Abstract
Objective:
Large-scale screening of the general population for islet autoantibodies (IAbs) to detect type 1 diabetes (T1D) has started worldwide. The standard screening method of separate radio-binding assay (RBA) for each IAb is an inefficient bottleneck. Furthermore, most positive results by RBA in screening of general population individuals without a clinical diagnosis of T1D are low-affinity and not predictive of future diabetes.
Research Design and Methods:
We have developed and validated a novel 6-Plex assay based on electrochemiluminescence (ECL) technology that combines in a single well high-affinity IAbs (to insulin, GAD, IA-2, and ZnT8), transglutaminase autoantibodies for celiac disease, and severe acute respiratory syndrome coronavirus 2 antibodies. The Autoimmunity Screening for Kids (ASK) provided 880 serum samples, from 828 children aged 1–17 years without diabetes who were previously tested for IAbs using single ECL assays and RBA assays.
Results:
Levels of all six antibodies in the 6-Plex ECL assay correlated well with respective single ECL assay levels. Similar to single ECL assays, the 6-Plex ECL assay positivity was congruent with the RBA in 95% (35/37) of children who later developed T1D and in 88% (105/119) high-risk children with multiple IAbs. In contrast, only 56% (86/154, P < 0.0001) of children with persistent single IAb by RBA were found to be positive by 6-Plex ECL assay. Of 555 samples negative for all IAbs by RBA, few (0.2%–0.5%) were positive at low levels in the 6-Plex ECL assay.
Conclusions:
The study demonstrated that the 6-Plex ECL assay compares favorably to the standard RBAs in terms of disease specificity for general population screening in children. The 6-Plex ECL assay was therefore adopted as the primary screening tool in the general population screening ASK program with advantages of high efficiency, low cost, and low serum volume.
Keywords: Type 1 diabetes, Autoantibodies, Multiplex ECL assay, General population
Introduction
The incidence of type 1 diabetes (T1D) is rapidly increasing by 3%–5% annually and has doubled in the last 20 years,1,2 especially in young children. In the United States, 1.6 million people have T1D, and it is an estimate based on our ongoing study of Autoimmunity Screening for Kids (ASK) that as many have multiple islet autoantibodies (IAbs) or pre-T1D (unpublished data) without meeting diagnostic criteria for T1D based on glycemic status. IAbs frequently appear years before clinical disease and are currently the most reliable biomarkers for T1D prediction and clinical diagnosis.
The presence of 2 or more IAbs, including antibodies to insulin (IAA), glutamic acid decarboxylase (GADA), islet antigen 2 (IA-2A), and zinc transporter 8 (ZnT8A) predicts development of clinical T1D in nearly all affected children.3 Children at risk for T1D need to be identified before the onset of symptoms to prevent life-threatening diabetic ketoacidosis, identify individuals for current and upcoming trials to prevent T1D, and define the underlying mechanisms of islet autoimmunity and its triggers.
While worldwide prevention efforts for T1D are underway and multiple candidate interventions are being proposed to abrogate or slow progression to diabetes among IAb-positive individuals, mass screening of the general population for eligible participants with radio-binding assay (RBA) where each IAb is measured individually remains a laborious and inefficient bottleneck. In addition, although the standard RBA has high assay sensitivity and specificity, a large proportion of IAb positivity detected by RBA in screening, especially in those individuals positive for a single IAb, were of low affinity with low or no disease risk, resulting in an overall low-positive predictive value (PPV).4
We have recently developed and validated nonradioactive IAb assays using electrochemiluminescence (ECL) detection with exceptional sensitivity and specificity for all four IAbs, including IAA, GADA, IA-2A, and ZnT8A, compared to the standard RBA.4–8 The ECL assay discriminates high-affinity autoantibodies from low-affinity autoantibodies. Using the ECL assay for each IAb allows for removal of the low affinity positivity generated by RBA without decreasing the sensitivity for detection of cases with single or multiple IAbs who were followed to clinical disease. In 2016, the ASK study was launched from the Barbara Davis Center to screen for T1D and celiac disease among general population children in the greater Denver metropolitan area.9
The ECL assay has been applied in screening, performed in parallel with RBA, and revealed its significant advantage of higher disease specificity.4–8 More recently, using ECL detection on a platform from Meso Scale Discovery (MSD), we have successfully developed a multiplexed ECL assay10,11 using the technology of U-Plex plates from MSD to combine multiple autoantibodies in one single well on a 96-well plate. In this study, we present the validation study data of a multiplex ECL assay combining 6 autoantibody assays in 1 well (6-Plex ECL assay), including all 4 major IAbs, autoantibodies to transglutaminase (TGA) for celiac disease, and Covid-19 antibodies (Covid-19A) using selected samples from the ASK study.
We compared the multiplex ECL assay with RBA and their corresponding single ECL assay. The 6-plex ECL assay was arranged to perform in a blinded manner. The purpose of this validation study was to decide whether to accept the multiplex ECL assay as the initial screening tool in the ASK study.
Research Design and Methods
Subjects
The ASK study screens general population children between ages 1 and 17 years for T1D and celiac disease with an optional severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody test. Children with positive autoantibodies at screening are retested for confirmation, and confirmed positive children are followed every 3–6 months through the ASK follow-up and monitoring protocol. From 2016 to March 2021, over 26,800 children have been screened. Both standard RBA and single ECL assays for IAA, GADA, IA-2A, ZnT8A, and TGA were performed in parallel on all ASK samples until December 2019, except for ECL-ZnT8A, which was not available initially. Retrospective analysis of ECL-ZnT8A was conducted on all ASK subjects positive for RBA-ZnT8A upon initial screening and longitudinal follow-up samples.
Since March 2020, all new participants and follow-up subjects of the ASK study were also offered screening for Covid-19A to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein with an ECL assay.12 In the present study, 880 ASK study samples from 828 children were selected for the validation of 6-Plex ECL assay. The IAb-positive samples (n = 325) are from 273 children, including 119 at high risk for T1D development who were positive for multiple IAbs or developed clinical T1D and 154 with low risk for T1D development who were positive for a single IAb. Samples from 555 children who tested negative for all IAb were included in the analysis. All subjects have provided written consent and studies were approved by the University of Colorado Institutional Review Board.
6-Plex ECL assay
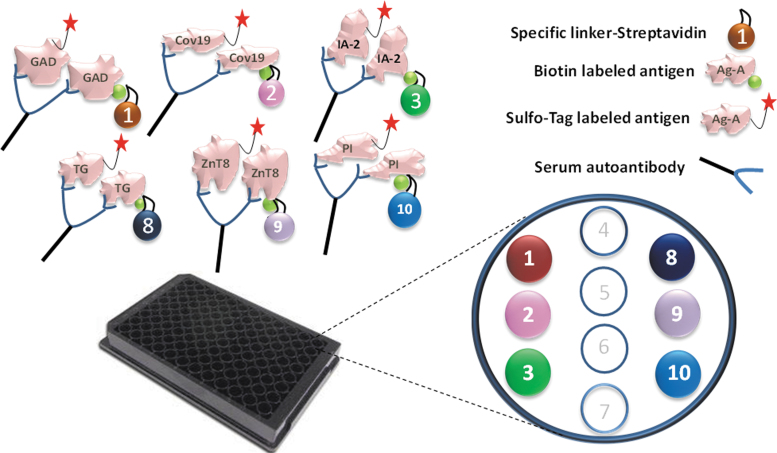
The method of 6-Plex ECL assay is similar to our previously published multiplex ECL assay.11 In brief, 6 different streptavidin conjugated linkers were bound to 6 corresponding biotinylated antigen proteins. The layout of the 6 active linker spots is shown in Figure 1. Linkers 1, 2, 3, 8, 9, and 10 were bound to GAD65, RBD of SARS-CoV-2 spike protein, IA-2, TG, ZnT8, and insulin in separated tubes, respectively, by incubating at room temperature for 0.5 h. Next, stop solution was added, and incubation occurred at room temperature for 30 min. Then 6 linker-coupled antigens were combined, and 6 sulfo-tag labeled antigen proteins were added. The 6 antigen-linker mixtures were incubated with preheated sera at room temperature for 2 h and then at 4°C overnight.
FIG. 1.
Illustration of multiplex ECL assay. The autoantibodies in serum will link the sulfo-tagged antigen to the biotinylated antigen coupled with specific linker, which will be captured by the plate. The specifically coupled linker numbers for six different antigen proteins are illustrated. Detection of plate captured sulfo-tagged antigens are accomplished with ECL. ECL, electrochemiluminescence.
The next day, the incubates were added to a precoated U-Plex (MSD) plate and incubated at room temperature for 1 h. The plate was then washed, 2X reader buffer (MSD) was added, and the plate was counted in a MSD sector SQ120.
RBA for IAbs and TGA
RBA assays for the four major IAbs and TGA were previously published.13–16 The cutoffs for these autoantibody RBAs were set at around 99th percentile of normal controls. In the most recent IASP (Islet Autoantibody Standardization Program) workshop of the year 2020, the sensitivities and specificities were 78% and 99% for GADA, 72% and 100% for IA-2A, 62% and 99% for IAA, and 74% and 100% for ZnT8A, respectively.
Single ECL assay
Single ECL assays for the four major IAbs, TGA, and Covid-19A were published elsewhere.5,7,8,12,17 The upper limits of normal were established 99th percentile or above. In the most recent IASP workshop of the year 2020, the sensitivity and specificity for ECL assays were 78% and 100% for GADA, 72% and 100% for IA-2A, 66% and 99% for IAA, and 74% and 100% for ZnT8A, respectively.
IAbs cases
The criteria set as cases for IAbs is positive for multiple IAbs or confirmed single IAb at both screening and confirmation visits or T1D diagnosed.
TGA cases
The criteria for cases of TGA are confirmed positive for TGA in both screening and confirmation visits.
Statistics
All statistical analyses were performed using GraphPad Prism software (version 8.0; GraphPad Software Institute, San Diego, CA) and SAS v9.4 (SAS Institute, Inc., Cary, NC). Pearson's correlation analysis was performed to access the strength of correlation in levels between two assays. Percentiles corresponding to antibody levels were calculated separately by their IAbs or TGA case status, where percentile of antibody value equals to number of antibody values below that value divided by total number of antibody values and times by 100. Scatter plots were constructed by plotting the antibody levels on the y axis and the corresponding percentiles on the x axis by their IAbs or TGA case status.
The optimal cutoff value of each autoantibody assay was determined by drawing a horizontal line on the graph where it corresponded to at least 99% percentile in the control group and also the lowest percentile in the case group, which indicated the highest sensitivity. Sensitivity, specificity, PPV, and negative predictive value for IAbs or TGA were calculated against the results of standard RBA, either single or multiple IAbs as positive cases (Table 1). A P value of <0.05 at two-sided test was considered statistically significant.
Table 1.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value in Multiplex Electrochemiluminescence Assay, Correlated to Each Corresponding Islet Autoantibody Positivity by Radio-Binding Assay
| Cutoff | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| GADA | 0.010 | 81% | 99% | 92% | 82% |
| IA-2A | 0.007 | 89% | 99% | 89% | 72% |
| ZnT8A | 0.007 | 83% | 99% | 83% | 74% |
| IAA | 0.006 | 69% | 99% | 80% | 74% |
| TGA | 0.008 | 96% | 100% | 89% | 100% |
GADA, glutamic acid decarboxylase; IA-2A, islet antigen 2; IAA, including antibodies to insulin; NPV, negative predictive value; PPV, positive predictive value; TGA, transglutaminase; ZnT8A, zinc transporter 8.
Results
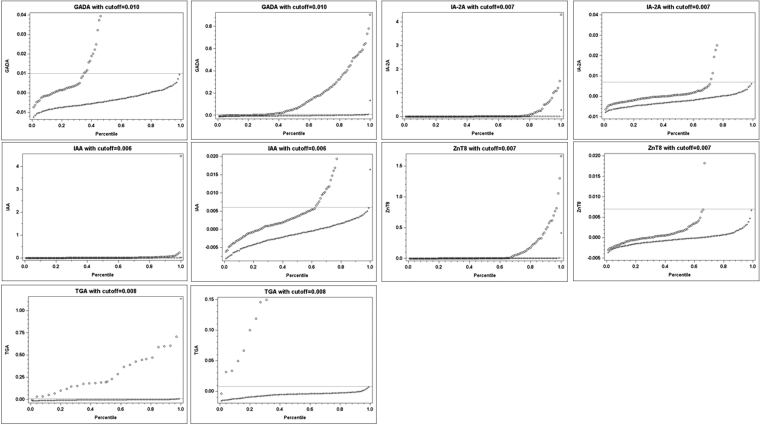
The 6-Plex ECL assay was performed in blinded manner and the data were analyzed after the results were submitted to the ASK study committee. Based on the scatter plots in Figure 2, the cutoffs of index values for 6-Plex ECL assay were set at 99.5th to 99.8th percentiles of 555 normal controls with 0.010 for GADA, 0.007 for IA-2A, 0.007 for ZnT8A, 0.006 for IAA, and 0.008 for TGA, respectively (Table 1). The cutoff value for Covid-19A in 6-Plex ECL assay is 0.050, equal to an index value of 5.0 in single ECL assay. These index cutoff values were used for all analyses of the positivity in the 6-Plex ECL assay and for the comparisons with their corresponding single ECL assays and RBAs.
FIG. 2.
Scatter plots of antibody levels versus their corresponding percentiles. Scatter plots were constructed by plotting the antibody levels on the y axis and the corresponding percentiles on the x axis by their IAbs or TGA case status. A horizontal line on the graph corresponded to at least 99th percentile in the control group. The mark “o” represents for the cases and “+” for the controls. Two plots were presented for each antibody, right panel with whole y axis range and left panel with amplified low range of y axis. IAbs, islet autoantibodies.
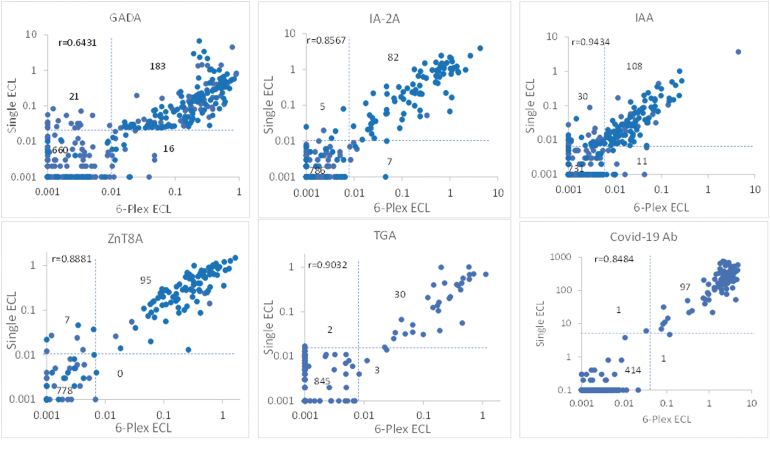
Among overall 273 children with 325 samples positive for IAbs by RBA, 70% of children (191/273) were positive in 6-Plex ECL assay and 74% (203/273) positive in single ECL assay. Two ECL assays, multiplex and single ECL, were congruent to each other for both positivity and levels as shown in Figure 3. Discordance between ECL assay and RBA was mainly from those with single IAb. Among 119 children with high risk who either developed clinical T1D or were positive for multiple IAbs by RBA, 6-Plex ECL assay results were concordant with RBA in 105 (88%) children, showing a similar positivity pattern. Of the remaining 14 children who were negative in 6-Plex ECL assay, 13 were also found negative in the single ECL assays, and 1 was slightly positive for single ECL-ZnT8A at a low level. Of the 39 children who developed T1D, 6-Plex ECL assay results were concordant with RBA in 37 (95%).
FIG. 3.
Correlation between the autoantibody levels measured by 6-Plex ECL assay and single ECL assays. Dotted lines represent the cutoff for each assay, respectively.
Two children who were negative by 6-Plex ECL assay were low level positive by RBA for a single IAb (one for IAA only and one for IA-2A only) and were both negative by single ECL assays. In contrast, among the children with single IAb by RBA, only 56% (86/154, P < 0.0001) were detected positive by 6-Plex ECL assay and a large proportion (68/154) were not confirmed by 6-Plex ECL assay.
Among 555 children negative for all IAbs by RBA, none had multiple IAbs, and only 9 samples were single IAb positive at low levels in the 6-Plex ECL assay, one (0.2%) for GADA, 2 (0.4%) for IA-2A, 3 (0.5%) for IAA, and 3 (0.5%) for ZnT8A. The positivity of both TGA and Covid-19A in the 6-Plex ECL assay was well correlated with their single ECL assays and with RBA-TGA. Only a few samples at low levels were discordant with each other.
Of 273 cases positive for IAbs, 50 cases with multiple clinical visit samples were included in the analyses to test the assay consistency, including 32 cases with persistent multiple IAbs and 18 cases with a persistent single IAb by RBA. Of these 50 cases, all cases showed consistent results in the 6-Plex ECL assay. Of the 32 cases that were persistently multiple IAb-positive by RBA, 30 were consistently positive, and 2 were consistently negative by 6-Plex ECL assay. Of the 18 cases with a persistent single IAb by RBA, 13 were consistently positive and 5 were consistently negative by 6-Plex ECL assay. The seven persistently positive cases by RBA that were consistently negative by 6-Plex ECL assay were also found consistently negative by single ECL assay.
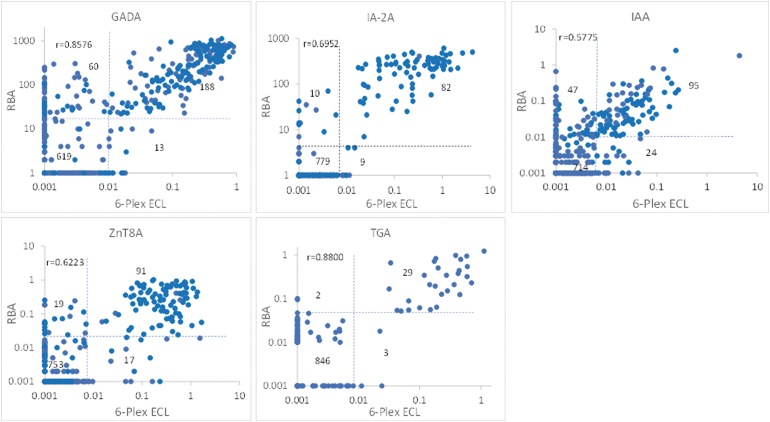
Overall, a large proportion of single IAb-positive cases by RBA, especially for GADA and IAA, were negative in both the 6-Plex ECL and single ECL assays. The levels of all autoantibodies in 6-Plex ECL assay were well correlated with their corresponding 6 single assays, either with single ECL assays as shown in Figure 3 or with single RBA in Figure 4. The correlation coefficients between 6-Plex and single ECL assays were r = 0.6431 for GADA, r = 0.8567 for IA-2A, r = 0.9434 for IAA, r = 0.8881 for ZnT8A, r = 0.9032 for TGA, and r = 0.8484 for Covid-19A (P < 0.0001 for all). The correlation coefficients between 6-Plex ECL assay and RBAs were r = 0.8576 for GADA, r = 0.6952 for IA-2A, r = 0.5775 for IAA, r = 0.6223 for ZnT8A, and r = 0.8800 for TGA (P < 0.0001 for all).
FIG. 4.
Correlation between the autoantibody levels measured by 6-Plex ECL assay and single RBA. Dotted lines represent the cutoff for each assay, respectively. RBA, radio-binding assay.
Between the 6-Plex ECL assay and RBAs, there were some discordant results, especially for GADA and IAA. Most of these samples were single IAb positive by RBA and were also negative in the single ECL assay. Between 6-Plex ECL and single ECL assay, only a small number of samples were discordant, and they were all at low levels.
Discussion
Several large scale national and international screening programs for T1D are in progress, including general population screening efforts in children living in Europe and the United States.9,18 Importantly, multiple candidate interventions are under investigation to abrogate or slow progression to clinical T1D among IAb-positive individuals. All four biochemically defined IAbs, including IAA, GADA, IA-2A, and ZnT8A are important in prediction and evaluation of risk of progression to T1D in both relatives of patients with T1D and general population. Measurement of these four IAbs using current standard RBA with single IAb measurement are laborious and inefficient for a large-scale screening initiative. While significant progress has been made in standardization of IAb assays and more efficient technologies, the cost and logistic complexity of multiplexing methods preclude their wide-spread use in population-based screening given the limitation that the currently used multiplex ECL assay still depends on the MSD platform.
A combined assay, 3-Screen ICA™ enzyme linked immunosorbent assay (ELISA) combining 3 IAbs (GADA, IA-2A, and ZnT8A) has been applied to an ongoing Fr1da study in Germany,19 a general population screening in young children. While 3-Screen ELISA format successfully achieved some goals of screening in the general population with high efficiency and low cost, a major limitation20 is the lack of IAA. It is very important to include IAA since IAA is usually the first IAb to appear and has a high prevalence in young children. Furthermore, 3-Screen ELISA is not a true multiplexing assay as it cannot distinguish which of the 3 IAbs are present from a positive signal. The performance of the multiplex assay has been repeated in multiple cohorts and it will need to be analytically and clinically validated.
It has been reported by multiple study groups and well documented that single IAb with low-affinity detected by current standard RBA present a low risk for T1D development.4,5,7,8,21–24 In the screening of both relatives of T1D patients and the general population, a large proportion of positivity by RBA was found to be due to low-affinity IAbs,4,7,8,22,23 which widely appeared among participants with only a single IAb. This is similar to findings from recent studies in adult-onset diabetes and the LADA patient cohort.25,26 Using the ECL assay in multiple clinical studies, including DAISY, TrialNet, TEDDY, and ASK, has demonstrated that the ECL assay is a high-affinity antibody assay and able to discriminate high-affinity IAbs from low-affinity IAbs generated by RBA.
The ECL assay also demonstrated similar sensitivity as RBA in newly diagnosed T1D patient cohorts and individuals with multiple IAbs at high risk for T1D development. In contrast, only 20% to 40% of samples positive for a single IAb by RBA were also positive in the ECL assay; those IAb positive by RBA but negative by ECL assay were of low affinity.4,5,7,8,26 Multiplex ECL assay technology was built on the platform of the ECL assay, and it inherited this unique advantage of high-affinity detection. Similar to single ECL assays, the 6-Plex ECL assay in the present study correlated well with RBA for identifying individuals at high risk for T1D development, including participants with multiple IAbs and/or those who developed clinical T1D during follow-up.
The 6-Plex assay also confirmed the separation of individuals at high risk for T1D development from those at low risk for T1D development with single IAb. The majority of single IAb detected by RBA, which represents a large proportion of IAb positivity in screening, was found to be of low affinity with low disease risk and resulted in overall low predictive value. A high-affinity IAb assay such as ECL assay is needed to highly discriminate disease-specific IAbs with high affinity from the low affinity with low-risk prediction.
There are some limitations as we discussed in our previous study for a multiplexed ECL assay.10 A small number (<1%) of 6-Plex ECL assay sample results were false positives without a clear underlying reason. These false positives can be removed by confirmation assay using single ECL assay. For routine laboratory quality assurance/control, all positive results are recommended to be confirmed with their corresponding single ECL assays to remove the false positives from the multiplex ECL assay. The optimal assay conditions for each antibody assay, for example, serum volume used, and final dilution of serum in the incubation with labeled antigen, could be varied for different antibody assays. For the multiplex antibody assays incubated in a single well, the assay condition might not be able to be adjusted to the best condition for each autoantibody assays.
It will be important to test the assay conditions for each individual antibody assay on an U-Plex plate before combining them into a multiplex assay. In our present study, the 6-Plex ECL assay missed two T1D cases that were positive by RBA and more work needs to be done to improve this assay sensitivity. Prozone phenomenon, which is when a false negative results from a high antibody level interfering with the antigen-antibody binding, was not observed in the present study as described in a previous study with a different combination of autoantibodies in a multiplex ECL assay. The population in this study is limited to children in the United States and extended validations to adult population and other ethnic children should be done in the future.
As a result of this validation study, the 6-Plex ECL assay has been formally accepted as the first-line IAb screening assay to be used for assessing T1D risk in general population ASK subjects. The samples with one or more IAb positive in the 6-Plex ECL assay will be confirmed by RBA for all four IAbs to look for the possibility of multiple IAbs by RBA. The samples with TGA positive in 6-Plex ECL assay will also be confirmed by RBA. With significantly reducing in both labor and reagents, the cost of 6-Plex ECL assay is reduced to <30% of original six single RBA when a plate reader equipment is available. We expect a large data set created with 6-Plex ECL assay for analysis from the study.
In conclusion, the 6-Plex ECL assay illustrated its very good performance for measuring all six antibodies combined, identical to their corresponding single ECL assays with high sensitivity and specificity. The multiplex assay inherited an important unique feature of being a high-affinity antibody assay from the ECL assay platform, which results in 6-Plex ECL results having significantly higher disease specificity than the current standard RBA measurements. Most importantly, the multiplexing feature makes large scale screening much more feasible in the general population as it is highly efficient, low cost, and requires a small volume of serum.
Authors' Contributions
L.H., X.J., C.G.R., K.W., D.M., F.D., B.S., A.S., K.S. researched data and edited the article. M.R. and L.Y. designed study, researched data, and wrote article. M.R. and L.Y. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The study was supported by JDRF grant 2-SRA-2018-533-S-B, 2-SRA-2020-965-S-B, 1-SRA-2017-564-M_N, NIH grant DK32083, and Diabetes Research Center (DRC) grant P30 DK116073.
References
- 1. TEDDY Study Group: The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vehik K, Hamman RF, Lezotte D, et al. : Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007;30:503–509. [DOI] [PubMed] [Google Scholar]
- 3. Ziegler AG, Rewers M, Simell O, et al. : 1. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miao D, Steck A, Zhang L, et al. : And the type 1 diabetes TrialNet study group. Electrochemiluminescence Assays for Insulin and GAD Autoantibodies Improve Prediction of Type 1 Diabetes Risk. Diabetes Tech Ther 2015;17:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu L, Miao D, Scrimgeour L, et al. : Distinguishing persistent insulin autoantibodies with differential risk: non-radioactive bivalent proinsulin/insulin autoantibody assay. Diabetes 2012;61:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu L, Dong F, Fuse M, et al. : Proinsulin/insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care 2013;36:2266–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao D, Guyer KM, Dong F, et al. : GAD65 autoantibodies detected by electrochemiluminescence (ECL) assay identify high risk for type 1 diabetes. Diabetes 2013;62:4174–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia X, He L, Miao D, et al. : High-affinity ZnT8 autoantibodies by electrochemiluminescence assay improve risk prediction for type 1 diabetes. J Clin Endocrinol Metab 2021;3:dgab575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McQueen RB, Rasmussen CG, Waugh K, et al. : Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care 2020;43:1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu Y, Zhao Z, Waugh K, et al. : High-throughput multiplexed autoantibody detection to screen type 1 diabetes and multiple autoimmune diseases simultaneously. EBioMedicine 2019;47:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia X, He L, Gu Y, et al. : A high-throughput electrochemiluminescence 7-Plex assay simultaneously screening for type 1 diabetes and multiple autoimmune diseases. J Vis Exp 2020;29:159. [DOI] [PubMed] [Google Scholar]
- 12. Jia X, Gesualdo P, Geno Rasmussen C, et al. : Prevalence of SARS-CoV-2 antibodies in children and adults with type 1 diabetes. Diabetes Technol Ther 2021;23:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu L, Robles DT, Abiru N, et al. : Early expression of anti-insulin autoantibodies of man and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonifacio E, Yu L, Williams AK, et al. : Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wenzlau JM, Juhl K, Yu L, et al. : The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bao F, Yu L, Babu S, et al. : One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease associated transglutaminase autoantibodies. J Autoimmun 1999;13:143–148. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Z, Miao D, Waugh K, et al. : Higher sensitivity and earlier identification of celiac disease autoimmunity by a non-radioactive assay for transglutaminase autoantibodies. J Immunol Res 2016;2016:2904563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Insel RA, Dunne JL, Ziegler AG: General population screening for type 1 diabetes: has its time come? Curr Opin Endocrinol Diabetes Obes 2015;22:270–276. [DOI] [PubMed] [Google Scholar]
- 19. Ziegler AG, Haupt F, Scholz M, et al. : 3 Screen ELISA for high-throughput detection of beta cell autoantibodies in capillary blood. Diabetes Technol Ther 2016;18:687–693. [DOI] [PubMed] [Google Scholar]
- 20. Zhao Z, Yu L: High-throughput screening in general population for type 1 diabetes. Diabetes Technol Ther 2016;18:674–676. [DOI] [PubMed] [Google Scholar]
- 21. Achenbach P, Koczwara K, Knopff A, et al. : Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004;114:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlosser M, Koczwara K, Kenk H, et al. : In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia 2005;48:1830–1832. [DOI] [PubMed] [Google Scholar]
- 23. Mayr A, Schlosser M, Grober N, et al. : GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 2007;56:1527–1533. [DOI] [PubMed] [Google Scholar]
- 24. Siljander H, Harkonen T, Hermann R, et al. : Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes Metab Res Rev 2009;25:615–622. [DOI] [PubMed] [Google Scholar]
- 25. Achenbach P, Hawa MI, Krause S, et al. ; Action LADA consortium. Autoantibodies to N-terminally truncated GAD improve clinical phenotyping of individuals with adult-onset diabetes: action LADA 12. Diabetologia 2018;61:1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu Y, Jia X, Vartak T, et al. ; Action LADA consortium and the Diabetes in Young Adults (DiYA) Study Group: Improving clinical utility of GAD65 autoantibodies by electrochemiluminescence assay and clinical phenotype when identifying autoimmune adult-onset diabetes. Diabetologia 2021;64:2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]