Abstract
Background
Improved understanding of the epidemiology and mortality risk factors of extrapulmonary tuberculosis (EPTB) may facilitate successful diagnosis and management.
Methods
We analyzed national surveillance data from Ukraine to characterize EPTB subtypes (ie, localized in different anatomic sites). We calculated annual reported incidence, stratified by age, sex, and human immunodeficiency virus (HIV) status. Using Cox regression, we estimated mortality risk factors.
Results
Between January 2015 and November 2018, 14 062 adults/adolescents (≥15 years) and 417 children (<15 years) had EPTB with or without concomitant pulmonary TB. The most commonly reported EPTB subtypes were pleural, peripheral lymph node, and osteoarticular. Most EPTB subtype notifications peaked at age 30–39 years and were higher in males. In adults/adolescents, most peripheral TB lymphadenitis, central nervous system (CNS) TB, and abdominal TB occurred in those with untreated HIV. CNS TB notifications in people without HIV peaked before age 5 years. Adults/adolescents with CNS TB (adjusted hazard ratio [aHR]: 3.22; 95% CI: 2.89–3.60) and abdominal TB (aHR: 1.83; 95% CI: 1.59–2.11) were more likely to die than those with pulmonary TB. Children with CNS TB were more likely to die (aHR: 88.25; 95% CI: 43.49–179.10) than those with non-CNS TB. Among adults/adolescents, older age and HIV were associated with death. Rifampicin resistance was associated with mortality in pleural, peripheral lymph node, and CNS TB.
Conclusions
We identified the most common EPTB subtypes by age and sex, patterns of EPTB disease by HIV status, and mortality risk factors. These findings can inform diagnosis and care for people with EPTB.
Keywords: epidemiology, public health surveillance, children, adolescents, tuberculous meningitis
Improved understanding of extrapulmonary tuberculosis may facilitate diagnosis and management. This analysis of national surveillance data from Ukraine describes age- and sex-stratified notification rates and identifies mortality risk factors for the most common extrapulmonary tuberculosis subtypes in adults/adolescents and children.
Most individuals with tuberculosis (TB) have pulmonary disease, but when the immune system fails to contain Mycobacterium tuberculosis within the lungs, M. tuberculosis can seed other organs and cause extrapulmonary TB (EPTB) [1]. Accordingly, EPTB occurs more commonly, but not exclusively, in immunocompromised people [2]. Extrapulmonary TB may involve 1 or more sites, either with or without concomitant pulmonary TB. Due to nonspecific symptoms, the paucibacillary nature of some subtypes (ie, localized in different anatomic sites), and limited capacity for invasive sampling in resource-constrained settings, EPTB can be challenging to diagnose [3].
Data on EPTB epidemiology, treatment outcomes, and mortality risk factors remain limited. Sixteen percent of the 7.1 million incident TB cases reported to the World Health Organization (WHO) in 2019 were exclusively extrapulmonary [4]. However, because this figure does not include people with concomitant pulmonary disease and because EPTB is likely underdiagnosed, the true incidence is unknown. The WHO does not stratify EPTB incidence by age, sex, human immunodeficiency virus (HIV) status, or subtype; nor does the WHO report EPTB treatment outcomes [4]. Few studies have disaggregated pediatric EPTB data [5–17] or identified risk factors for EPTB mortality [9, 10]. Yet, these indicators are critical for identifying shortcomings in detection and patient characteristics associated with disease and death.
To fill these gaps, we analyzed programmatic data from the National TB Program (NTP) in Ukraine, where EPTB comprises just under 10% of TB case notifications (ie, reported incidence) [18, 19]. For the most commonly reported EPTB subtypes in Ukraine, we aimed to (1) describe the notification rate, stratified by age and sex, and calculate the proportion with HIV coinfection; (2) estimate the mortality risk compared with pulmonary TB; and (3) identify mortality risk factors.
METHODS
Setting
In 2019, Ukraine had an estimated TB incidence of 77 per 100 000 [4]. According to Ukrainian NTP guidelines, evaluation for pulmonary TB should include respiratory specimen collection for smear microscopy; mycobacterial culture; Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA); drug susceptibility test (DST) to rifampicin, isoniazid, ethambutol, pyrazinamide, and streptomycin; posterior-anterior and lateral chest radiographs (CXRs); and HIV testing [20]. Ukraine’s NTP has no guidelines for diagnosing EPTB.
In Ukraine, patients with drug-susceptible pulmonary TB receive 2 months of isoniazid, rifampicin, pyrazinamide, and ethambutol (intensive phase), followed by 4 months of isoniazid and rifampicin (continuation phase). Patients with drug-susceptible EPTB receive the same regimen—with the exception of those with central nervous system (CNS) TB and TB osteomyelitis, for whom the continuation phase is 7 to 10 months. Patients with rifampicin-resistant TB (RR-TB) of any site (pulmonary or EPTB) receive 20-month regimens composed of 4 or more drugs to which their infecting strain is susceptible. Corticosteroids are given for 6 or more weeks at treatment initiation for all CNS TB [20].
Data Collection
On 9 November 2018, we downloaded de-identified data from eTB Manager, an online electronic case registry established in 2013 to facilitate the reporting of TB cases to Ukraine’s NTP. Other than hospitals and clinics in Russian-controlled areas of Donetsk and Luhansk, all medical facilities, both public (which manage the vast majority of TB cases) and private, enter cases into eTB Manager. Variables collected for each patient include age, sex, urban (cities) versus rural (suburbs, towns, villages, or farms) residence, alcohol dependence, injection drug use, history of incarceration, homelessness, history of migration (immigration, refugee status, or internal displacement), unemployment, work in healthcare, HIV and antiretroviral therapy (ART) status at treatment initiation, new versus recurrent TB, rifampicin susceptibility, anatomic site(s) of TB, diagnostic method (microbiologically confirmed vs clinically diagnosed), and treatment outcome. Substance use is self-reported.
Definitions
We defined EPTB as TB diagnosed at sites other than the lung parenchyma, intrathoracic lymph nodes, or airway. Due to imprecision of the “airway” category, we could not differentiate laryngeal TB from TB of the intrathoracic airway. Central nervous system TB included cases labeled as TB meningitis, tuberculoma, or “nervous system TB not otherwise specified.” Abdominal TB encompassed TB of the intraabdominal lymph nodes, peritoneum, and/or abdominal organs. In eTB Manager, miliary TB is defined as radiographic abnormalities in more than 1 lung field and is not restricted to cases with widespread millet-like lesions; therefore, we did not consider miliary TB in our analysis.
We defined recurrent TB as disease in a patient who was previously treated for TB, with the most recent regimen having ended 6 or more months ago with an outcome of cure or treatment completion. Microbiological confirmation included smear, culture, and/or Xpert MTB/RIF positivity from any involved organ. eTB Manager does not report specific sample type, but in cases involving only 1 site we assumed the sample was taken from that site. Clinical diagnosis was based on symptoms, exam and imaging findings, and contact with a known TB case. Rifampicin susceptibility was either confirmed by the patient’s own DST or presumed based on the source case’s DST. We defined death as all-cause mortality or transfer to palliative care during treatment.
Inclusion and Exclusion Criteria
The unit of analysis was the incident TB case. A patient could contribute more than 1 case if 6 or more months passed between the cure or treatment completion of 1 regimen and the initiation of a subsequent regimen. As data collection was incomplete during the first 2 years of eTB Manager’s implementation (2013–2014), we excluded cases in which the patient started TB treatment before 1 January 2015. We analyzed only EPTB subtypes for which 500 or more cases, with or without concomitant involvement of the lungs or other extrapulmonary sites, were reported.
Statistical Analysis
We conducted 3 sets of analyses. First, for each EPTB subtype, we calculated the average annual notification rate per 100 000, disaggregated by sex and age, between 2015 and 2017. We excluded 2018 because we lacked the complete year’s data. The notification rates included both cases with TB exclusively in that site as well as those with involvement of other sites. Age- and sex-disaggregated population estimates were obtained from the World Bank [21]. Due to the lack of age- and sex-stratified population estimates for people with HIV, we could not calculate TB incidence for this group, but we reported the proportion of TB cases with HIV coinfection. We did not calculate 95% confidence intervals (CIs) because notification rates were meant to be descriptive, not inferential.
Second, using multivariable Cox proportional hazards regression, we estimated the hazard of death associated with each EPTB subtype and the number of diseased sites (including lungs). For each age group, we built 2 regression models. In model 1, we included explanatory variables indicating if each EPTB subtype was present, plus a variable for the number of sites involved. The reference group was exclusively pulmonary TB. In model 2, we further adjusted for all plausible confounders and effect modifiers reported in eTB Manager: age, sex, new versus recurrent TB, microbiological versus clinical diagnosis, rifampicin susceptibility, HIV/ART status, urban versus rural residence, substance use, homelessness, employment, history of incarceration, and migration status. In the pediatric group, we examined death associated with CNS TB only because no more than 1 death was reported for all other EPTB subtypes. The reference group was any non-CNS, including pulmonary, TB in only 1 site. We adjusted for the same confounders and effect modifiers as for the adult/adolescent group except for substance use, employment, and incarceration, which were not recorded for children.
Third, in the adult/adolescent group, we used Cox regression to identify demographic and clinical risk factors for death associated with TB in each anatomic site, compared to a lack of involvement of that site. Due to small numbers in the pediatric group, we compared mortality risk factors using Fisher’s exact test.
For Cox regression in the second and third sets of analysis, we checked the proportional hazards assumption for each explanatory variable by plotting and visually inspecting log-log survival estimates against time for categorical variables. We used time-varying coefficients to stratify explanatory variables if the resulting log-log–transformed survival curves were not reasonably parallel. We then built multivariable models using backward selection. We started with a full model with all explanatory variables, which were then removed one by one in descending order of P value. The goodness of fit of the successive, nested models was compared using the likelihood ratio test until a statistically significant difference (P < .05) was detected. The final models were those with the fewest parameters but whose fit did not significantly differ from the larger models. We used backward selection for its computational simplicity and the advantage of assessing the joint predictive ability of all available explanatory variables. For the resulting multivariable model, we verified the proportional hazards assumption by examining the Schoenfeld residuals.
Because only 2203 (1.9%) of 114 609 patients in the entire dataset contributed more than 1 case, we did not adjust for clustering on the individual.
We conducted analyses using Excel for Microsoft 365 (Microsoft Corporation, Redmond, WA, USA) and R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics Approval
This study used nonidentifiable data and, therefore, was deemed exempt from institutional review board (IRB) approval by Bogomolets National Medical University (Kyiv City, Ukraine), the Public Health Center of the Ministry of Health (Kyiv City, Ukraine), and Boston University (Boston, MA, USA). The IRB of the Lifespan Health System (Providence, RI, USA) approved this study and waived informed consent.
RESULTS
Extrapulmonary Tuberculosis Notifications
During the study period, Ukraine’s NTP recorded 112 339 TB cases in patients 15 years and older. Of these cases, 98 277 (87.5%) were exclusively pulmonary, 7328 (6.5%) were exclusively extrapulmonary, and 6734 (6.0%) were both pulmonary and extrapulmonary. Of 2251 incident TB cases recorded in patients younger than 15 years old, 1834 (81.5%) were exclusively pulmonary, 324 (14.4%) were exclusively extrapulmonary, and 93 (4.1%) were both. Tuberculosis of the eye, skin, ear, adrenal glands, and unspecified site each comprised fewer than 500 cases and thus were excluded from further analyses. Tables 1 and 2 provide the total notifications and characteristics of the EPTB subtypes.
Table 1.
Demographic and Clinical Characteristics of Adults and Older Adolescents, Stratified by Site of Tuberculosis Disease
| Disease Site or Number of Sites Affected, n (% of total cases) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Lung | Pleura | Peripheral Lymph Node | Bone/Joint | CNS | Abdomen | GU System | 1 Site | 2+ Sites | |
| Total cases | 112 339 | 105 011 | 6803 | 2369 | 2093 | 937 | 771 | 605 | 105 595 | 6744 |
| Age, mean (SD), years | 43.3 (14.0) |
43.3 (13.9) |
41.6 (13.5) |
39.7 (11.2) |
51.5 (14.5) |
39.0 (10.0) |
40.3 (10.2) |
46.5 (15.7) |
43.4 (14.1) |
41.7 (12.1) |
| Male sex | 80 384 (71.6) |
76 037 (72.4) |
4805 (70.6) |
1417 (59.8) |
1326 (63.4) |
576 (61.5) |
496 (64.3) |
331 (54.7) |
75 571 (71.6) |
4813 (71.4) |
| Urban residencea | 68 722 (61.9) |
63 789 (61.5) |
4104 (60.8) |
1719 (73.2) |
1438 (69.1) |
652 (70.0) |
556 (73.4) |
415 (69.6) |
64 410 (61.8) |
4312 (64.4) |
| Alcohol dependence | 16,649 (14.8) |
16288 (15.5) |
955 (14.0) |
175 (7.4) |
106 (5.1) |
147 (15.7) |
93 (12.1) |
22 (3.6) |
15 524 (14.7) |
1125 (16.7) |
| Injection drug use | 4476 (4.0) |
4105 (3.9) |
310 (4.6) |
269 (11.4) |
151 (7.2) |
133 (14.2) |
98 (12.7) |
16 (2.6) |
3901 (3.7) |
575 (8.5) |
| Homelessness | 3366 (3.0) |
3303 (3.1) |
204 (3.0) |
40 (1.7) |
18 (0.9) |
20 (2.1) |
22 (2.9) |
3 (0.5) |
3119 (3.0) |
247 (3.7) |
| Unemployed status | 53 739 (47.8) |
50 850 (48.4) |
3519 (51.7) |
1389 (58.6) |
724 (34.6) |
563 (60.1) |
408 (52.9) |
210 (34.7) |
49 743 (47.1) |
3996 (59.3) |
| Healthcare worker | 1270 (1.1) |
1132 (1.1) |
101 (1.5) |
16 (0.7) |
39 (1.9) |
9 (1.0) |
7 (0.9) |
13 (2.1) |
1213 (1.1) |
57 (0.8) |
| History of incarceration | 4750 (4.2) |
4605 (4.4) |
210 (3.1) |
52 (2.2) |
37 (1.8) |
32 (3.4) |
21 (2.7) |
5 (0.8) |
4537 (4.3) |
213 (3.2) |
| History of migration | 1279 (1.1) |
1202 (1.1) |
67 (1.0) |
29 (1.2) |
34 (1.6) |
16 (1.7) |
18 (2.3) |
7 (1.2) |
1190 (1.1) |
89 (1.3) |
| Recurrent TB | 27 557 (24.5) |
26 485 (25.2) |
985 (14.5) |
328 (13.8) |
388 (18.5) |
164 (17.5) |
131 (17.0) |
175 (28.9) |
26 347 (25.0) |
1210 (17.9) |
| Additional site(s) of TB disease | 6744 (6.0) |
6522 (6.2) |
4060 (59.7) |
1291 (54.5) |
444 (21.2) |
648 (69.2) |
481 (62.4) |
132 (21.8) |
NA | NA |
| Microbiologically confirmed TBb | 73 955 (65.8) |
72 776 (69.3) |
3324 (48.9) |
880 (37.1) |
339 (16.2) |
424 (45.3) |
311 (40.3) |
192 (31.7) |
69 654 (66.0) |
4301 (63.8) |
| Rifampicin-resistant TBc | 24 373 (21.7) |
24 096 (22.9) |
909 (13.4) |
303 (12.8) |
99 (4.7) |
159 (17.0) |
92 (11.9) |
53 (8.8) |
23 049 (21.8) |
1324 (19.6) |
| HIV/ART statusd | ||||||||||
| Without HIV | 84 411 (75.1) |
79 601 (75.8) |
4596 (67.6) | 322 (13.6) |
1658 (79.2) |
152 (16.2) | 146 (18.9) |
486 (80.3) |
81 328 (77.0) |
3083 (45.7) |
| With HIV/on ART | 5284 (4.7) |
4644 (4.4) |
344 (5.1) |
403 (17.0) |
202 (9.7) |
150 (16.0) |
140 (18.2) |
35 (5.8) |
4659 (4.4) |
625 (9.3) |
| With HIV/not on ART | 19 436 (17.3) |
17 764 (16.9) |
1738 (25.5) |
1616 (68.2) |
165 (7.9) |
619 (66.1) |
470 (61.0) |
63 (10.4) |
16 495 (15.6) |
2941 (43.6) |
| HIV status unknown | 3208 (2.9) |
3002 (2.9) |
125 (1.8) |
28 (1.2) |
68 (3.2) |
16 (1.7) |
15 (1.9) |
21 (3.5) |
3113 (2.9) |
95 (1.4) |
| Treatment outcome | ||||||||||
| Cure/ treatment completion | 67 083 (59.7) |
61 877 (58.9) |
4244 (62.4) |
1320 (55.7) |
1328 (63.4) |
237 (25.3) |
375 (48.6) |
473 (78.2) |
63 817 (60.4) |
3266 (48.4) |
| Deathe | 14 733 (13.1) |
14 028 (13.4) |
847 (12.5) |
414 (17.5) |
213 (10.2) |
510 (54.4) |
243 (31.5) |
34 (5.6) |
13 242 (12.5) |
1491 (22.1) |
| Treatment failure | 2001 (1.8) |
1976 (1.9) |
89 (1.3) |
26 (1.1) |
12 (0.6) |
9 (1.0) |
10 (1.3) |
4 (0.7) |
1877 (1.8) |
124 (1.8) |
| Transfer | 681 (0.6) |
660 (0.6) |
33 (0.5) |
8 (0.3) |
9 (0.4) |
7 (0.7) |
4 (0.5) |
2 (0.3) |
640 (0.6) |
41 (0.6) |
| Loss to follow-up | 7250 (6.5) |
6948 (6.6) |
395 (5.8) |
118 (5.0) |
81 (3.9) |
25 (2.7) |
30 (3.9) |
22 (3.6) |
6847 (6.5) |
403 (6.0) |
| Treatment in process | 20 591 (18.3) |
19 522 (18.6) |
1195 (17.6) |
483 (20.4) |
450 (21.5) |
149 (15.9) |
109 (14.1) |
70 (11.6) |
19 172 (18.2) |
1419 (21.0) |
Abbreviations: ART, antiretroviral therapy; CNS, central nervous system; DST, drug susceptibility test; GU, genitourinary; HIV, human immunodeficiency virus; NA, not applicable; TB, tuberculosis.
Data missing for 1360 participants.
Microbiological confirmation means that acid-fast bacilli were detected by smear microscopy and/or Mycobacterium tuberculosis was detected by culture and/or Xpert MTB/RIF (Cepheid) from any anatomic site. For patients with no other site of disease, the percentages of microbiologically confirmed cases were as follows: pulmonary, 69.6%; pleural, 21.9%; peripheral lymphadenitis, 17.7%; osteoarticular, 5.1%; CNS, 24.2%; abdominal, 13.5%; and GU system, 19.7%.
Rifampicin susceptibility was either confirmed by a DST of the patient’s own M. tuberculosis isolate or presumed based on a DST of the source case’s isolate.
HIV and ART statuses were determined at the time of TB treatment initiation.
Death was defined as all-cause mortality or transfer to palliative care before the end of the prescribed treatment. Of the patients with an outcome of death, the numbers (%) who were transferred to palliative care in each category were as follows: all, 1821 (12.4%); lung, 1810 (12.9%); pleura, 47 (5.5%); peripheral lymph node, 7 (1.7%); bone/joint, 5 (2.3%); CNS, 7 (1.4%); abdomen, 6 (2.5%); and GU, system 2 (5.9%).
Table 2.
Demographic and Clinical Characteristics of Children, Stratified by Site of Tuberculosis Disease
| Disease Site or Number of Sites Affected, n (% of total cases) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Lung | Bone/Joint | Pleura | Peripheral Lymph Node | CNS | Abdomen | GU System | 1 Site | 2+ Sites | |
| Total cases | 2251 | 1927 | 123 | 96 | 89 | 53 | 11 | 3 | 2155 | 96 |
| Age group | ||||||||||
| 0–1 year | 460 (20.4) | 414 (21.5) | 29 (23.6) | 4 (4.2) | 12 (13.5) | 27 (50.9) | 0 (0.0) | 0 (0.0) | 430 (20.0) | 30 (31.2) |
| 2–4 years | 532 (23.6) | 459 (23.8) | 38 (30.9) | 12 (12.5) | 17 (19.1) | 15 (28.3) | 2 (18.2) | 1 (33.3) | 512 (23.8) | 20 (20.8) |
| 5–9 years | 654 (29.1) | 561 (29.1) | 21 (17.1) | 31 (32.3) | 33 (37.1) | 5 (9.4) | 3 (27.3) | 1 (33.3) | 638 (29.6) | 16 (16.7) |
| 10–14 years | 605 (26.9) | 493 (25.6) | 35 (28.5) | 49 (51.0) | 27 (30.3) | 6 (11.3) | 6 (54.5) | 1 (33.3) | 575 (26.7) | 30 (31.2) |
| Male sex | 1172 (52.1) | 1006 (52.2) | 61 (49.6) | 64 (66.7) | 42 (47.2) | 30 (56.6) | 3 (27.3) | 1 (33.3) | 1119 (51.9) | 53 (55.2) |
| Urban residencea | 1262 (56.5) | 1070 (56.1) | 86 (70.5) | 53 (55.2) | 46 (52.9) | 30 (56.6) | 5 (45.5) | 3 (100.0) | 1216 (56.9) | 46 (48.9) |
| History of migration | 29 (1.3) | 26 (1.3) | 2 (1.6) | 1 (1.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 28 (1.3) | 1 (1.0) |
| Recurrent TB | 53 (2.4) | 41 (2.1) | 7 (5.7) | 1 (1.0) | 4 (4.5) | 2 (3.8) | 0 (0.0) | 1 (33.3) | 49 (2.3) | 4 (4.2) |
| Microbiologically confirmed TBb | 516 (22.9) | 455 (23.6) | 14 (11.4) | 29 (30.2) | 13 (14.6) | 36 (67.9) | 3 (27.3) | 2 (66.7) | 474 (22.0) | 23 (24.0) |
| Additional site(s) of TB disease | 96 (4.3) | 91 (4.7) | 16 (13.0) | 31 (32.3) | 18 (20.2) | 28 (52.8) | 5 (45.5) | 0 (0.0) | NA | NA |
| Rifampicin-resistant TBc | 241 (10.7) | 209 (10.8) | 13 (10.6) | 14 (14.6) | 7 (7.9) | 17 (32.1) | 1 (9.1) | 1 (33.3) | 218 (10.1) | 23 (24.0) |
| HIV/ART statusd | ||||||||||
| Without HIV | 1918 (85.2) | 1647 (85.5) | 97 (78.9) | 89 (92.7) | 68 (76.4) | 40 (75.5) | 9 (81.8) | 3 (100.0) | 1840 (85.4) | 78 (81.2) |
| With HIV/on ART | 74 (3.3) | 65 (3.4) | 0 (0.0) | 3 (3.1) | 6 (6.7) | 3 (5.7) | 0 (0.0) | 0 (0.0) | 70 (3.2) | 4 (4.2) |
| With HIV/not on ART | 93 (4.1) | 81 (4.2) | 3 (2.4) | 1 (1.0) | 10 (11.2) | 4 (7.5) | 2 (18.2) | 0 (0.0) | 84 (3.9) | 9 (9.4) |
| HIV status unknown | 166 (7.4) | 134 (7.0) | 23 (18.7) | 3 (3.1) | 5 (5.6) | 6 (11.3) | 0 (0.0) | 0 (0.0) | 161 (7.5) | 5 (5.2) |
| Treatment outcome | ||||||||||
| Cure/treatment completion | 1713 (76.1) | 1472 (76.4) | 88 (71.5) | 67 (69.8) | 70 (78.7) | 19 (35.8) | 7 (63.6) | 2 (66.7) | 1662 (77.1) | 51 (53.1) |
| Deathe | 42 (1.9) | 26 (1.3) | 1 (0.8) | 1 (1.0) | 1 (1.1) | 27 (50.9) | 1 (9.1) | 0 (0.0) | 25 (1.2) | 17 (17.7) |
| Treatment failure | 11 (0.5) | 10 (0.5) | 1 (0.8) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (0.5) | 1 (1.0) |
| Transfer | 10 (0.4) | 8 (0.4) | 1 (0.8) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (0.4) | 1 (1.0) |
| Loss to follow-up | 21 (0.9) | 18 (0.9) | 0 (0.0) | 1 (1.0) | 3 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (0.9) | 1 (1.0) |
| Treatment in process | 454 (20.2) | 393 (20.4) | 32 (26.0) | 24 (25.0) | 15 (16.9) | 7 (13.2) | 3 (27.3) | 1 (33.3) | 429 (19.9) | 25 (26.0) |
Homelessness not included because only three children experienced homelessness; all had pulmonary TB only.
Abbreviations: ART, antiretroviral therapy; CNS, central nervous system; DST, drug susceptibility test; GU, genitourinary; HIV, human immunodeficiency virus; NA, not applicable; TB, tuberculosis.
Data missing for 19 participants.
Microbiological confirmation means that acid-fast bacilli were detected by smear microscopy and/or Mycobacterium tuberculosis was detected by culture and/or Xpert MTB/RIF (Cepheid) from any anatomic site. For patients with no other site of disease, the percentages of microbiologically confirmed cases were as follows: pulmonary, 22.6%; pleural, 32.3%; peripheral lymphadenitis, 8.5%; osteoarticular, 8.4%; CNS, 68.0%; abdominal, 0.0%; and GU system, 66.7%.
Rifampicin susceptibility was either confirmed by a DST of the patient’s own M. tuberculosis isolate or presumed based on a DST of the source case’s isolate.
HIV and ART status were determined at the time of TB treatment initiation.
Death was defined as all-cause mortality or transfer to palliative care before the end of the prescribed treatment. Only 1 child, who had both pulmonary and CNS TB, was transferred to palliative care.
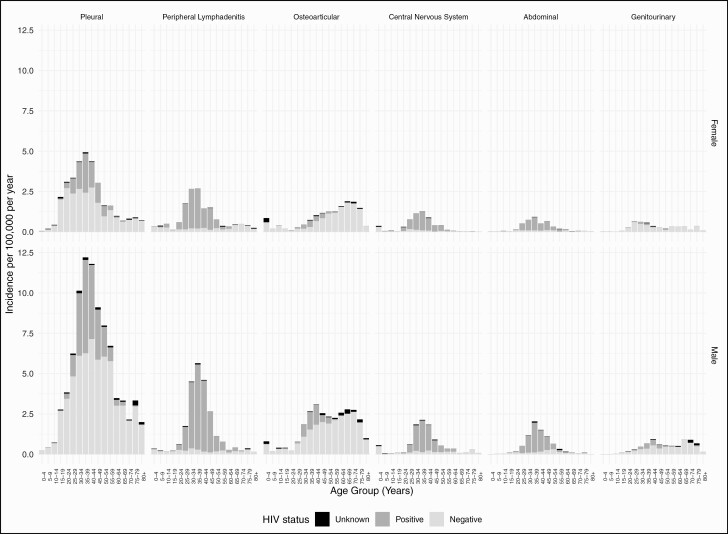
The average annual notification rate per 100 000 was 63.6 for pulmonary TB, 3.9 for pleural TB, 1.4 for peripheral TB lymphadenitis, 1.3 for osteoarticular TB, 0.6 for CNS TB, 0.5 for abdominal TB, and 0.4 for genitourinary (GU) TB. In patients aged 15 years and older, males comprised more than 70% of pulmonary and pleural TB cases and 55–64% of all other EPTB cases (Table 1). In patients younger than 15 years old, the sex distribution of cases was more even (Table 2). Most EPTB subtype notifications peaked between 30 and 39 years of age, while notifications of osteoarticular and male GU TB peaked in later life (Figure 1, Supplementary Table 1). In adults/adolescents, 61–68% of peripheral TB lymphadenitis, CNS TB, and abdominal TB occurred in those with untreated HIV; an additional 16–18% occurred in those with treated HIV (Table 1). Among people without HIV, CNS TB notifications peaked before 5 years of age (Figure 1).
Figure 1.
Average annual incidence of extrapulmonary tuberculosis, Ukraine: 2015–2017. Abbreviation: HIV, human immunodeficiency virus.
Proportion of Diagnoses That Were Microbiologically Confirmed
In adults/adolescents, microbiological confirmation was attained in less than 50% of EPTB cases compared to 69% of pulmonary TB cases (Table 1). Extrapulmonary TB subtypes with the highest percentage of microbiological confirmation were pleural and CNS TB (45–49%). The subtype with the lowest percentage was osteoarticular TB (16%). In children, we observed less than 30% microbiological confirmation in pulmonary TB and all EPTB subtypes other than GU (but only 3 cases were reported) and CNS TB (Table 2). Microbiological confirmation occurred in 36 (67.9%) of 53 CNS cases and in 31 (67.4%) of the subset of 46 meningitis cases.
Mortality
Of 14 062 adults/adolescents with EPTB, 2179 (15.5%) died. Compared with adults/adolescents with pulmonary TB only, those with CNS TB (adjusted hazard ratio [aHR]: 3.22; 95% CI: 2.89–3.60) and abdominal TB (aHR: 1.83; 95% CI: 1.59–2.11) were more likely to die (Table 3). Of 417 children with EPTB, 31 (7.4%) died. CNS TB was the only EPTB subtype associated with more than 1 pediatric death. Children with CNS TB, compared with those without CNS involvement, had an adjusted mortality hazard of 88.25 (95% CI: 43.49–179.10). Having more than 1 diseased site was associated with death in patients aged 15 years and older but not those younger than 15 years old.
Table 3.
Hazard of Death Associated With Extrapulmonary Tuberculosis Subtype and Number of Disease Sites
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| aHR (95% CI) | P | aHR (95% CI) | P | |
| Adults/adolescents (≥15 years old) | ||||
| Exclusively pulmonary | Ref | Ref | ||
| Pleural | .74 (.67–.82) | <.001 | .89 (.81–.99) | .03 |
| Peripheral lymphadenitis | .91 (.81–1.01) | .08 | .84 (.74–.94) | .002 |
| Osteoarticular | .66 (.57–.76) | <.001 | .78 (.68–.90) | <.001 |
| CNS | 3.45 (3.09–3.84) | <.001 | 3.22 (2.89–3.60) | <.001 |
| Abdominal | 1.91 (1.66–2.20) | <.001 | 1.83 (1.59–2.11) | <.001 |
| GU | .38 (.27–.53) | <.001 | .45 (.32–.64) | <.001 |
| 2+ sites involved | 1.65 (1.50–1.81) | <.001 | 1.17 (1.06–1.29) | .001 |
| Children (<15 years old) | ||||
| Non-CNS in one site | Ref | Ref | ||
| CNS | 85.48 (39.79–183.61) | <.001 | 88.25 (43.49–179.10) | <.001 |
| 2+ sites involved | 1.29 (0.62–2.67) | .49 | … | … |
Abbreviations: aHR, adjusted hazard ratio; ART, antiretroviral therapy; CI, confidence interval; CNS, central nervous system; EPTB, extrapulmonary tuberculosis; GU, genitourinary; HIV, human immunodeficiency virus; Ref, reference; TB, tuberculosis.
Model 1 included only variables indicating the presence of each EPTB subtype and the number of diseased sites.
In adults/adolescents, model 2 further adjusted for age, sex, new vs recurrent TB, microbiological vs clinical diagnosis, rifampicin susceptibility, HIV/ART status, urban vs rural residence, alcohol dependence, injection drug use, homelessness, unemployment, work in healthcare, history of incarceration, and history of migration. In children, model 2 further adjusted for age group, rifampicin susceptibility, and new vs recurrent TB. In the adult/adolescent group, we analyzed age as a continuous variable; in the pediatric group, we analyzed age as a categorical variable to account for nonlinear changes in TB risk and clinical presentation that occur in childhood [18, 26].
Risk Factors for Death
Among adults/adolescents, older age was associated with death for all EPTB subtypes (Supplementary Tables 2–7). Compared with patients without HIV, those with HIV had higher mortality, with similar hazards regardless of ART status. In patients with osteoarticular, CNS, or abdominal TB, HIV was associated with death only after the first 45–60 days of TB treatment. Rifampicin resistance was associated with mortality in TB of the pleura, peripheral lymph nodes, and CNS. In univariable regression, microbiological confirmation was associated with death for every EPTB subtype except for osteoarticular TB; however, it was eliminated from all final models during backward selection.
Among 53 children with CNS TB, 27 (50.9%) died. No associations were observed between demographic or clinical characteristics and death, but the numbers were small (Supplementary Table 8).
DISCUSSION
We have characterized the notification rates and mortality risk of the most common EPTB subtypes in Ukraine. Within each EPTB subtype, we identified risk factors for death. Our findings contribute to the scarce literature on EPTB epidemiology—particularly in pediatrics. Similar to other population-level studies, we observed that the pleura and peripheral lymph nodes were the most common EPTB sites and that patients with osteoarticular and GU TB tended to be older than other patients with TB [5–14]. Also, like most other population-level studies, we observed a higher notification of EPTB in males than females, although the male-to-female ratio was lower for EPTB than for pulmonary TB (only 1 study from Lebanon reported more pulmonary and EPTB cases in females than males) [5–14]. Our study clarifies that the sex difference begins in adolescence. With respect to children and adolescents, our data are consistent with 3 well-known patterns: the peak incidence of CNS TB in the first 5 years of life; the rapid rise in pleural TB incidence during adolescence, particularly in males; and the rarity of GU TB in childhood [1, 15–17, 22, 23]. The consistency of these findings across diverse settings strengthens the validity of these epidemiological patterns.
HIV infection is known to increase EPTB risk, but its association with particular EPTB subtypes has not been well characterized [24–26]. We found that most adults aged 20 years and older with CNS, abdominal, or peripheral lymph node TB had HIV. In contrast, the proportion of other EPTB patients with HIV was comparable to that of patients with pulmonary TB. Other studies also have shown associations between poorly controlled HIV and TB of the abdomen, peripheral lymph nodes, and CNS, vis-à-vis cases of osteoarticular or pleural TB [25, 26]. These findings are unsurprising since dissemination to the CNS and abdomen occurs after host failure to contain M. tuberculosis in the lungs, whereas pleural TB often represents a hyperinflammatory response to an adjacent pulmonary lesion [1, 22]. However, reasons for the weaker relationship between HIV infection and TB in bones/joints or the GU system, which also results from dissemination, remain unclear.
Our findings highlight 4 potential shortcomings in TB diagnosis in Ukraine. First, the high frequency (67%) of microbiological confirmation in childhood TB meningitis cases raises concerns for under- and/or delayed detection of this most lethal and debilitating form of TB [27]. Tuberculosis meningitis is a paucibacillary disease, and microbiological confirmation by culture, the most sensitive assay, is estimated to occur in only 35% of pediatric cases [27]. Many children with culture-negative TB meningitis may die before diagnosis. The high frequency of microbiological confirmation—together with the 51% risk of death we observed (compared to a 19% pooled risk of death in a meta-analysis [27])—also may reflect diagnosis in advanced stages of the disease. Second, the higher mortality from abdominal TB among adults/adolescents in Ukraine compared with other cohorts also suggests delayed diagnosis and treatment of this EPTB subtype [28, 29]. Third, notification rates of osteoarticular TB exceeded rates in other settings, and only 11–16% of cases were microbiologically confirmed [6–11, 14]. Osteoarticular TB comprised nearly 30% of childhood EPTB cases in Ukraine and occurred more frequently than peripheral TB lymphadenitis, whereas in other population-level studies, peripheral TB lymphadenitis was the EPTB subtype most commonly diagnosed in childhood and osteoarticular TB comprised only 4–11% of childhood EPTB cases [15–17]. These observations raise concern for overdiagnosis, which leads to unnecessary prolonged treatment and neglect of the true osteoarticular pathology. At the same time, in children, skeletal TB sometimes develops as a late complication of untreated pulmonary TB [30]. Thus, delayed detection of pulmonary TB may have contributed to the relatively high proportion, compared with other pediatric cohorts, of EPTB cases that were osteoarticular. Finally, we observed that children comprised only 2% of all notified TB cases during the study period. Given Ukraine’s TB incidence, children are expected to comprise approximately 8% of the country’s TB cases [31]. This discrepancy raises suspicion for underdiagnosis and/or underreporting of childhood TB.
Our results have implications for clinical management. Most critically, the high hazards of death associated with CNS TB in patients of all ages and with abdominal TB in adults/adolescents—which strongly suggest delayed treatment—underscore the need for clinicians to maintain a high index of suspicion and low threshold for empirical treatment for these EPTB subtypes. Additionally, our characterization of EPTB incidence—stratified by age, sex, HIV status, and EPTB subtype—and risk factors for death helps clinicians identify the patients who are most vulnerable to developing and dying from specific EPTB subtypes. Patients with EPTB with risk factors for death require close treatment monitoring, and further research is needed to pinpoint the mechanisms that lead to clinical decline in order to optimize management. For instance, we observed an association between death and HIV in all EPTB subtypes other than GU. However, this association was significant only after the first 45–60 days of treatment for osteoarticular, CNS, and abdominal TB. Potential reasons for the late occurrence of death include immune reconstitution inflammatory syndrome (IRIS) or non–TB-related complications of HIV, but additional studies are needed to understand these findings.
This study had limitations. Ukraine’s NTP lacks guidelines for EPTB diagnosis; moreover, patients already diagnosed with pulmonary TB may have had limited or no evaluation for concomitant EPTB. Therefore, misdiagnoses may have occurred. We cannot exclude the possibility that diagnostic bias on the part of clinicians (ie, differential clinical suspicion and threshold for diagnosing certain EPTB subtypes based on HIV status) may have contributed to the discrepancies in EPTB notifications between patients with and without HIV. The imprecision of certain eTB Manager categories precluded our ability to identify cases of miliary TB or laryngeal TB. The use of a source case’s DST to classify patients (who lack their own DST) as having rifampicin-susceptible or RR-TB may be problematic when several years pass between the initial M. tuberculosis infection and the development of disease, which generally occurs in GU TB and sometimes occurs in osteoarticular TB [1, 30]. We lacked data on HIV viral load or CD4 count to further characterize patients’ immune status. We had no information on regimen adherence, which may have influenced associations between certain covariates and death. Substance use was self-reported, likely leading to an underestimation of its prevalence. Finally, clerical errors were possible since these were programmatic data.
Despite these limitations, we have characterized EPTB distribution by age, sex, and HIV status; highlighted potential shortcomings in EPTB diagnosis in Ukraine; and identified risk factors for death from EPTB. These findings help elucidate the epidemiology and treatment outcomes of these often-neglected forms of TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study conceptualization: S. K., H. E. J., C. R. H., S. S. C. Data collection: M. D., I. T., I. V., V. P. Data analysis: S. K., T. L., S. S. C. Interpretation of results: S. K., H. E. J., M. D., E. J. C., N. R. R., S. S. C. Manuscript writing: S. K., S. S. C. All authors have reviewed and approved the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant numbers K01TW010829 to S. S. C. and R03AI164123 to H. E. J.) and the Ministry of Health of Ukraine (grant number 0121U107800 to M. D.).
Contributor Information
Sara Khalife, Department of Pediatrics, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Helen E Jenkins, Department of Biostatistics, Boston University School of Public Health, Boston, Massachusetts, USA.
Mariia Dolynska, Department of Tuberculosis and Pulmonology, Bogomolets National Medical University, Kyiv City, Ukraine.
Iana Terleieva, Public Health Center of the Ministry of Health, Kyiv City, Ukraine.
Iurii Varchenko, Public Health Center of the Ministry of Health, Kyiv City, Ukraine.
Tao Liu, Department of Biostatistics, Brown University School of Public Health, Providence, Rhode Island, USA.
E Jane Carter, Department of Medicine, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
C Robert Horsburgh, Department of Biostatistics, Boston University School of Public Health, Boston, Massachusetts, USA; Department of Medicine, Boston University School of Medicine, Boston, Massachusetts, USA; Departments of Epidemiology and Global Health, Boston University School of Public Health, Boston, Massachusetts, USAand.
Natasha R Rybak, Department of Medicine, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Vasyl Petrenko, Department of Tuberculosis and Pulmonology, Bogomolets National Medical University, Kyiv City, Ukraine.
Silvia S Chiang, Department of Pediatrics, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA; Center for International Health Research, Rhode Island Hospital, Providence, Rhode Island, USA.
References
- 1. Wallgren A. The time-table of tuberculosis. Tubercle 1948; 29:245–51. [DOI] [PubMed] [Google Scholar]
- 2. Yang Z, Kong Y, Wilson F, et al. . Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis 2004; 38:199–205. [DOI] [PubMed] [Google Scholar]
- 3. Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR.. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014; 44:435–46. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Global Tuberculosis Report 2020. Geneva, Switzerland: World Health Organization, 2020. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports. Accessed 18 August 2021. [Google Scholar]
- 5. Kipp AM, Stout JE, Hamilton CD, Van Rie A.. Extrapulmonary tuberculosis, human immunodeficiency virus, and foreign birth in North Carolina, 1993–2006. BMC Public Health 2008; 8:107. doi: 10.1186/1471-2458-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR.. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 2009; 49:1350–7. [DOI] [PubMed] [Google Scholar]
- 7. Ducomble T, Tolksdorf K, Karagiannis I, et al. . The burden of extrapulmonary and meningitis tuberculosis: an investigation of national surveillance data, Germany, 2002 to 2009. Euro Surveill 2013; 18:20436. doi: 10.2807/ese.18.12.20436-en. [DOI] [PubMed] [Google Scholar]
- 8. Gomes T, Reis-Santos B, Bertolde A, Johnson JL, Riley LW, Maciel EL.. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect Dis 2014; 14:9. doi: 10.1186/1471-2334-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA.. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect 2018; 7:102. doi: 10.1038/s41426-018-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holden IK, Lillebaek T, Andersen PH, Bjerrum S, Wejse C, Johansen IS.. Extrapulmonary tuberculosis in Denmark from 2009 to 2014: characteristics and predictors for treatment outcome. Open Forum Infect Dis 2019; 6:ofz388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang W, Yu J, Du J, et al. . The epidemiology of extrapulmonary tuberculosis in China: a large-scale multi-center observational study. PLoS One 2020; 15:e0237753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Son L, Hulland E, Cookson ST, Castro KG, Yaacoub H.. Epidemiology and risk factors for extrapulmonary tuberculosis in Lebanon. Int J Tuberc Lung Dis 2020; 24:414–9. [DOI] [PubMed] [Google Scholar]
- 13. Sbayi A, Arfaoui A, Janah H, Koraichi SE, Quyou A.. Epidemiological characteristics and some risk factors of extrapulmonary tuberculosis in Larache, Morocco. Pan Afr Med J 2020; 36:381. doi: 10.11604/pamj.2020.36.381.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreiro L, Ruano-Ravina A, Otero-Mallo R, et al. . Recent epidemiological trends in extrapulmonary TB in Galicia, Spain. Int J Tuberc Lung Dis 2021; 25:373–81. [DOI] [PubMed] [Google Scholar]
- 15. Lobato MN, Cummings K, Will D, Royce S.. Tuberculosis in children and adolescents: California, 1985 to 1995. Pediatr Infect Dis J 1998; 17:407–11. [DOI] [PubMed] [Google Scholar]
- 16. Phongsamart W, Kitai I, Gardam M, Wang J, Khan K.. A population-based study of tuberculosis in children and adolescents in Ontario. Pediatr Infect Dis J 2009; 28:416–9. [DOI] [PubMed] [Google Scholar]
- 17. Teo SS, Tay EL, Douglas P, Krause VL, Graham SM.. The epidemiology of tuberculosis in children in Australia, 2003–2012. Med J Aust 2015; 203:440. doi: 10.5694/mja15.00717. [DOI] [PubMed] [Google Scholar]
- 18. Public Health Center of the Ministry of Health of Ukraine. Tuberculosis in Ukraine: analytical and statistical reference book. Kyiv City, Ukraine: Ministry of Health, 2017. Available at: https://www.phc.org.ua/sites/default/files/uploads/files/PATH_booklet_003-4.pdf. Accessed 23 November 2021. [Google Scholar]
- 19. European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2020–2018 Data. Stockholm, Sweden: European Centre for Disease Prevention and Control, 2020. Available at: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2020-2018-data. Accessed 23 November 2021. [Google Scholar]
- 20. Ministry of Health of Ukraine. Order of the Ministry of Health of Ukraine No. 620: On Approval and Implementation of Medical-Technological Documents on Standardization of Medical Aid for Tuberculosis. Kyiv, Ukraine, 2014. https://zakon.rada.gov.ua/rada/show/v0620282-14#Text. Accessed 20 December 2021. [Google Scholar]
- 21. The World Bank. Data: health, nutrition and population statistics: population estimates and projections. Available at: http://datatopics.worldbank.org/hnp/popestimates. Accessed 15 August 2021.
- 22. Marais BJ, Gie RP, Schaaf HS, et al. . The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 23. Weber HC, Beyers N, Gie RP, Schaaf HS, Fish T, Donald PR.. The clinical and radiological features of tuberculosis in adolescents. Ann Trop Paediatr 2000; 20:5–10. [DOI] [PubMed] [Google Scholar]
- 24. Jaryal A, Raina R, Sarkar M, Sharma A.. Manifestations of tuberculosis in HIV/AIDS patients and its relationship with CD4 count. Lung India 2011; 28:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kingkaew N, Sangtong B, Amnuaiphon W, et al. . HIV-associated extrapulmonary tuberculosis in Thailand: epidemiology and risk factors for death. Int J Infect Dis 2009; 13:722–9. [DOI] [PubMed] [Google Scholar]
- 26. Leeds IL, Magee MJ, Kurbatova EV, et al. . Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Infect Dis 2012; 55:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiang SS, Khan FA, Milstein MB, et al. . Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:947–57. [DOI] [PubMed] [Google Scholar]
- 28. Cho JK, Choi YM, Lee SS, et al. . Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis 2018; 18:699. doi: 10.1186/s12879-018-3635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jullien S, Jain S, Ryan H, Ahuja V.. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst Rev 2016; 11:CD012163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lincoln EM, Sewell EM.. Tuberculosis in Children. New York, NY: McGraw-Hill Book Company, Inc, 1963. [Google Scholar]
- 31. Jenkins HE, Tolman AW, Yuen CM, et al. . Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383:1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.