Abstract
Background
Introduction of antiretroviral therapy (ART) has been associated with a decline in human immunodeficiency virus (HIV)-related mortality, although HIV remains a leading cause of death in sub-Saharan Africa. We describe all-cause mortality and its predictors in people living with HIV (PLWH) in the African Cohort Study (AFRICOS).
Methods
AFRICOS enrolls participants with or without HIV at 12 sites in Kenya, Uganda, Tanzania, and Nigeria. Evaluations every 6 months include sociobehavioral questionnaires, medical history, physical examination, and laboratory tests. Mortality data are collected from medical records and survivor interviews. Multivariable Cox proportional hazards models were used to calculate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for factors associated with mortality.
Results
From 2013 through 2020, 2724 PLWH completed at least 1 follow-up visit or experienced death. Of these 58.4% were females, 25.8% were aged ≥ 50 years, and 98.3% were ART-experienced. We observed 11.42 deaths per 1000 person-years (95% CI: 9.53–13.68) with causes ascertained in 54% of participants. Deaths were caused by malignancy (28.1%), infections (29.7%), and other non-HIV related conditions. Predictors of mortality included CD4 ≤ 350 cells/µL (aHR 2.01 [95% CI: 1.31–3.08]), a log10copies/mL increase of viral load (aHR 1.36 [95% CI: 1.22–1.51]), recent fever (aHR 1.85[95% CI: 1.22–2.81]), body mass index < 18.5 kg/m2 (aHR 2.20 [95% CI: 1.44–3.38]), clinical depression (aHR 2.42 [95% CI: 1.40–4.18]), World Health Organization (WHO) stage III (aHR 2.18 [95% CI: 1.31–3.61]), a g/dL increase in hemoglobin (aHR 0.79 [95% CI: .72–.85]), and every year on ART (aHR 0.67 [95% CI: .56–.81]).
Conclusions
The mortality rate was low in this cohort of mostly virally suppressed PLWH. Patterns of deaths and identified predictors suggest multiple targets for interventions to reduce mortality.
Keywords: HIV, mortality, Africa South of the Sahara, acquired immunodeficiency syndrome, cause of death
Among PLWH in East Africa and Nigeria, mortality rate was 11.42/1000 person-years. Malignancy, infections, and non-HIV related conditions were common causes. Factors associated with increased mortality included low CD4, high viral load, low BMI, recent fever, and advanced WHO stage.
With the introduction of antiretroviral therapy (ART), there has been a steady worldwide decline in deaths directly attributable to human immunodeficiency virus (HIV) [1, 2], including about a 40% reduction in the World Health Organization (WHO) African region between 2010 and 2018 [3]. Data from 7 demographic surveillance sites estimated HIV-related mortality to be 45–88 deaths per 1000 person-years (PY) before the era of widely available ART and 14–46/1000 PY after the mass rollout of ART programs in sub-Saharan Africa. However, even in 2019, an estimated 690 000 people died from HIV-related illnesses out of which 64% were from Sub-Saharan Africa [4], and 5.6% of all deaths in the WHO African region were attributable to HIV [5]. The maturity of cohorts on ART and improvements in program management, including the recent test and treat strategy, are likely to further reduce HIV-related mortality [6], as people living with HIV (PLWH) are initiated on ART earlier in their disease course [7].
Although many studies of PLWH have found that HIV-related causes of death are the most common, non-HIV-related causes are increasing, particularly non-AIDS cancers [8–11]. However, ascertaining causes of death in limited resource environments remains a challenge. For example, in a 10-year HIV cohort from Uganda that started enrolment in 2004, almost one-third of causes of death were unknown [10]. Understanding the causes of mortality is important to target programs to improve overall health and longevity for populations living with HIV.
Several predictors of mortality among PLWH have been previously described. A study in Tanzania conducted from 2003 to 2006, identified moderate and severe anemia and severe malnutrition as predictors [12], whereas in a Kenyan cohort of PLWH initiating ART, predictors included low body mass index (BMI) and low CD4 counts [13]. In a Ugandan study conducted between 2004 and 2013, identified predictors included male gender, older age, low education status, unemployment, and advanced immunodeficiency [14]. Most of these studies examined baseline predictors at the start of ART without incorporating longitudinal data to reflect the viral suppression and immune reconstitution that occur with successful ART. There is a paucity of information on factors associated with mortality in cohort studies that conduct activities in a public health setting in the context of the UNAIDS 90-90-90 and 95-95-95 strategies [15]. Understanding predictors of mortality may inform patient management by focusing clinicians on key factors that require attention. In addition, these same factors can be useful for prioritizing program activities toward decreasing mortality.
We examined all-cause mortality and its predictors in a cohort of PLWH followed between 2013 and 2020, the majority of whom were virally suppressed at the time of analysis. We also characterized the most common causes of death in this population.
METHODS
Study Design and Participants
The African Cohort Study (AFRICOS) is an ongoing prospective study that follows people with and without HIV at 12 clinical care sites embedded in 5 HIV care programs supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) in Kayunga, Uganda; South Rift Valley, Kenya; Kisumu West, Kenya; Abuja and Lagos, Nigeria; and Mbeya, Tanzania. The study, established in 2013, longitudinally assesses the impact of clinical practices, biological factors and socio-behavioral issues on HIV infection and disease progression in an African context as previously described [16]. Briefly, for this analysis, adult participants aged ≥ 18 years with and without HIV were recruited in a roughly 5:1 ratio from client lists at participating PEPFAR clinics, new diagnoses from PEPFAR counseling and testing programs, and serodifferent partners of participants. PLWH were started on ART according to routine practices, which since 2017 have included “test and start” guidance. Every 6 months, sociodemographic and behavioral factors were collected via detailed questionnaires, a study clinician took a medical history and performed a physical examination and standardized laboratory evaluations were conducted. Participants who missed a scheduled study visit were followed up by study personnel via mobile phone contacts and home visits. Information on deaths was routinely collected from hospital records, including autopsy reports where available, or from relatives of the deceased.
Written informed consent was obtained from all study participants before conduct of any study activities. The study was approved by institutional review boards of the Makerere University School of Public Health, the Tanzania National Institute of Medical Research, the Kenya Medical Research Institute, the Nigerian Ministry of Defense, and the Walter Reed Army Institute of Research.
Laboratory Evaluations
HIV RNA was quantified assessed using several different platforms over the course of the study: Roche Cobas Ampliprep/Cobas TaqMan HIV Type 1 (HIV-1) Test, v2.0 (linear range 20–10 0000 000 or 48–10 000 000 copies/mL); Roche High Pure/COBAS TaqMan HIV-1 Test, v2.0 (linear range 34–10 000 000 copies/mL); COBAS® AmpliPrep/COBAS® TaqMan® 48 HIV-1 Test (linear range 48–10 000 000 copies/mL); or Abbott Real Time HIV-1 Viral Load assay (linear range 40–10 000 000 copies/mL). CD4 T-cell count was assessed using BD FACSCount, BD FACSCalibur, BD FACSCanto II, or BD FACSPresto. Full hemogram was assessed using Beckman Coulter AcT5 Diff and Sysmex ×1000i.
Statistical Analyses
These analyses included enrolled participants who completed at least 1 follow-up study visit or experienced death after enrollment. All data were obtained from the most recent study visit available except for WHO staging, which was recorded at study enrollment. Data were collected between January 2013 and March 2020.
Selected sociodemographic data were analyzed as frequency counts with corresponding percentages. In the survival analyses, PLWH were considered to have become at risk for death at study enrollment. Participants without HIV who seroconverted after enrollment were considered to have become at risk for death at HIV diagnosis. Participants who did not experience death were right censored at their most recent study visit. Unadjusted and adjusted Cox proportional hazards regression models were run to establish risk factors associated with mortality at the most recent visit by estimating hazard ratios (HRs) and associated 95% confidence intervals (CIs). The final multivariable model was selected using the backward stepwise method, systematically eliminating from the model factors with P > .2. Statistical significance for the final multivariable model was considered at P < .05. In Cox proportional hazards modeling, duration on ART was observed not to follow the proportionality assumption when modeled as an interaction term with time; This was corrected by modeling duration on ART as a time-varying covariate. Sensitivity analyses between the final multivariable model where the failure event was death and a second model where the failure event was death or loss to follow-up were performed. ART-naive participants were considered to have been on ART for zero days. Participants with undetectable HIV RNA were considered to have 0 log10copies/mL. BMI was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2). Depression was assessed using Center for Epidemiological Studies Depression (CES-D) scale, with a score > 16 considered diagnostic of depression [17]. Having any malignancy or any infection at the most recent visit was analyzed to determine their association with risk of death. Malignancies and infections reported as the cause of death were also analyzed.
Mortality rates were computed by dividing the total number of deaths by the total number of person-years of follow up and multiplying by 1000 to obtain rates per 1000 person-years. Bivariate analyses comparing risk of mortality between PLWH and participants without HIV were also run. In addition, for PLWH with a known cause of death, we performed bivariate analyses to compare socio-demographic and clinical factors between those with HIV related and non-HIV related causes of death. Missing data were excluded from analyses for those factors with nonresponse and missing data points. Statistical analyses were conducted in STATA 13.0 (StataCorp, College Station, Texas, USA).
RESULTS
Study Population
Of the 3540 participants enrolled in the cohort, 3206 were eligible for these analyses including 2724/2937 (92.8%) PLWH and 482/603 (79.9%) without HIV.
The 2724 PLWH had a median age 38.8 years (interquartile range [IQR], 31.8–46.3) at enrolment and 42.8 years (35.5–50.3) at the most recent visit. The majority of participants were female (58.4 %), married (57.0%), non-Catholic Christians (67.4%), had no or primary level education (58.0%), and were not employed (60.7%; Table 1).
Table 1.
Sociodemographics of Participants Living With Human Immunodeficiency Virus (HIV)
| Total N (%) | Dead n (%) | Alive n (%) | Mortality Unadjusted HR (95% CI) | P value | |
|---|---|---|---|---|---|
| Number of participants | 2724 | 118 | 2606 | … | … |
| Site | |||||
| Kayunga, Uganda | 503 (18.5) | 37 (7.4) | 466 (92.6) | 1.00 | |
| South Rift Valley, Kenya | 944 (34.7) | 33 (3.5) | 911 (96.5) | 0.47 (.29–.75) | .002 |
| Kisumu West, Kenya | 500 (18.4) | 24 (4.8) | 476 (95.2) | 0.64 (.38–1.07) | .087 |
| Mbeya, Tanzania | 500 (18.4) | 14 (2.8) | 486 (97.2) | 0.44 (.24–.82) | .009 |
| Abuja and Lagos, Nigeria | 277 (10.2) | 10 (3.6) | 267 (96.4) | 0.53 (.26–1.06) | .074 |
| Age at most recent visit | |||||
| 18–24 years | 119 (4.4) | 5 (4.2) | 114 (95.8) | 1.00 | |
| 25–39 years | 961 (35.3) | 40 (4.2) | 921 (95.8) | 0.63 (.25–1.61) | .339 |
| 40–49 years | 940 (34.5) | 45 (4.8) | 895 (95.2) | 0.68 (.27–1.72) | .415 |
| ≥50 | 704 (25.8) | 28 (4.0) | 676 (96.0) | 0.55 (.21–1.42) | .213 |
| Sex | |||||
| Male | 1134 (41.6) | 58 (5.1) | 1076 (94.9) | 1.00 | |
| Female | 1590 (58.4) | 60 (3.8) | 1530 (96.2) | 0.72 (.50–1.03) | .076 |
| Marital status, n = 2723 | |||||
| Single | 317 (11.6) | 14 (4.4) | 303 (95.6) | 1.35 (.76–2.42) | .311 |
| Married | 1549 (57.0) | 60 (3.9) | 1489 (96.1) | 1.00 | |
| Divorced/Separated | 404 (14.8) | 25 (6.2) | 379 (93.8) | 1.66 (1.04–2.64) | .033 |
| Widowed | 437 (16.1) | 18 (4.1) | 419 (95.9) | 1.04 (.61–1.75) | .890 |
| Other | 16 (0.6) | 0 (0.0) | 16 (100.0) | … | … |
| Religion | |||||
| Catholic Christian | 680 (25.0) | 38 (5.6) | 642 (94.4) | 1.59 (1.07–2.37) | .023 |
| Non catholic Christian | 1837 (67.4) | 66 (3.6) | 1771 (96.4) | 1.00 | |
| Muslim | 184 (6.8) | 11 (6.0) | 173 (94.0) | 1.70 (.90–3.22) | .104 |
| Traditionalist | 19 (0.7) | 3 (15.8) | 16 (84.2) | 4.83 (1.52–15.40) | .008 |
| Other | 4 (0.1) | 0 (0.0) | 4 (100.0) | … | … |
| Highest level of school, n = 2722 | |||||
| No school | 108 (4.0) | 6 (5.6) | 102 (94.4) | 1.00 | |
| Primary | 1469 (54.0) | 64 (4.4) | 1405 (95.6) | 0.80 (.35–1.85) | .603 |
| Secondary | 803 (29.5) | 37 (4.6) | 766 (95.4) | 0.84 (.36–1.99) | .695 |
| Vocational/university | 340 (12.5) | 10 (2.9) | 330 (97.1) | 0.56 (.20–1.54) | .260 |
| Other | 2 (0.1) | 0 (0.0) | 2 (100.0) | … | |
| Currently employed, n = 2723 | |||||
| No | 1653 (60.7) | 69(4.2) | 1584 (95.8) | 1.00 | |
| Yes | 1070 (39.3) | 48(4.5) | 1022 (95.5) | 1.05 (.73–1.52) | .797 |
For these mortality analyses, only PLWH with at least 2 documented study visits, including the enrolment visit and/or a reported death event were considered, n = 2724. Unadjusted Cox proportional hazards regression models were run to identify socio-demographic factors associated with mortality at the most recent visit by estimating HRs and associated 95% CIs. For factors with non-response and missing data points, only available data were analyzed.
Abbreviations: CI, confidence interval; HR, hazard ratio; PLWH, people living with HIV.
The majority were ART experienced (98.3%) at their most recent visit. The median duration on ART was 5.1 years (IQR, 3.1–9.0). The majority, 73.4% (1998/2721) had CD4 > 350 cells/µl, and an undetectable viral load (83.5%, n = 2268/2716), whereas 45.3% (1228/2710) were on first-line regimens of NRTI, and dolutegravir at the most recent visit. Only 8.5% (232/2716) and 16.5% (448/2716) of participants had a viral load ≥ 1000 copies/mL and ≥ 50 copies/mL, respectively.
Mortality
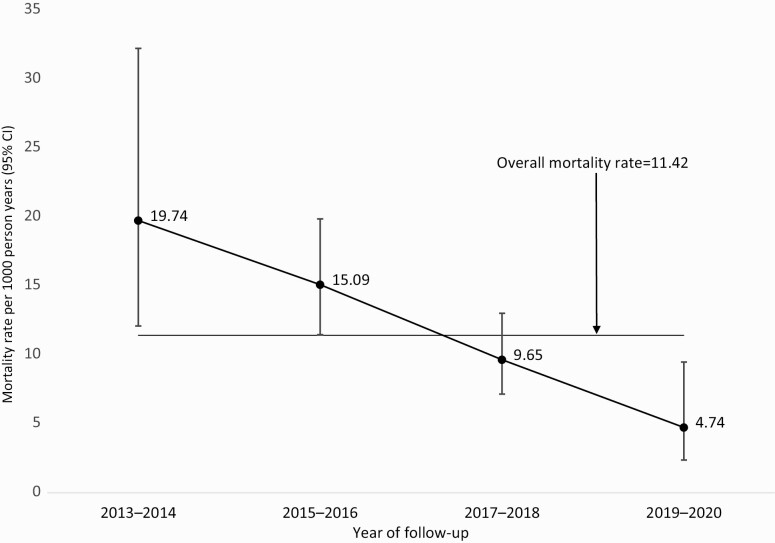
Overall, there were 123 deaths in the cohort over 12 030 PY of observation, yielding a mortality rate of 10.22/1000 PY. The median time from study enrolment to death was 1.3 years (IQR, 0.5–2.5). Among PLWH, 118 deaths were observed with an overall mortality rate of 11.42/1000 PY (95% CI: 9.53–13.68) while in people without HIV, the mortality rate was 2.95/1000 PY (95% CI: 1.23–7.09). The mortality rate for PLWH gradually reduced from 19.74/1000 PY in 2013–2014 to 4.74/1000 PY in 2019–2020 (P < .001; Figure 1). The risk of death for PLWH varied across sites. The Uganda site had significantly higher mortality rate compared to South Rift Valley, Kenya and Mbeya, Tanzania sites (Supplementary Table 1). There were no significant differences in mortality rates across different age groups or gender (Table 1).
Figure 1.
Observed mortality rate among PLWH during study follow-up. Mortality rates were computed by dividing the total number of deaths by the total number of person-years of follow-up and multiplying by 1000 to obtain rates per 1000 PY. Among PLWH, 118 deaths were observed with an overall mortality rate of 11.42/1000 PY (95% CI: 9.53–13.68). Mortality rate for PLWH gradually reduced from 19.74/1000 PY in 2013–2014 to 4.74/1000 PY in 2019–2020 (P < .001). Abbreviations: CI, confidence interval; PLWH, people living with HIV; PY, person-years.
Causes of Death
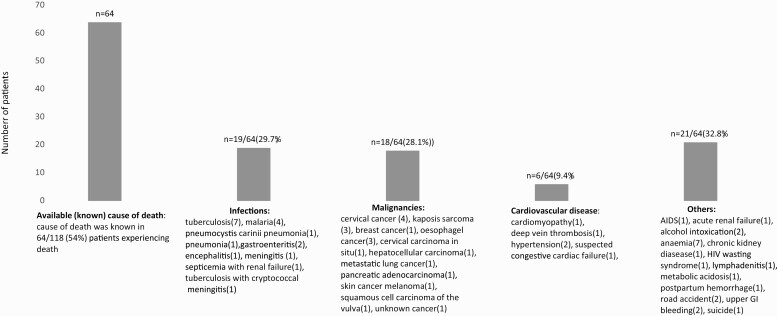
The cause of death was available for 54% (64/118) of PLWH. Only 8 autopsies were performed to identify the cause of death. The most frequent causes of death included infection in 19/64 (29.7%) and malignancy in 18/64 (28.1%; Figure 2). A slightly higher proportion of deaths (53.1%) were from non-HIV related causes. Compared to participants with HIV related causes of death, participants with non-HIV related causes of death were significantly older (P = .020), had been longer on ART (P = .007), had lower viral load (P = .002), and higher median hemoglobin (g/dL) (P = .035). Interestingly, a higher proportion (73.9%) of those with CD4 ≤ 350 cells/µL had non-HIV related deaths compared to 42.5% with CD4 ≥ 350 cells/ µL. HIV related deaths significantly reduced after year 2017 compared to the period 2013–2016 (P = .023); (Supplementary Table 2).
Figure 2.
Causes of death among PLWH. Cause of death was available for 54% (64/118) of PLWH. Only 8 autopsies were performed to identify cause of death. Most frequent causes of death included infection in 19/64 (29.7%) and malignancy in 18/64 (28.1%). A slightly higher proportion of deaths (53.1%) were from non-HIV related causes. Abbreviations: HIV, human immunodeficiency virus; PLWH, people living with HIV.
Five deaths were observed among participants without HIV with the cause of death being available in 3 participants: stroke, postpartum hemorrhage, and bacteremia.
Mortality Risk Factors
Site and sociodemographic factors including, religion, food insecurity, stigma, marital status, and use of traditional healers were associated with mortality in unadjusted models but were not significant in multivariable models (Table 2). In the multivariable model, independent predictors of mortality included CD4 ≤ 350 cells/µl (adjusted hazard ratio [aHR]: 2.01 [95% CI: 1.31–3.08]), a 1 log10copies/mL increase in viral load (aHR 1.36 [95% CI: 1.21–1.51], fever in the previous 6 months (aHR: 1.85 [95% CI: 1.22–2.81]), underweight (aHR: 2.20 [95% CI: 1.44–3.38]), WHO stage III (aHR 2.18 [95% CI 1.31–3.61]), clinical depression (aHR: 2.42 [95% CI: 1.40–4.18]), having any infection at the most recent visit (aHR:1.77 [95% CI:1.21–2.59]), a g/dL increase in hemoglobin (aHR:0.79 [95% CI .72–.85]) and every year on ART (aHR:0.67 [95% CI: .56–.81]).).
Table 2.
Bivariate and Multivariable Cox Proportional Hazards Regression Model for Mortality Risk Associated Factors
| Unadjusted Analyses | Adjusted Analyses | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Duration (years) on ART | 0.67 (.61–.73) | <.001 | 0.67 (.56–.81) | <.001 |
| ART status | ||||
| ART experienced | 1.00 | … | … | |
| ART naive | 6.32 (3.91–10.23) | <.001 | … | … |
| CD4 [cells/µL] | ||||
| >350 | 1.00 | 1.00 | ||
| ≤350 | 5.11 (3.52–7.42) | <.001 | 2.01 (1.31–3.08) | .001 |
| HIV RNA [log10copies/mL] | 2.00 (1.83–2.17) | <.001 | 1.36 (1.22–1.51) | <.001 |
| History of fever in the last 6 months | ||||
| No | 1.00 | 1.00 | ||
| Yes | 3.51 (2.43–5.08) | <.001 | 1.85 (1.22–2.81) | .004 |
| BMI | ||||
| Underweight | 3.58 (2.35–5.44) | <.001 | 2.20 (1.44–3.38) | <.001 |
| Normal weight | 1.00 | 1.00 | ||
| Overweight | 0.68 (.39–1.17) | .166 | 1.08 (.61–1.91) | .784 |
| Obese | 0.33 (.12–.91) | .032 | 0.44 (.14–1.42) | .171 |
| Hemoglobin (g/dL) | 0.69 (.65–.74) | <.001 | 0.79 (.72–.85) | <.001 |
| WHO classification | ||||
| Asymptomatic HIV infection only | 1.00 | 1.00 | ||
| WHO Stage I | … | … | … | … |
| WHO Stage II | 0.95 (.53–1.71) | .861 | 0.88 (.48–1.62) | .689 |
| WHO Stage III | 2.08 (1.26–3.45) | .004 | 2.18 (1.31–3.61) | .003 |
| WHO Stage IV | 1.91 (0.89–4.08) | .095 | 2.16 (.99–4.74) | .054 |
| Clinical depression | ||||
| No | 1.00 | 1.00 | ||
| Yes | 10.17 (6.82–15.17) | <.001 | 2.42 (1.40–4.18) | .001 |
| Any immediate family member diagnosed with HIV | ||||
| No | 1.00 | 1.00 | ||
| Yes | 5.34 (3.05–9.45) | <.001 | 2.32 (1.08–5.02) | .032 |
| Infectionsa | ||||
| No | 1.00 | 1.00 | ||
| Yes | 3.11 (2.16–4.48) | <.001 | 1.77 (1.20–2.59) | .004 |
| Malignanciesb | ||||
| No | 1.00 | … | … | |
| Yes | 27.89 (13.53–57.50) | <.001 | … | … |
| ART regimen | ||||
| NRTI, I | 0.02 (0.02–0.15) | <.001 | … | … |
| NRTI, NNRTI | 1.86 (1.03–3.36) | .041 | … | … |
| NRTI, PI | 1.00 | … | … | |
| Others | 1.26 (0.16–9.64) | .824 | … | … |
Unadjusted and adjusted Cox proportional hazards regression models were run to identify risk factors associated with mortality at the most recent visit by estimating HRs and associated 95% confidence intervals (CIs). All factors except WHO classification were assessed at participants’ most recent visit, WHO classification was only assessed at enrollment in this study. No deaths were observed among participants with WHO Stage I. Duration (years) on ART was modeled as a time varying covariate. Using backwards stepwise selection, factors with a P value > .2 were removed the multivariable model and statistical significance observed at P < .05. A total of 2705 participants with complete and non-missing data for all the adjusted factors, were considered in the adjusted model.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; I, integrase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WHO, World Health Organization.
Any infection reported at the most recent visit to include: bacteremia, bacterial vaginosis/trichomonas/chlamydia, candidiasis-oral/esophageal/vaginal, chronic hepatitis B, chronic hepatitis C, conjunctivitis, extrapulmonary cryptococcosis – central nervous system (CNS)/men, gastroenteritis, genital ulcer disease nitric oxide synthase (NOS), gingivitis/periodontitis/sinusitis/otitis media, herpes simplex infection, malaria, meningitis/encephalitis (bacterial/toxoplasma), pelvic inflammatory disease, pneumonia (non-cytomegalovirus [CMV]/non-pneumocystis pneumonia [PCP]), proctitis, pruritis (itch without rash), tuberculosis –pulmonary/ extra pulmonary, schistosomiasis, syphilis, tinea infection, upper respiratory infection, urinary tract infection, wound infection/ abscess/ cellulitis, wound infection/abscess/cellulitis.
Any malignancy present at the most recent visit to include anaplastic glioma/glioblastoma, brain tumor NOS, breast cancer, cancer of unknown primary (occult primary), cervical carcinoma, Kaposi’s sarcoma – gastrointestinal, Kaposi’s sarcoma– skin.
The final multivariable model was robust to sensitivity analyses that reclassified the failure event as either death or loss to follow-up (Supplementary Table 3).
DISCUSSION
Our study describes all-cause mortality and its predictors among PLWH across several sites in Uganda, Kenya, Tanzania, and Nigeria. Key study findings were a low overall mortality rate at 11.42/1000 PY with variations across study sites, non-HIV related causes of death contributing significantly to mortality and several, mostly biological predictors of mortality, including CD4, viral load, hemoglobin level, and low BMI. Variations in mortality across African countries has been reported in other studies [18]. The differences we observed could be attributed to differences in populations whether urban or rural, cultures and economies across the populations or tracing of losses to follow-up. Additionally, Kayunga, Uganda site had higher proportion of participants not on ART at enrolment compared to other sites with higher mortality observed in the first year of follow-up. Declining mortality rates observed as the study progressed are in line with studies that showed reduction in mortality over the years following the introduction of ART [2, 14]. In addition, mortality rates in our study are further impacted by concerted efforts of HIV programs toward UNAIDS 95-95-95 treatment targets [15], most recently the test and treat policy, better follow-up in a study environment, and the introduction of newer regimens that have improved viral suppression [19, 20]. Mortality among PLWH in 2019–2020 (4.74/1000 PY, 95% CI: 2.37–9.48) overlapped with that among participants without HIV (2.95/1000 PY, 95% CI: 1.23–7.09) further highlighting the success of efforts toward epidemic control.
In the multivariable model, mortality predictors identified included CD4 cell count/µL, log10 copies/mL of viral load, low BMI (Kg/m2) low hemoglobin (g/dL), and WHO staging as have similarly been reported elsewhere [12–14, 21]. Our study shows a harmful effect of depression on mortality. Several studies have shown depression to be associated with mortality in PLWH [22, 23]. One study found a risk for mortality to be 3.6 times higher among women living with HIV who had depressive symptoms [23]. This risk was determined to be greater than the mortality risk associated with not initiating ART. The same study showed a doubling of the risk among those with depressive symptoms and not on ART. Compared to other studies, the modest risk seen in our study could be because the majority of participants were on ART and virally suppressed. Routine screening for depression as part of comprehensive care in HIV programs may reduce the risk of mortality even further. Depression may accelerate HIV disease progression through biological and behavioral mechanisms to include alterations in glucocorticoids and catecholamine with effects on cellular immunity and poor social support, behavior, and nonadherence to medication [24, 25].
Several infections at the most recent visit were associated with an increased risk of death. Studies have implicated co-infections as a major driver of morbidity and mortality among PLWH [26–29]. In 2017, WHO reported that tuberculosis caused 1 in 3 of HIV-related deaths [30], and recommended that the 2 epidemics be handled concurrently within affected communities. Several studies have reported significant declines in tuberculosis incidence and mortality with use of antiretroviral treatment [31, 32]. Rapid scale-up of ART in Africa coupled with more recent tuberculosis prevention programs provide opportunities for reduction in tuberculosis mortality. In our study, only 7 of the 64 participants with known causes of death were due to tuberculosis. Tuberculosis screening and tuberculosis preventive therapy (TPT), which are routinely implemented in the cohort and efforts toward UNAIDS 95-95-95, are potential explanations for this observation. However, although the last few years have seen an emphasis on tuberculosis prevention in HIV programs, potential barriers that result in underutilization and scaling-up of TPT in resource limited settings must be overcome [33]. Manageable conditions like tuberculosis that were associated with mortality in our study indicate a need for programs to enhance screening and diagnosis of these comorbidities early enough to prevent mortality among PLWH.
In the era of antiretroviral therapy, varied causes of death have been reported to include non-HIV related conditions [34, 35]. The pattern of causes of death seen in our study is similar to what has been reported in other studies [10]. Among participants with a known cause of death, the highest mortality rate was attributed to infections, mostly tuberculosis, followed by malignancies, anemia, and cardiovascular disease highlighting a need to improve clinical acumen, early diagnosis, and preventive measures to counteract preventable deaths. Establishment of ART has led to a change in the epidemiology of cancer among PLWH [36, 37], as seen in our study where esophageal malignancies, pancreatic carcinoma, breast cancer, and melanoma were added to the list. Awareness of potential for the unusual malignancies and increasing cancer prevention and screening among this population is key in order to initiate treatment early and avert death. Cervical cancer was a common cause of death in our study and highlights the need for aggressive cervical cancer screening in HIV programs. Cardiovascular diseases as a cause of death has been seen in other studies and is likely to increase as people stay longer on ART [38].
The major limitation of our study was the inability to determine the cause of death in many of the participants. We used all possible means to obtain the cause of death to include hospital medical records when available, phone interviews or home visits. However, in 46% of participants, the cause of death could not be determined. In addition, there is a possibility of ascertainment bias resulting from potential misclassification of the causes of death in the study population. As has been noted in other resource limited settings [39], autopsy reports were uncommon among participants who died in this study, and verbal autopsies were not systematically performed to identify the cause of death. It is also possible that some participants who were lost to follow-up could have experienced death, but sensitivity analyses yielded similar results to the primary analysis. Because the analysis was cross-sectional at the most recent visit, it is possible that some of the factors identified as predictors of mortality such as anemia and low BMI may be the result of underlying disease leading to mortality rather than independent predictors. In addition, results from our study may not be generalizable to other populations, considering that people who enroll in cohort studies might be healthier and more engaged in care than the general population.
Our findings suggest low mortality among mostly virally suppressed PLWH as compared to prior studies, but mortality among PLWH was still more than 3 times higher than among contemporaneous comparators without HIV. Non-HIV related causes of death identified in the study serve as targets for interventions to reduce mortality. Viral suppression and concomitant immune reconstitution are critical deterrents of HIV-related mortality that are already prioritized by UNAIDS targets and have mostly been achieved within our cohort. More importantly, we identified low BMI and depression as independent predictors of mortality among PLWH that could be directly addressed through interventions such as the integration of nutritional and mental health services into comprehensive HIV care programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, local implementing partners, and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Mbeya Regional Referral Hospital, Defence Headquarters Medical Center, and the 68th Nigerian Army Reference Hospital.
The authors would also like to thank the AFRICOS Study Group—from the US Military HIV Research Program Headquarters Group: Danielle Bartolanzo, Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Steven Schech, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Ajay Parikh, Jaclyn Hern, Emma Duff, Kara Lombardi, Michelle Imbach, and Leigh Anne Eller; from the AFRICOS Uganda Group: Hannah Kibuuka, Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Claire Nakazzi Bagenda, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly, and Barbara Mukanza; from the AFRICOS South Rift Valley, Kenya Group: Jonah Maswai, Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, and Ignatius Kiptoo; from the AFRICOS Kisumu, Kenya Group: John Owuoth, Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo, and George Suja; from the AFRICOS Abuja, Nigeria Group: Michael Iroezindu, Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence C. Umeji, Onome Enas, Miriam Ayogu, Ijeoma Chigbu-Ukaegbu, Wilson Adai, Felicia Anayochukwu Odo, Rabi Abdu, Roseline Akiga, Helen Nwandu, Chisara Sylvestina Okolo, Ogundele Taiwo, Otene Oche Ben, Nicholas Innocent Eigege, Tony Ibrahim Musa, Juliet Chibuzor Joseph, Ndubuisi C. Okeke; from the AFRICOS Lagos, Nigeria Group: Zahra Parker, Nkechinyere Elizabeth Harrison, Uzoamaka Concilia Agbaim, Olutunde Ademola Adegbite, Ugochukwu Linus Asogwa, Adewale Adelakun, Chioma Ekeocha, Victoria Idi, Rachel Eluwa, Jumoke Titilayo Nwalozie, Igiri Faith, Blessing Irekpitan Wilson, Jacinta Elemere, Nkiru Nnadi, Francis Falaju Idowu, Ndubuisi Rosemary, Amaka Natalie Uzeogwu, Theresa Owanza Obende, Ifeoma Lauretta Obilor, Doris Emekaili, Edward Akinwale, and Inalegwu Ochai; from the AFRICOS Mbeya, Tanzania Group: Lucas Maganga, Emmanuel Bahemana, Samoel Khamadi, John Njegite, Connie Lueer, Abisai Kisinda, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Dorothy Mkondoo, Nancy Somi, Paschal Kiliba, Ephrasia Mwalongo, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, and Willyhelmina Olomi.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70-25.
Financial support. This work was supported by the President’s Emergency Plan for AIDS Relief via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the US Department of Defense (grant number W81XWH-18-2-0040). J. A. A. reports support from Military Infectious Disease Research Program, PEPFAR. T. A. C. reports support from US Army.
Contributor Information
Hannah Kibuuka, Makerere University Walter Reed Project, Kampala, Uganda.
Ezra Musingye, Makerere University Walter Reed Project, Kampala, Uganda.
Betty Mwesigwa, Makerere University Walter Reed Project, Kampala, Uganda.
Michael Semwogerere, Makerere University Walter Reed Project, Kampala, Uganda.
Michael Iroezindu, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; HJF Medical Research International, Abuja, Nigeria.
Emmanuel Bahemana, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; HJF Medical Research International, Mbeya, Tanzania.
Jonah Maswai, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; HJF Medical Research International, Kericho, Kenya.
John Owuoth, US Army Medical Research Directorate—Africa, Kisumu, Kenya; HJF Medical Research International, Kisumu, Kenyaand.
Allahna Esber, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Nicole Dear, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Trevor A Crowell, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Christina S Polyak, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Julie A Ake, US Military Human Immunodeficiency Virus (HIV) Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA.
AFRICOS Study Group:
Alexus Reynolds, Katherine Song, Mark Milazzo, Leilani Francisco, Steven Schech, Badryah Omar, Tsedal Mebrahtu, Elizabeth Lee, Kimberly Bohince, Ajay Parikh, Jaclyn Hern, Emma Duff, Kara Lombardi, Michelle Imbach, Leigh Anne Eller, Hannah Kibuuka, Michael Semwogerere, Prossy Naluyima, Godfrey Zziwa, Allan Tindikahwa, Claire Nakazzi Bagenda, Hilda Mutebe, Cate Kafeero, Enos Baghendaghe, William Lwebuge, Freddie Ssentogo, Hellen Birungi, Josephine Tegamanyi, Paul Wangiri, Christine Nabanoba, Phiona Namulondo, Richard Tumusiime, Ezra Musingye, Christina Nanteza, Joseph Wandege, Michael Waiswa, Evelyn Najjuma, Olive Maggaga, Isaac Kato Kenoly, Barbara Mukanza, Jonah Maswai, Rither Langat, Aaron Ngeno, Lucy Korir, Raphael Langat, Francis Opiyo, Alex Kasembeli, Christopher Ochieng, Japhet Towett, Jane Kimetto, Brighton Omondi, Mary Leelgo, Michael Obonyo, Linner Rotich, Enock Tonui, Ella Chelangat, Joan Kapkiai, Salome Wangare, Zeddy Bett Kesi, Janet Ngeno, Edwin Langat, Kennedy Labosso, Joshua Rotich, Leonard Cheruiyot, Enock Changwony, Mike Bii, Ezekiel Chumba, Susan Ontango, Danson Gitonga, Samuel Kiprotich, Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, Ignatius Kiptoo, John Owuoth, Valentine Sing’oei, Winne Rehema, Solomon Otieno, Celine Ogari, Elkanah Modi, Oscar Adimo, Charles Okwaro, Christine Lando, Margaret Onyango, Iddah Aoko, Kennedy Obambo, Joseph Meyo, George Suja, Michael Iroezindu, Yakubu Adamu, Nnamdi Azuakola, Mfreke Asuquo, Abdulwasiu Bolaji Tiamiyu, Afoke Kokogho, Samirah Sani Mohammed, Ifeanyi Okoye, Sunday Odeyemi, Aminu Suleiman, Lawrence C Umeji, Onome Enas, Miriam Ayogu, Ijeoma Chigbu-Ukaegbu, Wilson Adai, Felicia Anayochukwu Odo, Rabi Abdu, Roseline Akiga, Helen Nwandu, Chisara Sylvestina Okolo, Ogundele Taiwo, Otene Oche Ben, Nicholas Innocent Eigege, Tony Ibrahim Musa, Juliet Chibuzor Joseph, Ndubuisi C Okeke, Zahra Parker, Nkechinyere Elizabeth Harrison, Uzoamaka Concilia Agbaim, Olutunde Ademola Adegbite, Ugochukwu Linus Asogwa, Adewale Adelakun, Chioma Ekeocha, Victoria Idi, Rachel Eluwa, Jumoke Titilayo Nwalozie, Igiri Faith, Blessing Irekpitan Wilson, Jacinta Elemere, Nkiru Nnadi, Francis Falaju Idowu, Ndubuisi Rosemary, Amaka Natalie Uzeogwu, Theresa Owanza Obende, Ifeoma Lauretta Obilor, Doris Emekaili, Edward Akinwale, Inalegwu Ochai, Lucas Maganga, Emmanuel Bahemana, Samoel Khamadi, John Njegite, Connie Lueer, Abisai Kisinda, Jaquiline Mwamwaja, Faraja Mbwayu, Gloria David, Mtasi Mwaipopo, Reginald Gervas, Dorothy Mkondoo, Nancy Somi, Paschal Kiliba, Ephrasia Mwalongo, Gwamaka Mwaisanga, Johnisius Msigwa, Hawa Mfumbulwa, Peter Edwin, and Willyhelmina Olomi
References
- 1. Palella F, Jr., Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2. Reniers G, Slaymaker E, Nakiyingi-Miiro J, et al. Mortality trends in the era of antiretroviral therapy: evidence from the network for analysing longitudinal population based HIV/AIDS data on Africa (ALPHA). AIDS 2014; Supplement 4:S533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Global Health Observatory (GHO) data 2020 [cited 2020 30/7]. Number of deaths due to HIV/AIDS-situation and trends]. Available at: https://www.who.int/gho/hiv/epidemic_status/deaths_text/en/. Accessed 30 July 2020.
- 4. WHO. Global HIV programme. Available at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics. Accessed 10 August 2021.
- 5. WHO. Global health estimates: life expectancy and leading causes of death and disability. Available at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed 26 March 2021.
- 6. Song A, Liu X, Huang X, et al. From CD4-based initiation to treating all HIV-infected adults immediately: an evidence-based meta-analysis. Front Immunol 2018; 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esber AL, Coakley P, Ake JA, et al. Decreasing time to antiretroviral therapy initiation after HIV diagnosis in a clinic-based observational cohort study in four African countries. J Int AIDS Soc 2020; 23:e254–46-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saavedra A, Campinha-Bacote N, Hajjar M, et al. Causes of death and factors associated with early mortality of HIV-infected adults admitted to Korle-Bu Teaching Hospital. Pan Afr Med J 2017; 27:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2:e35–46. [DOI] [PubMed] [Google Scholar]
- 10. Kiragga AN, Mubiru F, Kambugu AD, Kamya MR, Castelnuovo B.. A decade of antiretroviral therapy in Uganda: what are the emerging causes of death? BMC Infect Dis 2019; 19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chimbetete C, Shamu T, Roelens M, Bote S, Mudzviti T, Keiser O.. Mortality trends and causes of death among HIV positive patients at Newlands Clinic in Harare, Zimbabwe. PLoS One 2020; 15:e0237904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunda DW, Nkandala I, Kilonzo SB, Kilangi BB, Mpondo BC.. Prevalence and risk factors of mortality among adult HIV patients initiating ART in rural setting of HIV care and treatment services in Northwestern Tanzania: a retrospective cohort study. J Sex Transm Dis 2017; 2017:7075601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silverman RA, John-Stewart GC, Beck IA, et al. Predictors of mortality within the first year of initiating antiretroviral therapy in urban and rural Kenya: a prospective cohort study. PLoS One 2019; 14:e02234–11-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubaihayo J, Tumwesigye NM, Konde-Lule J, et al. Trends and predictors of mortality among HIV positive patients in the era of highly active antiretroviral therapy in Uganda. Infect Dis Rep 2015; 7:5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. UNAIDS. Understanding fast-track accelerating action to end the AIDS epidemic by 2030. 2015. Available at https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf. Accessed 10 August 2021.
- 16. Ake JA, Polyak CS, Crowell TA, et al. Noninfectious comorbidity in the African Cohort Study. Clin Infect Dis 2019; 69:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401. [Google Scholar]
- 18. Johnson LF, Anderegg N, Zaniewski E, et al. Global variations in mortality in adults after initiating antiretroviral treatment: an updated analysis of the International epidemiology Databases to Evaluate AIDS cohort collaboration. AIDS 2019; 33:S283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 20. Nabitaka VM, Nawaggi P, Campbell J, et al. High acceptability and viral suppression of patients on Dolutegravir-based first-line regimens in pilot sites in Uganda: a mixed-methods prospective cohort study. PLoS One 2020; 15:e0232419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayalew MB. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. Aids Res Treat 2017; 2017:5415298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. So-Armah K, Gupta SK, Kundu S, et al. Depression and all-cause mortality risk in HIV-infected and HIV-uninfected US veterans: a cohort study. HIV Med 2019; 20:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesko CR, Todd JV, Cole SR, et al. Mortality under plausible interventions on antiretroviral treatment and depression in HIV-infected women: an application of the parametric g-formula. Ann Epidemiol 2017; 27:783–789.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry 2003; 54:295–306. [DOI] [PubMed] [Google Scholar]
- 25. Schuster R, Bornovalova M, Hunt E.. The influence of depression on the progression of HIV: direct and indirect effects. Behav Modif 2012; 36:123–45. [DOI] [PubMed] [Google Scholar]
- 26. Matthews PC, Geretti AM, Goulder PJ, Klenerman P.. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol 2014; 61:20–33. [DOI] [PubMed] [Google Scholar]
- 27. Crowell TA, Berry SA, Fleishman JA, et al. Impact of hepatitis coinfection on healthcare utilization among persons living with HIV. J Acquir Immune Defic Syndr 2015; 68:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crowell TA, Gebo KA, Balagopal A, et al. Impact of hepatitis coinfection on hospitalization rates and causes in a multicenter cohort of persons living with HIV. J Acquir Immune Defic Syndr 2014; 65:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tshitenge S, Ogunbanjo GA, Citeya A.. A mortality review of tuberculosis and HIV co-infected patients in Mahalapye, Botswana: does cotrimoxazole preventive therapy and/or antiretroviral therapy protect against death? Afr J Prim Health Care Fam Med 2018; 10:e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. WHO. HIV/AIDS: TB Causes 1 in 3 HIV Deaths. 2018. Available at: https://www.who.int/hiv/mediacentre/news/hiv-tb-patient-centred-care/en/. Accessed 6 October 2020. [Google Scholar]
- 31. Lawn SD, Kranzer K, Wood R.. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med 2009; 30:685–99, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Girardi E, Palmieri F, Cingolani A, et al. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 26:326–31. [DOI] [PubMed] [Google Scholar]
- 33. Pathmanathan I, Ahmedov S, Pevzner E, et al. TB preventive therapy for people living with HIV: key considerations for scale-up in resource-limited settings. Int J Tuberc Lung Dis 2018; 22:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mak DW-S, Hui EK, Chan L, Lam W, Lee MP, Lau SMJ.. Non-AIDS defining malignancies among HIV-infected patients in a tertiary referral center in Hong Kong. J Clin Oncol 2018; 36:1576. [Google Scholar]
- 35. Farahani M, Mulinder H, Farahani A, Marlink R.. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:636–50. [DOI] [PubMed] [Google Scholar]
- 36. Simard EP, Engels EA.. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010; 51:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiels MS, Engels EA.. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS 2017; 12:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freiberg MS, So-Armah K.. HIV and cardiovascular disease: we need a mechanism, and we need a plan. J Am Heart Assoc 2016; 4:e003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cox JA, Lukande RL, Kateregga A, Mayanja-Kizza H, Manabe YC, Colebunders R.. Autopsy acceptance rate and reasons for decline in Mulago Hospital, Kampala, Uganda. Trop Med Int Health 2011; 16:1015–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.