Abstract
Objective:
Evaluating the feasibility of closed-loop insulin delivery with a zone model predictive control (zone-MPC) algorithm designed for pregnancy complicated by type 1 diabetes (T1D).
Research Design and Methods:
Pregnant women with T1D from 14 to 32 weeks gestation already using continuous glucose monitor (CGM) augmented pump therapy were enrolled in a 2-day multicenter supervised outpatient study evaluating pregnancy-specific zone-MPC based closed-loop control (CLC) with the interoperable artificial pancreas system (iAPS) running on an unlocked smartphone. Meals and activities were unrestricted. The primary outcome was the CGM percentage of time between 63 and 140 mg/dL compared with participants' 1-week run-in period. Early (2-h) postprandial glucose control was also evaluated.
Results:
Eleven participants completed the study (age: 30.6 ± 4.1 years; gestational age: 20.7 ± 3.5 weeks; weight: 76.5 ± 15.3 kg; hemoglobin A1c: 5.6% ± 0.5% at enrollment). No serious adverse events occurred. Compared with the 1-week run-in, there was an increased percentage of time in 63–140 mg/dL during supervised CLC (CLC: 81.5%, run-in: 64%, P = 0.007) with less time >140 mg/dL (CLC: 16.5%, run-in: 30.8%, P = 0.029) and time <63 mg/dL (CLC: 2.0%, run-in:5.2%, P = 0.039). There was also less time <54 mg/dL (CLC: 0.7%, run-in:1.6%, P = 0.030) and >180 mg/dL (CLC: 4.9%, run-in: 13.1%, P = 0.032). Overnight glucose control was comparable, except for less time >250 mg/dL (CLC: 0%, run-in:3.9%, P = 0.030) and lower glucose standard deviation (CLC: 23.8 mg/dL, run-in:42.8 mg/dL, P = 0.007) during CLC.
Conclusion:
In this pilot study, use of the pregnancy-specific zone-MPC was feasible in pregnant women with T1D. Although the duration of our study was short and the number of participants was small, our findings add to the limited data available on the use of CLC systems during pregnancy (NCT04492566).
Keywords: Artificial pancreas, Closed loop control, Glucose control, Outpatient, Pregnancy, Type 1 diabetes
Introduction
It is well known that pregnancy in women with type 1 diabetes (T1D) can be associated with significant maternal and fetal morbidity and mortality. Maternal hyperglycemia has been linked to preeclampsia, medically indicated preterm delivery, labor abnormalities, need for cesarean delivery, and maternal birth trauma.1–4 Fetal and neonatal morbidity includes increased risk of congenital malformations, growth abnormalities (fetal growth restriction, small, or large for gestational age fetus or neonate), fluid abnormalities (oligo- and poly-hydramnios), stillbirth, birth trauma, neonatal hypoglycemia, hyperbilirubinemia, hypocalcemia, polycythemia, and neonatal intensive care admission.5–7 Pregestational and gestational elevated hemoglobin A1c (HbA1c), reduced time in target range, time in hyperglycemia, and episodes of severe maternal hypoglycemia have been associated with poorer outcomes.8–11 In addition to the importance of achieving HbA1c goals and glucose targets, maternal glycemic variability has been shown to be associated with adverse fetal outcomes.12
Optimal glycemic goals during pregnancy are still debated with respect to specific HbA1c and self-monitored blood glucose (SMBG) targets, despite the evidence supporting the relationship between glycemic control and pregnancy outcomes. Clinical guidelines note that the risk of hypoglycemia needs to be carefully balanced with the risk of hyperglycemia both for the mother and developing fetus13,14 The CONCEPTT trial revealed the benefits of continuous glucose monitoring (CGM) use during pregnancy.9 In 2019, the ADA published consensus guidelines for the use of CGM recommending that glycemic targets for pregnant women with T1D should be guided by a time in range (TIR) goal of >70% based on the glycemic target range of 63–140 mg/dL.15 Despite increased adoption of CGM16 and the use of more rapid insulin analogs17 to assist in glycemic management, many pregnant women still struggle to achieve TIR targets18 and reduce glycemic variability to optimize pregnancy outcomes. Questions remain regarding the benefits for and optimal management of women using sensor-augmented insulin pump therapy (SAP) during pregnancy,19 as well as the optimal ways to achieve the very narrow maternal glycemic goals and attain optimal maternal and fetal outcomes while minimizing hypoglycemia.
Limited data are available on closed-loop control (CLC) use in pregnancy.20–23 The two largest studies currently available from Stewart et al. reported results for 16 women using CLC compared with SAP.21,22 The CamAP System (CamDiab Ltd.) is a closed-loop system that bears CE mark for use in pregnant women with T1D. In the United States, there is currently no approved system for patient use during pregnancy outside of a research setting.
To address the unmet needs of pregnant women with T1D in the United States, we designed a zone model predictive control (zone-MPC) based CLC system specifically customized for use during pregnancy. The zone-MPC runs on the interoperable artificial pancreas system (iAPS),24 which consists of a CGM, an insulin pump, and an unlocked smartphone. Zone-MPC integrated in iAPS has been proven to be safe and effective for glycemic control in previous clinical trials.25,26 In this study, we report the first clinical trial results of the feasibility of a pregnancy-specific design of this system evaluated under supervised conditions.
Research Design and Methods
Trial conduct and oversight
This study (NCT04492566) was conducted in pregnant women with preexisting T1D at the Icahn School of Medicine at Mount Sinai (New York, NY), Mayo Clinic (Rochester, MN), and Sansum Diabetes Research Institute (Santa Barbara, CA) after approval by the Mayo Foundation IRB. Written informed consent was obtained from all participants. One REDCap electronic data capture tool (Mayo Clinic) was used to collect and manage the data from all sites.27,28
Participants and trial design
The eligibility criteria for participation included age ≥18 and ≤45 years, current use of an insulin pump, HbA1c ≤9%, 140/7 to 326/7 weeks gestation, singleton pregnancy without any other significant known pregnancy complications, and willingness to use bolus insulin for all meals and snacks containing ≥5 g of carbohydrate other than when treating hypoglycemia, use insulin aspart (Novolog) or insulin lispro (Humalog) for the CLC session, not to start any new noninsulin glucose-lowering agent during the trial, and to use study-provided devices and to abide by the study protocol. A complete list of eligibility criteria is given in the Supplementary Data.
The study consisted of up to 2 weeks run-in with participants using the study CGM, Dexcom G6 (Dexcom, Inc., San Diego, CA), and personal insulin pump therapy at home, followed by a 2-day supervised CLC period. All participants were provided and trained on the use of the CGM at the start of the run-in to reinforce proper use. The CGM was worn either on the abdomen, posterior upper arm, or buttock based on body habitus, skin findings, and participant preference. During this period, women were instructed to perform daily activities, food consumption and meal boluses as usual. Study clinicians could adjust the participants' treatment parameters (i.e., basal rates, carbohydrate ratios, insulin sensitivity factors) as needed to optimize pump settings based on sensor and pump data reviews before entering the CLC phase. At the end of this run-in period, data were downloaded before the initiation of the CLC study. Insulin pump settings were adjusted based on clinical judgment to address needs per gestational age during or at the end of the run-in phase. No adjustments were made during the CLC session unless otherwise stated.
During the CLC session, participants continued using the study CGM but switched to a research insulin pump (Tandem Diabetes Care, San Diego, CA). The devices connected wirelessly to the iAPS installed on an unlocked study phone (Google Pixel 2 or 3a).24 Participants were supervised by onsite study staff clinically trained in treating hypoglycemia and hyperglycemic emergencies per the clinical study protocol. Before initiating CLC and upon study completion, all enrolled participants at or beyond 23 weeks of gestational age had fetal heart rate recorded by a handheld fetal Doppler (CareFusion, Middleton, WI). Vital signs were recorded on admission and every 12 h.
Participants chose their meals without nutritional restrictions from nearby restaurants or markets and ate all meals at the supervised site. SMBG testing was performed before and 2 h after each meal. Hypoglycemia treatments occurred when either SMBG was <63 mg/dL or if participant wished to treat a glucose level above the 63 mg/dL threshold. For glucose levels <63 mg/dL, a repeat SMBG measurement was performed every 15 min after the rescue carbohydrate consumption until the hypoglycemic event was resolved. Additional testing was performed at bedtime. Participants were permitted to snack at any time, administer meal boluses per their home routine as well as exercise as per their typical routine and ingest additional carbohydrates for exercise, if needed.
Reportable events were categorized as those leading to hospitalization, occurring in association with a study device, severe hypoglycemia (defined as hypoglycemia requiring assistance owing to altered consciousness), diabetic ketoacidosis as defined by the Diabetes Control and Complications Trial, or hyperglycemia with ketonemia leading to injury or hospitalization.29 Owing to the more stringent glucose targets during pregnancy, moderate hyperglycemic events are reported for circumstances where the sensor glucose level was >180 mg/dL for more than 60 min without a dietary explanation.
Closed-loop system
The zone-MPC algorithm solves an optimization problem that minimizes the predicted glucose values' deviations from a time-dependent target zone.30 The cost for the glucose deviations above the zone are weighted by predicted glucose velocity and insulin on board (IOB) for enhanced hyperglycemia response as well as for limiting controller-induced hypoglycemia. The predicted glucose velocity is also used for assertive response to rapidly upward trending glucose. Finally, asymmetric costs for controller-delivered insulin deviating below and above the prescribed basal rate are induced to independently address hypoglycemia and hyperglycemia, respectively. The optimal insulin delivery plan is updated every 5 min based on new CGM data. Thus, the controller-delivered insulin can be highly time varying, in contrast to the preprogrammed basal rate, which is held constant for the specific times of the day. The optimization is subject to insulin-glucose dynamics and time-dependent insulin delivery and IOB constraints to prevent insulin stacking.
The pregnancy-specific zone-MPC was designed to address the tighter glucose control requirements in pregnancy. The target zones were redesigned to 80–110 mg/dL during the day and 80–100 mg/dL during the night from 12 to 4 AM, which are lower than the values used for the nonpregnant T1D population. Insulin delivery was more assertive when the blood glucose values were trending upwards while in 120–180 mg/dL range. Furthermore, postprandial control was intensified by relaxing the controller's IOB constraint. Details of these changes are provided elsewhere31 and summarized in Supplementary Data.
Meals were announced to the system by the user, and meal boluses were calculated based on the participants' prescribed bolus settings (i.e., carbohydrate ratio and insulin sensitivity factor) and user-estimated carbohydrate intake. Changes to meal bolus calculations were as follows: (1) full meal bolus was applied when blood glucose values were >70 mg/dL; (2) a correction was automatically added to the bolus to bring the glucose to reference glucose of 90 mg/dL if premeal glucose was >100 mg/dL; and (3) meal boluses were automatically reduced by 20% when mealtime glucose was <70 mg/dL. Note that the total insulin delivered during the CLC is the sum of controller-delivered insulin and user-requested bolus insulin.
A safety layer, Health Monitoring System, independent of the control algorithm, was embedded in the iAPS for improved safety against hypoglycemia through audiovisual advisory alarms and text messages.32 The system was set to produce an alert on the phone screen when it predicted a glucose value <65 mg/dL within the next 15 min. As the CLC system is an insulin-only system, hypoglycemia treatments were at the users' discretion. Users could also choose to administer additional correction boluses through the system.
Study endpoints
The primary endpoint was the percentage of time that CGM glucose level was in the 63–140 mg/dL target range. Secondary outcomes were the overnight percentage of time in the target range, 2-h postprandial percentage of time in the target range, percentage of time below thresholds of 63 and 54 mg/dL, and percentage of time >140, 180, and 250 mg/dL.14,15 Additional secondary outcomes were mean CGM glucose, glycemic variability assessed by both glucose standard deviation (SD) and coefficient of variation, the number of treatments for hypoglycemia, serious adverse events, serious adverse device events, adverse device effects, and unanticipated adverse device effects during the 2-day supervised study session. A root cause analysis of the event was performed to determine if the event was related to a component of the CLC system. Other outcome measures included active time in closed-loop, CGM use time, device issues, and total daily insulin delivery along with basal and bolus insulin delivery.
Statistical analysis
Statistical analyses were conducted on data from all participants. Glycemic outcomes were calculated based on the CGM data collected during the last week of run-in period for the home portion compared with the CGM data collected during the supervised study visit for the closed-loop portion. In addition to the overall glucose control measures, we assessed the overnight (midnight—6 AM) outcomes for both parts of the study. Controller-delivered insulin, user-requested insulin (meal and correction boluses), and total insulin during the CLC phase were calculated. The controller-delivered insulin was compared with the participants' total preprogrammed basal insulin profile over the same period. No comparisons were made for the observed basal, bolus, and total insulin between the run-in and CLC sessions owing to variable day-to-day factors (e.g., carbohydrate intake, physical activity).
Paired t-test was used to compare results between run-in versus CLC study periods. Exclusively for the CLC portion, we analyzed postprandial glucose control performance by evaluating the 2-h postprandial CGM readings in accordance with American Diabetes Association guidelines noting the importance of 1 and 2 h postprandial glucose values in this population.14 The performance was assessed based on the percentage of time in the target range within the first hour [0–1 h] and within the first 2 h [0–2 h] of meal intake. To assess full CGM trajectory of the early postprandial period, only main meals (i.e., breakfast, lunch, and dinner) consumed up to 2 h before the end of the CLC session were included in these analyses. Since there were multiple meals consumed per participant, linear mixed-effects regression models were used to account for the repeated measurements.
Results are presented as mean ± SD for descriptive statistics and paired t-test outcomes. Linear mixed-effect regression results are reported as the regression model estimate ± standard error. A two-sided significance level of 0.05 was used in all statistical analyses. There were no adjustments for multiple comparisons. Data processing was performed in MATLAB R2019b (MathWorks, Inc., Natick, MA), and we used R (R Core Team, 2019) for statistical analyses.
Results
Participant characteristics
Between August 2020 and June 2021, 11 participants were enrolled at age 30.6 ± 4.1 years with the following baseline characteristics at enrollment: gestational age of 20.7 ± 3.5 weeks, weight of 76.5 ± 15.3 kg, body mass index of 27.8 ± 4.5 kg/m2, and HbA1c of 5.6 ± 0.5% (38 ± 5 mmol/mol). All participants were on SAP before enrollment with a mean (±SD) total daily insulin dose of 51.2 ± 16.2 units. Baseline study participant characteristics are given in Table 1.
Table 1.
Demographics of Study Participants at Enrollment
| Number of participants | 11 |
| Age (years) | 30.6 ± 4.1 |
| Ethnicity | Not Hispanic or Latino (100%) |
| Race | White (100%) |
| Weight (kg) | 76.5 ± 15.3 |
| BMI (kg/m2) | 27.8 ± 4.5 |
| HbA1c | 5.6% ± 0.5% (38 ± 5 mmol/mol) |
| Systolic BP (mmHg) | 107.9 ± 8.2 |
| Diastolic BP (mmHg) | 65.8 ± 7.1 |
| Heart rate (bpm) | 86.8 ± 11 |
| Gestational age | 20.7 ± 3.5 |
| Gravida | 2.0 ± 1.3 |
| Parity | 0.8 ± 1.4 |
| Pump- | |
| Tandem t: slim x2 (Basal IQ) | 4 |
| Tandem t: slim x2 (Control IQ) | 2 |
| Tandem t: slim x2 (Manual mode) | 2 |
| Medtronic 670G (Manual mode) | 2 |
| Omnipod Eros | 1 |
| Duration of pump use | |
| <3 months | 1 |
| 1–5 years | 5 |
| 5–10 years | 1 |
| >10 years | 4 |
| CGM user at enrollment | 11 |
| Duration of CGM use, years | |
| 1–5 | 9 |
| 5–10 | 1 |
| >10 | 1 |
| Average total daily insulin-1 week before 48-h study (Units) | 51.2 ± 16.2 |
BMI, body mass index; CGM, continuous glucose monitor; HbA1c, hemoglobin A1c.
Study outcomes
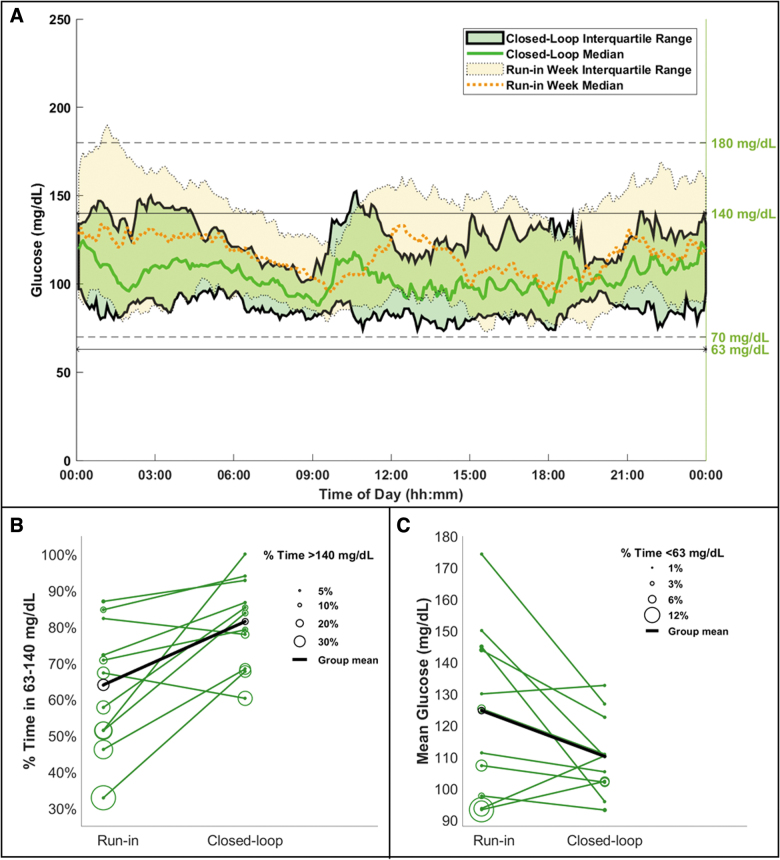
Supervised CLC sessions lasted 47.7 ± 2.5 h, and participants stayed in closed-loop 98.0% ± 1.3% of the time. CLC increased the percentage of time spent in the 63–140 mg/dL range (CLC: 81.5%, run-in: 64%, P = 0.007) by decreasing both time >140 mg/dL (CLC: 16.5%, run-in: 30.8%, P = 0.029) and time <63 mg/dL (CLC: 2.0%, run-in: 5.2%, P = 0.039). Furthermore, CLC improved the outcomes for times <54 mg/dL (CLC: 0.7%, run-in: 1.6%, P = 0.030), >180 mg/dL (CLC: 4.9%, run-in: 13.1%, P = 0.032), and in the broader glycemic range of 70–180 mg/dL (CLC: 90.1%, run-in: 77.6%, P = 0.003). Glycemic variability was less with CLC, as measured by both SD (CLC: 31.2 mg/dL, run-in: 42.6 mg/dL, P = 0.017) and coefficient of variation (CLC: 28.0%, run-in: 34.2%, P = 0.047). The only differences in overnight glucose control between therapies were time >250 mg/dL (CLC: 0%, run-in: 3.9%, P = 0.030) and glucose variability measured by glucose SD (CLC: 23.8 mg/dL, run-in: 42.8 mg/dL, P = 0.007). Detailed results and additional outcomes for both day-and-night and overnight alone are given in Table 2. Figure 1A provides the daily median and interquartile range of CGM measured glucose trajectories across all participants. Individual breakdowns of glycemic outcomes show that 9 of 11 participants had an improved outcome in at least one of the metrics as given in Figure 1B and C. For eight participants, CLC improved TIR, time <63 mg/dL, and time >140 mg/dL altogether.
Table 2.
Continuous Glucose Monitor Metrics Comparing the Performance of the Closed-Loop Period with the Run-In Period
| Sensor glucose metrics | Time | Closed-loop session (N = 11) | Run-in week (N = 11) | P |
|---|---|---|---|---|
| % Time 63–140 mg/dL | Day and night | 81.5 ± 12.3 | 64.0 ± 17.5 | 0.007** |
| Overnight | 75.2 ± 31.8 | 59.7 ± 19.8 | 0.167 | |
| % Time 70–180 mg/dL | Day and night | 90.1 ± 5.6 | 77.6 ± 10.4 | 0.003** |
| Overnight | 88.1 ± 13.7 | 75.9 ± 12.2 | 0.083* | |
| % Time <54 mg/dL | Day and night | 0.7 ± 1.4 | 1.6 ± 1.9 | 0.030** |
| Overnight | 0.2 ± 0.7 | 1.4 ± 2.3 | 0.132 | |
| % Time <63 mg/dL | Day and night | 2.0 ± 2.5 | 5.2 ± 6.0 | 0.039** |
| Overnight | 0.5 ± 1.5 | 4.9 ± 7.7 | 0.105 | |
| % Time <70 mg/dL | Day and night | 5.0 ± 4.2 | 9.2 ± 9.5 | 0.075* |
| Overnight | 2.2 ± 3.8 | 8.0 ± 11.0 | 0.115 | |
| % Time >140 mg/dL | Day and night | 16.5 ± 12.5 | 30.8 ± 20.5 | 0.029** |
| Overnight | 24.3 ± 31.6 | 35.4 ± 23.4 | 0.317 | |
| % Time >180 mg/dL | Day and night | 4.9 ± 5.1 | 13.1 ± 12.0 | 0.032** |
| Overnight | 9.6 ± 14.1 | 16.1 ± 13.9 | 0.346 | |
| % Time >250 mg/dL | Day and night | 0.3 ± 0.8 | 2.9 ± 4.2 | 0.061* |
| Overnight | 0 ± 0 | 3.9 ± 5.2 | 0.030** | |
| Mean glucose (mg/dL) | Day and night | 110.2 ± 12.6 | 124.7 ± 26.6 | 0.058* |
| Overnight | 118.8 ± 27.1 | 131.4 ± 30.8 | 0.130 | |
| SD glucose (mg/dL) | Day and night | 31.2 ± 9.1 | 42.6 ± 11.5 | 0.017** |
| Overnight | 23.8 ± 13.1 | 42.8 ± 14.4 | 0.007** | |
| CV glucose (%) | Day and night | 28.0 ± 6.5 | 34.2 ± 7.0 | 0.047** |
| Overnight | 19.4 ± 8.3 | 32.4 ± 8.9 | 0.366 |
The data are shown as mean ± SD.
0.05<P < 0.1.
P ≤ 0.05.
SD, standard deviation.
FIG. 1.
(A) Comparison of CGM glucose levels between CLC therapy (solid traces and green area) and participants' standard therapy (dashed traces and yellow area), (B) individual breakdown of time spent in the target range on the y-axis and time spent above the target range is represented by circles, (C) individual breakdown of mean CGM glucose on the y-axis and time spent below the target range is represented by circles. CGM, continuous glucose monitor; CLC, closed-loop control.
Participants had three main meals each day of the CLC session with the content and size of meals at their discretion. The meal amount entered into the meal bolus calculator was also decided by the participants. Combined with snacks, total carbohydrate entry over the CLC period was 272.5 ± 109.6 g per participant. Of the 66 meals consumed, 60 were eaten more than 2 h before the CLC session ended and were included in the analyses. For the meals included in the analyses, the carbohydrate content per meal ranged from 10 to 78 g, with an average of 39.6 ± 18.7 g. CGM glucose at mealtime before food ingestions was 98.8 ± 21.1 mg/dL. The average estimated TIR was 86.4% ± 3.9% for the first hour and 80.4% ± 4.9% for the first 2 h of the postprandial window.
Of all hypoglycemia treatments, 58% of them were required within 3 h of a meal bolus, and rescue carbohydrate intake ranged from 3 to 16 g. Episodes requiring additional consumption of carbohydrate were reported as a new event. Ten participants required treatment for hypoglycemia, and the number of treatments consumed per CLC session per participant ranged from 0 to 13, with an average of 4.7 ± 4.1, as detailed in the Supplementary Data. All treatments were self-administered by the participants.
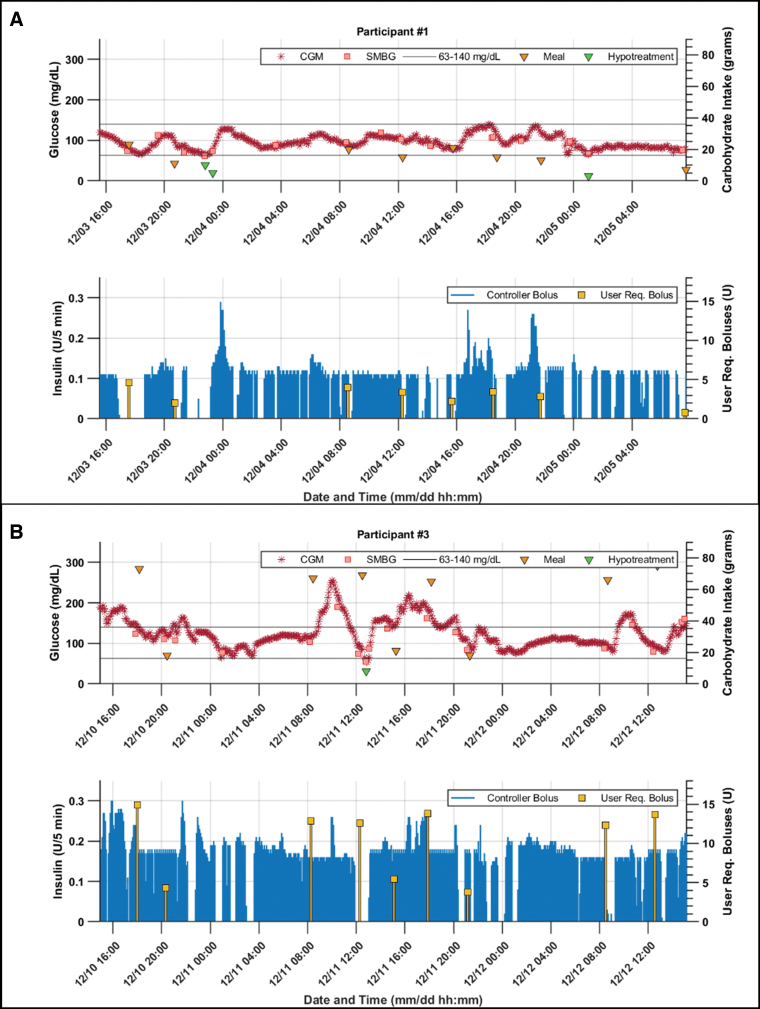
There was no significant difference between the controller-delivered insulin and the overall programmed basal insulin amounts (CLC: 45.2 ± 19.2 units, basal profile: 48.3 ± 21.3 units over the CLC session, P = 0.13). The average user-requested bolus during the CLC was 44.1 ± 20.7 units, and the total insulin delivered was 89.2 ± 37.2 units over the CLC session, averaged across participants. Detailed visuals for each participant's glucose profiles are provided in the Supplementary Data. Figure 2 provides a closer view of two participants' CLC data that are of particular interest as one had the highest TIR and low total carbohydrate intake, whereas the other one had the highest total carbohydrate intake among participants. Both participants achieved a TIR that was approximately twice of their TIR in the run-in week (100% vs. 51.8% in the participant given in Fig. 2A and 67.8% vs. 32.9% in participant given in Fig. 2B).
FIG. 2.
CLC session glucose, meal, and insulin delivery of (A) the participant with highest CGM time in the target 63–140 mg/dL range (100%) and low carbohydrate intake (125 g), and (B) the participant with highest carbohydrate intake (472 g). For both participants, the top figure shows the glucose on the left axis and carbohydrate intake amounts on the right axis; the bottom figure shows the controller decided micro-bolus deliveries on the left axis and the user requested boluses (i.e., meal and additional correction) are presented on the right axis. Note that participant numbers are assigned arbitrarily.
Safety and adverse events
One device issue occurred leading to a loss of CGM data for 95 min soon after a meal (Participant 6), which required re-entry of device settings on the study phone. The participant reverted to open loop during this time with transient hyperglycemia with a peak SMBG level of 259 mg/dL when reconnection was resumed. The participant administered a correction bolus and hyperglycemia resolved within 2 h of resumption of CLC insulin delivery. One episode of moderate hyperglycemia occurred overnight with the participant receiving intermittent occlusion alerts (Participant 5). SMBG and ketones were checked and were 185 mg/dL and 0.4 mM, respectively. The infusion site was changed at 3:30 AM, and by 4:20 AM CGM values dropped to 174 mg/dL. Another infusion set change occurred owing to irritation/itching at the infusion site with a CGM glucose level of 119 mg/dL. Both participants' overnight basal profiles were increased for the second night. No hospitalizations, episodes of ketoacidosis, or severe hypoglycemia occurred. The participant who consumed 13 hypoglycemic treatments during the study reported a long-standing history of preference for lower glucose levels and did not require assistance for any of her treatments.
Discussion
Our study reports the first CLC outcomes in pregnant women in the United States using a novel zone-MPC design specifically customized to meet the CGM TIR targets for pregnancy based on consensus guidelines.15 Compared with the 1-week run-in, there was an increased percentage of time in the 63–140 mg/dL range accompanied by decreased percentages above and below this range, during the supervised CLC session. Moreover, 9 of 11 participants had a greater TIR during CLC. Since every additional 5% improvement in maternal TIR during pregnancy can improve pregnancy outcomes,33 the glycemic control achieved by our CLC system has clinical significance. Equally important, no adverse safety events occurred, and sessions were completed without severe hyperglycemia or severe hypoglycemia despite the stricter glycemic targets. Six of 11 participants were already using an insulin pump with predictive low-glucose suspend features (i.e., Tandem Basal-IQ, or Tandem Control-IQ technology) during their run-in week, which had likely already helped decrease episodes of hypoglycemia in the run-in week.
For the early postprandial period, the high percentage in the target glucose range achieved by the pregnancy-specific zone-MPC is noteworthy because meal-related glucose control may become particularly challenging in pregnancy with T1D.34 The high postprandial performance may have resulted in more than half of the hypoglycemia treatments in the CLC session occurring within 3 h of a previous meal bolus. This may be owing to one or a combination of factors, such as inaccurate carbohydrate counting, suboptimal meal bolus parameters, and/or the CLC system's assertiveness level in the postprandial period. Further studies can provide better insight on this matter.
Previous reports of other CLC systems show a higher overnight performance compared with the day-and-night time performance. This was not the case for our study potentially owing to (1) late dinners—many from restaurants—with higher fat and protein content, without carbohydrate labeling making estimations for meal bolus more challenging, and (2) the conservative lower nighttime upper limit for micro-boluses (i.e., four times the prescribed basal rate per 5-min intervals) compared with the daytime limit (i.e., 1 unit per 5-min interval) to protect against nocturnal hypoglycemia.
Although the duration of our study was short and the number of participants was small, our findings are important because of the limited data available on the use of CLC systems during pregnancies complicated by T1D in an outpatient setting, and the significant glycemic improvements obtained with the CLC system. Few other CLC studies in an outpatient setting have been conducted in pregnant women with T1D. In an open-label, randomized, crossover study comparing a CLC system with sensor-augmented pump therapy worn overnight, Stewart et al. found that CLC users spent significantly more TIR (74.7% vs. 59.5%) and had lower overnight mean glucose levels (119 vs. 133 mg/dL).21
In a separate report comparing day-and-night CLC to sensor-augmented pump therapy among pregnant participants with T1D, the same research group found that the cohorts had comparable TIR (62.3% vs. 60.1%), mean CGM glucose, and proportions of time spent >140 mg/dL.22 A key difference was that the CLC group experienced less hypoglycemia, including less nocturnal hypoglycemia, a finding that can greatly impact the experience of self-care in T1D.22 Of note, compared with these studies, our participants had a lower average HbA1c at enrollment, and some participants were on SAP treatment that involved new advanced technologies (i.e., Tandem Basal-IQ and Control-IQ technologies).
Polsky and Akturk also reported a case series in which they described the experiences of three pregnant women with T1D utilizing off-label Medtronic 670G, the first CLC system to receive the FDA approval.23 They observed that with increased time spent in automated mode, along with the input of a premeal bolus and “fake carbohydrates,” there was increased time spent in the 70–180 mg/dL range. Our pregnancy-specific zone-MPC also led to a greater time in the 70–180 mg/dL range compared with the participants' SAP therapy.
Our dataset has limitations because of the small sample size related to COVID-19 pandemic restrictions as well as enrolling a group of women with low HbA1c concentrations (mean 5.6%) mostly in the second trimester of their pregnancy. In addition, this was not a randomized controlled trial, but instead compared a 1-week unsupervised run-in period to a 48 h supervised session. Another potential weakness is that some participants may have been more attentive to their carbohydrate intake, and low glucose levels that required treatment might have been addressed earlier owing to the supervision during the study compared with their normal care. Unlike the CLC period, the alerts placed into the CGM for hypoglycemia during run-in was at the discretion of individuals and may have impacted duration of individual hypoglycemia outcomes between the two sessions. It is important to note that we observed no systematic decrease or increase in the controller-delivered insulin compared with the prescribed basal profiles. Therefore, the consistent improvement in time below, above, and in the target range was obtained using CLC system-generated proactive timing of insulin delivery, rather than an overall increase in basal insulin use. Our study has strengths owing to its multicenter design, a range of gestational ages, use of various insulin pumps during the run-in, and participants consuming a free range of carbohydrate and meal choices, many of which were higher in fat or carbohydrate content than typically recommended, likely owing, in part, to meal options available during the study.
Conclusions
In conclusion, our pilot study demonstrates that a customized CLC system designed to specifically target glucose goals for pregnancy is feasible for women previously using insulin pumps and CGM. Initial studies enrolling women to assess the use and efficacy of the pregnancy-specific zone-MPC with iAPS in the home setting are currently underway (NCT04492566). Further studies are needed to evaluate this system in a larger group of women and sustained use throughout pregnancy.
LOIS-P Diabetes and Pregnancy Consortium
Harvard John A. Paulson School of Engineering and Applied Sciences, Harvard University, Boston, MA: Eyal Dassau (PI), Francis J. Doyle III, Basak Ozaslan; Icahn School of Medicine at Mount Sinai, New York: Carol J. Levy (PI), Barak Rosenn (PI), Camilla Levister (I), Grenye O'Malley (I), Dushyanthy Arasaratnam, Selassie Ogyaadu, AllyWang; Mayo Clinic, Rochester, MN: Yogish C. Kudva (PI), Donna Desjardins, Ravinder Jeet Kaur, Walter K Kremers, Corey Reid, Byron Smith, Shelly McCrady-Spitzer, Mari Charisse Trinidad; Sansum Diabetes Research Institute, Santa Barbara, CA: Jordan E. Pinsker (PI), Kristin Castorino (I), Mei Mei Church (I), Kristen Nelson, Jimena Perez, Molly Piper, Camilla Andre.
Supplementary Material
Acknowledgments
The work of the LOIS-P Diabetes and Pregnancy Consortium is dedicated to the memory of Dr. Lois Jovanovič, a pioneer in the field of diabetes and pregnancy, a tireless advocate for her patients, and a mentor to many of the co-authors. Statistical support was provided by Byron Smith, Mayo Clinic, and data science specialist Steven Worthington, at the Institute for Quantitative Social Science, Harvard University. The authors thank Clara Bakus for her help during the supervised CLC sessions at Sansum Diabetes Research Institution.
Authors' Contributions
G.O., K.C., Y.C.K., C.J.L., J.E.P., and E.D. were responsible for study design. B.O. designed, tested, and provided primary technical support for the CLC system with supervision of E.D., F.J.D. and assistance from S.D. B.O. conducted the statistical analyses in consultancy with S.D. and W.K.K. B.O., C.J.L. and Y.C.K. led writing the article and all co-authors contributed to the writing. C.J.L., E.D., B.R., J.E.P., and Y.C.K. were principal investigators at their respective sites. S.M.-S. managed the IRB process. C.R. set up the online platform for data access and management. C.L., M.M.C, D.D., M.P., S.O., R.J.K., and S.M.-S. conducted study visits and managed data collection at their sites. CR and BO was responsible for central management of data collection. All authors were responsible for reviewing and revising this article and assume responsibility and accountability for the results. E.D. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
C.J.L. has received research support from Insulet, Abbott Diabetes, Tandem Diabetes and Dexcom paid to her institution, and has received consulting fees from Dexcom. J.E.P. is currently an employee and shareholder of Tandem Diabetes Care, Inc., The work presented in the article was performed as part of his academic appointment at Sansum Diabetes Research Institute and is independent of his employment with Tandem Diabetes Care. G.O. receives research support from Tandem Diabetes, Insulet, Dexcom, and Abbot paid to her institution. K.C. receives research support provided to her institution from Dexcom, Abbott, Medtronic, Novonordisk, and Insulet. S.O. receives research support from Insulet, Dexcom, and Abbot paid to her institution. W.K.K. receives research funding from the NIH, DOD, AstraZeneca, Roche and Biogen all unrelated to this study. F.J.D. reports equity, licensed IP, and is a member of the Scientific Advisory Board of Mode AGC. Y.C.K. reports product support from Roche Diabetes, Dexcom, Tandem Diabetes and consulting fees from Novo Nordisk. E.D. reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this article was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. No other conflict of interest was reported.
Funding Information
Financial support for this study was provided by the National Institutes of Health (R01DK120358). Product support was provided by Dexcom, Inc. (AP-2020-014). REDCap data management was supported by the Research Computing Facility grant (UL1TR002377).
Supplementary Material
References
- 1. Maresh MJ, Holmes VA, Patterson CC, et al. : Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015;38:34–42. [DOI] [PubMed] [Google Scholar]
- 2. Holmes VA, Young IS, Patterson CC, et al. : Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care 2011;34:1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen O, Keidar N, Simchen M, et al. : Macrosomia in well controlled CSII treated type I diabetic pregnancy. Gynecol Endocrinol 2008;24:611–613. [DOI] [PubMed] [Google Scholar]
- 4. Ekbom P, Damm P, Feldt-Rasmussen B, et al. : Elevated third-trimester haemoglobin A1c predicts preterm delivery in type 1 diabetes. J Diabetes Complications 2008;22:297–302. [DOI] [PubMed] [Google Scholar]
- 5. Persson M, Norman M, Hanson U: Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 2009;32:2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evers IM, de Valk HW, Visser GH: Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 2004;328:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy HR, Bell R, Cartwright C, et al. : Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic-to-clinic variations: a prospective nationwide study. Diabetologia 2017;60:1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inkster ME, Fahey TP, Donnan PT, et al. : Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy Childbirth 2006;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feig DS, Donovan LE, Corcoy R, et al. : Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, et al. : Hypoglycaemia during pregnancy in women with Type 1 diabetes. Diabet Med 2012;29:558–566. [DOI] [PubMed] [Google Scholar]
- 11. Evers I, De Valk H, Mol B, et al. : Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia 2002;45:1484–1489. [DOI] [PubMed] [Google Scholar]
- 12. Dalfra MG, Sartore G, Cianni GD, et al. : Glucose variability in diabetic pregnancy. Diabetes Technol Ther 2011;13:853–859. [DOI] [PubMed] [Google Scholar]
- 13. Blumer I, Hadar E, Hadden DR, et al. : Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4227–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association: 15. Management of diabetes in pregnancy: standards of medical care in diabetes—2022. Diabetes Care 2022;45(Suppl 1):S232–S243. [DOI] [PubMed] [Google Scholar]
- 15. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy CJ, Foster NC, DuBose SN, et al. : Changes in device uptake and glycemic control among pregnant women with type 1 diabetes: data from the T1D exchange. J Diabetes Sci Technol 2021;15:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toledano Y, Hadar E, Hod M: Pharmacotherapy for hyperglycemia in pregnancy–the new insulins. Diabetes Res Clin Pract 2018;145:59–66. [DOI] [PubMed] [Google Scholar]
- 18. O'Malley G, Ozaslan B, Levy C, et al. : Longitudinal observation of insulin use and glucose sensor metrics in pregnant women with type 1 diabetes using continuous glucose monitors and insulin pumps: the LOIS-P Study. Diabetes Technol Ther 2021;23:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feig DS, Corcoy R, Donovan LE, et al. : Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care 2018;41:2471–2479. [DOI] [PubMed] [Google Scholar]
- 20. Murphy HR, Elleri D, Allen JM, et al. : Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stewart ZA, Wilinska ME, Hartnell S, et al. : Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med 2016;375:644–654. [DOI] [PubMed] [Google Scholar]
- 22. Stewart ZA, Wilinska ME, Hartnell S, et al. : Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care 2018;41:1391–1399. [DOI] [PubMed] [Google Scholar]
- 23. Polsky S, Akturk HK: Case series of a hybrid closed-loop system used in pregnancies in clinical practice. Diabetes Metab Res Rev 2020;36:e3248. [DOI] [PubMed] [Google Scholar]
- 24. Deshpande S, Pinsker JE, Zavitsanou S, et al. : Design and clinical evaluation of the interoperable artificial pancreas system (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther 2019;21:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forlenza GP, Deshpande S, Ly TT, et al. : Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care 2017;40:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deshpande S, Pinsker JE, Church MM, et al. : Randomized crossover comparison of automated insulin delivery versus conventional therapy using an unlocked smartphone with scheduled pasta and rice meal challenges in the outpatient setting. Diabetes Technol Ther 2020;22:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Minor BL, et al. : The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 30. Gondhalekar R, Dassau E, Doyle FJ, III: Velocity-weighting & velocity-penalty MPC of an artificial pancreas: improved safety & performance. Automatica 2018;91:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozaslan B, Deshpande S, Doyle FJ, III, Dassau E: Zone-MPC automated insulin delivery algorithm tuned for pregnancy complicated by type 1 diabetes. Front Endocrinol (Lausanne). 2022. Mar 22;12:768639. doi: 10.3389/fendo.2021.768639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harvey RA, Dassau E, Zisser H, et al. : Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy HR: Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia 2019;62:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy HR, Elleri D, Allen JM, et al. : Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.