INCIDENCE AND EPIDEMIOLOGY
Small-cell lung cancer (SCLC) is the most aggressive form of lung cancer. Although SCLC is characterised by rapid responses to chemotherapy (ChT) and sensitivity to radiotherapy (RT), due to early treatment resistance, the 5-year overall survival (OS) is <10%.1 The incidence of SCLC has decreased in recent decades, and with a prevalence of 1–5 per 10 000 people in the European community, SCLC has an orphan disease designation.2,3 SCLC is equally prevalent in males and females;2 however, the proportion of elderly (>70 years of age) patients with SCLC has increased from 23% in 1975 to 44% in 2010.4 Computed tomography (CT) screening does not improve survival of SCLC, as demonstrated in three trials [I, E].5,6 This is possibly related to the aggressiveness of SCLC, reflected both by the occurrence of SCLC as an interval cancer, i.e. diagnosed between two CT screenings, and the primarily late-stage screen-detected SCLC. As SCLC is highly related to tobacco smoking, smoking prevention or cessation are the most effective strategies to decrease the clinical impact of the disease [IV, A].
DIAGNOSIS AND PATHOLOGY/MOLECULAR BIOLOGY
Information regarding the diagnosis and molecular pathology/biology of SCLC can be found in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.annonc.2021.03.207.
Recommendations
SCLC is a high-grade neuroendocrine carcinoma with a typical morphology and should be diagnosed according to the World Health Organization criteria [IV, A].
For pathological diagnosis, histology is preferred over cytology [V, A].
Currently, no predictive biomarker is available and programmed death-ligand 1 (PD-L1) and tumour mutational burden (TMB) testing are not recommended in routine clinical practice [I, D].
STAGING AND RISK ASSESSMENT
The TNM (tumour—node—metastasis) staging classification 7th edition was adopted for SCLC, harbouring a higher prognostic value compared with the previously used subdivision in limited and extensive disease [IV, A].7 The description of disease stages according to the 8th edition TNM, and the median, 1-year and 2-year OS data are depicted in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.annonc.2021.03.207.8 However, in clinical trials, the terms ‘limited disease’, defined as the tumour being confined to one hemithorax and regional lymph nodes, and ‘extensive disease’ are used to define eligibility. For this reason, limited and extensive disease are used throughout this guideline.
The staging work-up for patients diagnosed with SCLC is shown in Table 1. A medical history, physical examination and laboratory tests should be carried out [V, A]. Attention should be drawn towards potential autoimmune-mediated paraneoplastic neurological symptoms,9 with their detection becoming increasingly important with the introduction of immunotherapy [V, C]. In non-metastatic disease, pulmonary function tests are also advised.10 Imaging consists of a chest and abdomen CT [IV, A]. In case of no metastases on CT scan, imaging should be complemented with a bone scintigraphy, or [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG—PET)—CT if available [V, B], and a magnetic resonance imaging (MRI) or a less sensitive brain CT scan if MRI is not available/possible [III, A].11 In patients with stage IV disease who are eligible but do not wish to undergo prophylactic cranial irradiation (PCI), a baseline MRI after ChT is recommended and serial MRIs are then advised as part of the follow-up [III, B].12 In case of an abnormal blood count or signs of blood—bone marrow infiltration, a bone marrow aspiration and biopsy are recommended in patients without known additional metastases in order to confirm bone marrow involvement [V, C]. The use of FDG—PET is still debated in SCLC; a review of small prospective series showed that 9% of patients were upstaged with FDG—PET and 4% were downstaged.13 In the majority of these series, pathological confirmation of metastatic sites was not obtained. As false-positive results have been reported using FDG—PET, the presence of a metastasis should be pathologically confirmed if it alters the treatment plan [II, C]. Of note, in the randomised CONVERT trial exploring different RT schedules in limited-stage SCLC, the outcomes of 57% of patients who were staged by PET—CT were not different to those who underwent staging by conventional CT scan.14 Given the limited evidence for PET—CT in SCLC, its role in the selection of patients for curative treatment remains controversial among those without metastases on CT. However, FDG—PET is recommended to assist in RT volume delineation [III, A]. In case a suspected solitary metastasis cannot be adequately diagnosed, or diagnosis significantly delays the start of treatment, the lesion can be re-evaluated after two cycles of ChT to confirm the diagnosis of metastatic disease. If pleural fluid/pericardial fluid is negative for metastasis, and if it is the only possible site of metastasis, treatment should be according to M0 status.
Table 1.
Diagnostic and staging work-up of SCLC
| History and clinical examination |
| Medical history (including smoking history and comorbidities) |
| PS |
| Physical examination |
| Assessment of paraneoplastic syndromes (especially when initiating immunotherapy) |
| Laboratory analysis |
| CBC, liver enzymes, sodium, potassium, calcium, glucose, LDH and renal functions tests should be carried out |
| Imaging |
| CT of the thorax and abdomen should be carried out in all patients; an FDG—PET—CT is optional |
| In case of a suspicion of bone metastasis and no other metastasis, a bone scintigraphy should be carried out unless FDG—PET is available Imaging of the brain (preferably MRI) is mandated in patients with stage I-III disease |
| MRI of the brain is recommended for patients with stage IV disease who are eligible for PCI but who choose not to undergo PCI |
| Tumour biopsy |
| A diagnosis of SCLC is preferably assessed based on histological examination of a biopsy |
| In case of planned surgery, invasive mediastinal staging is required |
| Functional assessment |
| Pulmonary function testing (FEV1, VC, DLCO) is required for patients with stage I-III SCLC who are candidates for surgery or RT |
| VO2 max assessment by cycle ergometry should be carried out if surgery is planned when pulmonary function tests are limited |
CBC, complete blood count; CT, computed tomography; DLCO, diffusing capacity of the lung for carbon monoxide; FDG, [18F]2-fluoro-2-deoxy-D-glucose; FEV1, forced expiratory volume; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PCI, prophylactic cranial irradiation; PET, positron emission tomography; PS, performance status; RT, radiotherapy; SCLC, small-cell lung cancer; VC, vital capacity; VO2 max, maximal oxygen uptake.
Poor prognostic factors in SCLC include impaired performance status (PS), weight loss, increased age, male sex, elevated lactate dehydrogenase (LDH) and low sodium [syndrome of inappropriate antidiuretic hormone secretion (SIADH)].15 In addition, a higher total gross tumour volume predicts a worse outcome in patients with locally advanced SCLC treated with chemoradiotherapy (CRT).16
Recommendations
Staging of SCLC should be according to the TNM 8th edition [IV, A].
Initial assessment should include smoking history, physical examination, complete blood count, liver enzymes, sodium, potassium, calcium, glucose, LDH, creatinine and lung function test (if localised disease) [V, A].
Attention should be drawn towards potential autoimmune-mediated paraneoplastic neurological symptoms [V, C].
A contrast-enhanced CT of the chest and abdomen is recommended [IV, A].
Imaging of the brain, preferably MRI, is recommended in localised disease [III, A].
Brain MRI is also recommended for stage IV patients not undergoing PCI [II, B].
FDG—PET is optional for staging in limited-stage disease. FDG—PET findings that modify treatment decisions should be pathologically confirmed [II, C]. However, FDG—PET is recommended to assist in RT volume delineation [III, A].
In limited-stage disease, additional bone scintigraphy is advised when no FDG—PET—CT has been carried out [V, B].
In limited-stage disease, a bone marrow aspiration and biopsy are advised in the case of abnormal blood counts suggesting bone marrow involvement [V, C].
In patients eligible for immunotherapy, attention should be paid to the detection of paraneoplastic disorders [V, C].
TREATMENT
Management of limited-stage disease
A proposed treatment algorithm for patients with stage I-III SCLC eligible for treatment of curative intent (selected limited-stage disease) is shown in Figure 1.
Figure 1. Treatment algorithm for SCLC in patients with limited-stage disease (i.e. stage I-III SCLC eligible for curative treatment).
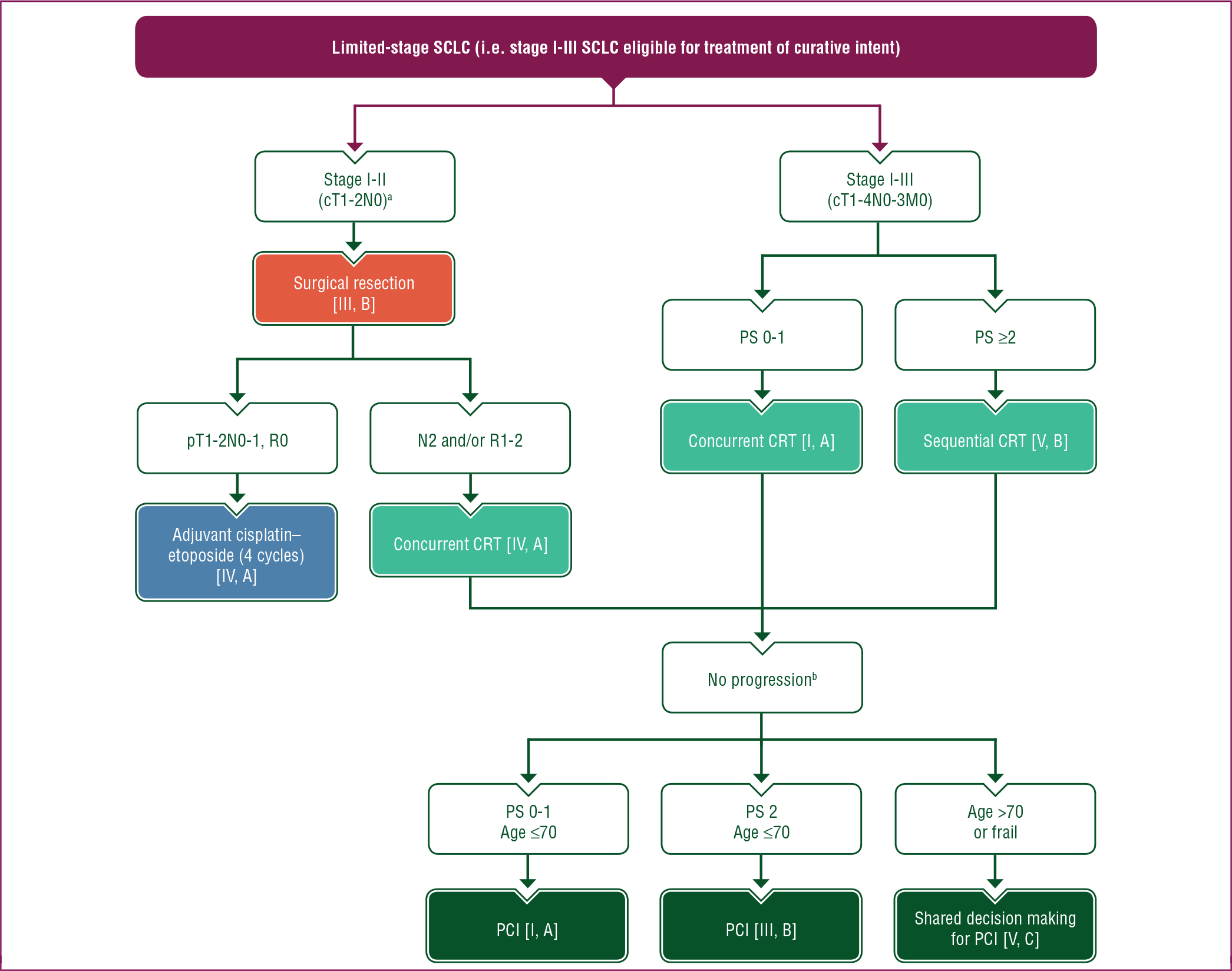
Purple: general categories or stratification; red: surgery; dark green: radiotherapy; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.
c, clinical; CRT, chemoradiotherapy; M, metastasis; MRI, magnetic resonance imaging; N, node; p, pathological; PCI, prophylactic cranial irradiation; PS, performance status; R, resection; SCLC, small-cell lung cancer; T, tumour.
a After extensive pathological mediastinal staging.
b The role of PCI is not well defined in patients with stage I-II SCLC, patients >70 years of age and frail patients. In these cases, shared decision making is recommended, including the option of brain MRI surveillance.
The role of surgery with multimodality treatment.
Indications and results of surgical resection for SCLC remain controversial and only a minority of patients with SCLC qualify for surgical resection. In 2017, a Cochrane systematic review concluded that currently available randomised controlled trials (RCTs) do not support a role for surgical resection in the management of stage I-III SCLC.17 However, the conclusions were of limited value due to the lack of contemporary data and the low quality of available evidence. In a recent retrospective analysis of 205 patients with SCLC who underwent resection, for those with pathological stages I and II, 5-year survival rates were 63.8% and 65.5%, respectively.18 In another analysis of the National Cancer Database, 507 patients with stage I/II SCLC undergoing lobectomy and adjuvant ChT were matched with patients receiving concurrent CRT.19 Median OS was 48.6 and 28.7 months, respectively, favouring the surgical approach (P < 0.0001). After extensive work-up, surgery, in the context of a multimodal treatment approach, may be considered in patients with clinical stages I and II disease (cT1–2N0) [III, B] and in those suspected of having a mixed histology of SCLC and non-small-cell lung cancer (NSCLC).20 SCLC may also be an incidental finding in patients undergoing surgery for a solitary pulmonary nodule, as seen in 4%−12% of cases.21
When considering surgical treatment of SCLC, extensive pathological mediastinal staging is required [IV, A].22,23 As with NSCLC, the aim of surgical treatment should be a complete (R0) resection according to the International Association for the Study of Lung Cancer criteria [III, A].24 Intraoperatively, a systematic nodal dissection should be carried out [IV, A]. Sublobular resection is not recommended [V, E]. Due to the aggressive nature of SCLC, the risk of progression to unresectable or incurable disease while awaiting surgery should be taken into account.
In an analysis of the National Cancer Database, the 5-year OS rate of 954 patients who underwent R0 resection for pT1–2N0M0 SCLC was 47%.25 A multivariate analysis showed that adjuvant ChT or ChT with PCI were associated with improved survival compared with no adjuvant therapy. Adjuvant ChT should therefore be administered after surgical resection of SCLC [IV, A]. In patients with unexpected, positive mediastinal lymph nodes (N2) or R1-R2 resection, adjuvant ChT must be combined with RT, preferably concurrently [IV, A].22
The role of induction ChT in patients with locally advanced SCLC has not been clearly established and this approach is not indicated for SCLC.20
The role of PCI is not well established, as discussed later.
Concurrent CRT: type of ChT and number of cycles.
The preferred ChT regimen for patients with limited-stage (stage I-III) SCLC is cisplatin plus etoposide [I, A].26 When cisplatin is not feasible, carboplatin plus etoposide is a possible alternative, with similar or slightly worse outcomes seen in small comparative studies [II, A].27 Standard dosing should be used, i.e. cisplatin 60–80 mg/m2 on day 1 and etoposide 100–120 mg/m2 on days 1, 2 and 3 of every 3-week cycle, with avoidance of dose reduction, especially during the first two cycles.28 The dose of cisplatin can also be split over 3 days (etoposide 100 mg/m2 on days 1–3 and cisplatin 25 mg/m2 on days 1–3) as this tends to be better tolerated and reduces the need for hydration.29 The use of granulocyte colony-stimulating factor (G-CSF) or granulocyte—macrophage colony-stimulating factor (GM-CSF) concomitant with CRT has been discouraged based on one randomised study of GM-CSF, but more recent data from the CONVERT trial have shown that these agents can be administered safely when indicated [II, B].29−31 The number of cycles is usually four; however, only small series have compared four with six cycles in localised SCLC.32
The success of introducing immune checkpoint inhibition with durvalumab as consolidation therapy after CRT for NSCLC has fostered interest in this approach in limited-stage SCLC.33 Consolidation treatment with nivolumab—ipilimumab in patients treated with CRT was investigated in the randomised phase II STIMULI trial.34 However, no improvement in progression-free survival (PFS) or OS was observed in patients treated with nivolumab—ipilimumab compared with the observational group.
A number of trials addressing the role of immunotherapy in this setting are ongoing (NCT02046733, NCT03540420, NCT03703297, NCT03811002).
RT schedule.
With the exception of patients with very early disease, those with T1–4N0–3M0 tumours and a PS of 0–1 should be treated with concurrent ChT and thoracic RT [I, A]. The current standard of care of twice-daily (b.i.d.) RT delivered concurrently with ChT is based on an RCT which demonstrated superiority in terms of survival for RT of 45 Gy b.i.d. in 30 fractions over 3 weeks versus 45 Gy once daily (o.d.) in 25 fractions over 5 weeks, both delivered concurrently with cisplatin plus etoposide.35 However, there has been a lack of consensus regarding the routine use of b.i.d. RT due to concerns regarding toxicity (one-third of patients developed grade ≥3 radiation oesophagitis in an historical study), debate about the RT schedule used in the control arm and practical/logistical issues. The CONVERT trial compared b.i.d. RT (45 Gy/30 fractions over 3 weeks) to a higher dose of o.d. RT (66 Gy/33 fractions over 6.5 weeks), both given concurrently with ChT (starting on cycle two).29 CONVERT is the first RCT providing outcomes data in patients staged with PET—CT using the TNM classification and treated with modern RT techniques (i.e. three-dimensional conformal RT or intensity-modulated RT without elective nodal irradiation).14 OS did not differ significantly between the two groups. OS achieved in both arms was higher and the toxicity much lower (>50% reduction) than previously reported in the literature. The 2- and 5-year OS were 56% and 34% in the b.i.d. group and 51% and 31% in the o.d. group, respectively. There was no difference in grade 3–4 oesophagitis or grade 3–4 radiation pneumonitis between the groups (19% versus 19% and 3% versus 2% in the b.i.d. and o.d. groups, respectively). In addition, a Norwegian phase II RCT, comparing 45 Gy/30 fractions b.i.d. with 42 Gy/15 fractions o.d., showed that there was no difference in major toxicity between the schedules and a numerically higher OS for treatment with 45 Gy b.i.d.36 Since CONVERT was designed to show superiority of o.d. RT and was not powered to show equivalence, the implication is that b.i.d. RT (45 Gy/30 fractions over 3 weeks) should remain as the standard of care in this group of patients [I, A]. When b.i.d. RT is not possible due to logistical reasons, o.d. RT (66 Gy/33 fractions over 6 weeks) is an alternative option. It should, however, be noted that the role of concurrent CRT is not as well defined in patients >70 years of age or in those who are frail.
Regarding the timing of RT and ChT, evidence from clinical trials suggest that thoracic RT should be initiated as early as possible, preferably starting on the first or second cycle of ChT. However, two recent meta-analyses investigating the timing of high-dose thoracic RT with ChT showed no difference in OS between earlier (≤30 days after starting ChT) and later (>30 days after starting ChT) RT initiation. Furthermore, a higher incidence of toxicity (haematological, oesophagitis and cardiac toxicity) was reported with early versus late thoracic RT.37,38 However, in the individual patient data meta-analysis, the hazard ratio (HR) was significantly in favour of earlier/shorter RT in trials where patients received ChT without a dose reduction or delay [HR 0.79; 95% confidence interval (CI) 0.69–0.91] [II, A].37 When the patient PS or dose to the organs at risk do not allow for the early administration of thoracic RT, it may be postponed until the start of the third ChT cycle [II, B]. Sequential CRT is an option for patients who are not considered candidates for concurrent CRT due to poor PS, comorbidities and/or disease volume [V, B].
RT treatment volume.
In sequential CRT, the optimal target volume remains an area of debate. An historical Southwest Oncology Group (SWOG) trial without contemporary imaging or RT techniques randomised patients achieving a partial response or stable disease after ChT to either wide-volume RT (pre-ChT tumour volume plus the mediastinum) or reduced-volume RT (post-ChT tumour volume with a margin of 2 cm) followed by further ChT.39 The local recurrence rate was similar in both arms. Therefore, it is recommended that the post-ChT primary tumour volume should be included in the radiation field [II, B].
No prospective studies are available on the nodal target volume after ChT. Thus, similar to NSCLC, including the involved nodal stations before ChT in the target volume is recommended [V, B].
Omission of elective node irradiation based on CT scans should be used with caution as this strategy may result in nodal failures.40 Whether selective node irradiation based on pre-treatment PET—CT scans can replace elective node irradiation has been addressed in two small single-arm studies.41,42 Both studies showed promisingly low nodal recurrence rates. Furthermore, in the CONVERT trial, elective node irradiation was omitted in all patients, with half of them staged using PET—CT.29 The incidence of isolated nodal failures has not been reported yet but the survival results are the best reported to date. Omission of elective node irradiation is therefore recommended in favour of selective node irradiation (i.e. involved nodes defined as FDG avid on PET—CT, enlarged on CT and/or biopsypositive) [III, A].
The role of PCI.
PCI significantly decreases the risk of symptomatic brain metastases and increases OS in patients with a complete remission (5.4% absolute improvement in 3-year OS).43 Patients with a PS of 0–1 and a response to CRT should therefore be offered PCI [I, A]. Patients often present with a PS >2 after CRT but very few have been included in PCI clinical trials and meta-analyses. In patients with a PS of 2, PCI can be considered [III, B].43 The recommended dose is 25 Gy in 10 daily fractions [I, A].44 However, it should be noted that the role of PCI is not as well defined in patients with stage I-II SCLC, who have a lower risk of developing brain metastases, and in those >70 years of age or who are frail. In such cases, shared decision-making is recommended [V, C]. There was no benefit of hippocampal-sparing PCI in terms of neurocognitive decline in a phase III trial (NCT01780675) which has been presented but not yet fully published. An additional ongoing trial is addressing this question (NCT01797159).
Management of extensive-stage disease
Patients with SCLC tend to be older (44% >70 years of age), have more comorbidities and have a poor PS at diagnosis. However, as rapid responses are expected, in many cases, treatment with ChT offers the best palliation.
A proposed treatment algorithm for patients with extensive-stage SCLC is shown in Figure 2.
Figure 2. Treatment algorithm for SCLC in patients with extensive-stage disease (i.e. stage IV or stage III SCLC not eligible for curative treatment).
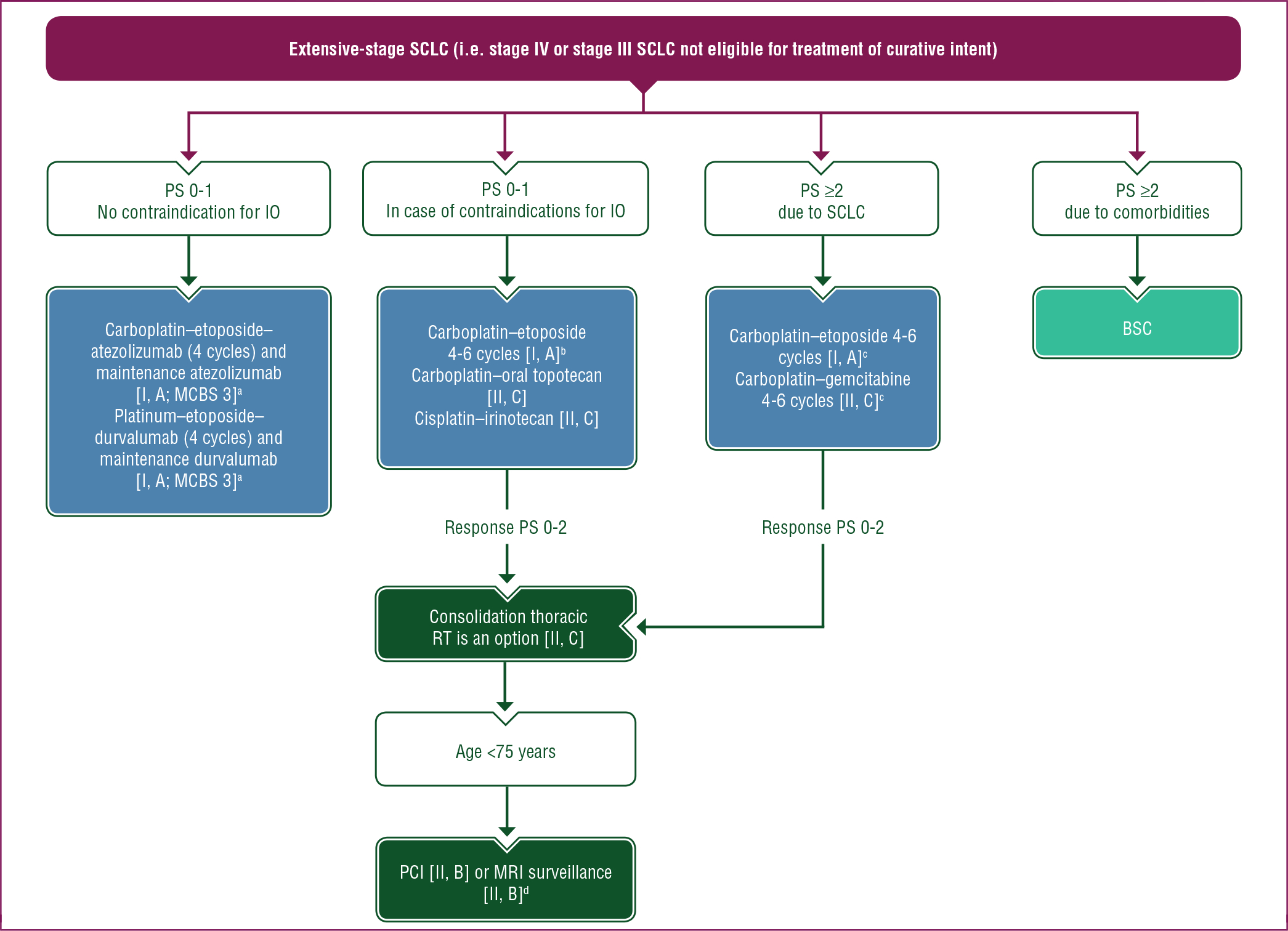
Purple: general categories or stratification; dark green: radiotherapy; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.
BSC, best supportive care; ChT, chemotherapy; G-CSF, granulocyte colony-stimulating factor; IO, immunotherapy; MCBS, ESMO-Magnitude of Clinical Benefit Scale; MRI, magnetic resonance imaging; PCI, prophylactic cranial irradiation; PS, performance status; RT, radiotherapy; SCLC, small-cell lung cancer.
a ESMO-MCBS v1.1 score for new therapy/indication approved by the EMA or FDA. The score has been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/scale-evaluation-forms-v1.0-v1.1/scale-evaluation-forms-v1.1).
b Carboplatin may be replaced by cisplatin in patients <70 years of age or based on the toxicity profile [II, C].
c In patients with a PS of ≥2, consider ChT dose reduction and/or G-CSF prophylaxis.
d No brain metastasis on MRI before PCI.
First-line ChT.
For decades, a platinum plus etoposide has been the preferred first-line treatment for extensive-stage SCLC, with a median OS of 9–10 months, PFS of 5–6 months and 1-year OS of ~35% [I, A].26 A meta-analysis of individual patient data showed no difference in OS between cisplatin and carboplatin.45 From this meta-analysis, it seems that in younger patients (<70 years), the outcome might be moderately better with cisplatin, although these subgroup analyses were exploratory. The toxicity profiles should also be considered in treatment decision making: cisplatin is associated with more non-haematological toxicity, such as nausea, vomiting and renal toxicity, whereas carboplatin leads to more myelosuppression. Therefore, in extensive-stage SCLC, cisplatin can be substituted by carboplatin [I, B]. However, for some patients, cisplatin might be preferred when taking age (<70 years of age), PS and toxicity profile into account [II, C]. Many RCTs have explored maintenance or continuation treatment in SCLC but none have shown improved outcomes compared with four to six cycles of a platinum plus etoposide [I, A].46
A study from the Japanese Cooperative Oncology Group (JCOG 9511) showed improved outcomes with the combination of cisplatin and irinotecan compared with cisplatin and etoposide (median OS: 12.8 versus 9.4 months).47 However, this could not be confirmed in a large, non-Asian study.48 A recent meta-analysis of 12 RCTs (7 written in Chinese) showed no difference in outcomes between cisplatin plus etoposide and cisplatin plus irinotecan in patients with ChT-naive, stage IV SCLC.49 Non-inferiority to platinum plus etoposide has been shown for platinum plus oral topotecan and for carboplatin plus gemcitabine in patients with a poor PS [II, C].50,51
First-line systemic treatment: the role of immunotherapy.
Immunotherapy has dramatically modified cancer treatment across several malignancies and has been an active area of investigation in SCLC.
Despite initial promising phase II trial results, the use of ipilimumab in combination with first-line platinum plus etoposide did not improve clinical outcomes compared with ChT in a phase III RCT.52
Recently, new standards of care were established in first-line therapy of extensive-stage SCLC based on two double-blind, phase III RCTs: IMpower133 and CASPIAN.53,54 IMpower133 evaluated the efficacy and safety of first-line atezolizumab (1200 mg, day 1) or placebo in combination with carboplatin [area under the curve (AUC) 5, day 1] and etoposide (100 mg/m2, days 1–3) every 3 weeks for four cycles followed by atezolizumab or placebo maintenance in treatment-naive patients with extensive-stage SCLC. PCI was permitted but consolidation thoracic RT was not. It met its co-primary endpoints of OS and investigator-assessed PFS at the first interim analysis. Median OS was 12.3 months (95% CI 10.8–15.9 months) for atezolizumab versus 10.3 months (95% CI 9.3–11.3 months) for placebo (HR 0.70; 95% CI 0.54–0.91; P = 0.0069). In the atezolizumab group, 34% of patients were alive at 18 months compared with 21% in the placebo group.55 Median PFS was 5.2 months (95% CI 4.4–5.6 months) for atezolizumab versus 4.3 months (95% CI 4.2–4.5 months) for placebo (HR 0.77; 95% CI 0.62–0.96; P = 0.017). Benefits were consistent across patient subgroups. Of importance, the modest PFS and OS benefits clearly emphasise the need for the identification of new predictive biomarkers, with exploratory analyses showing no predictive ability of blood TMB for this specific combination. CASPIAN is a three-arm trial evaluating durvalumab in patients with previously untreated, extensive-stage SCLC. Patients were randomised 1 : 1 : 1 to receive either platinum (carboplatin AUC 5–6 or cisplatin 75–80 mg/m2, day 1) plus etoposide (80–100 mg/m2, days 1–3) and durvalumab (1500 mg, day 1), with or without tremelimumab (75 mg, day 1), every 3 weeks for four cycles followed by durvalumab maintenance on day 1 every 4 weeks, or up to six cycles of platinum plus etoposide alone (control arm). PCI (used at the investigator’s discretion) was only allowed in the control arm. A statistically significant improvement in OS was reported with the addition of durvalumab to ChT, with a median OS of 12.9 months (95% CI 11.3–14.7 months) for durvalumab plus ChT versus 10.5 months (95% CI 9.3–11.2 months) for platinum plus etoposide alone (HR 0.75; 95% CI 0.62–0.91; P = 0.0032).56 OS at 18 months was 32.0% (95% CI 26.5% to 37.7%) for durvalumab versus 24.8% (95% CI 19.7% to 30.1%) in the ChT-only group. Benefits were consistent across patient subgroups and quality of life (QoL) was maintained.57 The addition of tremelimumab to durvalumab and the platinum doublet did not show any improvement in outcomes compared with ChT. With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting. However, it is important to stress that in both trials, only patients with a good clinical condition were enrolled (i.e. PS 0–1 and asymptomatic or treated brain metastases). Additionally, the median age of enrolled patients was relatively low (62–64 years). In stage IV SCLC, atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible ChT-naive patients with stage IV SCLC and a PS of 0–1 [I, A; ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) v1.1 score: 3 for atezolizumab and 3 for durvalumab]. In the recently reported KEYNOTE-604 trial, patients with extensive stage IV SCLC were randomised to receive platinum (carboplatin AUC 5 or cisplatin 75 mg/m2, day 1) and etoposide (100 mg/m2, days 1–3) with either pembrolizumab 200 mg or placebo for four cycles followed by pembrolizumab or placebo as maintenance therapy.58 PCI was optional in both arms. The study met its co-primary PFS endpoint (HR 0.75; 95% CI 0.61–0.91; P = 0.0023); however, the prespecified significance threshold for OS was not met (HR 0.80; 95% CI 0.64–0.98; P = 0.0164). The 2-year OS in the pembrolizumab group was 22.5% versus 11.2% in the ChT-only group.
The CheckMate 451 study showed no OS improvement of maintenance therapy with nivolumab plus ipilimumab or nivolumab alone compared with placebo (HR 0.92; 95% CI 0.75–1.12; P = 0.37 and HR 0.84; 95% CI 0.69–1.02).59
Thoracic RT in extensive-stage SCLC.
Two phase III RCTs have investigated the role of thoracic RT in extensive-stage SCLC. In the trial of Jeremic et al., a total of 210 patients were treated with three cycles of cisplatin plus etoposide.60 Patients with a complete response (CR) in distant metastases received either thoracic RT with concurrent daily carboplatin plus etoposide followed by two cycles of cisplatin plus etoposide or an additional four cycles of cisplatin plus etoposide. All patients with a CR in distant metastases also received PCI. Patients who received thoracic RT had significantly better survival rates than those who received only ChT (median OS 17 versus 11 months; 5-year survival 9.1% versus 3.7%, respectively; P = 0.041). Acute high-grade toxicity was higher in the RT group.
In the CREST trial, 495 patients with extensive-stage SCLC and a response to ChT were randomised to receive either PCI alone or PCI with thoracic RT (30 Gy/10–15 fractions).61 No significant improvement in 1-year OS (primary endpoint) was observed: 33% versus 28% for thoracic RT versus no thoracic RT. In a pre-planned secondary analysis, the 2-year OS was 13% versus 3% favouring thoracic RT (P = 0.004). An additional exploratory analysis showed that in patients with residual intrathoracic disease (a stratification factor), the OS was significantly longer in the thoracic RT group (HR 0.81; 95% CI 0.66–1.00; P = 0.044).62 No significant differences in toxicity were seen between the treatment arms. Thus, in patients with a PS of 0–2 who achieve a response after ChT, RT to the residual primary tumour and lymph nodes (30 Gy/10 fractions) is a treatment option [II, C]. There is a paucity of data on the integration of thoracic RT and immunotherapy; this should be explored in future research.
PCI in extensive-stage SCLC.
PCI is a standard treatment for patients with stage IV SCLC who are <75 years old, PS 0–2 and who have no progression after first-line ChT [II, B]. PCI reduces the occurrence of symptomatic brain metastases compared with observation and leads to a longer median survival. In the studies showing that PCI confers a survival benefit in patients with extensive-stage SCLC, patients were not pre-screened for brain metastases.43,63 A Japanese phase III RCT investigated the effectiveness of PCI in patients with extensive-stage SCLC.12 Overall, 224 patients without brain metastases on MRI after platinum-containing ChT were randomised to receive either PCI (25 Gy/10 fractions) and MRI follow-up or MRI follow-up only (every 3 months for 1 year and then at 18 and 24 months). The study was stopped early because of futility; no significant differences in OS or PFS were observed. The most common grade ≥3 adverse events after 3 months were loss of appetite (6% in the PCI group versus 2% in the observation group), malaise (3% versus 1%) and muscle weakness in the lower extremities (<1% versus 5%). No difference in the mini mental state examination score was observed between the two groups.
There are important differences between these studies, including the fact that brain MRI was not carried out for staging or follow-up in the trial reported by Slotman et al. RCTs comparing PCI and brain MRI surveillance versus MRI surveillance alone are in development. Thus, PCI (20 Gy/5 fractions and 25 Gy/10 fractions) is justified in the absence of staging or follow-up brain MRI assessments in patients <75 years of age and a PS of 0–2 who achieved a response after ChT [II, B]. There is a paucity of data on the integration of PCI and immunotherapy. In the IMpower133 study, PCI was allowed in the maintenance phase and 22 patients in each arm received PCI.53 In KEYNOTE-604, PCI was optional in both arms and 11.8% and 14.2% of patients in the pembrolizumab and placebo arms, respectively, received PCI.58 However, no details of the toxicity data for patients treated with PCI were provided. Additional research is therefore required regarding both the safety and efficacy of this approach.
Second-line therapy and beyond.
A proposed treatment algorithm for second-line therapy and beyond in patients with recurrent SCLC is shown in Figure 3.
Figure 3. Treatment algorithm for SCLC in patients with recurrent SCLC (i.e. second-line therapy and beyond).
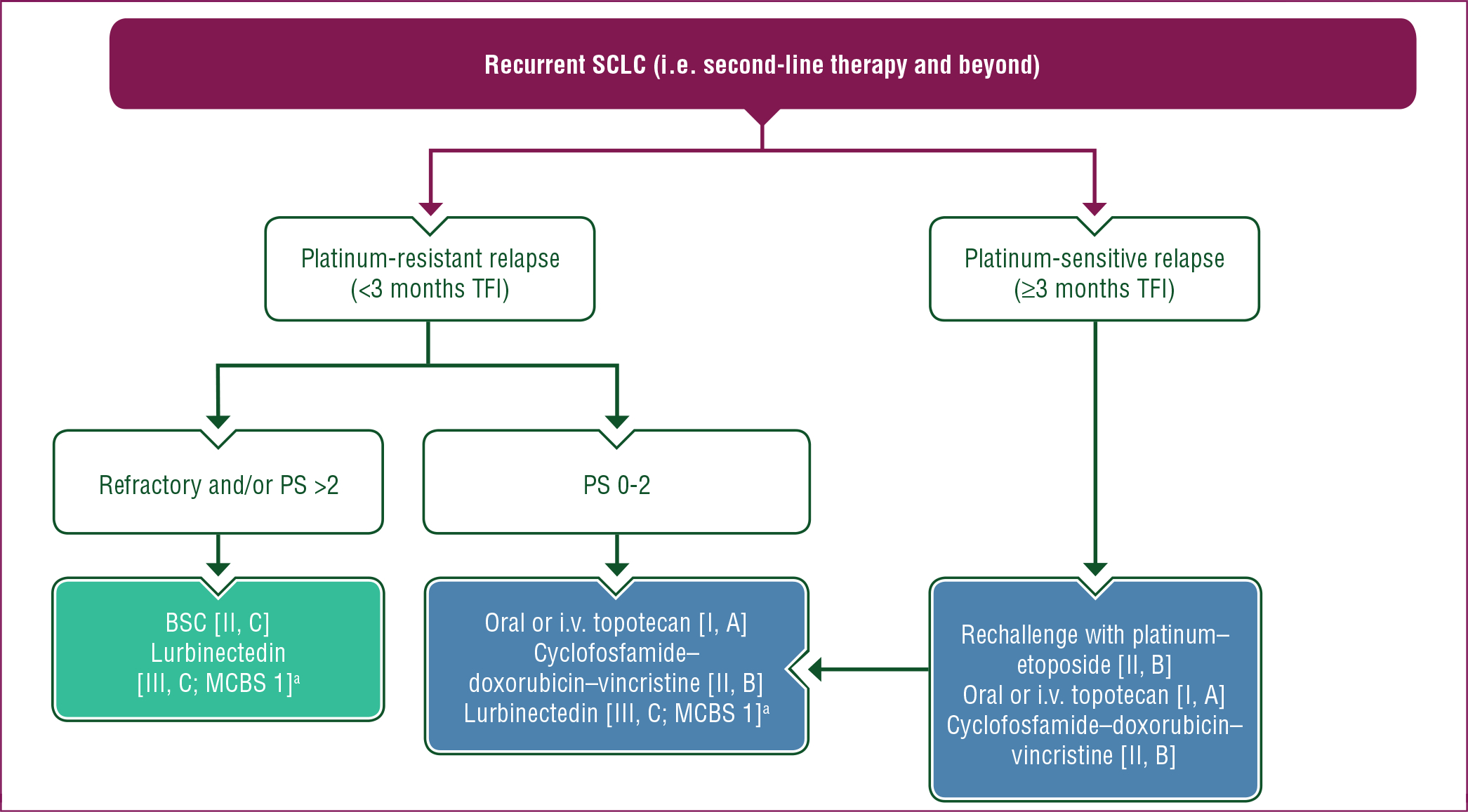
Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.
BSC, best supportive care; i.v., intravenous; MCBS, ESMO-Magnitude of Clinical Benefit Scale; PS, performance status; SCLC, small-cell lung cancer; TFI, treatment-free interval.
a ESMO-MCBS v1.1 score for new therapy/indication approved by the EMA or FDA. The score has been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/scale-evaluation-forms-v1.0-v1.1/scale-evaluation-forms-v1.1).
Standard treatment.
Although SCLC is remarkably sensitive to ChT, most patients relapse within 6 months. Response rates to second-line treatment depend on the treatment-free interval (TFI) and response to first-line platinum-based induction therapy.64 Response rates to second-line ChT are usually around 20%−30% in platinum-sensitive patients (i.e. TFI ≥3 months) and 15% in platinum-resistant patients (i.e. TFI <3 months). In platinum-refractory (i.e. patients not responding or progressing during ChT) and -resistant patients, outcomes are very poor and the clinical benefit of further systemic therapy is uncertain. For these patients, participation in a clinical trial or best supportive care (BSC) is recommended [II, C].
Topotecan is the only drug licensed in the European Union for use as second-line therapy in SCLC. Before topotecan development, anthracycline-based regimes were commonly used, including cyclophosphamide plus doxorubicin and vincristine (CAV). The first randomised trial with topotecan versus CAV showed similar objective response rates (ORRs), time to progression and OS between the two treatment arms and better tolerability with intravenous (i.v.) topotecan versus CAV.65 Subsequently, a phase III trial of oral topotecan demonstrated an improvement in OS versus BSC (median 25.9 versus 13.9 weeks for topotecan versus BSC, respectively; P = 0.0104), a slower decline in QoL and greater symptom control in patients with relapsed SCLC, of whom half had resistant disease.66 Oral and i.v. topotecan demonstrated similar efficacy in another phase III trial but with differing toxicity profiles.67 Either oral or i.v. topotecan is recommended for patients with platinum-resistant or -sensitive relapse, with CAV as an alternative option [II, B]. Another valid option in platinum-sensitive patients is rechallenge with first-line platinum plus etoposide [II, B].68 A phase III RCT recently showed comparable outcomes in patients with sensitive relapse when treated with either topotecan or rechallenge with carboplatin plus etoposide.69
Immunotherapy.
The efficacy of nivolumab and pembrolizumab as third-line monotherapies in stage IV SCLC was assessed in small phase I/II studies.70,71 In CheckMate 032, a single-arm, phase I/II study, 109 patients were treated with nivolumab (3 mg/kg) as third-line or later therapy. The ORR (primary outcome measure) was 11.9% (95% CI 6.5% to 19.5%).70 The median duration of response was 17.9 months (95% CI 3.0–42.1 months). In KEYNOTE-028, a single-arm, phase Ib study, 24 patients who had failed standard treatment were treated with pembrolizumab 10 mg/kg every 2 weeks.71 ORR was 33.3% (95% CI 15.6% to 55.3%). In addition, in KEYNOTE-158, an open-label, single-arm phase II study in advanced solid tumours, 76 SCLC patients who had failed standard first-line treatment (cohort G) were treated with pembrolizumab 200 mg every 3 weeks.72 ORR was 18.4%. These results led to the Food and Drug Administration (FDA) approvals of both nivolumab and pembrolizumab as monotherapies for the treatment of patients with stage IV SCLC who have progressed after platinum-based ChT and at least one other line of therapy. However, in late 2020/early 2021, the manufacturers of nivolumab and pembrolizumab both voluntarily withdrew the SCLC indication for their product following discussions with the FDA as both drugs failed to reach the OS endpoint in their phase III confirmatory trials (KEYNOTE-604, CheckMate 451 and CheckMate 331), a post-marketing requirement following accelerated approval by the FDA.73,74 In addition, the phase III RCT, CheckMate 331, comparing nivolumab to topotecan (or amrubicin) as second-line treatment in unselected (platinum-sensitive and -resistant) patients with stage IV SCLC and a PS of 0–1 failed to demonstrate an improvement in OS, PFS or ORR for nivolumab versus ChT.75 Limited efficacy was also seen in the phase II French Cooperative Thoracic Intergroup 16–03 trial evaluating atezolizumab in relapsed SCLC (N = 73); the disappointing ORR (2.3%) and median PFS (1.4 months) following immunotherapy precluded activation of the phase III part of the study.76
The combination of cytotoxic T-lymphocyte-associated protein 4 and PD-L1 blockade is being evaluated in several ongoing trials. CheckMate 032 investigated nivolumab plus ipilimumab in patients with SCLC who progressed after one or more prior regimens. Although a modest ORR of 19%−23% was reported, this increased to 46.2% in the highest tertile of tumours classified by TMB, with a median OS of 22 months.77,78 Preliminary results from the phase II BALTIC study evaluating the combination of durvalumab and tremelimumab in platinum-refractory or -resistant stage IV SCLC showed similar results with an ORR of 9.5% and a median OS of 6 months.79 These data require prospective validation and comparison with second-line ChT. In addition, the studies were carried out before the routine use of immune checkpoint inhibitors as first-line therapy. No data are available regarding rechallenge with immunotherapy in this setting.
Other systemic therapies in relapsed SCLC.
Paclitaxel, irinotecan and temozolomide have all shown a degree of activity, with ORRs in the order of 15%−29% in small phase II studies.80−83 In a phase III trial (JCOG 0605) comparing triplet ChT (cisplatin, etoposide and irinotecan) with topotecan as second-line treatment in highly selected, fit patients with SCLC who had relapsed ≥90 days after first-line therapy, the triplet regimen demonstrated superiority in terms of OS (median 18.2 versus 12.5 months, respectively; HR 0.67; P = 0.0079). However, the regimen has never been adopted because of the high proportion of grade ≥3 adverse events.84
Amrubicin failed to show a survival benefit versus topotecan in a phase III RCT, although a non-significant and modest improvement in OS was seen in a subset of platinum-refractory patients (HR 0.77; P = 0.047).85 Amrubicin is currently not available in Western countries.
Lurbinectedin, a selective inhibitor of RNA polymerase II, has recently been granted orphan drug status by the European Medicines Agency (EMA) as well as accelerated FDA approval for the treatment of SCLC. In a recent single-arm, phase II trial (PM1183-B-005–14, NCT02454972) of 105 patients with relapsed SCLC, single-agent lurbinectedin 3.2 mg/m2 given every 3 weeks showed promising activity as second-line therapy, with an ORR of 35.2% (22.2% in platinum-resistant and 45% in platinum-sensitive patients), median duration of response of 5.3 months and a manageable safety profile.86 Median OS was 9.3 months (95% CI 6.3–11.8 months). A randomised phase III trial (ATLANTIS, NCT02566993) of lurbinectedin at a dose of 2.0 mg/m2 plus doxorubicin versus investigator’s choice of CAV or topotecan has completed recruitment, and a recent press release reported that the trial failed to meet the prespecified superiority endpoint of OS.87 Lurbinectedin is a treatment option for patients progressing on or after first-line platinum-based ChT [III, C; ESMO-MCBS v1.1 score: 1].
Rovalpituzumab tesirine (Rova-T) is an antibody—drug conjugate targeting delta-like ligand 3 protein (DLL3). DLL3 is expressed in the majority of SCLCs whereas there is no or very limited expression in normal tissue, making it an interesting therapeutic target. However, a phase II study was less promising, with an ORR of 13.2% and an OS of 5.6 months in patients with DLL3-high SCLC.88 Enrolment of two phase III studies (NCT03061812, NCT03033511) was ceased after an interim analysis and development of Rova-T was halted. Drugs using bispecific T-cell engager (BiTE®) and chimeric antigen receptor T-cell approaches are in development and phase I trials are recruiting. Thus, although DLL3 is an interesting potential target, the efficacy of agents targeting DLL3 needs to be demonstrated.
Transformed SCLC
SCLC transformation is a known resistance mechanism in patients with epidermal growth factor receptor (EGFR)-mutated NSCLC who are treated with EGFR tyrosine kinase inhibitors.89 It occurs in 3%−5% of patients, especially in the presence of co-occurring RB1 and TP53 mutations.90 A retrospective analysis of 67 patients with EGFR-mutated SCLC showed a response rate of 54% to platinum—etoposide, with a median PFS of 3.4 months.91 Of 20 patients who were treated with a taxane, 10 (50%) had a response. However, none of the 17 patients who were treated with immunotherapy had a response. Thus, although responses appear inferior compared with those seen in de novo SCLC, both platinum—etoposide and taxanes are treatment options in patients with EGFR-mutated SCLC transformation [IV, B].
Recommendations
Surgery may be considered in patients with clinical stages I and II (cT1–2N0) SCLC in the context of a multimodal treatment concept and following a multidisciplinary board decision [III, B].
When considering surgical treatment for SCLC, pathological mediastinal staging is required [IV, A].
The aim of surgical treatment is to achieve an R0 resection [III, A].
Sublobular resection is not recommended for SCLC [V, E].
During surgery for SCLC, a systematic nodal dissection should be carried out [IV, A].
Adjuvant ChT should be given after surgical resection of SCLC [IV, A].
In patient with an R1-R2 resection or positive mediastinal lymph nodes (N2), adjuvant ChT should be combined with RT, preferably concurrently [IV, A].
The preferred ChT for patients with limited-stage (stage I-III) SCLC is cisplatin plus etoposide [I, A].
When cisplatin is contraindicated because of comorbidities, carboplatin plus etoposide is an alternative [II, A].
G-CSF is a treatment option to prevent haematological toxicity [II, B].
Patients with T1–4N0–3M0 tumours and a good PS (0–1) should be treated with concurrent ChT and thoracic RT [I, A].
The recommended dose fractionation schedule is 45 Gy b.i.d. in 30 fractions [I, A].
Thoracic RT should be initiated as early as possible, starting on the first or second cycle of ChT [II, A].
When the patient PS or dose to the organs at risk do not allow for the early administration of thoracic RT, it may be postponed until the start of the third cycle of ChT [II, B].
Sequential CRT is an option for patients who are not candidates for concurrent CRT due to poor PS, comorbidities and/or disease volume [V, B].
In case of response to ChT, the post-ChT primary tumour should be included in the radiation field [II, B].
In case of response to ChT, the pre-ChT nodal stations should be included in the radiation field [V, B].
Omission of elective node irradiation is recommended, in favour of selective node irradiation (i.e. involved nodes defined as FDG avid on PET—CT, enlarged on CT and/or biopsy-positive) [III, A].
Patients with stage III SCLC with a response after treatment (CRT) and a PS of 0–1 should be offered PCI [I, A]. PCI can be considered in patients with a PS of 2 [III, B].
The role of PCI is not as well defined in patients with stage I-II SCLC or in those >70 years of age or who are frail. In such cases, shared decision making is recommended [V, C].
The role of PCI or consolidation thoracic RT in combination with immunotherapy is not well defined in patients with extensive-stage SCLC due to a paucity of data. Treatment may be considered following a shared decision-making process [IV, C].
The recommended PCI regimen is 25 Gy/10 fractions [I, A].
An anti-PD-L1 inhibitor (atezolizumab [I, A; ESMO-MCBS v1.1 score: 3] or durvalumab [I, A; ESMO-MCBS v1.1 score: 3]) in combination with four cycles of a platinum and etoposide can be offered to all patients with treatment-naive extensive-stage SCLC, a PS of 0–1 and no contraindications for immunotherapy [I, A].
For immunotherapy-ineligible patients, the preferred first-line treatment of extensive-stage SCLC (PS 0–1 and PS 2 due to SCLC) is four to six cycles of a platinum plus etoposide [I, A].
In extensive-stage SCLC, cisplatin can be substituted with carboplatin [I, B].
For selected patients, considering age and toxicity profile, cisplatin might be preferred [II, C].
Cisplatin with irinotecan or oral topotecan are alternative treatment options [II, C].
In poor prognosis patients, gemcitabine plus carboplatin is an alternative treatment option [II, C].
In patients achieving a response after ChT and a PS of 0–2, RT to the residual primary tumour and lymph nodes (30 Gy/10 fractions) is a treatment option [II, C].
PCI (20 Gy/5 fractions and 25 Gy/10 fractions) is justified without prior MRI staging or follow-up in patients <75 years of age and a PS of 0–2 who achieved a response after ChT [II, B].
In patients with extensive-stage SCLC without brain metastases on brain MRI after ChT and who can be followed-up with regular brain MRI, PCI may be omitted [II, B].
Patients with platinum-refractory SCLC have a poor prognosis and participation in a clinical trial or BSC is recommended [II, C].
Either oral or i.v. topotecan is recommended for patients with platinum-resistant or -sensitive relapse; CAV is an alternative option [II, B].
Lurbinectedin [III, C; ESMO-MCBS v1.1 score: 1] is a treatment option for patients progressing on or after first-line platinum-based ChT [III, C].
In patients with platinum-sensitive SCLC, rechallenge with first-line platinum plus etoposide can be considered [II, B].
Both platinum—etoposide and taxanes are treatment options in patients with EGFR-mutated SCLC transformation [IV, B].
PERSONALISED MEDICINE
There are still no validated biomarkers that can be used for disease classification that have prognostic or predictive relevance or that can be used to inform medical treatment decisions. In addition, no targeted treatment has demonstrated activity in SCLC.
FOLLOW-UP, LONG-TERM IMPLICATIONS AND SURVIVORSHIP
Interval and duration of follow-up
Although no prospective trials are available regarding regular follow-up and its effect on survival, asymptomatic recurrences might be detected early with regular follow-up, with available treatments offered while the patient still has a good PS.92 CT scans every 2–3 months are recommended in patients with extensive-stage disease potentially qualifying for further treatments [V, C]. Patients with limited-stage disease who have received potentially curative treatment should undergo 3–6-monthly CT scans for 2 years with lengthening of intervals thereafter [V, C]. Regular brain MRIs (every 3 months in the first year and then every 6 months) are advised in patients who did not undergo PCI [II, C].12
Another reason for regular (long-term) follow-up is the early detection of second primaries. In one series, the cumulative relative risk for developing a second primary was 3.73 and was 6.83 for developing a secondary NSCLC.93 Yearly follow-up with a low-dose CT scan starting at the end of regular follow-up may be considered [V, C].
PCI: long-term toxicity
The long-term effects of PCI were studied in several randomised trials.94−96 In the PCI intergroup trial of 720 patients with non-metastatic SCLC, clinical neurological outcome and QoL were evaluated.95 There was no significant difference between the two groups over 3 years in any of the 17 selected items assessing QoL and neurological and cognitive functions. There was a mild deterioration over time of communication deficit, fatigue, intellectual deficit and memory. Age was a significant cofactor of neurocognitive decline and chronic neurotoxicity.96
In a recently reported Dutch-Flemish randomised phase III trial comparing standard PCI with hippocampus-sparing PCI, no differences in memory were observed.97
Neurocognitive decline after PCI may also be caused by other disorders, such as dementia and depression.98 In addition, some nutritional deficiencies, which may be exacerbated by systemic ChT (e.g. vitamin B and folate deficiency), may lead to cognitive impairment, dementia and depression. Thus, a thorough evaluation is needed before a diagnosis of post-RT cognitive decline can be made, especially in elderly patients with multiple comorbidities. PCI results in a mild decline in neurocognitive functioning in ~30% of patients. Severe deterioration requires an in-depth analysis looking for other treatable causes [IV, A].
Comorbidities and influence on long-term toxicity
Three-quarters of patients with SCLC have comorbidities, with half having two or more comorbidities. Cardiovascular and pulmonary diseases occur most frequently.99 Regular follow-up, paying attention to these comorbidities, could therefore be an option as this may improve survival. Pre-existing comorbidities, smoking habits and RT to the heart can all result in cardiac problems. Approximately 10% of patients with stage I-III SCLC experience cardiac problems and 3% die as a result.100
Smoking cessation
Continued smoking is associated with a higher risk of tumour recurrences, the development of second primaries, cardiovascular and cerebrovascular disease and all-cause mortality compared with those who stop smoking.101 Moreover, continued smoking is associated with a decreased QoL among survivors.102 Smoking cessation in patients already diagnosed with lung cancer improves PS and health-related QoL and may also improve survival.103 Therefore, smoking cessation is highly encouraged [IV, B].
Recommendations
Two- to three-monthly CT scans are recommended in patients with extensive-stage disease potentially qualifying for further treatments [V, C].
Six-monthly CT scans for 2 years with lengthening of intervals thereafter are recommended for patients with non-metastatic disease who have received potentially curative treatment [V, C].
Regular brain MRIs (every 3 months for the first year, then every 6 months) are advised in patients who have not undergone PCI [II, C].
As patients with a history of lung cancer are at high risk of developing a second primary, yearly follow-up with a low-dose CT starting from the end of regular follow-up may be considered [V, C].
Severe neurocognitive deterioration after PCI requires an in-depth analysis looking for other treatable causes [IV, A].
The occurrence of second malignancies, particularly if smoking is continued, is of concern in survivors and smoking cessation counselling is essential [IV, B].
METHODOLOGY
This Clinical Practice Guideline was developed in accordance with the ESMO standard operating procedures for Clinical Practice Guidelines development (http://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). The relevant literature has been selected by the expert authors. An ESMO-MCBS table with ESMO-MCBS scores is included in Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.03.207. ESMO-MCBS v1.1104 was used to calculate scores for new therapies/indications approved by the EMA and the FDA (https://www.esmo.org/Guidelines/ESMO-MCBS). The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2021.03.207.105 Statements without grading were considered justified standard clinical practice by the authors.
Supplementary Material
ACKNOWLEDGEMENTS
Corinne Faivre-Finn is supported by a grant from the NIHR Manchester Biomedical Research Centre. Manuscript editing support was provided by Angela Corstorphine of Kstorfin Medical Communications Ltd; this support was funded by ESMO.
FUNDING
No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Footnotes
DISCLOSURE
A-MCD reports receipt of honoraria for participation in advisory boards and/or lectures from Roche, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Pfizer, Bristol Myers Squibb (BMS), Amgen, Novartis, Merck Sharp & Dohme (MSD), Takeda and PharmaMar; research funding from BMS, AbbVie and Amgen. MF reports receipt of advisory board honoraria to institution from BMS, Takeda, AstraZeneca, Boehringer Ingelheim, MSD and Roche. AA reports receipt of honoraria for participation in advisory boards from AstraZeneca, Roche, MSD, BMS, Lilly, Takeda and Bayer; research grant from BMS. BB reports receipt of grants/research support to institute from AbbVie, Amgen, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cristal Therapeutics, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline (GSK), Ignyta, IPSEN, Inivata, Janssen, Merck KGaA, MSD, Nektar, Onxeo, OSE Immunotherapeutics, Pfizer, PharmaMar, Roche-Genentech, Sanofi, Servier, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma and Tolero Pharmaceuticals. CF-F reports participation in advisory boards for AstraZeneca; research funding from MSD and AstraZeneca. LEH reports research funding to institution from Roche, Boehringer Ingelheim and AstraZeneca; advisory board honoraria to institution from BMS, Lilly, Roche, Pfizer, Takeda, MSD, Amgen and Boehringer Ingelheim; speaker fees from MSD; travel/conference reimbursement from Roche and BMS; participation in mentorship program funded by AstraZeneca; fees for educational webinars from Quadia; fees to institution for interview sessions from Roche. SL reports consulting activities for MSD, AstraZeneca, BMS, Bayer and Eli Lilly. SP reports receipt of honoraria to institute for consultancy, advisory boards and/or lectures from AbbVie, Amgen, AstraZeneca, Bayer, Biocartis, Bioinvent, Blueprint Medicines, Boehringer Ingelheim, BMS, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, Roche, Foundation Medicine, Illumina, Janssen, MSD, Merck Serono, Merrimack, Mirati, Novartis, PharmaMar, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda and Vaccibody; institutional financial support for clinical trials from Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, BMS, Clovis, Roche, Illumina, MSD, Merck Serono, Novartis and Pfizer. NR reports receipt of speaker honoraria from MSD, BMS, Roche, Boehringer Ingelheim, Guardant Health, Pfizer, AbbVie, Ipsen, Novartis, AstraZeneca, Lilly, Takeda and Amgen; fees for organising educational events from Amgen and Roche; advisory panel honoraria from MSD, BMS, Roche, Boehringer Ingelheim, Guardant Health, Pfizer, AbbVie, Ipsen, Novartis, AstraZeneca, Lilly, Takeda and Amgen; sponsorship to attend scientific meetings from Boehringer Ingelheim, MSD and Roche; research grants from Novartis and Pfizer. CMR reports consultancy for AbbVie, Amgen, Ascentage, AstraZeneca, Bicycle, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Jazz, Lilly, Pfizer, PharmaMar, Syros and Vavotek; serves on scientific advisory boards for Bridge Medicines and Harpoon Therapeutics. DDR reports receipt of research grants to institute from BMS, AstraZeneca and Boehringer Ingelheim; advisory board honoraria to institute from BMS, AstraZeneca, Boehringer Ingelheim and Philips; funding to institute for investigator-initiated study from Olink. PEVS reports board membership of IASLC; treasurer of BACTS; fees to institution for serving as an external expert for AstraZeneca, MSD and National Cancer Institute, France. JV reports receipt of advisory board honoraria and/or lecture fees from Boehringer Ingelheim, AstraZeneca, MSD, Novartis, Roche, Pfizer and BMS; research grant to institute from MSD. MR reports receipt of honoraria for lectures and consultancy from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Lilly, Merck, MSD, Novartis, Pfizer, Roche and Samsung; funding for academic research from BMS and Boehringer Ingelheim.
Approved by the ESMO Guidelines Committee: February 2002, last update March 2021. This publication supersedes the previously published version—Ann Oncol. 2013;24(suppl 6):vi99-vi105.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society. 2019. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed October 20, 2020. [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. [DOI] [PubMed] [Google Scholar]
- 3.Breitling LP, Rinke A, Gress TM. Recent survival trends in high-grade neuroendocrine neoplasms and lung cancer. Neuroendocrinology. 2020;110(3–4):225–233. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Rahman O Changing epidemiology of elderly small cell lung cancer patients over the last 40 years; a SEER database analysis. Clin Respir J. 2018;12(3):1093–1099. [DOI] [PubMed] [Google Scholar]
- 5.Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369(10):920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva M, Galeone C, Sverzellati N, et al. Screening with low-dose computed tomography does not improve survival of small cell lung cancer. J Thorac Oncol. 2016;11(2):187–193. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2(12):1067–1077. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(3):300–311. [DOI] [PubMed] [Google Scholar]
- 9.Gozzard P, Woodhall M, Chapman C, et al. Paraneoplastic neurologic disorders in small cell lung carcinoma: a prospective study. Neurology 2015;85(3):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 11.Seute T, Leffers P, ten Velde GP, et al. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. 2008;112(8):1827–1834. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–671. [DOI] [PubMed] [Google Scholar]
- 13.Martucci F, Pascale M, Valli MC, et al. Impact of (18)F-FDG PET/CT in staging patients with small cell lung cancer: a systematic review and meta-analysis. Front Med (Lausanne). 2019;6:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manoharan P, Salem A, Mistry H, et al. (18)F-Fludeoxyglucose PET/CT in SCLC: analysis of the CONVERT randomized controlled trial. J Thorac Oncol. 2019;14(7):1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115(12):2721–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reymen B, Van Loon J, van Baardwijk A, et al. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85(5):1319–1324. [DOI] [PubMed] [Google Scholar]
- 17.Barnes H, See K, Barnett S, et al. Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst Rev. 2017;4(4):Cd011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Kallakury B, Chahine JJ, et al. Surgical resection of SCLC: prognostic factors and the tumor microenvironment. J Thorac Oncol. 2019;14(5):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakeam E, Acuna SA, Leighl NB, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer. 2017;109:78–88. [DOI] [PubMed] [Google Scholar]
- 20.Villaruz L, Karlovits BJ, Burns TF, et al. Small-cell lung cancer. In: LoCicero J III, Colson YL, Feins RH, et al., editors. Shields’ General Thoracic Surgery. Philadelphia, PA: Wolters Kluwer; 2019. p. 1308–1319. [Google Scholar]
- 21.Kreisman H, Wolkove N, Quoix E. Small cell lung cancer presenting as a solitary pulmonary nodule. Chest. 1992;101(1):225–231. [DOI] [PubMed] [Google Scholar]
- 22.Ernani V, Ganti AK. Surgery for limited-stage small cell lung cancer: ready for prime-time? J Thorac Dis. 2017;9(10):3576–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinchcombe TE. Current treatments for surgically resectable, limited-stage, and extensive-stage small cell lung cancer. Oncologist. 2017;22(12):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49(1):25–33. [DOI] [PubMed] [Google Scholar]
- 25.Yang CF, Chan DY, Speicher PJ, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. 2016;34(10):1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer. 2000;30(1):23–36. [DOI] [PubMed] [Google Scholar]
- 27.Karam I, Jiang SY, Khaira M, et al. Outcomes of small cell lung cancer patients treated with cisplatin-etoposide versus carboplatin-etoposide. Am J Clin Oncol. 2015;38(1):51–54. [DOI] [PubMed] [Google Scholar]
- 28.Arriagada R, Pignon JP, Le Chevalier T. Initial chemotherapeutic doses and long-term survival in limited small-cell lung cancer. N Engl J Med. 2001;345(17):1281–1282. [DOI] [PubMed] [Google Scholar]
- 29.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes F, Faivre-Finn C, Mistry H, et al. Safety of G-CSF with concurrent chemo-radiotherapy in limited-stage small cell lung cancer—secondary analysis of the randomised phase 3 CONVERT trial. Lung Cancer. 2021;153:165–170. [DOI] [PubMed] [Google Scholar]
- 31.Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol. 1995;13(7):1632–1641. [DOI] [PubMed] [Google Scholar]
- 32.Veslemes M, Polyzos A, Latsi P, et al. Optimal duration of chemotherapy in small cell lung cancer: a randomized study of 4 versus 6 cycles of cisplatin-etoposide. J Chemother. 1998;10(2):136–140. [DOI] [PubMed] [Google Scholar]
- 33.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 34.Peters S, Pujol JL, Dafni U, et al. LBA84 consolidation ipilimumab and nivolumab vs observation in limited stage SCLC after chemoradiotherapy: results from the ETOP/IFCT 4–12 STIMULI trial. Ann Oncol. 2020;31:S1211. [Google Scholar]
- 35.Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with oncedaily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265–271. [DOI] [PubMed] [Google Scholar]
- 36.Grønberg BH, Halvorsen TO, Fløtten Ø, et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55(5):591–597. [DOI] [PubMed] [Google Scholar]
- 37.De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27(10):818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Fang L, Wang X, et al. A meta-analysis of randomized controlled trials comparing early and late concurrent thoracic radiotherapy with etoposide and cisplatin/carboplatin chemotherapy for limited-disease small-cell lung cancer. Mol Clin Oncol. 2014;2(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kies MS, Mira JG, Crowley JJ, et al. Multimodal therapy for limited small-cell lung cancer: a randomized study of induction combination chemotherapy with or without thoracic radiation in complete responders; and with wide-field versus reduced-field radiation in partial responders: a Southwest Oncology Group Study. J Clin Oncol. 1987;5(4):592–600. [DOI] [PubMed] [Google Scholar]
- 40.De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol. 2006;80(3):307–312. [DOI] [PubMed] [Google Scholar]
- 41.van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77(2):329–336. [DOI] [PubMed] [Google Scholar]
- 42.Shirvani SM, Komaki R, Heymach JV, et al. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–484. [DOI] [PubMed] [Google Scholar]
- 44.Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): a randomised clinical trial. Lancet Oncol. 2009;10(5):467–474. [DOI] [PubMed] [Google Scholar]
- 45.Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30(14):1692–1698. [DOI] [PubMed] [Google Scholar]
- 46.Rossi A, Garassino MC, Cinquini M, et al. Maintenance or consolidation therapy in small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2010;70(2):119–128. [DOI] [PubMed] [Google Scholar]
- 47.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85–91. [DOI] [PubMed] [Google Scholar]
- 48.Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038–2043. [DOI] [PubMed] [Google Scholar]
- 49.Liu ZL, Wang B, Liu JZ, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin in patients with previously untreated extensive-stage small cell lung cancer: a meta-analysis. J Cancer Res Ther. 2018;14(Supplement):S1076–S1083. [DOI] [PubMed] [Google Scholar]
- 50.Eckardt JR, von Pawel J, Papai Z, et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2044–2051. [DOI] [PubMed] [Google Scholar]
- 51.Lee SM, James LE, Qian W, et al. Comparison of gemcitabine and carboplatin versus cisplatin and etoposide for patients with poor-prognosis small cell lung cancer. Thorax. 2009;64(1):75–80. [DOI] [PubMed] [Google Scholar]
- 52.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. [DOI] [PubMed] [Google Scholar]
- 53.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. [DOI] [PubMed] [Google Scholar]
- 54.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. [DOI] [PubMed] [Google Scholar]
- 55.Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. [DOI] [PubMed] [Google Scholar]
- 57.Goldman JW, Garassino MC, Chen Y, et al. Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study. Lung Cancer. 2020;149:46–52. [DOI] [PubMed] [Google Scholar]
- 58.Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owonikoko TK, Kim HR, Govindan R, et al. LBA1_PR—Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study. Ann Oncol. 2019;30:ii77. [Google Scholar]
- 60.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17(7):2092–2099. [DOI] [PubMed] [Google Scholar]
- 61.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385(9962):36–42. [DOI] [PubMed] [Google Scholar]
- 62.Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer—authors’ reply. Lancet. 2015;385(9975):1292–1293. [DOI] [PubMed] [Google Scholar]
- 63.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. [DOI] [PubMed] [Google Scholar]
- 64.Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol. 2012;7(5):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658–667. [DOI] [PubMed] [Google Scholar]
- 66.O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24(34):5441–5447. [DOI] [PubMed] [Google Scholar]
- 67.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092. [DOI] [PubMed] [Google Scholar]
- 68.Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol. 1987;23(9):1409–1411. [DOI] [PubMed] [Google Scholar]
- 69.Baize N, Monnet I, Greillier L, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1224–1233. [DOI] [PubMed] [Google Scholar]
- 70.Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823–3829. [DOI] [PubMed] [Google Scholar]
- 72.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. [DOI] [PubMed] [Google Scholar]
- 73.Merck. Merck provides update on KEYTRUDA® (pembrolizumab) indication in metastatic small cell lung cancer in the US. 2021. Available at: https://www.merck.com/news/merck-provides-update-on-keytruda-pembrolizumab-indication-in-metastatic-small-cell-lung-cancer-in-the-us/. Accessed March 19, 2021.
- 74.BMS. Bristol Myers Squibb statement on Opdivo (nivolumab) small cell lung cancer U.S. indication. 2020. Available at: https://news.bms.com/news/details/2020/Bristol-Myers-Squibb-Statement-on-Opdivo-nivolumab-Small-Cell-Lung-Cancer-US-Indication/default.aspx. Accessed March 19, 2021.
- 75.Spigel DR, Vicente D, Ciuleanu TE, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann Oncol. 2021;32(5):631–641. [DOI] [PubMed] [Google Scholar]
- 76.Pujol JL, Greillier L, Audigier-Valette C, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol. 2019;14(5):903–913. [DOI] [PubMed] [Google Scholar]
- 77.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. [DOI] [PubMed] [Google Scholar]
- 78.Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2019;35(2):329. [DOI] [PubMed] [Google Scholar]
- 79.Bondarenko I, Juan-Vidal O, Pajkos G, et al. 1665PD—Preliminary efficacy of durvalumab plus tremelimumab in platinum-refractory/resistant ED-SCLC from arm A of the phase II BALTIC study. Ann Oncol. 2018;29:viii596. [Google Scholar]
- 80.Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer. 1998;77(2):347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. 2006;26(1b):777–781. [PubMed] [Google Scholar]
- 82.Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18(4):1138–1145. [DOI] [PubMed] [Google Scholar]
- 83.Zauderer MG, Drilon A, Kadota K, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer. 2014;86(2):237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goto K, Ohe Y, Shibata T, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2016;17(8):1147–1157. [DOI] [PubMed] [Google Scholar]
- 85.von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. [DOI] [PubMed] [Google Scholar]
- 86.Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645–654. [DOI] [PubMed] [Google Scholar]
- 87.Jazz Pharmaceuticals. Jazz Pharmaceuticals and PharmaMar announce results of ATLANTIS phase 3 study evaluating Zepzelca™ in combination with doxorubicin for patients with small cell lung cancer following one prior platinum-containing line. 2020. Available at: https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-and-pharmamar-announce-results-atlantis. Accessed February 2, 2021.
- 88.Morgensztern D, Besse B, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25(23):6958–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Offin M, Chan JM, Tenet M, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcoux N, Gettinger SN, O’Kane G, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugiyama T, Hirose T, Hosaka T, et al. Effectiveness of intensive follow-up after response in patients with small cell lung cancer. Lung Cancer. 2008;59(2):255–261. [DOI] [PubMed] [Google Scholar]
- 93.Sagman U, Lishner M, Maki E, et al. Second primary malignancies following diagnosis of small-cell lung cancer. J Clin Oncol. 1992;10(10):1525–1533. [DOI] [PubMed] [Google Scholar]
- 94.Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer. 1997;33(11):1752–1758. [DOI] [PubMed] [Google Scholar]
- 95.Le Péchoux C, Laplanche A, Faivre-Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99–01, EORTC 22003–08004, RTOG 0212 and IFCT 99–01). Ann Oncol. 2011;22(5):1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Belderbos J, Ruysscher D, Jaeger K, et al. OC-0503 phase III trial of prophylactic cranial irradiation with or without hippocampus avoidance in SCLC. Radiother Oncol. 2019;133:S259. [Google Scholar]
- 98.De Ruysscher D, Niedermann G, Burnet NG, et al. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5(1):13. [DOI] [PubMed] [Google Scholar]
- 99.Aarts MJ, Aerts JG, van den Borne BE, et al. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer. 2015;16(4):282–291. [DOI] [PubMed] [Google Scholar]
- 100.Ferris MJ, Jiang R, Behera M, et al. Radiation therapy is associated with an increased incidence of cardiac events in patients with small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102(2):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Qi Y, Wampfler JA, et al. Effect of cigarette smoking on quality of life in small cell lung cancer patients. Eur J Cancer. 2012;48(11):1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dobson Amato KA, Hyland A, Reed R, et al. Tobacco cessation may improve lung cancer patient survival. J Thorac Oncol. 2015;10(7):1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cherny NI, Dafni U, Bogaerts J, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. [DOI] [PubMed] [Google Scholar]
- 105.Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 2001;33(2):139–144 (adapted from: [DOI] [PubMed] [Google Scholar]; Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;1918:1421). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


