Abstract
Introduction
Multiple clinical trials showed that 12 weeks of abrocitinib monotherapy was safe and effective for the treatment of moderate-to-severe atopic dermatitis (AD). The reversibility of pharmacologic activity after abrocitinib discontinuation was not described.
Methods
This post hoc analysis used data from a phase 2b study to evaluate maintenance of disease control during a 4-week drug-free follow-up period in patients with moderate-to-severe AD treated with once-daily abrocitinib (200 mg/100 mg) or placebo for 12 weeks. Proportions of patients who achieved and maintained 50% or 75% improvement in Eczema Area and Severity Index (EASI-50/EASI-75), an Investigator’s Global Assessment (IGA) score of 0/1, or at least a 4-point improvement in the pruritus numeric rating scale (pruritus NRS4) were determined. Biomarkers of Janus kinase inhibition and AD disease were measured in blood samples.
Results
Among week 12 responders to abrocitinib 200 mg, 77.4%, 42.3%, 21.1%, and 42.9% maintained their EASI-50, EASI-75, IGA, and pruritus NRS4 response at week 16; corresponding proportions of week 12 responders maintaining response to abrocitinib 100 mg were 51.9%, 35.0%, 33.3%, and 43.5%, respectively. Four weeks after abrocitinib discontinuation, all AD biomarkers reverted toward baseline levels, with high-sensitivity C-reactive protein and eosinophil percentage demonstrating the most complete recovery in patients treated with abrocitinib versus placebo.
Conclusion
Abrocitinib discontinuation resulted in rapid reversal of disease control consistent with reversal of suppression of pharmacodynamic and AD-specific biomarkers during the drug-free follow-up period. Maintenance of response was inversely related to the threshold of improvement. Patients with moderate-to-severe AD using continuous abrocitinib therapy would likely have the best long-term outcomes.
Trial Registration
ClinicalTrials.gov identifier NCT02780167.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00764-4.
Keywords: Abrocitinib, Atopic dermatitis, Biomarker, Discontinuation, Eczema, Pruritus
Key Summary Points
| In this clinical study, patients with eczema who were treated once daily with abrocitinib for 12 weeks showed improvement in the signs and symptoms of their disease. |
| This report looked at how long the beneficial effect of treatment lasted in the 4-week period after the drug was stopped. |
| The results of this analysis showed that the signs and symptoms of the disease returned quickly and suggest that patients need to take the drug continuously to keep their eczema under control. |
Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease characterized by dry, inflamed, itchy skin [1–4]. The prevalence of AD was reported to be between 2% and 10% in adults and between 15% and 30% in children in Western countries [1, 2].
Abrocitinib is an oral, once-daily, selective Janus kinase 1 (JAK1) inhibitor approved for the treatment of adults [5–8] and adolescents [5, 6] with moderate-to-severe AD. In a phase 2b study (NCT02780167), abrocitinib 200 mg or 100 mg showed clinically meaningful improvement in Investigator’s Global Assessment (IGA), 90% improvement on the Eczema Area and Severity Index (EASI-90), and a 4-point or greater improvement on the baseline pruritus numeric rating scale (pruritus NRS4) at week 12 [9, 10]. The efficacy and safety of once-daily abrocitinib (100 mg or 200 mg) in patients with moderate-to-severe AD were confirmed in three phase 3 studies (JADE MONO-1, NCT03349060; JADE MONO-2, NCT03575871; and JADE COMPARE, NCT03720470) [11–13].
Abrocitinib exerts pharmacologic action through reversible inhibition of JAK1 and is eliminated rapidly (mean t1/2 2.8–5.2 h after 10 days of once- or twice-daily administration) [14]. Therefore, associated pharmacologic effects are expected to diminish following treatment discontinuation. The durability of the response after discontinuation of abrocitinib was not reported. The objective of this analysis was to characterize the durability of response and the changes in pharmacodynamic and AD-specific biomarkers in the phase 2b study (NCT02780167) after abrocitinib discontinuation.
Methods
Study Design and Endpoints
A phase 2b, multicenter, randomized, double-blinded, placebo-controlled, parallel-group study was conducted between April 15, 2016, and April 4, 2017, at 58 centers in Australia, Canada, Germany, Hungary, and the USA (NCT02780167) to determine the efficacy and safety of once-daily abrocitinib (200 mg, 100 mg, 30 mg, or 10 mg) for patients with moderate-to-severe AD. Patients were randomly assigned 1:1:1:1:1 to receive abrocitinib (200, 100, 30, or 10 mg) or placebo once daily for 12 weeks, followed by 4 weeks of follow-up after discontinuation of study medication [9, 10]. Permitted concomitant AD medications were oral antihistamines and sponsor-provided emollient and sunscreen. The current analysis reports data for the placebo and abrocitinib 100- and 200-mg groups only.
The study was conducted in accordance with the Declaration of Helsinki, all International Council for Harmonization Good Clinical Practice Guidelines, and all local regulatory requirements following approval from the institutional review boards (IRBs) or ethics committees (ECs) at each study site. All patients provided written informed consent [9, 10]. A list of all IRBs and ECs is provided in the Supplementary Material.
Eligible patients were those aged 18–75 years with moderate-to-severe AD (IGA score ≥ 3, EASI score ≥ 12, percentage of affected body surface area ≥ 10) and an inadequate response or intolerance to topical medication [9].
Outcome Measures
In patients who achieved 50% or 75% improvement in EASI (EASI-50, EASI-75), IGA response (clear or almost clear and a 2-point or greater reduction from baseline), or pruritus NRS4 response at week 12, a post hoc analysis was performed to determine the proportions of patients who maintained EASI-50, EASI-75, IGA response, and pruritus NRS4 at week 16 (i.e., 4 weeks after treatment discontinuation). All patients with reported outcome measures at week 16 were analyzed. The proportions of patients who experienced worsened EASI and pruritus NRS scores, which were defined as exceeding baseline values by at least two intrapatient standard deviations, were also determined.
Biomarkers
Serum biomarkers were evaluated for the purpose of corroborating the findings on the outcome measures. Blood samples were collected during abrocitinib treatment and after discontinuation at week 12 to evaluate biomarkers of AD (serum interleukin [IL]-31 and thymus and activation-regulated chemokine [TARC] concentrations and blood eosinophil percentage). The concentration of high-sensitivity C-reactive protein (hs-CRP), which reflects both the pharmacodynamics of JAK inhibition and AD severity, was also evaluated [15–17].
The biomarker analysis used data only from patients who received at least one dose of study drug in the placebo and abrocitinib 200-mg and 100-mg groups. Descriptive analyses were performed using R 3.4.4 (R Core Team, Vienna, Austria).
Results
Baseline Characteristics
Of the 267 patients included in the phase 2b study, 164 in the full analysis set were treated with abrocitinib 200 mg (n = 54), abrocitinib 100 mg (n = 55), or placebo (n = 55) [9, 10] and were included in this post hoc analysis. The mean age for patients receiving placebo, abrocitinib 100 mg, and abrocitinib 200 mg was 42.6, 41.1, and 38.7 years, respectively, and the median disease duration was 25.6, 23.8, and 19.6 years, respectively (Table 1) [9, 10]. Most participants were White (70.1%) and had moderate disease (IGA-3, 59.1%); 52.1% were women [9, 10].
Table 1.
Baseline demographics and clinical characteristics [9]
| Abrocitinib 200 mg | Abrocitinib 100 mg | Placebo | |
|---|---|---|---|
| Safety analysis set, N | 55 | 56 | 56 |
| Age, mean (SD), years | 38.7 (17.6) | 41.1 (15.6) | 42.6 (15.1) |
| Male sex, n (%) | 28 (50.9) | 31 (55.4) | 21 (37.5) |
| Race, n (%) | |||
| White | 37 (67.3) | 40 (71.4) | 40 (71.4) |
| Black | 13 (23.6) | 7 (12.5) | 10 (17.9) |
| Asian | 5 (9.1) | 8 (14.3) | 4 (7.1) |
| Other | 0 | 1 (1.8) | 2 (3.6) |
| Disease duration, median (range), years | 19.6 (1.9–68.8) | 23.8 (1.1–66.7) | 25.6 (1.1–67.1) |
| Full analysis set, N | 54 | 55 | 55 |
| IGA, n (%) | |||
| Moderate (3) | 34 (63.0) | 29 (52.7) | 34 (61.8) |
| Severe (4) | 20 (37.0) | 26 (47.3) | 21 (38.2) |
| EASI, mean (SD) | 24.6 (13.5) | 26.7 (11.8) | 25.4 (12.9) |
EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, SD standard deviation
Maintenance of Response After Discontinuation of Abrocitinib
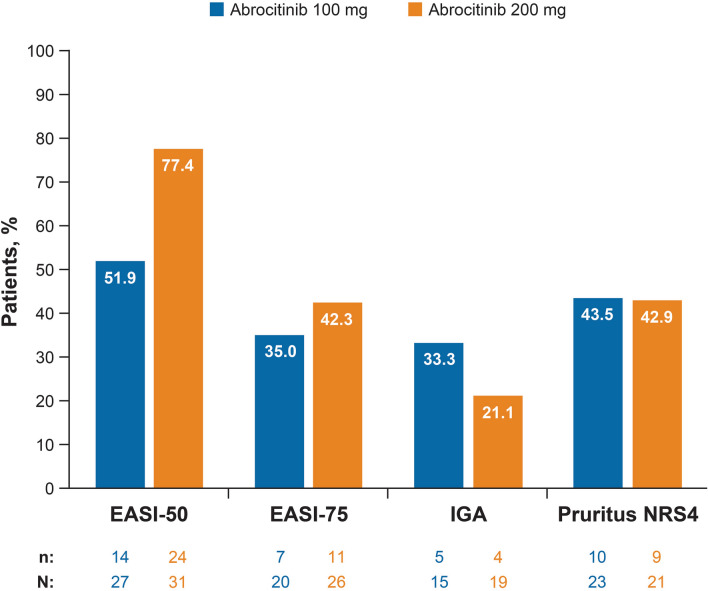
At week 12, there were 49 IGA responders. Of those, 3 (6.1%) received placebo, 16 (32.7%) received abrocitinib 100 mg, and 21 (42.9%) received abrocitinib 200 mg. (The remaining 9 patients were treated with abrocitinib 10 mg or 30 mg and are not analyzed here.) Maintenance of response at week 16 was observed in 100% (2/2), 33.3% (5/15), and 21.1% (4/19) of the patients receiving placebo, abrocitinib 100 mg, and abrocitinib 200 mg, respectively, who had data at that visit. Maintenance rates for EASI-50 or EASI-75 at week 16 were higher than those of IGA response, with the highest maintenance rates observed for EASI-50 (Fig. 1; Table 2).
Fig. 1.
Proportions of week 12 EASI or pruritus NRS4 responders who maintained response at week 16. EASI-50/EASI-75, 50% or 75% improvement in EASI; IGA response, IGA response of clear (0) or almost clear (1); pruritus NRS4, 4-point or greater improvement in pruritus NRS from baseline. EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment 0/1, pruritus NRS4 pruritus numerical rating scale, n week 12 responders who maintained the response at week 16, N week 12 responders who were evaluable at week 16 (i.e., week 12 responders who maintained and who did not maintain the response at week 16)
Table 2.
Response to abrocitinib treatment at week 12 and maintenance of response at week 16
| Responders at week 12, n (%) | Maintained response at week 16, n | Did not maintain response at week 16, n | Total assessed for response maintenance, n | Maintained response at week 16, % | |
|---|---|---|---|---|---|
| EASI-50 (N = 109)a | |||||
| Placebo | 14 (12.8) | 8 | 0 | 8 | 100 |
| Abrocitinib 100 mg | 30 (27.5) | 14 | 13 | 27 | 51.9 |
| Abrocitinib 200 mg | 38 (34.9) | 24 | 7 | 31 | 77.4 |
| EASI-75 (N = 75)a | |||||
| Placebo | 8 (10.7) | 6 | 1 | 7 | 85.7 |
| Abrocitinib 100 mg | 22 (29.3) | 7 | 13 | 20 | 35.0 |
| Abrocitinib 200 mg | 31 (41.3) | 11 | 15 | 26 | 42.3 |
| IGA (N = 49)a | |||||
| Placebo | 3 (6.1) | 2 | 0 | 2 | 100.0 |
| Abrocitinib 100 mg | 16 (32.7) | 5 | 10 | 15 | 33.3 |
| Abrocitinib 200 mg | 21 (42.9) | 4 | 15 | 19 | 21.1 |
| Pruritus NRS4 (N = 91)a | |||||
| Placebo | 13 (14.3) | 5 | 1 | 6 | 83.3 |
| Abrocitinib 100 mg | 25 (27.5) | 10 | 13 | 23 | 43.5 |
| Abrocitinib 200 mg | 28 (30.8) | 9 | 12 | 21 | 42.9 |
EASI-50, EASI-75 50% and 75% improvement in the Eczema Area and Severity Index, respectively, IGA Investigator’s Global Assessment, pruritus NRS4 4-point or greater improvement in baseline pruritus numeric rating scale score
aThe number of total responders at week 12 includes patients who received abrocitinib 10 mg and 30 mg
At week 12, there were 91 pruritus NRS4 responders. Of those, 13 (14.3%) received placebo, 25 (27.5%) received abrocitinib 100 mg, and 28 (30.8%) received abrocitinib 200 mg. Maintenance of response at week 16 was observed in 83.3% (5/6), 43.5% (10/23), and 42.9% (9/21), respectively, of the patients who had data at that visit (Table 2).
Maintenance of Response Below Predose Baseline
Most patients who achieved a response at week 12 maintained EASI and pruritus NRS scores below baseline at week 16. This includes all week 12 responders who received abrocitinib 200 mg and 80.0% of EASI-75 responders (16/20) and 87.0% of pruritus NRS4 responders (20/23) who received abrocitinib 100 mg.
Worsening of Response After Treatment Discontinuation
Worsening of EASI and pruritus NRS scores at week 16 was defined as a score increase over the week 12 values by at least two baseline intrapatient standard deviations (EASI, ≥ 14; pruritus NRS, ≥ 1). Such a worsening was observed in 10.0% of patients (2/20) and 4.3% of patients (1/23) who had attained week 12 EASI-75 and pruritus NRS4, respectively, with abrocitinib 100 mg. None of the patients who had attained week 12 EASI-75 (n = 26) or pruritus NRS4 (n = 21) with abrocitinib 200 mg experienced such a worsening.
Assessment of Biomarkers After Discontinuation of Abrocitinib
On the basis of the changes from baseline, hs-CRP was more sensitive to abrocitinib treatment than to placebo. Throughout the 12-week dosing period, the median serum concentration of hs-CRP was the lowest for abrocitinib 200 mg, followed by abrocitinib 100 mg, while it was relatively unchanged for placebo (Supplementary Fig. 1). At week 12, the median percentage changes from baseline in hs-CRP serum concentration were − 41.2% (abrocitinib 200 mg), − 53.0% (abrocitinib 100 mg), and 36.4% (placebo).
Throughout the 12-week dosing period, the median levels of IL-31, eosinophil percentage, and TARC concentration were lower in patients who received abrocitinib 200 mg than in patients who received abrocitinib 100 mg or placebo (Supplementary Fig. 1). At week 12, all three treatment groups had decreases in serum levels of these biomarkers from baseline. The median percentage change (decrease) from baseline was particularly marked for abrocitinib 200 mg for IL-31, and for both abrocitinib dose levels for eosinophil percentage. At week 12, the median percentage changes from baseline IL-31 concentration were − 73.3% for patients treated with abrocitinib 200 mg, − 35.0% for patients treated with abrocitinib 100 mg, and 6.2% for patients who received placebo. Those values were − 32.3%, − 19.0%, and − 4.2%, respectively, for eosinophil percentage and − 26.1%, − 12.8%, and − 18.0%, respectively, for TARC concentration.
Four weeks after discontinuation (i.e., at week 16), the median serum concentration of hs-CRP, the biomarker most sensitive to abrocitinib treatment, increased for both 200-mg and 100-mg dosages (reaching − 16.8% and − 12.8% median percentage change from baseline, respectively), which represents a substantial increase from week 12. In contrast, the median serum concentration of hs-CRP in the placebo group at week 16 was relatively unchanged from week 12, with the same median percentage change from baseline (36.4%).
IL-31 serum concentration, eosinophil percentage, and TARC serum concentration also increased, approaching baseline levels within 4 weeks after discontinuation of both abrocitinib dosages (Supplementary Fig. 1). At week 16, the median percentage changes from baseline for IL-31 were − 20.9% (abrocitinib 200 mg), − 42.7% (abrocitinib 100 mg), and − 6.2% (placebo). For eosinophil percentage, those values were − 7.4%, − 1.1%, and 4.0%, respectively, and for TARC, − 4.8%, − 16.7%, and − 4.6%, respectively (Supplementary Fig. 1).
Discussion
As measured by thresholds of clinically meaningful changes in skin clearance (EASI-75 or IGA response) and itch relief (pruritus NRS4), most patients showed a reversal of response 4 weeks after discontinuation of abrocitinib. Overall, the maintenance of response was inversely related to the stringency of the endpoint. All EASI-75 and pruritus NRS4 responders who received abrocitinib 200 mg and nearly all EASI-75 and pruritus NRS4 responders who received abrocitinib 100 mg maintained disease activity below baseline. Some patients had week 16 values exceeding baseline but no rebound, which was defined as exceeding baseline scores by two or more standard deviations. These results indicate that discontinuation of abrocitinib was associated with rapid reversal of disease control and suggest that patients may experience maximum clinical benefit when abrocitinib is used without interruption. Eventual discontinuation of abrocitinib may be possible in patients who achieve sustained disease control, but tapering should not be done prematurely.
The rapid reversal of disease control after discontinuation of abrocitinib is corroborated by the trend of AD biomarkers to revert toward predose baseline levels after the last abrocitinib dose. All four biomarkers in patients with moderate-to-severe AD followed this trend, with the most complete recovery noted with hs-CRP and eosinophil percentage.
This study has several limitations, including the post hoc nature of the analyses and the small sample sizes. The small sample size of responders at week 12 of treatment also precluded our ability to clarify whether changes in biomarker levels are correlated with durability of response. Thus, the data should be interpreted with caution.
Conclusion
In this phase 2b study conducted in patients with moderate-to-severe AD, biomarker response to abrocitinib rapidly reversed after treatment discontinuation. However, measures of AD severity remained at or below baseline and did not rebound after discontinuation. Patients with moderate-to-severe AD who are prescribed abrocitinib are likely to receive the best outcome when taking abrocitinib without disruption.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored by Pfizer Inc., which also funded the journal’s Rapid Service fee. The study was designed by the sponsor and data were collected and analyzed by the sponsor. The data were reviewed by the authors and the manuscript was reviewed and approved by the authors at all stages of development. Writing and editorial support (ApotheCom) were funded by Pfizer Inc.
Medical Writing and Editorial Assistance
Editorial/medical writing support under the guidance of the authors was provided by Marianna Johnson, PhD, and Kristine De La Torre, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4).
Author Contributions
Hernan Valdez and Gary Chan contributed to the conceptualization of this analysis. Gary Chan and the sponsor’s programming group performed or supported the formal analysis. Gary Chan, Melinda J. Gooderham, Jonathan I. Silverberg, Robert Bissonnette, and Elena Peeva contributed to the writing of the first draft. All authors (Melinda J. Gooderham, Giampiero Girolomoni, Julian O. Moore, Jonathan I. Silverberg, Robert Bissonnette, Seth Forman, Elena Peeva, Pinaki Biswas, Hernan Valdez, and Gary Chan) contributed to the data interpretation, critically reviewed the manuscript, and approved the submitted version.
List of Investigators
Australia: Rodney Sinclair, George Varigos, Kurt Gebauer, Lynda Spelman, Michael Freeman, Diana Rubel, Ktut Arya, Shireen Sidhu. Canada: Catherine Maari, Melinda Gooderham, Charles Lynde, Yves Poulin, Kim Papp, Mani Raman, Vincent Ho, Jerry Tan, Sheetal Sapra, Marni Wiseman, Ginette Girard. Germany: Diamant Thaci, Athanasios Tsianakas, Margrit Simon. Hungary: Lajos Kemeny, Ivan Orojan, Noemi Bakos, Gabriella Nagy. United States of America: Ellen Frankel, James Solomon, Paul Yamauchi, George Schmieder, Matthew Zook, Richard Beasley, Stacy Smith, Shondra Smith, Emil Tanghetti, Stephen Schleicher, Lawrence Sher, Jeffrey Weinberg, Jerry Bagel, Josep Genebriera-Delamo, David Pariser, Scott Fretzin, Melody Stone, Seth Forman, George Murakawa, Emily Becker, Craig Teller, Paul Getz, Scott Guenthner, Iftikhar Hussain, Mark Ling, Weily Soong, Bruce Strober, Laura Ferris, Robert Nossa, Chien Fang, Neil Sadick, Neal Bhatia.
Disclosures
Melinda J. Gooderham has received grants, personal fees, honoraria, and/or nonfinancial support from Pfizer Inc., AbbVie, Amgen, AkrosPharma, Arcutis, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Dermira, Dermavant, Eli Lilly and Company, Galderma, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Merck, Novartis, Roche, Sanofi Genzyme, Regeneron, Sun Pharma, UCB, and Bausch Health (Valeant). Giampiero Girolomoni has been principal investigator in clinical trials sponsored by and/or and has received personal fees from Pfizer Inc., AbbVie, Abiogen, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Celltrion, Eli Lilly and Company, Genzyme, LEO Pharma, Novartis, OM Pharma, Regeneron, Samsung, Sandoz, and UCB. Julian O. Moore has no conflicts of interest to disclose. Jonathan I. Silverberg is an investigator for AbbVie, Celgene, Eli Lilly and Company, GSK, Kiniksa, LEO Pharma, Menlo Therapeutics, Realm Therapeutics, Regeneron, Roche, and Sanofi; a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Asana Biosciences, Dermira, Dermavant, Eli Lilly and Company, Galderma, GSK, Glenmark, Incyte, Kiniksa, LEO Pharma, MedImmune, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron, and Sanofi; a speaker for Regeneron and Sanofi; and is on advisory boards for Pfizer Inc., Dermira, LEO Pharma, and Menlo Therapeutics. Seth Forman has no conflicts of interest to disclose. Robert Bissonnette is an advisory board member, consultant, speaker and/or investigator for and receives honoraria and/or grants from Pfizer Inc., AbbVie, Arcutis, Arena Pharma, Aristea, Asana BioSciences, Bellus Health, Bluefin Biomedicine, Boehringer Ingelheim, CARA, Dermavant, Eli Lilly and Company, EMD Serono, Evidera, Galderma, GSK, Inmagene Bio, Incyte, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Ralexar, RAPT, Regeneron, Respivant, Sanofi-Genzyme, Sienna, Target RWE, and Vyne Therapeutics. Robert Bissonnette is also an employee and shareholder of Innovaderm Research. Elena Peeva, Pinaki Biwas, Hernan Valdez, and Gary Chan are employees and shareholders of Pfizer Inc.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki, all International Council for Harmonization Good Clinical Practice Guidelines, and all local regulatory requirements following approval from the IRBs or ECs at each study site. A list of all IRBs and ECs is provided in the Supplementary Material. All patients provided written informed consent to participate in this study.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22.e12. doi: 10.1016/j.anai.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42. doi: 10.1186/s41687-019-0128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–706.e2697. doi: 10.1016/j.jaip.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–52.e543. doi: 10.1016/j.anai.2017.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cibinqo (abrocitinib), 100 mg film-coated tablets [prescribing information]. Kent, UK: Pfizer Limited; September 2021. https://www.medicines.org.uk/emc/product/12873/smpc. Accessed 22 Feb 2022.
- 6.Pfizer Inc. Japan’s MHLW approves Pfizer’s CIBINQO® (abrocitinib) for adults and adolescents with moderate to severe atopic dermatitis. September 30, 2021. https://www.pfizer.com/news/press-release/press-release-detail/japans-mhlw-approves-pfizers-cibinqor-abrocitinib-adults. Accessed 22 Feb 2022.
- 7.Cibinqo (abrocitinib) [summary of product characteristics]. Amsterdam, the Netherlands: European Medicines Agency; December 17, 2021. https://www.ema.europa.eu/en/documents/product-information/cibinqo-epar-product-information_en.pdf. Accessed 22 Feb 2022.
- 8.Cibinqo (abrocitinib) tablets [prescribing information]. New York, NY: Pfizer Inc.; January 2022: https://cdn.pfizer.com/pfizercom/USPI_Med_Guide_CIBINQO_Abrocitinib_tablet.pdf. Accessed 22 Feb 2022.
- 9.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson EL, Wollenberg A, Bissonnette R, et al. Patient-reported symptoms and disease impacts in adults with moderate-to-severe atopic dermatitis: results from a phase 2b study with abrocitinib. Dermatitis. 2021;32(1S):S53–S61. doi: 10.1097/DER.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 11.Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 14.Peeva E, Hodge MR, Kieras E, et al. Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: a phase 1, randomized, placebo-controlled, dose-escalation study. Br J Clin Pharmacol. 2018;84(8):1776–1788. doi: 10.1111/bcp.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22(6):453–467. doi: 10.1093/intimm/dxq027. [DOI] [PubMed] [Google Scholar]
- 16.Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115(4):640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 17.Febvre-James M, Lecureur V, Fardel O. Potent repression of C-reactive protein (CRP) expression by the JAK1/2 inhibitor ruxolitinib in inflammatory human hepatocytes. Inflamm Res. 2020;69(1):51–62. doi: 10.1007/s00011-019-01293-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.