of Ocimum basilicum in graded level of Cr(VI) amended soil (0, 25, 50, 100 mg/kg). B Effect of bioinoculants on Cr(VI) bioavailability of Ocimum basilicum in Cr(VI) amended soil (100 mg kg–1) with graded level of vermicompost doses (0, 2.5, 5.0 and 10 ton/ha)
Vermicompost experiment
The consistent reduction of bioavailable Cr(VI) was observed with increased vermicompost doses and maximum reduction was noticed at highest doses of vermicompost application (10 t ha–1). We found a 34% higher reduction in bioavailable Cr(VI) was observed when compared to the pots untreated with vermicompost.. The inoculation of MB alone in co-inoculation further showed in reduction of bioavailable Cr(VI). As compare to the control, we found 75% and 76% higher Cr reduction in the treatments of MB and MB + GF, respectively; however, no significant effects of GF alone or in combination with MB were noticed on reduction in bioavailable Cr (Fig. 3b).
Effect on Cr uptake in Ocimum basilicum
Bioinoculants experiment
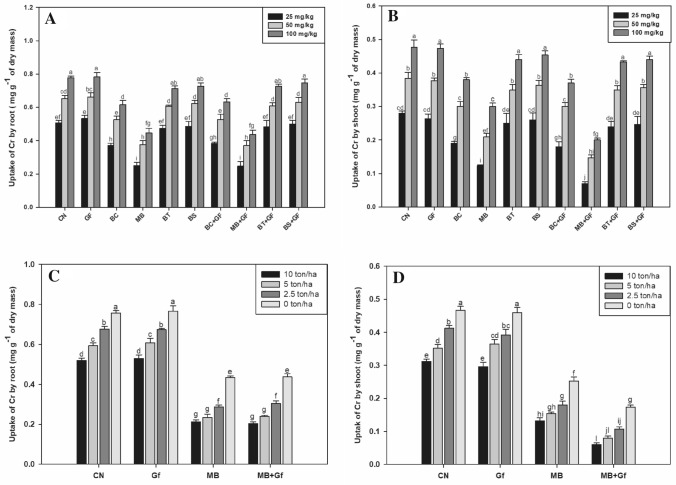
The uptake of Cr was significantly higher in control plants as compared to that of the plants treated (bacterial inoculums) ones. We observed that as compared to control, the maximum reduction in Cr uptake in roots were observed in plants inoculated with MB alone or in combination with GF; although presence of GF did not made any significant differences in uptake of Cr(VI) in plant roots. While we found that the Cr(VI) uptake was significantly lower in shoots when inoculated together with MB (Fig. 4a, b). Considering the uptake of Cr in control as 100%, the uptake of Cr in roots at 100 mg kg–1 of Cr(VI) was decreased almost by 42% and 44% in the corresponding treatments of MB and MB + GF. Likewise, 37% and 58% decrease were found in Cr uptake by aerial part of plants in aforesaid treatment was observed, respectively (Fig. 4a, b).
Fig. 4.
Effect of bioinoculants on (A) uptake of Cr by root and (B) uptake of Cr by shoot of Ocimum basilicum in graded level of Cr(VI) amended soil (0, 25, 50, 100 mg/kg). Effect of bioinoculants on (C) uptake of Cr by root and (D) uptake of Cr by shoot of Ocimum basilicum in Cr(VI) amended soil (100 mg kg–1) with graded level of vermicompost doses (0, 2.5, 5.0 and 10 ton/ha). Error bars shown as standard error of mean (SE), different letters above the error bars show significant difference at P ≤ 0.05
Vermicompost experiment
In vermicompost experiment, we found that with increased gradient of vermicompost the Cr uptake by plant roots and shoot system were proportionally decreased. It was observed that maximum impact was seen at the highest doses (10 t ha–1) of vermicompost (31% and 33%, respectively). Furthermore, we observed 27% decreased Cr uptake in the plants inoculated with MB + GF treatment. Likewise, we also found that there was 73% lower Cr uptake in the aerial part of plants of the same mentioned treatment (Fig. 4c, d).
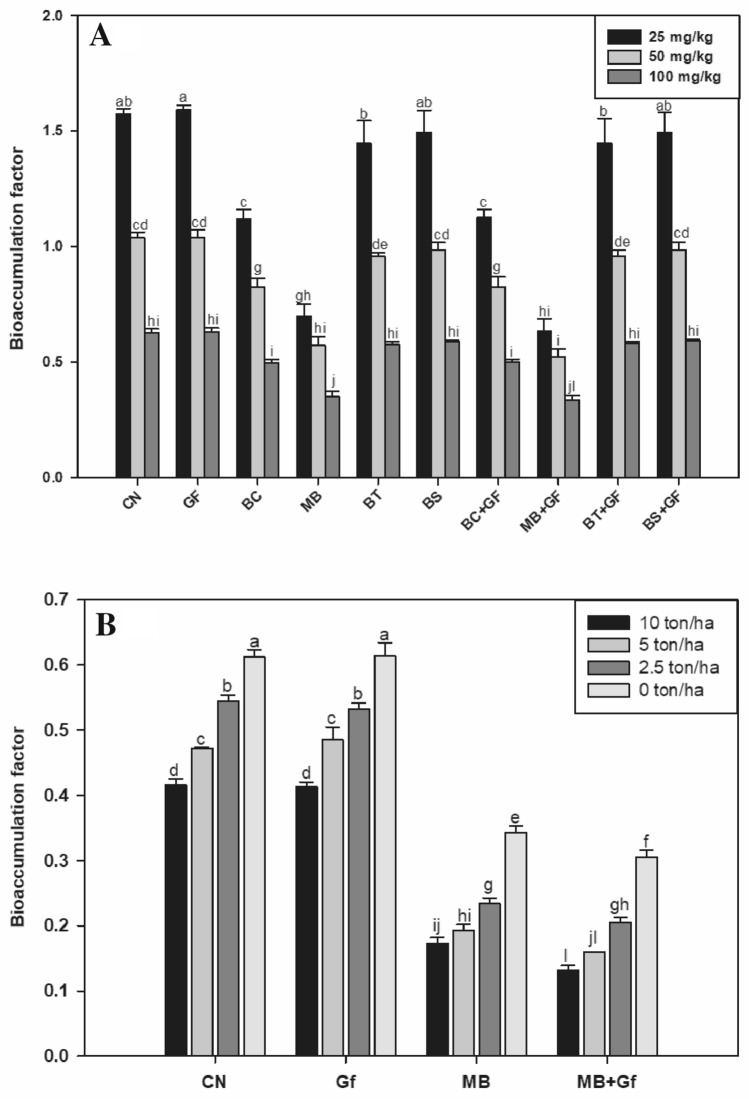
Effect on BAF
Bioinoculants experiment
The data, related to BAF for root and shoot, are presented in Fig. 5. The minimum bioaccumulation of Cr was observed in MB and MB + GF treated plants, although both at par statistically. Considering the control value as 100%, the Cr accumulation by MB + GF and MB was found to be reduced by 45% and 42% in roots, respectively, at 100 mg kg–1 of Cr(VI) (Fig. 5a).
Fig. 5.
A Effect of bioinoculants on bioaccumulation factor in graded level of Cr(VI) amended soil (0, 25, 50, 100 mg/kg). B Effect of bioinoculants on bioaccumulation factor in Cr(VI) amended soil (100 mg kg–1) with graded level of vermicompost doses (0, 2.5, 5.0 and 10 ton/ha)
Vermicompost experiment
The inverse relationship between BAF and vermicompost doses was noticed. From vermicompost Experiment, it was observed that, at highest doses of vermicompost there was 32% less Cr accumulation observed as compared to the plants not treated with vermicompost. Co-inoculation of MB with Gf considerably decreased (78%) the Cr accumulation in plants (Fig. 5b).
Effect on translocation factor
Bioinoculants experiment
The TF values < 1, suggested restricted transport of Cr from root to shoot. At 25 mg kg–1 of Cr(VI) supplementation, a significant decrease in Cr transportation was observed to the plants inoculated either alone with MB or in co-inoculated form (MB + GF). Further augment of Cr(VI) concentration in soil, had no significant decrease in transportation efficiency among all treatment except in MB + GF inoculated plants. We observed that at higher concentration both MB and GF alone are not effective in restricting the translocation, but the plants treated in co-inoculated form showed relatively lower transport of Cr to the aerial parts. As compared to control, a 29% reduced translocation of Cr from root to aerial parts were found in MB + GF treated plants (100 mg kg–1 of Cr(VI) (Fig. 6a).
Fig. 6.
A Effect of bioinoculants on translocation factor in graded level of Cr(VI) amended soil (0, 25, 50, 100 mg/kg). B Effect of bioinoculants on translocation factor in Cr(VI) amended soil (100 mg kg–1) with graded level of vermicompost doses (0, 2.5, 5.0 and 10 ton/ha)
Vermicompost experiment
The transportation of Cr from root to shoot was also not impacted by MB and GF treatments alone in respite of vermicompost doses. However, we found a reduced transport of Cr in plants inoculated with MB + GF. A maximum of 52% reduced Cr transport was observed in the combined treatment of MB + GF along with vermicompost dose of 10 t ha–1. Nevertheless, we also found that vermicompost at all the applied doses alone did not produce any impact on the translocation of Cr from roots to shoots (Fig. 6b).
Effect on uptake of NPK
Bioinoculants experiment
Data related to uptake of NPK are shown in Table 2. We found maximum uptake of N was observed in MB + GF inoculated plants. There was an increment in N uptake by 32% as compared to that of non-inoculated control. Similar trends were observed in uptake of P (38%) and K (19%) of the same treatments (Table 2).
Table 2.
NPK analysis of plant samples of Ocimum basilicum (bioinoculants experiment)
| Treatments | Uptake of NPK by plants (%) | |||
|---|---|---|---|---|
| 0 mg kg‒1 | 25 mg kg‒1 | 50 mg kg‒1 | 100 mg kg‒1 | |
| Nitrogen (N) | ||||
| CN | 1.892de# | 1.791hi | 1.630no | 1.537p |
| GF | 2.138a | 1.849fg | 1.659 mn | 1.573op |
| BC | 1.942bc | 1.949bc | 1.709jl | 1.687lm |
| MB | 2.053ab | 2.009ab | 1.929bc | 1.953bc |
| BT | 1.994ab | 1.820gh | 1.646mn | 1.607no |
| BS | 1.879ef | 1.756ij | 1.656mn | 1.617no |
| BC + GF | 2.063ab | 2.052ab | 1.934bc | 1.897cd |
| MB + GF | 2.150a | 2.085ab | 2.083ab | 2.023ab |
| BT + GF | 2.120a | 2.043ab | 1.683lm | 1.633no |
| BS + GF | 1.841fg | 1.871ef | 1.680lm | 1.643no |
| Phosphorous (P) | ||||
| CN | 0.756gh | 0.738i | 0.661mn | 0.581q |
| GF | 0.905b | 0.793ef | 0.690jl | 0.612p |
| BC | 0.868c | 0.778fg | 0.701j | 0.628op |
| MB | 0.860c | 0.805e | 0.709j | 0.649no |
| BT | 0.838d | 0.753hi | 0.676lm | 0.615p |
| BS | 0.803e | 0.757gh | 0.672lm | 0.615p |
| BC + GF | 0.926ab | 0.802e | 0.767gh | 0.675 lm |
| MB + GF | 0.941a | 0.831d | 0.807e | 0.801e |
| BT + GF | 0.947a | 0.766gh | 0.741i | 0.652 mn |
| BS + GF | 0.912b | 0.767gh | 0.745hi | 0.654 mn |
| Potassium (K) | ||||
| CN | 2.080gh | 2.033hi | 1.920pq | 1.873q |
| GF | 2.353a | 2.160de | 1.950 mn | 1.900q |
| BC | 2.230cd | 2.210de | 2.010jl | 1.933op |
| MB | 2.223de | 2.220de | 2.160de | 2.043hi |
| BT | 2.127ef | 2.057gh | 1.947no | 1.887q |
| BS | 2.120fg | 2.053gh | 1.967lm | 1.873q |
| BC + GF | 2.350a | 2.210de | 2.073gh | 2.080gh |
| MB + GF | 2.377a | 2.370a | 2.327ab | 2.237bc |
| BT + GF | 2.340a | 2.123ef | 2.017ij | 1.890q |
| BS + GF | 2.333ab | 2.097gh | 2.017ij | 1.917pq |
#Value showed in each column followed by different letters is significantly different at P ≤ 0.01 and the column value is the mean of four replicates
Vermicompost experiment
The application of vermicompost doses increased the uptake of NPK content and as expected it was maximum at doses of 10 t ha–1 of vermicompost. The nutrient uptake (N, P and K) uptake improved significantly (14%, 19% and 8%, respectively) as compared to the plants not treated with vermicompost. The consortia of MB + GF further improved N, P and K content in O. basilicum an increment of 36%, 27% and 17% uptake in N, P and K was observed, respectively, as compared to that of control (Table 3).
Table 3.
NPK analysis of plant samples of Ocimum basilicum (vermicompost experiment)
| Treatments | Uptake of NPK by plants (%) | |||
|---|---|---|---|---|
| 0 t ha‒1 | 2.5 t ha‒1 | 5 t ha‒1 | 10 t ha‒1 | |
| Nitrogen (N) | ||||
| CN | 1.557 h# | 1.633gh | 1.703fg | 1.770f |
| GF | 1.563h | 1.673fg | 1.740f | 1.873e |
| MB | 1.950de | 1.990cd | 2.043bc | 2.113ab |
| MB + GF | 2.043bc | 2.070bc | 2.133ab | 2.187a |
| Phosphorous (P) | ||||
| CN | 0.601 l | 0.649ij | 0.683 h | 0.714fg |
| GF | 0.622jl | 0.675hi | 0.731ef | 0.766d |
| MB | 0.635j | 0.687gh | 0.748de | 0.761de |
| MB + GF | 0.809c | 0.856b | 0.873b | 0.925a |
| Potassium (K) | ||||
| CN | 1.907i | 1.963gh | 2.037f | 2.057f |
| GF | 1.913hi | 1.977g | 2.127e | 2.183cd |
| MB | 2.033f | 2.073f | 2.150de | 2.237bc |
| MB + GF | 2.190cd | 2.270b | 2.337a | 2.370a |
#Value showed in each column followed by different letters is significantly different at P ≤ 0.01 and the column value is the mean of four replicates
Microbial population estimation
In bioinoculants experiment, we found that with augmentation of Cr(VI) in soil, a constant decreased Gf colonization was observed. However, the population of GF and its colonization in bioinoculants treated plants improved significantly. The maximum increase in number of GF spores and its colonization in roots was recorded in plants inoculated with co-inoculation of MB + GF. It was found that a 95% increased spore population was observed in the pots soil treated with MB + GF (Table 4). Similarly we observed an increment of 86% colonization in the roots same aforementioned treatment.). However, we also observed that the bacterial population was not affected by either singly or in combination treatments with GF. In the vermicompost experiment, the application of vermicompost doses further increased the GF population and it was found maximum on application of 10 t ha–1 doses of vermicompost. There was also an increased spore population of 125%, was found in the soil treated with aforementioned bioinoculants as compared to that of the plants inoculated with GF alone. Like GF treatment, application of vermicompost (10 t ha–1), also improved the population of MB (52%) as compared to the plants not supplemented with vermicompost (Table 5).
Table 4.
Mean population of microbes in the root zone soil of Ocimum basilicum at the time of harvesting
| Treatment | Root zone microbial Population | ||
|---|---|---|---|
| SUCR inocula (CFU × 105 g–1 soil) | Arbuscular mycorrhizal fungi | ||
| No. of spore (50 g–1 soil) | Percent root colonization | ||
| 0 mg kg–1 | |||
| CN | ─ | ─ | ─ |
| BC | 1.52ab# | ─ | ─ |
| MB | 1.66ab | ─ | ─ |
| BT | 1.41cd | ─ | ─ |
| BS | 1.31ef | ─ | ─ |
| BC + GF | 1.50bc | 236ab | 66ab |
| MB + GF | 1.69a | 243a | 65ab |
| BT + GF | 1.43cd | 230ab | 69a |
| BS + GF | 1.28ef | 229ab | 66ab |
| GF | ─ | 241ab | 68ab |
| 25 mg kg–1 | |||
| CN | ─ | ─ | ─ |
| BC | 1.30ef | ─ | ─ |
| MB | 1.59ab | ─ | ─ |
| BT | 1.27ef | ─ | ─ |
| BS | 1.21fg | ─ | ─ |
| BC + GF | 1.32de | 214bc | 60cd |
| MB + GF | 1.57ab | 230ab | 62bc |
| BT + GF | 1.30ef | 186ef | 56ef |
| BS + GF | 1.19fg | 178fg | 52fg |
| GF | ─ | 162gh | 45hi |
| 50 mg kg–1 | |||
| CN | ─ | ─ | ─ |
| BC | 1.26ef | ─ | ─ |
| MB | 1.51ab | ─ | ─ |
| BT | 1.17fg | ─ | ─ |
| BS | 1.10gh | ─ | ─ |
| BC + GF | 1.22fg | 197de | 50fg |
| MB + GF | 1.49bc | 222ab | 56de |
| BT + GF | 1.26ef | 154hi | 47gh |
| BS + GF | 1.13fg | 138ij | 43i |
| GF | ─ | 122lm | 35j |
| 100 mg kg–1 | |||
| CN | ─ | ─ | ─ |
| BC | 0.81j | ─ | ─ |
| MB | 1.08hi | ─ | ─ |
| BT | 0.67jl | ─ | ─ |
| BS | 0.58 l | ─ | ─ |
| BC + GF | 0.81j | 172fg | 44i |
| MB + GF | 1.04i | 205cd | 52fg |
| BT + GF | 0.70jl | 135jl | 32jl |
| BS + GF | 0.55 l | 124lm | 30jl |
| GF | ─ | 105m | 28 l |
#Value showed in each column followed by different letters is significantly different at P ≤ 0.01 and the column value is the mean of three replicates
Table 5.
Mean population of microbes in the root zone soil of Ocimum basilicum at the time of harvesting
| Treatment | Root Zone microbial Population | ||
|---|---|---|---|
| MB (CFU × 105 g–1 soil) | Arbuscular mycorrhizal fungi | ||
| No. of spore (50 g–1 soil) | Percent root colonization | ||
| 0 ton/ha | |||
| CN | – | – | – |
| MB | 1.09d# | – | – |
| GF | – | 110e | 29e |
| MB + GF | 1.10d | 214b | 49c |
| 2.5 ton/ha | |||
| CN | – | – | – |
| MB | 1.27c | – | – |
| GF | – | 150d | 39d |
| MB + GF | 1.24cd | 227b | 55b |
| 5 ton/ha | |||
| CN | – | – | – |
| MB | 1.46b | – | – |
| GF | – | 133cd | 45c |
| MB + GF | 1.43b | 225b | 57ab |
| 10 ton/ha | |||
| CN | – | – | – |
| MB | 1.66a | – | – |
| GF | – | 167c | 50c |
| MB + GF | 1.69a | 248a | 61a |
#Value showed in each column followed by different letters is significantly different at P ≤ 0.01 and the column value is the mean of three replicates
Discussion
Cr(VI) stress is emerging as a major challenge to be tackled along with climate change, population expansion. There is a direct impact of Cr(VI) stress on living being and proper attention is required for devising ecofriendly management strategy. In last decade researchers have tried to manage Cr(VI) stress by isolating and enriching rhizosphere with Cr(VI) reducing rhizobacteria which could be a wise and useful option for improving plant growth yield in Cr(VI)-contaminated soil (Marandi et al. 2020; Zayed and Terry 2003; Mohanty and Patra 2011; Soni et al. 2014a, b). However, there is required more efficient management strategy with an integrated approach for better management of Cr(VI) stress. In the lines of this our group looked some of the efficient options and model plants which might be employed for the management of toxicity and mitigation of Cr(VI) stress. From our previous studies we have developed ideas and demonstrated that vermicompost improves the performance of beneficial microbes (Singh et al. 2013a, b, c) and presence of chromate reducing bacteria improves AMF colonization and in turn improved plant growth (Soni et al. 2014a). Vermicompost apart from acting as a source of nutrients also have ability to chelate (Pande et al. 2007) and reduce Cr(VI) (Kozˇuh et al. 2000) toxicity. The compound such as humic substances and polysaccharides present in vermicompost act as a natural chelating agent which may link heavy metals (Bianchi and Ceccanti 2010) and helps in mitigation. However, we were looking for the model plant along with integrated management approach, which will not only mitigate the Cr(VI) stress, but products obtained can also be fit to be used for. Therefore, we devised a study, selecting O. basilicum as a model plant and hypothesized that vermicompost as a substrate will improve the population of chromate reducing rhizobacteria and in turn mycorrhiza colonization leading to enhanced growth of plants in chromate polluted soils besides this O. basilicum being an aromatic plant may act as a good model as its secondary metabolite (essential oil) are not impacted by Cr(VI) stress, except may be in quantity.
The screening results for the better performing strains revealed that only MB and its combination with GF were effectively mitigating the Cr stress. While treatments with other microbes, i.e., BC, BF alone and in combination were lower in responses (Tables S1–S9, supplementary files) as compared to that of MB. Since all the strains cannot be assessed for biopotentials due to technical handling we narrowed our studies to the most efficiently performing strains. Such selection criteria for microbial selection based on performance is not only followed in abiotic stress management but also in biotic stress management (Soni et al. 2014a; Tiwari et al. 2021). The results from our studies clearly demarcated that the plant growth (root length and shoot height) and yield (dry biomass) were found to be reduced when plants were cultivated under Cr(VI) stress. The impact of severity further increases with increase in the concentration of Cr(VI) as our results revealed the same with gradient concentration used in. The major impact of toxicity occurs on the root system of the plants. Our studies also demonstrated that root growth and development is hampered. The reduction in plants root growth could be due to disruption in cell division process which causes time lengthening of cell cycle. Apart from aforementioned, the direct Cr contact with root may damage the plasma membrane of root cells and consequently leakage of cell content might be increasing the severity, leading to collapse of root system (Castro et al. 2007). We also found that there was drastic reduction of plant growth yield in untreated plants, which increased with concentration gradient. The reduction in plant height and dry biomass could be due to reduction in nutrient uptake and further might be a possibility that transport of water and nutrients system have collapsed due to poor developed root system which is essential for supporting plant growth and development (Shankar et al. 2005). Moreover, Cr transport to aerial parts may also have a direct impact on cellular metabolism of shoots causing reduction of plant growth (Shanker et al. 2005). Besides this, Cr also interacts with endogenous phytohormones and inactivates them (Moya et al. 1995). Our studies also found that the decline in yield (dry biomass) was also increased with exposure to increased gradient concentrations of Cr(VI). The decrease in dry biomass production might also be due to the oxidative damage (due to ROS generation) of the photosynthetic and mitochondrial apparatus/processes which plays vital role in growth development and yield (Dixit et al. 2002). However, we have found that with the application of bioinoculants and vermicompost along with mycorrhiza have potentials to manage toxicity of Cr(VI) and even have contributed to growth yield and development. Our results demonstrated that with application of chromium tolerant microbes such as MB, in O. basilicum potentially helped in mitigating toxic impacts of Cr(VI) and supported growth. This could be due to conversion of toxic Cr(VI) to relatively nontoxic Cr(III) in rhizospheric environment thus providing relatively favorable conditions for growth of plant roots GF and thus facilitating nutritional uptake (Soni et al. 2014a). We also found that combination, co inoculation are better options for the stress management, not only related to abiotic stress but even biotic stress can be managed better with co-inoculation of one or more microbes in synergism (Tiwari et al. 2017, 2021). Here we also found that combination of microbes MB + GF performed better in management of Cr(VI) stress than comparison to singly inoculated treatments. Our result suggest that the application of MB can lower the chromium toxicity to the plant by reducing the bioavailability of toxic Cr(VI). The reduced Cr(VI) toxicity levels in soil may provide the favourable environment for the growth, proliferation, and colonization of AMF resulting in improved growth and yield of crop plants. Moreover, improved colonization of AMF can lower down the heavy metal toxicity to plants by immobilizing the metals and further restrict the translocation of Cr(VI) to aerial parts.
Further on, application of vermicompost as we said previously has self-potentials of heavy metals (Jadia and Fulekar 2008) including Cr(VI) stress management (Koka et al. 2019). We found that microbial co-inoculation performed much better and improved growth, yield and nutrient content then comparison to microbes alone or vermicompost treatments in alone. Our results further supported the model of integrated approach of management, as demonstrated that combination of vermicompost + MB + GF have showed much improvement in plant growth and yield then comparison to any other treatments. This might be due to vermicompost acted as a substrate for proliferation of bacterial population, thus enriched the rhizosphere with chromate reducing bacteria, second vermicompost itself helped in GF multiplication and colonization, which is well known for its potentials in abiotic stress management (Khan et al. 2001). The effective microbial treatments (MB + GF) also enhanced nutrient uptake (NPK) in treated plants. This could be due to the reason that they might have reduced toxicity of the Cr (VI) which directly impacted in better root system development and nutrient absorption. Apart from this vermicompost itself has been found as a supplement for plant nutrients (Kumar and Sharma 2018), not only this they maintain moisture content of the soil, They have adsorbing capability for heavy metals thus may reduce their availability to the plants reducing severity and toxicity (Matos and Arruda 2003; Jordão et al. 2009, 2010, 2011; Koka et al. 2019). However, the heavy metal adsorption capacity of vermicompost depends on its particle size, porosity, as well as the molecular structure of the adsorbed compound (Lamim et al. 1998). In our study they performed well which supports the previous results that vermicompost itself have Cr(VI) mitigating potentials.
However, the most commonly reported mechanisms for metal ion sorption are electrostatic interaction, ion exchange, complexation and precipitation (Barrera et al. 2006) which might have worked in our experiment and helped in mitigation of Cr(VI) stress. There is also another strategy of management by vermicompost which employs humic and fulvic acid present in them (Chaab et al. 2016), have demonstrated potentials which can also reduce the Cr(VI) and may indirectly help in improving root growth (Kozˇuh et al. 2000). Moreover, there could be also a possibility of alleviation of plant toxicity due to Cr chelation by vermicompost (Pande et al. 2007). Therefore, these all factors might have contributed well in Cr(VI) stress management on O. basilicum as demonstrated by our results. Our results also showed there is decline in the bioavailability of Cr(VI) which might be due to the higher amount of Cr reducing metabolites produced by MB (Soni et al. 2013) although the possibility of adsorption of Cr(VI) by these strains cannot be neglected (Kanga et al. 2007). Not only this the integrated management approach involving vermicompost might also be a major player and contributed well in reducing the bioavailable Cr(VI) in soil. As our results showed lower bioavailable Cr(VI) in combined treatments. Therefore, possibility of vermicompost cannot be neglected in reducing the bio-availability of Cr in soils. Furthermore, the application of vermicompost also reduced the uptake of Cr. This could be due to the direct conversion of available Cr(VI) into non available forms either by reduction or chelation of Cr by vermicompost. There are reported literature which also emphasized that microflora and organic compounds such as vermicompost plays critical role in, the conversion of Cr(VI) into Cr(III) which also gets further enhancement with texture, and conditions, i.e., pH, moisture, temperature, and oxidation–reduction conditions, in the particular soil (Kozˇuh et al. 2000; Polti et al. 2009). Therefore, we predict that these factors might have influenced Cr(VI) reduction and showed direct impact on Cr uptake by plant cells in soil.
We have found that with the treatment of bioinoculants there is reduction of translocation factor. They performed better in combination treatments. The decrease in translocation of Cr from root to shoot could be due to immobilization of Cr in vacuole of root cells (Shanker et al. 2005). Furthermore, we also found that the application of vermicompost also reduced the translocation of Cr from root to shoot. The decrease in translocation may be because of improved of root growth due to reduced chromate toxicity or indirectly by increase in population of rhizobacteria and mycorrhiza. All these factors might have contributed in lowering the uptake and toxicity. There is also a well reported mechanism of mycorrhizal association benefits plants by coping with abiotic stresses. The presence of mycorrhiza in roots will immobilize metals in several ways such as binding of heavy metals to chitin present in fungal cell walls. In addition, fungal vesicles may be involved in storing toxic metals and thereby avoiding their translocation to upper parts of the plants (Gother and Paszkowski 2006).Since vermicompost enhanced the mycorrhization and colonization, they might have played role in lowering the toxicity by sorting or immobilizing metals and reducing translocation thus lowering the toxicity to the plants.
Conclusions
This study clearly demonstrated the successful role of vermicompost in reducing Cr toxicity in plants. The experimental evidence showed that vermicompost consequently reduced Cr(VI) toxicity with gradient doses of application. It further decreased the bioavailability and uptake of Cr to the upper parts of the plants. The vermicompost not only directly but even indirectly supported management of Cr(VI) stress by supporting the proliferation of chromate reducing rhizobacteria such as MB, that further decreased the bioavailable and toxic form of Cr(VI) and consequently contributed to the higher herb yield. Furthermore, the integrated approach also improved mycorrhization and contributed in synergistic management of Cr(VI) stress. With the application of the integrated management approach, a new model for management of Cr(VI) stress has been paved. This model will not only benefit the Cr(VI) stress management in O. basilicum but will also guide framing such approaches in other crops and plants too.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the Director, CSIR–Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing necessary facilities and encouragement during the course of investigation, Kundan Wasnik for soil analysis (N, P and K analysis) and the Indian Council of Medical Research (ICMR), New Delhi, India, for providing financial support to SKS. Authors are thankful to Dr. Alok Kalra and all supporting staffs of MTD lab for their extensive support and help during the entire tenure of experimental work.
Author contributions
SKS and RS designed the experiment and conducted the experiment; ST and RS helped in data analysis and editing of the original draft. SKS and ST prepared the final draft. All authors have read and agreed with the present version of the manuscript.
Funding
This work was supported by the Indian Council of Medical Research grant no. 3/1/3/JRF-2010/MPD-5 (31756) received to SKS.
Declarations
Conflicts of interest
The authors declare that there is no conflict of interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
All authors have seen the latest version of the manuscript and agree to its publication.
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sumit K. Soni, Email: sumit.soni@icar.gov.in
Sudeep Tiwari, Email: sudeep@post.bgu.ac.il.
References
- Abaidoo RC, Keraita B, Drechsel P, Dissanayake P, Maxwell AS (2010) Soil and crop contamination through wastewater irrigation and options for risk reduction in developing countries. In Soil Biology and Agriculture in the Tropics; Springer: Berlin, Germany. 275–21
- Asmatullah Qureshi SN, Shakoori AR. Hexavalent chromium induced congenital abnormalities in chick embryos. J Appl Toxicol. 1998;18(3):167–171. doi: 10.1002/(SICI)1099-1263(199805/06)18:3<167::AID-JAT492>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG. Cytotoxicity and oxidative mechanism of different forms of chromium. Toxicology. 2002;180:5–22. doi: 10.1016/S0300-483X(02)00378-5. [DOI] [PubMed] [Google Scholar]
- Barrera H, Ureña-Núñez F, Bilyeu B, Barrera-Díaz C. Removal of Chromium and toxic ions present in mine drainage by Ectodermis of Opuntia. J Hazard Mater. 2006;136:846–853. doi: 10.1016/j.jhazmat.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Bharti N, Baghel S, Barnawal D, Yadav A, Kalra A. The greater effectiveness of Glomus mosseae and Glomus intraradices in improving productivity, oil content and tolerance of salt stressed menthol mint (Mentha arvensis) J Sci Food Agric. 2013;93:2154–2161. doi: 10.1002/jsfa.6021. [DOI] [PubMed] [Google Scholar]
- Bianchi V, Ceccanti B. A three components system (TRIAS) in the phytoremediation of polluted environmental matrices. Toxicol Environ Chem. 2010;92(3):477–493. doi: 10.1080/02772240903036154. [DOI] [Google Scholar]
- Breeher L, Gerr F, Fuortes L. A case report of adult lead toxicity following use of Ayurvedic herbal medication. J Occup Med Toxicol. 2013;8(1):26–30. doi: 10.1186/1745-6673-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd GI, Dixon DG, Glick BR. Plant growth promoting bacteria that decreases heavy metal toxicity in plants. Can J Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- Castro RO, Trujillo MM, Bucio JL, Cervantes C, Dubrovsky J. Effects of dichromate on growth and root system architecture of Arabidopsis thaliana seedlings. Plant Sci. 2007;172:684–691. doi: 10.1016/j.plantsci.2006.11.004. [DOI] [Google Scholar]
- Chaab A, Moezzi A, Sayyad G, Chorom M. Effect of compost and humic acid in mobility and concentration of cadmium and chromium in soil and plant. Global J Environm Sci Manag. 2016;2(4):389–396. doi: 10.22034/gjesm.2016.02.04.008. [DOI] [Google Scholar]
- Cheung KH, Gu JD. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad. 2007;59:8–15. doi: 10.1016/j.ibiod.2006.05.002. [DOI] [Google Scholar]
- Codd R, Irwin JA, Lay PA. Sialoglycoprotein and carbohydrate complexes in chromium toxicity. Curr Opi Chem Biol. 2003;17(2):213–219. doi: 10.1016/S1367-5931(03)00017-6. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Gawarammana IB, Archer JR, House IM, Shaw D, Wood DM. Heavy metal poisoning from ayurvedic traditional medicine: an emerging problem? Int J Environ Health. 2008;2(3/4):463–473. doi: 10.1504/IJENVH.2008.020936. [DOI] [Google Scholar]
- Dixit V, Pandey V, Shyam R. Cr ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ. 2002;25:687–690. doi: 10.1046/j.1365-3040.2002.00843.x. [DOI] [Google Scholar]
- Edwards CA, Fletcher KE. Interaction between earthworms and microorganisms in organic matter breakdown. Agric Ecosyst Environ. 1988;24:235–247. doi: 10.1016/0167-8809(88)90069-2. [DOI] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Singh SPA. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut. 2005;133:365–371. doi: 10.1016/j.envpol.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Gianinazzi S, Huchette O, Gianinazzi-Pearson V (2008) New outlooks in mycorrhiza applications. In: Baar J, Estaun V, Ortas I, Orfanoudakis M, Alifragis D (Eds) Proceedings of the COST870 meeting “Mycorrhiza application in sustainable agriculture and natural systems”, 17– 19 September 2008. Thessaloniki, Greece, pp 20–22
- Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- Glick BR, Patten CL, Holguin G, Penrose GM. Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria. London: Imperial College Press; 1999. [Google Scholar]
- Gother V, Paszkowski U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta. 2006;223:1115–1119. doi: 10.1007/s00425-006-0225-0. [DOI] [PubMed] [Google Scholar]
- Grayer RG, Kite GC, Goldstone FJ, Bryan SE, Paton A, Putievsky E. Infraspecific taxonomy and essential oil chemotypes in basil, Ocimum basilicum. Phytochemistry. 1996;43:1033–1039. doi: 10.1016/S0031-9422(96)00429-3. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Verma SK, Khan K, Verma RK. Phytoremediation using aromatic plants: A sustainable approach for remediation of heavy metals polluted sites. Environ Sci Technol. 2013;47(18):10115–10116. doi: 10.1021/es403469c. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, GuptaV K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and Chromium uptake in Helianthus annuus (L.) J Hazard Mater. 2019;377:391–398. doi: 10.1016/j.jhazmat.2019.05.054. [DOI] [PubMed] [Google Scholar]
- Gupta R, Tiwari S, Saikia KS, Shukla V, Singh R, Singh SP, Kumar APV, Pandey R. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma. 2014 doi: 10.1007/s00709-014-0657-5. [DOI] [PubMed] [Google Scholar]
- Jackson ML (1973) Soil chemical analysis (Indian ed). Prentice Hall of India Pvt Ltd, New Delhi
- Jadia CD, Fulekar MH. Phytoremediation: the application of Vermicompost to remove zinc, cadmium, copper, nickel and lead by sunflower plant. Environ Eng Manag J. 2008;7(5):547–558. doi: 10.30638/eemj.2008.078. [DOI] [Google Scholar]
- Jiménez B. Irrigation in developing countries using wastewater. Int Rev Environ Strateg. 2006;6:229–250. [Google Scholar]
- Jordão CP, Fernandes RBA, de Lima RK, de Souza NB, de Barros PM. Zn(II) adsorption from synthetic solution and kaolin wastewater onto vermicompost. J Hazard Mater. 2009;162:804–811. doi: 10.1016/j.jhazmat.2008.05.104. [DOI] [PubMed] [Google Scholar]
- Jordão CP, Fernandes RBA, De Lima RK, De Barros PM, Fontes MPF, De Paula Souza FM. A study on Al(III) and Fe(II) ions sorption by cattle manure vermicompost. Water Air Soil Pollut. 2010;210:51–61. doi: 10.1007/s11270-009-0223-5. [DOI] [Google Scholar]
- Jordão CP, Pereira WL, Carari DM, Fernandes RBA, de Almeida RM, Fontes MPF. Adsorption from Brazilian soils of Cu(II) and Cd(II) using cattle manure vermicompost. Int J Environ Stud. 2011;68:719–736. doi: 10.1080/00207233.2011.587953. [DOI] [Google Scholar]
- Kalra A, Chandra M, Awasthi A, Singh AK, Khanuja SPS. Natural compound enhancing growth and survival of rhizobial inoculants in vermicompost based formulation. Biol Fertil Soil. 2010;46:521–524. doi: 10.1007/s00374-010-0443-2. [DOI] [Google Scholar]
- Kanga SY, Lee JU, Kim KW. Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem Engin J. 2007;36:54–58. doi: 10.1016/j.bej.2006.06.005. [DOI] [Google Scholar]
- Karuppanapandian T, Sinha PB, Haniya AK, Manoharan K. Chromium-induced accumulation of peroxide content, stimulation of antioxidative enzymes and lipid peroxidation in green gram (Vigna radiata L. cv. Wilczek) leaves. Afr J Biotechnol. 2009;8(3):475–479. [Google Scholar]
- Katz SA, Salem H. The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol. 1992;13(3):217–224. doi: 10.1002/jat.2550130314. [DOI] [PubMed] [Google Scholar]
- Khan AG. Relationship between Chromium biomagnification accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environ Int. 2001;26:417–423. doi: 10.1016/S0160-4120(01)00022-8. [DOI] [PubMed] [Google Scholar]
- Khan MS, Zaidi A. Synergistic effects of the inoculation with plant growth promoting rhizobacteria and an arbuscular mycorrhizal fungus on the performance of wheat. Turk J Agric for. 2007;31:355–362. [Google Scholar]
- Koka RK, Sharma PK, Behera JK, Chalageri G. Remediation of chromium toxicity by FYM and vermicompost in Rice (Oryza sativa) Int J Curr Microbiol App Sci. 2019;8(2):1906–1922. doi: 10.20546/ijcmas.2019.802.222. [DOI] [Google Scholar]
- Kozuh N, Stupar J, Gorenc B. Reduction and oxidation process of Cr in soils. Environ Sci Technol. 2000;34:112–119. doi: 10.1021/es981162m. [DOI] [Google Scholar]
- Krishna RK, Philip L. Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater. 2005;121:109–117. doi: 10.1016/j.jhazmat.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sharma PK. Augmentation of nitrogen and phosphorous mineralization in chromium contaminated soils using organic amendments. Int J Chem Stud. 2018;6(2):3417–3422. [Google Scholar]
- Lamim SSM, Jordão CP, Brune W, Pereira JL, Bellato CR. Physical and chemical characterization of vermicompost from bovine manure and evaluation of competitive retention of copper and zinc. Quim Nova. 1998;21:271–283. [Google Scholar]
- Losi ME, Frankenberger WT. Chromium-resistant microorganisms isolated from evaporation ponds of a metal processing plant. Water Air Soil Pollut. 1994;74:405–413. doi: 10.1007/BF00479803. [DOI] [Google Scholar]
- Mabbett AN, Macaskie LE. A novel isolate of Desulfovibrio sp. with enhanced ability to reduce Cr (VI) Biotechnol Lett. 2001;23:683–687. doi: 10.1023/A:1010352417399. [DOI] [Google Scholar]
- Mamaril JC, Paner ET, Alpante BM. Biosorption and desorption studies of Chromium (iii) by free and immobilized Rhizobium (BJVr 12) cell biomass. Biodegradation. 1997;8:275–285. doi: 10.1023/A:1008213712910. [DOI] [Google Scholar]
- Marandi AK, Das S, Samantaray DP. Improvement of rice plant productivity by native Cr(VI) reducing and plant growth promoting soil bacteria Enterobacter cloacae. Chemosphere. 2020;240:124895. doi: 10.1016/j.chemosphere.2019.124895. [DOI] [PubMed] [Google Scholar]
- Matos GD, Arruda MAZ. Vermicompost as an adsorbent for removing metal ions from laboratory effluents. Process Biochem. 2003;39:81–88. doi: 10.1016/S0032-9592(02)00315-1. [DOI] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol. 1990;11:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Mohanty M, Patra HK. Attenuation of Chromium toxicity by bioremediation technology. Rev Environ Contam Toxicol. 2011;210:1–34. doi: 10.1007/978-1-4419-7615-4_1. [DOI] [PubMed] [Google Scholar]
- Moya JL, Ros R, Picazo I. Heavy-metal hormone interactions in rice plants: effects on growth, net photosynthesis, and carbohydrate distribution. J Plant Growth Regul. 1995;14:61–67. doi: 10.1007/BF00203115. [DOI] [Google Scholar]
- Oliveira H. Chromium as an environmental pollutant: insights on induced plant toxicity. J Botany. 2012 doi: 10.1155/2012/375843. [DOI] [Google Scholar]
- Padalia RC, Verma RS, Upadhyay RK, Chauhan A, Singh VR. Productivity and essential oil quality assessment of promising accessions of Ocimum basilicum L. from north India. Ind Crops Prod. 2017;97:79–86. doi: 10.1016/j.indcrop.2016.12.008. [DOI] [Google Scholar]
- Pande P, Chand S, Yadav VK, Anwar M, Patra DD. Influence of Chromium with vermicompost on growth and accumulation by Brahmi. Commun Soil Sci Plant Anal. 2007;38:2815–2829. doi: 10.1080/00103620701663057. [DOI] [Google Scholar]
- Papassiopi N, Kontoyianni A, Vaxevanidou K, Xenidis A. Assessment of Chromium biostabilization in contaminated soils using standard leaching and sequential extraction techniques. Sci Total Environ. 2009;407:925–936. doi: 10.1016/j.scitotenv.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Patra RC, Malik B, Beer M, Megharaj M, Naidu R. Molecular characterization of Chromium (VI) reducing potential in Gram positive bacteria isolated from contaminated sites. Soil Biol Biochem. 2010;42:1857–1863. doi: 10.1016/j.soilbio.2010.07.005. [DOI] [Google Scholar]
- Polti MA, Garcıa RO, Amoroso MJ, Abate CM. Bioremediation of Chromium(VI) contaminated soil by Streptomyces sp. MC1. J Basic Microbiol. 2009;49:285–292. doi: 10.1002/jobm.200800239. [DOI] [PubMed] [Google Scholar]
- Puzon GJ, Petersen JN, Roberts AG, Kramer DM, Xun L. A bacterial flavin reductase system reduces chromates (III)–NAD+ complex. Biochem Biophy Res. 2002;294:76–81. doi: 10.1016/S0006-291X(02)00438-2. [DOI] [PubMed] [Google Scholar]
- Rai V, Kakkar P, Khatoon S, Rawat AKS, Mehrotra S. Heavy metal accumulation in some herbal drugs. Pharm Biol. 2001;39:384–387. doi: 10.1076/phbi.39.5.384.5898. [DOI] [Google Scholar]
- Rai UN, Tripathi RD, Vajpayee P, Jha VN, Ali MB. Bioaccumulation of toxic metals (Cr, Cd, Pb, and Cu) by seeds of Euryale ferox Salisb. (Makhana) Chemosphere. 2002;46:267–272. doi: 10.1016/S0045-6535(01)00087-X. [DOI] [PubMed] [Google Scholar]
- Rajkumar M, Freitas H. Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere. 2008;71:834–842. doi: 10.1016/j.chemosphere.2007.11.038. [DOI] [PubMed] [Google Scholar]
- Rajkumar M, Nagendran R, Lee KJ, Lee WH. Characterization of a novel Cr6+ reducing Pseudomonas sp. with plant growth-promoting potential. Curr Microbiol. 2005;50:266–271. doi: 10.1007/s00284-005-4470-4. [DOI] [PubMed] [Google Scholar]
- Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ. Influence of plant growth promoting bacteria and Cr6+on the growth of Indian mustard. Chemosphere. 2006;62:741–748. doi: 10.1016/j.chemosphere.2005.04.117. [DOI] [PubMed] [Google Scholar]
- Rtidel H, Terytze K. Determination of extractable Chromium(VI) in soil using a photometric method. Chemosphere. 1999;39:697–708. doi: 10.1016/S0045-6535(99)00134-4. [DOI] [Google Scholar]
- Saikia KS, Tiwari S, Pandey R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita Biol Control. 2013;65:225–234. doi: 10.1016/j.biocontrol.2013.01.014. [DOI] [Google Scholar]
- Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008;300(8):915–923. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S. Chromium toxicity in plants. Environ Int. 2005;31:735–753. doi: 10.1016/j.envint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Singh R, Divya S, Awasthi A, Kalra A. Technology for efficient and successful delivery of vermicompost colonized bioinoculants in Pogostemon cablin (patchouli) Benth. World J Microbiol Biotechnol. 2012;28:323–333. doi: 10.1007/s11274-011-0823-2. [DOI] [PubMed] [Google Scholar]
- Singh R, Kalra A, Ravish BS, Divya S, Paramaeswarn TN, Srinivas KVNS, Bagyaraj DJ. Effect of potential bioinoculants and organic manures on root-rot and wilt, growth, yield and quality of organically grown Coleus forskohlii in semiarid tropical region of Bangalore (India) Plant Pathol. 2012;61:700–708. doi: 10.1111/j.1365-3059.2011.02567.x. [DOI] [Google Scholar]
- Singh R, Soni SK, Awasthi A, Kalra A. Evaluation of vermicompost doses for management of root-rot disease complex in Coleus forskohlii under organic field conditions. Aust Plant Pathol. 2012;41:397–403. doi: 10.1007/s13313-012-0134-6. [DOI] [Google Scholar]
- Singh R, Singh R, Soni SK, Patel RP, Kalra A. Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crops Prod. 2013;45:335–342. doi: 10.1016/j.indcrop.2013.01.003. [DOI] [Google Scholar]
- Singh R, Singh R, Soni SK, Singh SP, Chauhan UK, Kalra A. Vermicompost from biodegraded distillation waste improves soil properties and essential oil yield of Pogostemon cablin (patchouli) Benth. Appl Soil Ecol. 2013;70:48–56. doi: 10.1016/j.apsoil.2013.04.007. [DOI] [Google Scholar]
- Singh R, Soni SK, Kalra A. Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza. 2013;23:35–44. doi: 10.1007/s00572-012-0447-x. [DOI] [PubMed] [Google Scholar]
- Snow ET, Xu LS. Chromium(III) bound to DNA templates promotes increased polymerase processivity and decreased fidelity during replication in vitro. Biochemistry. 1991;30:11238–11245. doi: 10.1021/bi00111a007. [DOI] [PubMed] [Google Scholar]
- Soni SK, Singh R, Awasthi A, Singh M, Kalra A. In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environ Sci Pollut Res. 2013;20:1661–1674. doi: 10.1007/s11356-012-1178-4. [DOI] [PubMed] [Google Scholar]
- Soni SK, Singh R, Singh M, Awasthi A, Wasnik K, Kalra A. Pretreatment of Cr (VI)-amended soil with chromate-reducing rhizobacteria decreases plant toxicity and increases the yield of Pisum sativum. Arch Environ Contam Toxicol. 2014;66(4):616–627. doi: 10.1007/s00244-014-0003-0. [DOI] [PubMed] [Google Scholar]
- Soni SK, Singh R, Ngpoore NK, Niranjan A, Singh P, Mishra A. Isolation and characterization of endophytic fungi having plant growth promotion traits that biosynthesizes bacosides and withanolides under in vitro conditions. Braz J Microbiol. 2021;52:1791–1805. doi: 10.1007/s42770-021-00586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni SK, Singh R, Awasthi A, Kalra A. A Cr(VI)-reducing Microbacterium sp strain SUCR140 enhances growth and yield of Zea mays in Cr(VI) amended soil through reduced chromium toxicity and improves colonization of arbuscular mycorrhizal fungi. Environ Sci Pollut Res. 2014;21(3):1971–1979. doi: 10.1007/s11356-013-2098-7. [DOI] [PubMed] [Google Scholar]
- Soni SK, Mishra MK, Mishra M, Kumari S, Saxena S, Shukla V, Tiwari S, Shirke P (2022) Papaya leaf curl virus (PaLCuV) infection on papaya (Carica papaya L.) Plants alters anatomical and physiological properties and reduces bioactive components. Plants 11 (5), 10.3390/plants11050579 [DOI] [PMC free article] [PubMed]
- Tappero R, Peltier E, Grafe M, Heidel K, Ginder-Vogel M, Livi KJT, Rivers ML, Marcus MA, Chaney RL, Sparks DL. Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol. 2007;175:641–654. doi: 10.1111/j.1469-8137.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- Telci I, Bayram E, Yilmaz G, Avci B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.) Biochem Syst Ecol. 2006;34:489–497. doi: 10.1016/j.bse.2006.01.009. [DOI] [Google Scholar]
- Tiwari S, Pandey S, Singh PC, Pandey R. Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind Crops Prod. 2017 doi: 10.1016/j.indcrop.2016.12.030. [DOI] [Google Scholar]
- Tiwari S, Pandey R, Gross A. Identification of rhizospheric microorganisms that manages root knot nematode and improve oil yield in sweet basil (Ocimum basilicum L.) Agronomy. 2021;11(3):570. doi: 10.3390/agronomy11030570. [DOI] [Google Scholar]
- USEPA (1998) Toxicological review of hexavalent chromium. Chemical Abstract Service (CAS) No. 18540–29–9
- Vernay P, Gauthier MC, Hitmi A. Interaction of bioaccumulation of heavy metal Chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere. 2007;68:1563–1575. doi: 10.1016/j.chemosphere.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Vincent JB. The biochemistry of chromium. J Nutr. 2000;130:715–718. doi: 10.1093/jn/130.4.715. [DOI] [PubMed] [Google Scholar]
- Vivas A, Vörös I, Biró B, Campos E, Barea JM, Azcón R. Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environ Pollut. 2003;126:179–189. doi: 10.1016/S0269-7491(03)00195-7. [DOI] [PubMed] [Google Scholar]
- Vivas A, Barea JM, Azcón R. Interactive effect of Brevibacillus brevis and Glomus mosseae, both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soil. Environ Pollut. 2005;134:257–266. doi: 10.1016/j.envpol.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Vivas A, Barea JM, Biró B, Azcón R. Effectiveness of autochthonous bacterium and mycorrhizal fungus on Trifolium growth, symbiotic development and soil enzymatic activities in Zn contaminated soil. J Appl Microbiol. 2006;100:587–598. doi: 10.1111/j.1365-2672.2005.02804.x. [DOI] [PubMed] [Google Scholar]
- Wani PA, Zaidi A, Khan MS. Cr reducing and plant growth promoting potential of Mesorhizobium species under Chromium stress. Bioremed J. 2009;13:121–129. doi: 10.1080/10889860903124289. [DOI] [Google Scholar]
- WHO (1996) Guidelines for drinking-water quality. Health criteria and other supporting information, 2nd ed., WHO, Geneva, 2: 940–949
- Wise SS, Elmore LW, Holt SE, Little JE, Anto nucci PG, Bryant BH, Pierce WSJ, Telomerase mediated lifespan extension of human bronchial cells does not affect hexavalent chromium induced cytotoxicity or genotoxicity. Mol Cell Biochem. 2004;255(1–2):103–112. doi: 10.1023/B:MCBI.0000007266.82705.d9. [DOI] [PubMed] [Google Scholar]
- Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant Soil. 2003;249:139–156. doi: 10.1023/A:1022504826342. [DOI] [Google Scholar]
- Zheljazkov VD, Nielsen NE. Effect of heavy metals on peppermint and cornmint. Plant Soil. 1996;178:59–66. doi: 10.1007/BF00011163. [DOI] [Google Scholar]
- Zheljazkov VD, Nielsen NE. Studies on the effect of heavy metals (Cd, Pb, Cu, Mn, Zn and Fe) upon the growth, productivity and quality of Lavender (Lavandula angustifolia Mill.) J Essent Oil Res. 1996;8:259–274. doi: 10.1080/10412905.1996.9700612. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.