Abstract
Melasma is a prevalent chronic relapsing pigmentary disorder that affects photoexposed areas, especially in women of childbearing age. Although there is currently no curative treatment available for melasma, this manuscript critically reviews the knowledge regarding photoprotection, topical and oral therapies, and procedures such as peelings, laser, and microneedling that represent the main strategies for control and prevention of this disease. As the pathogenesis of melasma is not entirely understood, there are prospects for the development of new therapeutic strategies that might act on the pathways that promote sustained pigmentation rather than merely decreasing melanin synthesis and removing melanin from the epidermis.
Keywords: Melasma, Peeling, Sunscreen, UV radiation, Laser
Key Summary Points
| Melasma is a frequent relapsing pigmentary disorder that significantly impacts quality of life, and no curative treatment is yet available. |
| Photoprotection against UVB, UVA, and blue–violet visible light (e.g., broad-spectrum tinted sunscreen) is essential for successful treatment, improving pigmentation, preventing relapse, and preventing disease in high-risk individuals. |
| Topical therapies leading to tyrosinase inhibition are the mainstay of treatment; hydroquinone and triple combination cream are the reference drugs. |
| Oral therapies (e.g., tranexamic acid) and microneedling can be associated with topical therapies to synergistically increase their efficacy. |
| Superficial peelings and low-fluence (e.g., Q-switched) laser therapy increase melanin removal from the epidermis, accelerating clinical results. |
Introduction
Melasma affects photoexposed areas, especially in women of reproductive age, but no curative treatment is available, significantly impacting the quality of life, including low self-esteem that leads to social contingencies, anxiety, and depressive symptoms [1–3]. While melasma induced by pregnancy or photosensitizing drugs has a better outcome after the causative factor ceases, most patients experience a chronic disorder, with seasonal variation and relapses after successful treatment, requiring post-treatment maintenance. In general, melasma fades with aging, and it disappears in most women after menopause, although extrafacial melasma can persist longer [4, 5]. Despite the high prevalence of melasma and the variety of treatments available, there are few large-sample randomized controlled trials to support a robust hierarchy of treatments. This review aims to present and critically discuss the main treatment strategies for melasma.
Sun Protection
Along with pregnancy, direct sun exposure is the leading risk factor for melasma, reported by 27–51% of patients as a trigger and 84% as a factor of clinical impairment [6, 7]. Patients with melasma usually report occupational or intentional sun exposure. Thus, rigorous sun protection measures should be conditionally recommended for any patient submitted to treatment and post-treatment maintenance.
As melanogenesis in melasma can be induced by UVB, UVA, or short-wavelength visible light (VL), patients with melasma must be aware of the type of sun exposure in ordinary, daily situations. Large amounts of radiation reach the skin even in the shade or near windows, while UVA and VL pass through windshields and window glass. Moreover, these wavelengths occur at melanogenic levels from 08:30 until 17:30 in intertropical areas [8, 9].
As most sunscreens underperform in real life due to the low amount of product used or insufficient reapplications, especially for UVA and VL protection, behavioral measures and self-awareness regarding sun exposure should be reinforced for patients with melasma [10–12]. Additionally, dark-skinned people are less adherent to routine sun protection, sunscreen use, and reapplication, although they are more affected by melasma than fair-skinned individuals, thereby hindering the effectiveness of treatment [13].
Among behavioral measures for sun avoidance, topical sunscreens play a significant role in melasma treatment and should be tailored for the best performance in pigmentary disorders [14]. Nevertheless, the photoprotectors used in commercial topical sunscreens differ broadly among countries due to different regulatory recommendations from health agencies.
The UVB protection offered by sunscreens is determined worldwide by the in vivo sun protection factor (SPF). For routine daily activities, SPF-30 sunscreen can prevent skin redness and photoaging [15, 16]. Nonetheless, most patients usually apply up to 50% of the recommended layer of topical sunscreens on their skin, so products with higher SPF values (e.g., ≥ 50) are recommended for patients with pigmentary disorders [17].
UVA radiation is highly melanogenic and reaches high levels early in the morning (even on cloudy days), passing through ordinary glass, but does not induce erythema. UVA is the main waveband that demands attention in melasma treatment and post-treatment maintenance. Protection against UVA is usually determined by the in vivo persistent pigment darkening (PPD) factor. However, different regulatory agencies use various nomenclatures for UVA protection [15]. As the accomplishment of high PPD values compromises the cosmetic nature of topical sunscreens, the PPD range of 20–30 is indicated in pigmentary disorders to guarantee patient adherence.
Although VL radiation (especially in the high-energy blue–violet range) can induce melanogenesis, there is still no validated method for assessing the efficacy of sunscreens to protect against this waveband [15, 18]. Tinted sunscreens are indicated to protect against VL, while iron oxide salts seem to provide better performance in melasma. Moreover, although intense heat leads to melanogenesis, there is no systematic study on solar heat (infrared radiation [IR]) in melasma, nor are validated methods to assess IR protection available. Thus, workers submitted to intense heat as cooks, bakers, metallurgists, glassmakers, and drivers should be advised of the role of intense heat in the chronicity of melasma.
Despite the importance of sun protection in the treatment of melasma, few systematic studies have explored sunscreens in treating and preventing melasma relapse. The assessment of placebo groups in randomized controlled trials for melasma allows for measuring the efficacy of broad-spectrum tinted sunscreens, accounting for a 10–20% melasma area and severity index (MASI) reduction in 8 weeks [19]. After 12 weeks of using a broad-spectrum sunscreen (SPF19 PA+++), there was a 26.9% reduction in the MASI in India [20]. Notably, the use of tinted (with iron oxide) broad-spectrum sunscreen reduced the post-treatment relapse of melasma in 39 French women [21].
Regarding high-risk populations, 200 women from Morocco during early pregnancy were solicited to use a broad-spectrum sunscreen (SPF 69, PPD 28). The incidence of melasma was 2.7% after 12 months of follow-up, lower than expected (53%) in this high-risk population [22]. Furthermore, 68 Mexican patients with melasma applied topical 4% hydroquinone (HQ) for 8 weeks; however, they were randomized to use an ordinary SPF-50 sunscreen or a broad-spectrum tinted sunscreen with iron oxide, which promoted greater UVA and VL protection. The mean reduction of MASI was 62% for the ordinary sunscreen group and 78% for the broad-spectrum and near-VL sunscreens [23].
Cosmetic makeup can be recommended to camouflage melasma. However, the photoprotection provided by multifunctional products (e.g., moisturizers, powders) is not as good as that offered by standard topical sunscreens, which should be applied under the makeup. Indeed, there is still no robust evidence that adding topical antioxidants (e.g., vitamin C, vitamin E, Polypodium leucAtomos) or depigmenting agents (e.g., butylresorcinol, glabridin) to the composition of topical sunscreens increases the efficacy of treatments for melasma [17]. Thus, the role of oral antioxidants (misnamed “oral photoprotectors”) is also discussed below.
Finally, some cosmetics, photosensitizing drugs (e.g., phenothiazines, sulfonamides, sulfonylureas, thiazide diuretics, tetracyclines, tricyclic antidepressants, promethazine, piroxicam), and steroid hormones (e.g., contraceptive pills, estrogen replacement therapies) increase the melanogenic effect of sun exposure and should be substituted to enable successful melasma treatment [24–27]. Since melasma has a definitive genetic background, adoption of preventive measures such as sun protection and avoidance of contraceptive pills should be recommended in adolescent girls from families with melasma [28].
Topical (Self-Applied) Treatments
Hydroquinone and Triple Combination (TC)
HQ is a phenolic compound with antioxidant properties that interferes with melanogenesis by inhibiting tyrosinase. It also inhibits DNA and RNA synthesis and can alter the formation of melanosomes, causing selective damage to melanocytes. These properties suppress the melanocyte metabolic process, inducing a gradual decrease in melanin production. The effectiveness and toxicity of topical HQ depend on the final product’s concentration, vehicle, and chemical stability.
Compounding pharmacies in USA can make up to 12% HQ; over-the-counter HQ (3% and 2%) products are no longer available. Generally, HQ is used as 2–5% cream and can be applied twice a day when starting therapy, but is usually prescribed for use at bedtime. The depigmenting effect can be seen after 5–8 weeks, and the treatment can be continued for up to 1 year [29]. In melasma, the mean reduction of the modified MASI (mMASI) by treatment with a broad-spectrum sunscreen and topical 4% HQ is expected to be 30–40% after 8 weeks and 45–55% after 16 weeks [30, 31].
The efficacy of HQ is optimized when associated with retinoids and topical corticosteroids. In 1975, Kligman and Willis described the use of the association of 0.1% tretinoin, 5% HQ, and 0.1% dexamethasone in skin lightening [32]. Currently, the association of 0.05% tretinoin with 4% HQ and 0.01% fluocinolone acetonide (triple combination [TC]) is considered the gold-standard topical treatment for melasma owing to its potent and rapid whitening effect [33, 34]. All measures of effectiveness and safety indicate that intermittent use of TC for up to 6 months is recommended. In melasma, the reduction of mMASI by treatment with a broad-spectrum sunscreen and topical TC is expected to be 30–40% after 8 weeks and 50–60% for 16 weeks [35–37].
The use of HQ and TC can promote adverse effects, such as allergic and irritative contact dermatitis, confetti-like leukoderma, redness, and telangiectasias. Exogenous ochronosis was reported due to prolonged use of high concentrations of HQ. Although topical HQ has been prohibited in some countries, there is no substantial evidence of risk from topical use for treatment of melasma. However, neither HQ nor tretinoin should be used during pregnancy [38–40].
Azelaic Acid
Azelaic acid, or 9-carbonodicarboxilic acid, is a nonphenolic compound derived from the yeast Pityrosporum ovale . It is a weak tyrosinase inhibitor of melanocytes and mitochondrial oxidoreductases, reducing the DNA synthesis of normal and hyperfunctioning melanocytes. Azelaic acid is safe for use in pregnant and breastfeeding patients and is generally well tolerated since the stinging and itching that follows its application fade in a few minutes. Azelaic acid is generally used as 20% cream twice daily for melasma, reducing the MASI by 50% after 8 weeks [41].
Niacinamide
Niacinamide, or nicotinamide, is a biologically active form of niacin (vitamin B3) found in the root of many vegetables and yeast. It is an essential precursor of coenzymes such as nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleophosphate (NADPH) molecules with a potent antioxidant action [42]. In vitro studies have shown that niacinamide significantly decreases the transfer of melanosomes to keratinocytes, interfering in the cell signaling pathway between keratinocytes and melanocytes, decreasing melanogenesis [43]. Topical niacinamide stabilizes the epidermal barrier function with increased protein and ceramide synthesis and keratinocyte differentiation, improving skin microrelief and attenuating fine wrinkles. Moreover, it promotes an antiinflammatory effect on the skin [44]. Niacinamide is safe for use in pregnant and breastfeeding patients and is generally applied alone as 4–5% cream twice daily or in association with other lighteners, such as kojic acid and arbutin. In melasma, 4% niacinamide has led to a 62% reduction of hemi-MASI (hMASI) after 8 weeks [45].
Cysteamine
l-Cysteamine is an aminothiol compound with antioxidant and depigmenting properties due to the inhibition of peroxidase and tyrosinase enzymes. It also acts as a chelator of iron and copper and increases intracellular glutathione levels. l-Cysteamine can be found naturally as an intracellular degradation product of l-cysteine.
Two randomized, double-blind, placebo-controlled studies with 50 and 53 participants tested a fine layer of 5% cysteamine cream on the face at night, leaving it up to 3 hours for 16 weeks, achieving reductions of the MASI by 50% and 59%, respectively [46, 47]. Moreover, a randomized, evaluator-blinded clinical trial including 40 Brazilian women with facial melasma who underwent broad-spectrum tinted sunscreen and nightly application of 5% cysteamine or 4% HQ for 16 weeks resulted in an MASI reduction of 32% and 52%, respectively [31]. Another comparison of cysteamine and 4% HQ for 16 weeks in Australia resulted in 21% and 32% mMASI reduction, respectively [48]. Furthermore, in a randomized trial of 50 women with melasma, 5% cysteamine was compared with a modified TC (4% HQ, 0.05% retinoic acid, and 0.1% betamethasone) for 16 weeks, achieving a 51% and 42% reduction of MASI, respectively.
Thiamidol
Thiamidol (isobutylamido thiazolyl resorcinol) is a new, potent tyrosinase inhibitor proven to prevent UVB-induced hyperpigmentation in human skin [49]. After 24 weeks of thiamidol 0.2% applied twice daily, 23 women with facial melasma experienced a mean MASI reduction of 26%. A randomized controlled trial including 50 Brazilian women with melasma compared a double layer of thiamidol 0.2% twice daily versus nightly 4% HQ for 12 weeks with a broad-spectrum tinted sunscreen. The reduction of mMASI was 43% and 33%, respectively.
Other Topical Agents
Several promising topical products are under consideration for melasma treatment [50]. However, few have been tested in randomized controlled trials using MASI scores as the primary outcome, limiting the evaluation of their efficacy and the definition of their role among available treatments.
The most active presentation of vitamin C is l-ascorbic acid, usually prescribed for melasma at concentrations of 5–20%. As it is highly unstable, several compounds have been developed for skin bleaching and melasma [51]. Vitamin C inhibits melanogenesis by interacting with the copper in tyrosinase, depleting dopaquinone. It is commonly associated with other lighteners, such as kojic acid and arbutin.
Kojic acid is obtained from various fungi such as Aspergillus oryzae , Penicillium spp., and Acetobacter spp. It also binds to the copper in tyrosinase, blocking its activity and eumelanogenesis. In the treatment of melasma, kojic acid is used in a concentration ranging from 1% to 4% in combination with other lighteners. Its effect begins after 2–4 weeks of continuous use, with progressive improvement up to 6 months [52].
Arbutin is an HQ derivative extracted from the grape Uva ursi folium, also found in the leaves of blueberries and cranberries. It inhibits tyrosinase and prevents the maturation of melanosomes. In melasma, it is usually used at concentrations ranging from 1% to 6% in combination with other lighteners and procedures [53].
Glycolic acid is an alpha hydroxy acid whose effect is dependent on its pH and concentration. It reduces the cohesion of corneocytes at the junction of the stratum corneum and granulosum, which leads to thinning of the epidermis with continued use. This agent facilitates the penetration of other active principles and promotes melanin’s dispersion by increasing keratinocyte cell turnover [54]. For the treatment of melasma, glycolic acid is usually associated with other lighteners, such as kojic acid and HQ, in creams at concentrations from 4% to 10% [55]. The main adverse effects arise from the primary irritation. There may also be temporary erythema and burning shortly after application.
Clobetasol is a potent topical steroid that promotes rapid clearing of melasma pigmentation. The mechanism of depigmentation promoted by corticosteroids has not yet been elucidated, and the clearance promoted is usually of short duration. Moreover, the cream cannot be administered for extended periods due to its local side effects, although clobetasol can be used for a short cycle followed by another treatment with a better safety profile. Sequential treatment with topical clobetasol for up to 8 weeks followed for topical bleaching agents can promote a faster result when compared with the usual bleaching agent [56, 57].
Ten patients with facial melasma from India were treated with 0.05% clobetasol propionate cream topically twice daily for 4 weeks, followed by use once daily associated with sunscreen for another 4 weeks. All participants presented pigmentation fading after 2 weeks, which was more noticeable after 4–6 weeks. However, three (30%) had to stop the treatment after 4 weeks because of local atrophy and telangiectasia [57].
In a split-face trial performed in India, participants with facial melasma were prescribed twice-daily application of 20% azelaic acid cream to half the face for 24 weeks. On the other half of the face, they applied 0.05% clobetasol propionate cream for 8 weeks only, followed by 20% azelaic cream for the next 16 weeks. For the 30 patients who completed the study, the side that received the sequential therapy presented substantial lightening, with mild and transient side effects [56].
Topical tranexamic acid (TXA) performs worse than its oral and intradermal regimens. Moreover, TXA has a variety of formulations, vehicles, and regimens of treatment that increase the variability of the results and hinder a suitable evaluation of its role in melasma treatment [58]. A vehicle-controlled study using liposomal 5% TXA for 12 weeks in Thailand resulted in a 51% reduction in MASI, despite no difference between groups [58].
A randomized split-face study of 100 Egyptian women compared liposomal 5% TXA cream twice daily with nightly 4% HQ for 12 weeks, which resulted in an hMASI reduction of 46% and 45%, respectively [59]. Another randomized trial with 60 Iranian women compared 5% TXA twice daily with 3% HQ for 12 weeks, resulting in a 52% and 48% mMASI reduction, respectively [60]. Furthermore, a randomized study compared topical TXA with a modified TC for 8 weeks in India, resulting in 5% and 30% MASI reduction, respectively [61].
Metformin inhibits melanogenesis by reducing intracellular cyclic adenosine monophosphate (cAMP) accumulation, but its low lipophilicity obligates its use in high concentrations for topical efficacy [62]. Topical metformin 30% lotion twice daily was compared with TC for 8 weeks in a randomized controlled study involving 40 Egyptian patients with melasma. The decrease in MASI was 56% and 57%, respectively [63]. Meanwhile, there is no robust evidence of the association of melasma with insulin resistance [64].
Methimazole is an oral antithyroid drug that has recently attracted attention due to its depigmenting effect by inhibiting peroxidase, but it has also been shown to exert inhibitory effects on tyrosinase in experimental studies. Topically, it is noncytotoxic to melanocytes, and it does not promote changes in serum thyroid stimulating hormone (TSH) levels [65]. A double-blind study evaluated the efficacy and safety of 5% methimazole once a day versus 4% HQ for 8 weeks in 50 Iranian women with melasma. The reduction of mMASI was 25% and 76%, respectively [66].
Melatonin is a hormone synthesized and secreted by the pineal gland, with a powerful antioxidant role that regulates the circadian rhythm. It reduces UV-induced free radicals and inhibits the alpha-melanocyte-stimulating hormone [67]. Since systemic oxidative stress is increased in patients with melasma, serum levels of melatonin and catalase are significantly lower [68]. After 90 days of topical melatonin 5% twice daily combined with sunscreen for 90 days, the MASI decreased by 31% compared with HQ 4% and sunscreen, which achieved a decrease of 37% [69]. As sleep disorders are a major source of oxidative stress, oral melatonin can play a special role in the treatment of patients who refer poor sleep quality [70].
Malassezin is a natural indole compound produced by Malassezia furfur , reported to induce melanocyte apoptosis, dose-dependent induction of apoptotic markers, and decreased melanin synthesis in melanocyte cultures. In vitro tests also demonstrated that malassezin is not a tyrosinase inhibitor, but a highly active agonist of the aryl hydrocarbon receptor with key homeostasis functions that may impact the mechanism of pigmentation. In a double blind, dosing range study, the group that used topic malassezin twice a day for 8 weeks had a 30.13% reduction in the severity of hyperpigmentation, compared with 16.67% in the placebo group. There were no relapses during 8 weeks of follow-up [71, 72].
In 2017, Cohen proposed a novel (topically administered) double therapy for melasma that combine an anti-estrogen and a vascular endothelial growth factor inhibitor. The anti-estrogen could be either a selective estrogen receptor modulator (such as tamoxifen or raloxifene) or an aromatase inhibitor (such as anastrozole, letrozole, or exemestane). The vascular endothelial growth factor inhibitor would be bevacizumab [73–75]. Investigation of this novel approach in clinical trials is warranted.
Systemic Treatments
Tranexamic Acid (TXA)
TXA is a synthetic lysine-analog antifibrinolytic agent that competitively inhibits the activation of plasminogen to plasmin. It promotes the inhibition of tyrosinase activity by blocking the interaction of melanocytes and keratinocytes via the inhibition of the plasminogen/plasmin system. TXA also decreases mast cell activity and inhibits fibroblast growth factors [76, 77].
In a randomized controlled trial with 44 women, oral 250 mg TXA or placebo was given twice daily for 3 months, leading to a 49% reduction of MASI, compared with 18% in those who used sunscreen alone [78]. However, there was a notable relapse of melasma in the 3 months that followed TXA interruption.
Oral TXA also improves the performance of adjuvant creams such as TC cream in melasma, with earlier clinical response [79]. In a randomized trial, the group that received oral TXA, 250 mg, twice daily for 60 days associated with a broad-spectrum sunscreen and TC cream presented a 64% reduction of mMASI, while the group that received a broad-spectrum sunscreen and TC presented only a 12% mMASI reduction [80, 81]. A relapse of melasma was observed in the months following TXA interruption, even with sunscreen and TC maintenance.
Regarding TXA dosage, patients with severe melasma were randomized to receive a daily dose of 500 mg, 750 mg, 1000 mg, or 1500 mg for 8 weeks. All four doses were effective, and there were no relevant differences in the MASI or melanin index reductions among them [82], although faster results were achieved with higher dosages [83]. As long as the prothrombotic effects are dose dependent, the lower dose offers a favorable result, as fewer patient side effects and risks are preferable. Despite its off-label indication, TXA is usually prescribed as 250 mg capsules twice daily for 2–4 months in melasma.
A retrospective analysis enrolled 561 patients with different treatment regimens. Adverse events occurred in 40 (7.1%). Most were transient, but one (0.2%) developed deep vein thrombosis 6 weeks after starting therapy. She was later diagnosed with familial protein S deficiency [84].
The primary reported side effects of TXA are back pain, musculoskeletal pain, oligomenorrhea, headache, urticarial rash, and abdominal cramps. Contraindications to TXA are renal dysfunction, malignancy, cardiovascular or respiratory disease, current anticoagulant therapy, (personal or familiar) history of thromboembolic disease (including deep vein thrombosis, pulmonary embolism, arterial thrombosis, stroke, and subarachnoid hemorrhage), and pregnancy. Other factors (e.g., hormonal contraception or replacement therapy, smoking, and long-distance travel) should be considered as potential exclusion criteria. There are no biochemical markers of TXA-related thrombotic events, so careful anamnesis should be performed to identify the main risk factors [85].
Pycnogenol
Pycnogenol is a standardized extract obtained from the bark of the French maritime pine Pinus pinaster. It contains monomeric phenolic compounds (catechin, epicatechin, and taxifolin) and condensed flavonoids (procyanidins), with antioxidant and antiinflammatory activities capable of stimulating the synthesis of the enzyme-induced nitric oxide synthase and antityrosinase activity leading to the suppression of melanin biosynthesis. In vitro, pycnogenol has antioxidant properties that are more potent than vitamins E and C. In addition, it recycles vitamin C, regenerates vitamin E, and reduces the skin erythema induced by UVB [86]. The usual dosage prescribed is 75–150 mg daily in melasma, divided into two to three doses for 2–4 months. The side effects are negligible.
Oral pycnogenol 50 mg twice daily has been suggested to treat melasma, reducing 58% of MASI in 31 Brazilian women after 90 days [87]. Another case series enrolled 30 Chinese women with melasma, who took 25 mg pycnogenol three times daily for 30 days, leading to a 32% mean area reduction and a 22% intensity reduction of facial melasma [86]. Furthermore, a randomized controlled trial evaluated the addition of 150 mg oral pycnogenol (or placebo) to a broad-spectrum tinted sunscreen and TC in 44 women with melasma. At 60 days, the reduction of mMASI was superior for the group who took oral pycnogenol, at 49% versus 34% [37].
Other Systemic Agents
Polypodium leucatomos (PL) is an over-the-counter medication, marketed as Heliocare in USA. It is a fern of the Polypodiaceae family, native to Central and South America. It has been described as an antiinflammatory supplement for treating inflammatory dermatoses and reducing sunburn severity. Its exact mechanism of action is unknown, but it is a potent antioxidant and seems to work to maintain the extracellular matrix’s structural integrity, which is usually affected by ultraviolet radiation [88, 89]. These properties have led to the hypothesis of its efficacy for the treatment of melasma. However, a trial involving 40 Hispanic women with moderate to severe melasma using sunscreen and randomized to receive oral placebo or 720 mg PL extract daily for 12 weeks revealed no difference in MASI reduction [90].
Another study included 33 Asian women with melasma using topical 4% HQ cream and broad-spectrum sunscreen randomized to take PL 480 mg/day or placebo for 12 weeks. At 56 days, the decrease in MASI was more significant in the PL group (49.4%) than placebo (32.6%). Nevertheless, the reduction in the melanin index, erythema index, quality of life scores, and blinded global photographic improvement did not differ between the groups [91]. These contradictory results warrant new systematic studies to investigate the efficacy of PL in treating melasma.
Carotenoids are natural pigments present in several plants that act as stabilizers of unstable molecules due to their ability to reversibly chelate reactive oxygen species and other free radicals, absorbing the energy of these molecules and dissipating it in the form of heat. In the skin, photoprotection by carotenoids occurs by direct absorption of UVA, UVB, and VL by acting against the action of UVR on exposed molecules, which become unstable and potentially hazardous to the DNA molecules and cellular proteins. In addition, they can activate antioxidant and antiproliferative enzymatic pathways in response to UVR exposure [92]. Oral fucoxanthin (10 mg/kg) reduced experimentally UVB-induced skin pigmentation in guinea pigs, probably due to suppression of proinflammatory cytokine prostaglandin E2 (PGE2) synthesis and receptors involved in melanogenesis [85]. Despite the plausibility of its use, there have been no randomized controlled trials of oral carotenoids in melasma to date.
Glutathione has been purposed to treat melasma due to its antioxidant properties, leading to tyrosinase inhibition. Moreover, it increases intracellular cysteine levels and N-acetylcysteine, shifting melanogenesis from eumelanin to pheomelanin. It is prescribed worldwide for skin discoloration by an oral or intravenous route. A randomized placebo-controlled study with 60 young Thai medical students taking 250 mg twice daily oral glutathione for 4 weeks provided a consistent decrease in the melanin index and a reduction of lentigines [93]. Thus, randomized controlled studies using oral antioxidants with glutathione or N-acetylcysteine for melasma are warranted.
Oral lycopene-rich tomato extract proved to increase the serum levels of superoxide dismutase and contribute with topical 4% hydroquinone to mMASI reduction, after 12 weeks [94].
Chemical Peelings
Chemical peelings accelerate the turnover of epidermal keratinocytes, classified as superficial, medium, and deep according to the skin layer and histological level reached. The effects can include epidermal remodeling, keratinocytes from the corneal layer, melanin elimination, and dermal reorganization due to the inflammatory process and production of cytokines, which stimulate fibroblasts and increase collagen and elastin synthesis [95]. Moreover, there are many indications for medical peelings in cosmetic dermatology. With melasma, they are usually associated with medical care, such as TC and HQ plus a broad-spectrum tinted sunscreen, and other procedures, such as microneedling, for better results [96, 97].
Chemical peels are not the first-line therapy, but they represent an adjuvant measure. Notably, superficial peeling in serial sessions should be performed for melasma. The effects are dependent on keratinocyte turnover and melanin distribution [98, 99]. The most commonly used agents for chemical peeling are (1) 15% or 20% trichloroacetic acid (TCA) in aqueous solution, gently applied with gauze and not removed or neutralized; (2) 30%, 50%, or 70% glycolic acid (GA) in gel, applied with gloved fingers and removed (after erythema or stinging) or neutralized (if frosting) with 10% sodium bicarbonate; (3) 20% or 30% salicylic acid (SA) in ethanol, applied with gauze and not removed; and (4) Jessner’s solution (JS), which is composed of 14 g resorcinol, 14 g salicylic acid, and 14 mL lactic acid (85%) in ethyl alcohol 95%, applied with gauze and not removed.
The results may be mild, moderate, or marked, but no additional reduction in skin pigmentation may be observed. GA is the agent most widely cited in literature about chemical peelings and melasma [100]. Full-strength lactic acid (LA) solution, in concentrations of 92% or 82%, was reported in two studies as an alternative to be applied with gloved fingers just over the lesional area and removed after erythema or stinging. Its efficacy seemed similar to that of JS [101, 102].
Retinoic acid (RA) or tretinoin, in concentrations of 1%, 3%, and 5% in ethanol/propylene glycol, was first reported in 2001 as an agent for serial superficial peelings and indicated for melasma and photoaging [103, 104]. It is a yellow-colored dispersion and should be maintained on the skin for up to 6 h (washed at home), and a brown dye is added for facial use with no influence on the peelings’ effect. The expectation is to increase keratinocyte turnover and eliminate preformed melanin within the epidermis. However, RA is a volatile formulation, degraded when exposed to VL, UV, and O2. Thus, a powerful antioxidant system is needed.
To date, the best vehicle and ideal concentration for RA remain unknown. Pharmacotechnical studies have shown greater retention of high-strength formulations in the corneal layer when compared with traditional 0.05% RA cream. Thus, high concentrations have no apparent advantage since they are more difficult to homogenize, increasing the risk of crystallization and reducing skin penetration [105].
An evidence-based review, including 40 studies and 2912 patients, concluded that GA peeling is not more effective than HQ 4% [106]. Furthermore, another review, including grades of recommendation, concluded that all superficial peelings might be used in melasma as an additional or maintenance therapy, with mild to moderate efficacy. The authors also demonstrated no significant advantage of one agent over another and recommended a choice based on comfort level, the experience of the dermatologist, and the safest strength [107].
In contrast, a recent network meta-analysis evaluated the efficacy of all currently available treatments for melasma. Chemical peels were not cited as a therapeutic option due to the paucity of high-quality randomized controlled trials [108]. Adverse effects are rare, but the primary concern is the risk of hyperpigmentation, especially in dark-skinned individuals [99].
Twenty-one randomized and comparative studies about chemical peels for melasma were identified (Table 1). In general, no relevant differences were observed in the comparisons. Nonetheless, it is important to highlight the high heterogeneity and limitations of the studies, such as (1) low to moderate methodological quality, (2) variation in a selected population, (3) a lack of inclusion and evaluation in different skin types, (4) small sample sizes, (5) variable designs, (6) types of chemical peels, (7) deficient efficacy measures, (8) short-term or no follow-up, (9) attrition rate, (10) heterogeneities in the grouped results, and (11) poor conclusions. Nevertheless, GA is considered more effective and safer than TCA peeling for melasma [99, 106], while JS and RA peels are low-cost and safe adjuvant procedures for melasma. Moreover, modified JS (17% LA, 17% SA, 8% citric acid, and ethanol) may reduce allergic reactions to resorcinol and post-inflammatory hyperpigmentation [109]. However, additional well-designed studies are needed.
Table 1.
Randomized and controlled trials, prospective and comparative studies about chemical peelings in melasma
| Author, year | Study design | Population | Interventions, efficacy parameters duration, follow-up | Results |
|---|---|---|---|---|
| Kalla, 2001 | NR, comparative | N = 100 Indian patients M:F = 1:1.6 2 groups (A, B) |
A = 6855–75% GA B = 32 10–15% TCA Parameters not specified Till significant improvement Follow-up = 3 months |
TCA: faster response, but more relapse and hyperpigmentation |
| Hurley, 2002 | R, IB, split-face | N = 21 Hispanic patients (18 completed) phototypes IV and V; 2 groups (A, B) |
A: 4% HQ cream twice daily—8 weeks B: 4% HQ cream twice daily + 20%—30% GA, every 2 weeks (20% for 4 weeks, 30% for 4 weeks) MASI, Mexameter, subjective 8 weeks No follow-up |
Significant reduction in both groups, with no difference |
| Sarkar, 2002 | Open, pilot, comparative, prospective |
N = 40 Indian patients Photo types III-V M:F = 18:22 2 groups (1, 2) |
1 = 20: MKF daily + serial GA (30–40%), 3 weeks interval 2 = 20: MKF daily 21 weeks No follow-up |
Significant improvement in both groups, more rapid and greater in group 1 |
| Khunger, 2004 | Open, pilot, split-face |
N = 10 female Indian patients |
1% RA daily X 70% GA, weekly, 12 weeks mMASI, photograph No follow-up | Significant decrease in both sides, with no difference |
| Dogra, 2006 | R | N = 50 female Pakistan patients 2 groups (A, B) |
A = 50% GA B = 20% TCA + 5% HQ and 0.025% RA 3 peels, every 3 weeks MASI, photographs No follow-up |
Same efficacy, tolerability to GA better than to TCA |
| Macedo, 2006 | Open, comparative, split-face prospective | N = 8 female Brazilian patients |
10% GA/4% HQ cream on both sides of the face + 4 bi-weekly 70% GA to one side X peeling vehicle on the other side, Clinical pictures assessed by 3 independent dermatologists No follow-up |
Similar improvement on both sides; 70% GA did not produce additional reduction in pigmentation |
| Sharquie, 2006 | NR, split-face, comparative |
N = 30 Iraqi women (26) and men (4) 24 completed (20 women, 4 men) phototype IV 2 groups (1, 2) |
1 = 15—92% LA 2 = 15 JS every 3 weeks Till desired response 2 to 5 sessions MASI Follow-up = 6 months |
Significant improvement on both sides, with no difference; no side effects |
| Erbil, 2007 | RCT |
N = 25; 15 = cases 10 = controls |
Cases: serial GA 20 → 35 → 50 → 70% every other week + daily 20% AA cream (b.i.d.) + adapalene 0.1% gel Controls: daily 20% AA cream (b.i.d.) + 0.1% adapalene gel MASI 20 weeks 20 weeks |
Good response for all, significantly better for group peels, with transient erythema and PIH in 3 patients |
| Soliman, 2007 | R, comparative |
N = 30 female patients, phototypes III and IV 2 groups (A, B) |
A = 15 20% TCA B = 15 20% TCA + topical 5% ascorbic acid + 0.05% RA gel and 4% HQ cream once daily MASI and patients’ global response 6 weeks 12 and 16 weeks |
Improvement and maintenance during follow-up A = 10 patients (67%) B = 13 patients (87%) No significant |
| Garg, 2008 | R, SB |
N = 60 Indian patients phototype IV (50 completed) 3 groups (A, B, C) M:F = 1:6.5 |
A = 15 GA 20% → 50% B = 17 0.025% RA + GA 20% → 50% C = 18 2% HQ + GA 20% → 50%) 2-weeks interval, MASI No follow-up |
All improved, most significant in C A > B > C |
| Safoury, 2009 | NR, SB, prospective split-face |
N = 20 women phototypes III and IV |
15% TCA on both sides X mJS on one Side 10 days interval MASI 10 weeks 8 weeks |
Significantly higher improvement with combination, but significant discomfort |
| Kumari, 2010 | NR, comparative |
N = 40 Indian women 2 groups (1, 2) |
1 = 20 12% GA or 0.1% RA cream, at night, previously, for 2 weeks + 20–35% GA 2 = 20 10–20% TCA every 15 days MASI Photographs 12 weeks No follow-up |
Similar efficacy MASI reduction: 79% for GA 73% for TCA No difference Fewer side effects for GA compared to TCA |
| lknur, 2010 | Single-blind, randomized, right-left of the face | N = 31 Turkish patients (24 completed) | 12 serial peel GA X AFA. 2 weeks interval. mMASI 6 months. No follow-up |
Significant decrease of mMASI No difference AFA—less irritating |
| Kodali, 2010 | RCT, split-face, prospective | N = 20 Latin American women (18 completed) |
20%—30% SA every 2 weeks 4 peels on one side of the face X 4% HQ cream to both sides, twice daily Narrowband reflectance spectrophotometry No follow-up |
Significant reduction in pigment intensity, no difference between sides 20% to 30% SA peels are were not effective for melasma |
| Faghihi, 2011 | RCT, DB, split-face | N = 63 patients (male and female) |
70% GA X 1% RA peel, 4 sessions 2 weeks interval, MASI 8 weeks No follow-up |
No difference RA 1% more tolerable |
| Puri, 2012 | NR, comparative |
N = 30 Indian patients M:F = 1:6.5 2 groups (A, B) |
A: 15% TCA B: 35% GA peel every 3 weeks MASI 15 weeks No follow-up |
Same efficacy, TCA: more side effects |
| Sobhi, 2012 | SB, right-left comparison |
N = 14 Egyptian women, phototypes IV-V |
Right side: 70% GA 6 sessions X Left side: nanosome vitamin C applied by iontophoresis MASI Photographs evaluated by two single-blinded physicians No follow-up |
Both sides improved Nanosome vitamin C was better Few and transient side effects |
| Mahajan, 2015 | RCT, DB |
N = 40 Indian patients (38 completed) 2 groups (1, 2) |
1 = 20 just TC 2 = 20 serial GA peel 2-weeks interval + 20% AA cream MASI, digital photography, VAS 12 weeks 12 weeks |
Significant reduction in MASI with no difference between groups |
| Sarkar, 2016 | R, comparative |
N = 90 Indian patients 3 groups (A, B, C) |
Before peels: 4% HQ + 0.05% RA cream, 4 weeks A = 35% GA B = SM 20% AS / 10% mandelic acid C = phytic acid peels. every 14 days MASI Photography 12 weeks Follow-up = 20 weeks |
MASI reduction: A = 62.36% B = 60.98% C = 44.71% 35% GA and SM were equally efficacious and safe and more effective than phytic acid peels SM was better tolerated |
| Choudhary, 2017 | R, parallel control, comparative, SB |
N = 36 female patients 12, melasma 12, photomelanosis 12, post acne pigmentation, 3 groups (1, 2, 3) (4 patients each) |
1. 20% SA every 2 weeks 2. 50% GA every 3 weeks 3. 15% TCA every 3 weeks 6 sessions Investigator’s Global Improvement Assessment, Patient’s satisfaction grading scale based in photographs No follow-up |
Improvement (all together) marked = 27.77% (10/36) moderate = 36.11% (13/36) mild = 36.11% (13/36) No difference All well tolerated |
| Abdel-Meguid, 2017 | RCT, IB, split face, right-left, assessor-blinded | N = 24 female Egyptian patients, phototypes IV-V |
20%-25% TC + JS X 20%-25% TCA alone 6 sessions 2 weeks interval MASI photographs No follow-up |
Significant decrease in MASI for both regimens; JS + TCA: more effective |
RCT randomized controlled trial, R randomized, NR non-randomized, SB single-blind, DB double-blind, IB investigator-blind, TC triple combination (2%, HQ, 0.05%, RA, 0.01%); Fluocinolone), AA azelaic acid, mMASI modified Melasma Area and Severity Index, VAS Visual Analog Scale, PIH post-inflammatory hyperpigmentation, MKF modified Kligman formula, M:F Male:Female
Microneedling
Microneedling was first developed to treat acne scars and skin laxity. Nevertheless, an improvement in post-inflammatory hyperpigmentation and melasma was perceived in these patients [110]. When performed with a roller, providing a moderate skin injury (diffuse erythema and some bleeding spots) promoted an early improvement of melasma [111]. This technique, associated with TC and broad-spectrum sunscreen for 8 weeks (Fig. 1), better promoted a superior mMASI reduction (48%) than TC alone and proved to prevent further relapsing [81, 112].
Fig. 1.
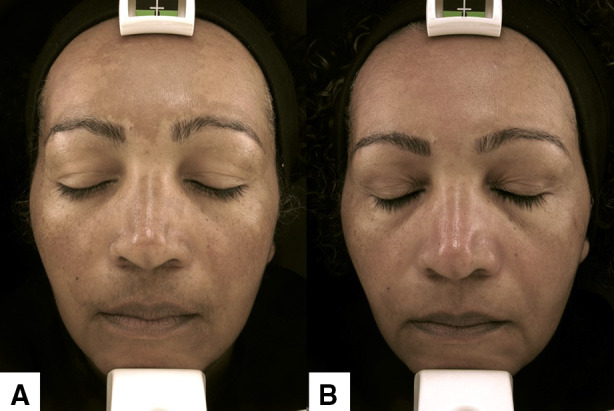
Facial melasma in a 48-year-old type V Fitzpatrick woman treated with tinted sunscreen, triple combination at bedtime, oral tranexamic acid 250 mg every 12 h and two microneedling sessions with a 30-day interval between sessions. A: before and B: after 2 months of treatment
The mechanism of microneedling action is not yet entirely known. After the procedure, an early keratinocyte proliferation occurs, increasing melanin transcutaneous elimination. In addition, microneedling improves patterns of skin photoaging, such as solar elastosis and pendulum melanocytes, while restructuring damaged basal membranes [113]. Microneedling is also a safe procedure to perform on skins of color [106].
Many studies have cited microneedling to increase topical bleaching agent penetration. However, the best endpoint for penetration is not unanimous. Furthermore, the bleaching effect of the product depends on the skin penetration and frequency of use. Therefore, the punctual drug delivery of a bleacher on a biweekly or monthly basis of microneedling was not beneficial. On the contrary, most of these agents were not developed for intradermic use. Thus, their safety profile is unknown. In addition, the lateral and deep penetration of these substances after microneedling occurs erratically, making the adverse effects of a possible systemic absorption unpredictable [114].
Radiofrequency microneedle was reported as an effective treatment for refractory melasma, although no large randomized controlled studies have been performed [115–117].
Intradermotherapy and Platelet-Rich Plasma
A significant challenge in treating melasma is the low permeability of water-soluble substances through the hydrophobic layer of the stratum corneum. With intradermotherapy, medications are delivered directly into the dermis, resulting in a higher drug concentration in the target area with smaller volumes. Consequently, the effect is greater and longer-lasting, with fewer adverse events. For intradermal injection, substances must be solubilized in sterile water, and mixtures should be avoided, with different substances injected via separate syringes.
TXA is the most studied substance in intradermotherapy for melasma. Injections are performed by a 28-gauge to 30-gauge syringe at 1 mm depth and 1 cm distance between each point. In clinical trials, 3–12 sessions were performed with an interval of 1–6 weeks between them. There was a reduction in the severity score, which varied between 33.3% and 81.2%. Different concentrations of TXA were used (4–100 mg/cc), but higher concentrations did not appear to improve the skin lightening. Notably, lower concentrations relapsed more, while higher concentrations were more painful. Intradermotherapy with TXA appears to lighten the skin more slowly but has similar efficacy to oral treatment, with a better safety profile [118]. Although intradermotherapy is likely to promote melasma improvement, controlled studies are scarce despite the wide use of vitamin C and glutathione products.
Platelet-rich plasma (PRP) seems to be another promising technique for treating melasma, although only a few controlled studies have compared PRP with standard therapies. No consensus exists regarding a standardized method of PRP preparation or the concentration of platelets. Platelets contain platelet-growth factor, transforming-growth factor (TGF) β1 and β2, and epidermal growth factor. These substances act on pigment metabolism and inflammation and can repair the altered dermal structures of melasma.
In a meta-analysis of PRP for melasma involving 395 adults in ten clinical trials, there was a significant reduction in the mMASI score after the procedure [119]. In addition to intradermal injection, PRP can be used after microneedling, with similar results between the two techniques. The number of PRP sessions ranged from three to six, held monthly or semi-monthly. Minimal side effects were seen, such as temporary erythema, edema, and pain at the injection site.
Laser and Light Technologies
Multiple clinical trials have explored the use of laser and light technologies to treat melasma. However, due to the heterogeneity of the study design (clinimetric parameters used as outcomes, the number of sessions, treatment intervals, and duration of follow-up), the diversity of device settings, and melasma characteristics, comparing these results is complicated [120]. In clinical practice, the laser that targets melanin most used for treating melasma is the Q-switched Nd-YAG in toning mode. The Q-switching technique produces pulses with an extremely high peak of energy delivered in a short time (nanoseconds). The result is destruction of melanin by a photoacoustic effect [121, 122]. The toning mode, in turn, uses low fluence (up to 3j/cm2) and larger spot size in sessions with short intervals (1–6 weeks). Hence, there is no destruction of melanocytes or keratinocytes in the epidermis but rather the intracellular melanosomes (subcellular selective photothermolysis), leading to less inflammation and consequently less post-inflammatory hyperchromia and hypochromia in confetti. However, even with this safer technique, there are reports of dyschromia after procedures with short intervals [123].
Recently, the industry has developed lasers whose pulse duration is picoseconds at different wavelengths: 532, 755, and 1064 nm. The energy, delivered in an even shorter time, reduces the photothermal effect and increases the photoacoustic effect in the treated tissue. However, the superiority of picosecond lasers compared with nanosecond lasers in the treatment of melasma may not be well established [124, 125].
Besides pigment, lasers that target water are also used for treating melasma. Ablative fractional lasers (CO2 and Er:YAG) vaporize the keratinocytes with more melanin and the hyperfunctioning melanocytes from melasma. Moreover, the healing of the skin around the coagulation columns improves the photoaged environment of the upper dermis of melasma, possibly reducing the paracrine stimulus to the melanocytes.
However, ablative lasers have a high thermal effect, resulting in dyschromia. Thus, short pulses with low energy density are recommended to reduce this complication [126]. Nonablative lasers, in turn, produce columns of coagulative damage within the dermis, keeping the stratum corneum intact, which generates less inflammation. One nonablative laser, the 1927 nm thulium device, appears to be promising for treating melasma because its penetration is around the dermal–epidermal junction [127].
Hypervascularization is an epiphenomenon of the ionizing radiation damage in melasma while participating in the maintenance of hyperpigmentation. Hence, a pulsed dye laser (585 nm) can be used in melasma, especially when telangiectasias are visualized. When treating the vessels, the pulsed dye laser decreases the stimulus to melanocytes and subsequently the recurrence of the spots. Due to the risk of post-inflammatory hyperchromia, it should not be used in higher (≥ IV) phototypes [128].
Dermabrasion
Superficial mechanical abrasion of the skin using a micromotor and a rotating diamond probe has been proposed to cure melasma. Indeed, gentle dermabrasion restores the whole epidermis and upper dermis. Although transient post-inflammatory hyperpigmentation is expected, long-term residual hypochromia is verified after dermabrasion for acne scars. For example, a long-term follow-up of 410 Thai women with melasma treated with dermabrasion evidenced a 95% sustained effect for 5 years and a low rate of adverse effects. However, these results were never repeated through a controlled trial [129]. Nevertheless, microdermabrasion with aluminum oxide has no direct role in melasma treatment since it only affects the stratum corneum but may facilitate the permeation of products [130].
Conclusions
The treatment of melasma is challenging, as the spots are not cleared completely in most patients and recurrences are frequent. The first-line treatment is rigorous daily sun protection and the association of tinted sunscreen with topical bleaching agents, which may have a different action on melanogenesis.
In severe and/or resistant cases, oral drugs should be prescribed. Tranexamic acid has the best evidence for treating melasma, however it is a thrombophilic drug and thus should be prescribed in select patients. There is also accumulating evidence for the use of pycnogenol as a systemic adjuvant in the treatment of melasma.
Procedures also are helpful to treat melasma. Microneedling is a low-cost technique with few adverse effects that complements topical treatment. Chemical peels act mainly via epidermal renewal. Lasers and light-based technologies should be used with maximum care as heat induces post-inflammatory melanogenesis.
The combination of topical bleaching agents with oral medications and procedures can provide faster results, although strategies for the prevention of further relapses are poorly studied.
Future Research
Intense research is being carried out on new and more effective melasma treatments and the combination of different mechanisms of action of the available drugs. Strategies that reduce local and systemic oxidative stress, stabilize upper dermis mast cells, decrease melanogenesis without melanocyte toxicity, remove epidermal melanin without inflammatory stimulus, revert senescence, and induce autophagy are considered promising. Thus, developing effective treatment and preventive strategies in melasma is a work in progress that depends on a better understanding of its pathogenesis. Meanwhile, sun protection associated with treatments that reduce melanogenesis, increase epithelial melanin turnover, and repair the upper dermis is the mainstay of therapeutic strategies.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All named authors contributed equally to this manuscript.
Disclosures
Daniel P. Cassiano, Ana Cláudia C. Espósito, Carolina N. da Silva, Paula B. Lima, Joana A. F. Dias, Karime Hassun, Luciane D. B. Miot, Hélio A. Mio, and Ediléia Bagatin have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The patient in Fig. 1 gave informed consent for the publication of the image.
Contributor Information
Daniel P. Cassiano, Email: danielpcassiano@uol.com.br
Ana Cláudia C. Espósito, Email: anaclaudiaesposito@gmail.com
Carolina N. da Silva, Email: carol_nunhez@hotmail.com
Paula B. Lima, Email: paulabassolima@hotmail.com
Karime Hassun, Email: karime@uniderma.com.br.
Luciane D. B. Miot, Email: luciane.miot@unesp.br
Hélio A. Miot, Email: heliomiot@gmail.com
Ediléia Bagatin, Email: edileia_bagatin@yahoo.com.br.
References
- 1.Esposito MCC, Esposito ACC, Jorge MFS, D'Elia MPB, Miot HA. Depression, anxiety, and self-esteem in women with facial melasma: an Internet-based survey in Brazil. Int J Dermatol. 2021;60(9):e346–e347. doi: 10.1111/ijd.15490. [DOI] [PubMed] [Google Scholar]
- 2.Pollo CF, Miot LDB, Meneguin S, Miot HA. Factors associated with quality of life in facial melasma: a cross-sectional study. Int J Cosmet Sci. 2018;40(3):313–316. doi: 10.1111/ics.12464. [DOI] [PubMed] [Google Scholar]
- 3.Maranzatto CF, Miot HA, Miot LD, Meneguin S. Psychometrican analysis and dimensional structure of the Brazilian version of melasma quality of life scale (MELASQoL-BP) An Bras Dermatol. 2016;91(4):422–428. doi: 10.1590/abd1806-4841.20165014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritter CG, Fiss DV, Borges da Costa JA, de Carvalho RR, Bauermann G, Cestari TF. Extra-facial melasma: clinical, histopathological, and immunohistochemical case-control study. J Eur Acad Dermatol Venereol. 2013;27(9):1088–94. [DOI] [PubMed]
- 5.Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782. doi: 10.1590/abd1806-4841.20143063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinot C, Cheffai S, Latreille J, Dhaoui MA, Youssef S, Jaber K, et al. Aggravating factors for melasma: a prospective study in 197 Tunisian patients. J Eur Acad Dermatol Venereol. 2010;24(9):1060–1069. doi: 10.1111/j.1468-3083.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- 7.Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27(2):151–156. doi: 10.1111/j.1468-3083.2011.04430.x. [DOI] [PubMed] [Google Scholar]
- 8.Alcantara GP, Esposito ACC, Olivatti TOF, Yoshida MM, Miot HA. Evaluation of ex vivo melanogenic response to UVB, UVA, and visible light in facial melasma and unaffected adjacent skin. An Bras Dermatol. 2020;95(6):684–690. doi: 10.1016/j.abd.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazeto I, Esposito ACC, Cassiano DP, Miot HA. Sun exposure (UVB, UVA, and blue-violet visible light) in ordinary daily situations. Int J Dermatol. 2021. [DOI] [PubMed]
- 10.Schalka S, dos Reis VM, Cuce LC. The influence of the amount of sunscreen applied and its sun protection factor (SPF): evaluation of two sunscreens including the same ingredients at different concentrations. Photodermatol Photoimmunol Photomed. 2009;25(4):175–180. doi: 10.1111/j.1600-0781.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 11.Nii D, Esposito AC, Peres G, Schmitt JV, Miot H. Tinted sunscreens lead to a smaller amount of the product applied on the face. Int J Dermatol. 2020;59(12):e438–e439. doi: 10.1111/ijd.15151. [DOI] [PubMed] [Google Scholar]
- 12.Henderson SI, King KL, Karipidis KK, Tinker RA, Green AC. Effectiveness, compliance and application of sunscreen for solar ultraviolet radiation protection in Australia. Public Health Res Pract. 2022;32(1). [DOI] [PubMed]
- 13.Tsai J, Chien AL. Photoprotection for Skin of Color. Am J Clin Dermatol. 2022;23(2):195–205. doi: 10.1007/s40257-021-00670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passeron T, Lim HW, Goh CL, Kang HY, Ly F, Morita A, et al. Photoprotection according to skin phototype and dermatoses: practical recommendations from an expert panel. J Eur Acad Dermatol Venereol. 2021;35(7):1460–1469. doi: 10.1111/jdv.17242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Addor FAS, Barcaui CB, Gomes EE, Lupi O, Marcon CR, Miot HA. Sunscreen lotions in the dermatological prescription: review of concepts and controversies. An Bras Dermatol. 2022;97(2):204–222. doi: 10.1016/j.abd.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan LL, Lim HW, Mohammad TF. Sunscreens and photoaging: a review of current literature. Am J Clin Dermatol. 2021;22(6):819–828. doi: 10.1007/s40257-021-00632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgado-Carrasco D, Piquero-Casals J, Granger C, Trullas C, Passeron T. Melasma: The need for tailored photoprotection to improve clinical outcomes. Photodermatol Photoimmunol Photomed. 2022:(ahead of print). [DOI] [PMC free article] [PubMed]
- 18.Lim HW, Kohli I, Granger C, Trullas C, Piquero-Casals J, Narda M, et al. Photoprotection of the skin from visible light-induced pigmentation: current testing methods and proposed harmonization. J Invest Dermatol. 2021;141(11):2569–2576. doi: 10.1016/j.jid.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Dias JAF, Lima PB, Cassiano DP, Esposito ACC, Bagatin E, Miot LDB, et al. Oral ketotifen associated with famotidine for the treatment of facial melasma: a randomized, double-blind, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2022;36(2):e123–e125. doi: 10.1111/jdv.17692. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar R, Ghunawat S, Narang I, Verma S, Garg VK, Dua R. Role of broad-spectrum sunscreen alone in the improvement of melasma area severity index (MASI) and Melasma Quality of Life Index in melasma. J Cosmet Dermatol. 2019;18(4):1066–1073. doi: 10.1111/jocd.12911. [DOI] [PubMed] [Google Scholar]
- 21.Boukari F, Jourdan E, Fontas E, Montaudie H, Castela E, Lacour JP, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72(1):189–90 e1. [DOI] [PubMed]
- 22.Lakhdar H, Zouhair K, Khadir K, Essari A, Richard A, Seite S, et al. Evaluation of the effectiveness of a broad-spectrum sunscreen in the prevention of chloasma in pregnant women. J Eur Acad Dermatol Venereol. 2007;21(6):738–742. doi: 10.1111/j.1468-3083.2007.02185.x. [DOI] [PubMed] [Google Scholar]
- 23.Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, Fuentes-Ahumada C, Torres-Alvarez B. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30(1):35–42. doi: 10.1111/phpp.12086. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar R, Puri P, Jain RK, Singh A, Desai A. Melasma in men: a clinical, aetiological and histological study. J Eur Acad Dermatol Venereol. 2010;24(7):768–772. doi: 10.1111/j.1468-3083.2009.03524.x. [DOI] [PubMed] [Google Scholar]
- 25.Kar SK. Melasma: A rare adverse effect of clomipramine. Indian J Pharmacol. 2016;48(4):453–454. doi: 10.4103/0253-7613.186203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locci-Molina N, Wang A, Kroumpouzos G. Melasma improving spontaneously upon switching from a combined oral contraceptive to a hormone-releasing intrauterine device: a report of four cases. Acta Derm Venereol. 2015;95(5):624–625. doi: 10.2340/00015555-2013. [DOI] [PubMed] [Google Scholar]
- 27.Prabha N, Mahajan VK, Mehta KS, Chauhan PS, Gupta M. Cosmetic contact sensitivity in patients with melasma: results of a pilot study. Dermatol Res Pract. 2014;2014:316219. doi: 10.1155/2014/316219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmo NF, Ramos GB, Salomao H, Werneck RI, Mira MT, Miot LDB, et al. Complex segregation analysis of facial melasma in Brazil: evidence for a genetic susceptibility with a dominant pattern of segregation. Arch Dermatol Res. 2018;310(10):827–831. doi: 10.1007/s00403-018-1861-5. [DOI] [PubMed] [Google Scholar]
- 29.Grimes PE. Melasma Etiologic and therapeutic considerations. Arch Dermatol. 1995;131(12):1453–1457. doi: 10.1001/archderm.1995.01690240119022. [DOI] [PubMed] [Google Scholar]
- 30.Lima PB, Dias JAF, Cassiano DP, Esposito ACC, Miot LDB, Bagatin E, et al. Efficacy and safety of topical isobutylamido thiazolyl resorcinol (Thiamidol) vs. 4% hydroquinone cream for facial melasma: an evaluator-blinded, randomized controlled trial. J Eur Acad Dermatol Venereol. 2021;35(9):1881–7. [DOI] [PubMed]
- 31.Lima PB, Dias JAF, Cassiano D, Esposito ACC, Bagatin E, Miot LDB, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59(12):1531–1536. doi: 10.1111/ijd.15146. [DOI] [PubMed] [Google Scholar]
- 32.Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111(1):40–48. doi: 10.1001/archderm.1975.01630130042004. [DOI] [PubMed] [Google Scholar]
- 33.Cestari T, Arellano I, Hexsel D, Ortonne JP. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23(7):760–772. doi: 10.1111/j.1468-3083.2009.03251.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira Cestari T, Hassun K, Sittart A, de Lourdes VM. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6(1):36–39. doi: 10.1111/j.1473-2165.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad Nasrollahi S, Sabet Nematzadeh M, Samadi A, Ayatollahi A, Yadangi S, Abels C, et al. Evaluation of the safety and efficacy of a triple combination cream (hydroquinone, tretinoin, and fluocinolone) for treatment of melasma in Middle Eastern skin. Clin Cosmet Investig Dermatol. 2019;12:437–444. doi: 10.2147/CCID.S202285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennitz A, Kinberger M, Avila Valle G, Passeron T, Nast A, Werner RN. Self-applied topical interventions for melasma: a systematic review and meta-analysis of data from randomized, investigator-blinded clinical trials. Br J Dermatol. 2022:(ahead of print). [DOI] [PubMed]
- 37.Lima PB, Dias JAF, Esposito ACC, Miot LDB, Miot HA. French maritime pine bark extract (pycnogenol) in association with triple combination cream for the treatment of facial melasma in women: a double-blind, randomized, placebo-controlled trial. J Eur Acad Dermatol Venereol. 2021;35(2):502–508. doi: 10.1111/jdv.16896. [DOI] [PubMed] [Google Scholar]
- 38.Levitt J. The safety of hydroquinone: a dermatologist's response to the 2006 Federal Register. J Am Acad Dermatol. 2007;57(5):854–872. doi: 10.1016/j.jaad.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Bhawan J, Grimes P, Pandya AG, Keady M, Byers HR, Guevara IL, et al. A histological examination for skin atrophy after 6 months of treatment with fluocinolone acetonide 0.01%, hydroquinone 4%, and tretinoin 0.05% cream. Am J Dermatopathol. 2009;31(8):794–8. [DOI] [PubMed]
- 40.O'Donoghue JL, Beevers C, Buard A. Hvdroquinone: assessment of genotoxic potential in the in vivo alkaline comet assay. Toxicol Rep. 2021;8:206–214. doi: 10.1016/j.toxrep.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farshi S. Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J Cosmet Dermatol. 2011;10(4):282–287. doi: 10.1111/j.1473-2165.2011.00580.x. [DOI] [PubMed] [Google Scholar]
- 42.Gehring W. Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol. 2004;3(2):88–93. doi: 10.1111/j.1473-2130.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J Investig Dermatol Symp Proc. 2008;13(1):20–24. doi: 10.1038/jidsymp.2008.8. [DOI] [PubMed] [Google Scholar]
- 44.Kimball AB, Kaczvinsky JR, Li J, Robinson LR, Matts PJ, Berge CA, et al. Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: results of a randomized, double-blind, vehicle-controlled trial. Br J Dermatol. 2010;162(2):435–441. doi: 10.1111/j.1365-2133.2009.09477.x. [DOI] [PubMed] [Google Scholar]
- 45.Navarrete-Solis J, Castanedo-Cazares JP, Torres-Alvarez B, Oros-Ovalle C, Fuentes-Ahumada C, Gonzalez FJ, et al. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol Res Pract. 2011;2011:379173. doi: 10.1155/2011/379173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farshi S, Mansouri P, Kasraee B. Efficacy of cysteamine cream in the treatment of epidermal melasma, evaluating by Dermacatch as a new measurement method: a randomized double blind placebo controlled study. J Dermatolog Treat. 2018;29(2):182–189. doi: 10.1080/09546634.2017.1351608. [DOI] [PubMed] [Google Scholar]
- 47.Mansouri P, Farshi S, Hashemi Z, Kasraee B. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173(1):209–217. doi: 10.1111/bjd.13424. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen J, Remyn L, Chung IY, Honigman A, Gourani-Tehrani S, Wutami I, et al. Evaluation of the efficacy of cysteamine cream compared to hydroquinone in the treatment of melasma: a randomised, double-blinded trial. Australas J Dermatol. 2021;62(1):e41–e46. doi: 10.1111/ajd.13432. [DOI] [PubMed] [Google Scholar]
- 49.Vachiramon V, Kositkuljorn C, Leerunyakul K, Chanprapaph K. Isobutylamido thiazolyl resorcinol for prevention of UVB-induced hyperpigmentation. J Cosmet Dermatol. 2021;20(3):987–992. doi: 10.1111/jocd.13615. [DOI] [PubMed] [Google Scholar]
- 50.Cohen PR. Approach to skin lightening in patients with melasma. J Drugs Dermatol. 2018;17(9):1018. [PubMed] [Google Scholar]
- 51.Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int J Dermatol. 2004;43(8):604–7. [DOI] [PubMed]
- 52.Zachary CM, Wang JV, Saedi N. Kojic acid for melasma: popular ingredient in skincare products. Skinmed. 2020;18(5):271–273. [PubMed] [Google Scholar]
- 53.Boo YC. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants (Basel). 2021;10(7):1129. doi: 10.3390/antiox10071129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergfeld W, Tung R, Vidimos A, Vellanki L, Remzi B, Stanton-Hicks U. Improving the cosmetic appearance of photoaged skin with glycolic acid. J Am Acad Dermatol. 1997;36(6 Pt 1):1011–1013. doi: 10.1016/S0190-9622(97)80290-3. [DOI] [PubMed] [Google Scholar]
- 55.Draelos ZD, Yatskayer M, Bhushan P, Pillai S, Oresajo C. Evaluation of a kojic acid, emblica extract, and glycolic acid formulation compared with hydroquinone 4% for skin lightening. Cutis. 2010;86(3):153–158. [PubMed] [Google Scholar]
- 56.Sarkar R, Bhalla M, Kanwar AJ. A comparative study of 20% azelaic acid cream monotherapy versus a sequential therapy in the treatment of melasma in dark-skinned patients. Dermatology. 2002;205(3):249–254. doi: 10.1159/000065851. [DOI] [PubMed] [Google Scholar]
- 57.Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatology. 1994;188(2):170. doi: 10.1159/000247129. [DOI] [PubMed] [Google Scholar]
- 58.Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, Nakakes A. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14(3):150–4. [DOI] [PubMed]
- 59.El-Husseiny R, Rakha N, Sallam M. Efficacy and safety of tranexamic acid 5% cream vs hydroquinone 4% cream in treating melasma: a split-face comparative clinical, histopathological, and antera 3D camera study. Dermatol Ther. 2020;33(6):e14240. doi: 10.1111/dth.14240. [DOI] [PubMed] [Google Scholar]
- 60.Janney MS, Subramaniyan R, Dabas R, Lal S, Das NM, Godara SK. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12(1):63–67. doi: 10.4103/JCAS.JCAS_40_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahu PJ, Singh AL, Kulkarni S, Madke B, Saoji V, Jawade S. Study of oral tranexamic acid, topical tranexamic acid, and modified Kligman's regimen in treatment of melasma. J Cosmet Dermatol. 2020;19(6):1456–1462. doi: 10.1111/jocd.13430. [DOI] [PubMed] [Google Scholar]
- 62.Lehraiki A, Abbe P, Cerezo M, Rouaud F, Regazzetti C, Chignon-Sicard B, et al. Inhibition of melanogenesis by the antidiabetic metformin. J Invest Dermatol. 2014;134(10):2589–2597. doi: 10.1038/jid.2014.202. [DOI] [PubMed] [Google Scholar]
- 63.AboAlsoud ES, Eldahshan RM, AbouKhodair Mohammed H, Elsaie ML. Safety and efficacy of topical metformin 30% cream versus triple combination cream (Kligman's formula) in treating melasma: a randomized controlled study. J Cosmet Dermatol. 2022;21(6):2508–2515. doi: 10.1111/jocd.14953. [DOI] [PubMed] [Google Scholar]
- 64.Jia X, Qi B, Li Y, Yan H, Guo J, Mu Q. Commentary on "Metabolic Syndrome in Melasma: A case-control study". J Cosmet Dermatol. 2022. [DOI] [PubMed]
- 65.Kasraee B, Safaee Ardekani GH, Parhizgar A, Handjani F, Omrani GR, Samani M, et al. Safety of topical methimazole for the treatment of melasma. Transdermal absorption, the effect on thyroid function and cutaneous adverse effects. Skin Pharmacol Physiol. 2008;21(6):300–5. [DOI] [PubMed]
- 66.Gheisari M, Dadkhahfar S, Olamaei E, Moghimi HR, Niknejad N, Najar NN. The efficacy and safety of topical 5% methimazole vs 4% hydroquinone in the treatment of melasma: a randomized controlled trial. J Cosmet Dermatol. 2020;19(1):167–172. doi: 10.1111/jocd.12987. [DOI] [PubMed] [Google Scholar]
- 67.Juhasz MLW, Levin MK. The role of systemic treatments for skin lightening. J Cosmet Dermatol. 2018;17(6):1144–1157. doi: 10.1111/jocd.12747. [DOI] [PubMed] [Google Scholar]
- 68.Sarkar R, Devadasan S, Choubey V, Goswami B. Melatonin and oxidative stress in melasma - an unexplored territory; a prospective study. Int J Dermatol. 2020;59(5):572–575. doi: 10.1111/ijd.14827. [DOI] [PubMed] [Google Scholar]
- 69.Hamadi SA, Mahammed MM, Aljaf AN, Ali A. The role of topical and oral melatonin in management of melasma patients. J Assoc Arab Univ Bas Appl Sc. 2010;8:30–42. [Google Scholar]
- 70.Sampaio Xerfan EM, Andersen ML, Tomimori J, Tufik S, da Silva FA. Melasma and the possible interaction with sleep quality. J Clin Aesthet Dermatol. 2020;13(11):12. [PMC free article] [PubMed] [Google Scholar]
- 71.Grimes P, Bhawan J, Howell M, Desai S, Coryell E, Einziger M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21(2):141–145. doi: 10.36849/JDD.6596. [DOI] [PubMed] [Google Scholar]
- 72.Grimes PE, Bhawan J, Howell MD, Desai S, Coryell E, Nashawati R, et al. A novel proof-of-concept study assessing the lightening effects and safety of malassezin for treatment of facial hyperpigmentation. J Am Acad Dermatol. 2022:(ahead of print). [DOI] [PubMed]
- 73.Cohen PR. Melasma treatment: A novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor. Med Hypotheses. 2017;101:1–5. doi: 10.1016/j.mehy.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Cohen PR. Topical anti-estrogen therapy to treat melasma. J Clin Aesthet Dermatol. 2017;10(6):16. [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen PR. Melasma treatment with combined chemical peels and a novel topical agent containing an antiestrogen and a vascular endothelial growth factor inhibitor. Dermatol Surg. 2018;44(4):592–593. doi: 10.1097/DSS.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 76.Tan AWM, Sen P, Chua SH, Goh BK. Oral tranexamic acid lightens refractory melasma. Australas J Dermatol. 2017;58(3):e105–e108. doi: 10.1111/ajd.12474. [DOI] [PubMed] [Google Scholar]
- 77.Wohltmann W. JAAD Game Changers: Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2019;81(6):1458. doi: 10.1016/j.jaad.2019.06.1295. [DOI] [PubMed] [Google Scholar]
- 78.Del Rosario E, Florez-Pollack S, Zapata L, Jr, Hernandez K, Tovar-Garza A, Rodrigues M, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78(2):363–369. doi: 10.1016/j.jaad.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 79.Cohen PR. Systemic treatments for melasma: adjuvant therapy with a novel topical agent. Int J Dermatol. 2018;57(3):e20–e21. doi: 10.1111/ijd.13887. [DOI] [PubMed] [Google Scholar]
- 80.Cohen PR. Melasma treatment with oral tranexamic acid and a novel adjuvant topical therapy. Cutis. 2018;102(2):106. [PubMed] [Google Scholar]
- 81.Cassiano D, Esposito ACC, Hassun K, Bagatin E, Lima M, Lima EVA, et al. Efficacy and safety of microneedling and oral tranexamic acid in the treatment of facial melasma in women: an open, evaluator-blinded, randomized clinical trial. J Am Acad Dermatol. 2020;83(4):1176–1178. doi: 10.1016/j.jaad.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Zhu CY, Li Y, Sun QN, Takada A, Kawada A. Analysis of the effect of different doses of oral tranexamic acid on melasma: a multicentre prospective study. Eur J Dermatol. 2019;29(1):55–58. doi: 10.1684/ejd.2018.3494. [DOI] [PubMed] [Google Scholar]
- 83.Chowdhary B, Mahajan VK, Mehta KS, Chauhan PS, Sharma V, Sharma A, et al. Therapeutic efficacy and safety of oral tranexamic acid 250 mg once a day versus 500 mg twice a day: a comparative study. Arch Dermatol Res. 2021;313(2):109–117. doi: 10.1007/s00403-020-02078-x. [DOI] [PubMed] [Google Scholar]
- 84.Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75(2):385–392. doi: 10.1016/j.jaad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Bala HR, Lee S, Wong C, Pandya AG, Rodrigues M. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44(6):814–825. doi: 10.1097/DSS.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 86.Ni Z, Mu Y, Gulati O. Treatment of melasma with pycnogenol. Phytother Res. 2002;16(6):567–571. doi: 10.1002/ptr.1085. [DOI] [PubMed] [Google Scholar]
- 87.Pinto CAS, Delfes MFZ, Reis LM, Garbers LE, Torre DS, Passos PCVR. The use of pycnogenol in the treatment of melasma. Surg Cosm Dermatol. 2015;7(3):218–222. [Google Scholar]
- 88.Middelkamp-Hup MA, Pathak MA, Parrado C, Goukassian D, Rius-Diaz F, Mihm MC, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51(6):910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 89.Zattra E, Coleman C, Arad S, Helms E, Levine D, Bord E, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175(5):1952–1961. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed AM, Lopez I, Perese F, Vasquez R, Hynan LS, Chong B, et al. A randomized, double-blinded, placebo-controlled trial of oral Polypodium leucotomos extract as an adjunct to sunscreen in the treatment of melasma. JAMA Dermatol. 2013;149(8):981–983. doi: 10.1001/jamadermatol.2013.4294. [DOI] [PubMed] [Google Scholar]
- 91.Goh CL, Chuah SY, Tien S, Thng G, Vitale MA, Delgado-Rubin A. Double-blind, placebo-controlled trial to evaluate the effectiveness of polypodium leucotomos extract in the treatment of melasma in asian skin: a pilot study. J Clin Aesthet Dermatol. 2018;11(3):14–19. [PMC free article] [PubMed] [Google Scholar]
- 92.Stahl W, Sies H. beta-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96(5):1179S–S1184. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 93.Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: a randomized, double-blind, placebo-controlled study. J Dermatolog Treat. 2012;23(2):97–102. doi: 10.3109/09546631003801619. [DOI] [PubMed] [Google Scholar]
- 94.Avianggi HD, Indar R, Adriani D, Riyanto P, Muslimin M, Afriliana L, et al. The effectiveness of tomato extract on superoxide dismutase (SOD) and severity degree of patients with melasma. Ital J Dermatol Venerol. 2022;157(3):262–269. doi: 10.23736/S2784-8671.22.07152-3. [DOI] [PubMed] [Google Scholar]
- 95.Lee KC, Wambier CG, Soon SL, Sterling JB, Landau M, Rullan P, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81(2):313–324. doi: 10.1016/j.jaad.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 96.Azzam OA, Leheta TM, Nagui NA, Shaarawy E, Hay RM, Hilal RF. Different therapeutic modalities for treatment of melasma. J Cosmet Dermatol. 2009;8(4):275–281. doi: 10.1111/j.1473-2165.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- 97.Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb). 2017;7(3):305–318. doi: 10.1007/s13555-017-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I, Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3(7):32–43. [PMC free article] [PubMed] [Google Scholar]
- 99.Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5(4):247–253. doi: 10.4103/0974-2077.104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erbil H, Sezer E, Tastan B, Arca E, Kurumlu Z. Efficacy and safety of serial glycolic acid peels and a topical regimen in the treatment of recalcitrant melasma. J Dermatol. 2007;34(1):25–30. doi: 10.1111/j.1346-8138.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 101.Sharquie KE, Al-Tikreety MM, Al-Mashhadani SA. Lactic acid chemical peels as a new therapeutic modality in melasma in comparison to Jessner's solution chemical peels. Dermatol Surg. 2006;32(12):1429–1436. doi: 10.1111/j.1524-4725.2006.32352.x. [DOI] [PubMed] [Google Scholar]
- 102.Singh R, Goyal S, Ahmed QR, Gupta N, Singh S. Effect of 82% lactic acid in treatment of melasma. Int Sch Res Notices. 2014;2014:407142. doi: 10.1155/2014/407142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuce LC, Bertino MC, Scattone L, Birkenhauer MC. Tretinoin peeling. Dermatol Surg. 2001;27(1):12–14. [PubMed] [Google Scholar]
- 104.Khunger N, Sarkar R, Jain RK. Tretinoin peels versus glycolic acid peels in the treatment of Melasma in dark-skinned patients. Dermatol Surg. 2004;30(5):756–60; discussion 60. [DOI] [PubMed]
- 105.Raminelli ACP, Rodrigues-Oliveira AF, Yokota R, Sumita JM, Oliveira-Silva D, Wambier CG, et al. Cutaneous absorption of tretinoin in 0.05% cream and 5% chemical peel formulas. J Am Acad Dermatol. 2020;83(5):1483–5. [DOI] [PubMed]
- 106.Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013;14(5):359–376. doi: 10.1007/s40257-013-0038-4. [DOI] [PubMed] [Google Scholar]
- 107.Sarma N, Chakraborty S, Poojary SA, Rathi S, Kumaran S, Nirmal B, et al. Evidence-based review, grade of recommendation, and suggested treatment recommendations for melasma. Indian Dermatol Online J. 2017;8(6):406–442. doi: 10.4103/idoj.IDOJ_187_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y, Wu S, Wu H, Liang X, Guo D, Zhuo F. Comparison of the efficacy of melasma treatments: a network meta-analysis of randomized controlled trials. Front Med (Lausanne). 2021;8:713554. doi: 10.3389/fmed.2021.713554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Safoury OS, Zaki NM, El Nabarawy EA, Farag EA. A study comparing chemical peeling using modified Jessner's solution and 15% trichloroacetic Acid versus 15% trichloroacetic acid in the treatment of melasma. Indian J Dermatol. 2009;54(1):41–45. doi: 10.4103/0019-5154.48985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lima EA. Microneedling in facial recalcitrant melasma: report of a series of 22 cases. An Bras Dermatol. 2015;90(6):919–921. doi: 10.1590/abd1806-4841.20154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cassiano DP, Esposito ACC, Hassun KM, Lima EVA, Bagatin E, Miot HA. Early clinical and histological changes induced by microneedling in facial melasma: a pilot study. Indian J Dermatol Venereol Leprol. 2019;85(6):638–641. doi: 10.4103/ijdvl.IJDVL_44_19. [DOI] [PubMed] [Google Scholar]
- 112.Bailey AJM, Li HO, Tan MG, Cheng W, Dover JS. Microneedling as an adjuvant to topical therapies for melasma: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;86(4):797–810. doi: 10.1016/j.jaad.2021.03.116. [DOI] [PubMed] [Google Scholar]
- 113.Cassiano DP, Esposito ACC, Hassun KM, Lima M, Lima EVA, Miot LDB, et al. Histological changes in facial melasma after treatment with triple combination cream with or without oral tranexamic acid and/or microneedling, a randomised clinical trial. Indian J Dermatol Venereol Leprol. 2022:1–10. [DOI] [PubMed]
- 114.Sasaki GH. Micro-needling depth penetration, presence of pigment particles, and fluorescein-stained platelets: clinical usage for aesthetic concerns. Aesthet Surg J. 2017;37(1):71–83. doi: 10.1093/asj/sjw120. [DOI] [PubMed] [Google Scholar]
- 115.Gulfan MCB, Wanitphakdeedecha R, Wongdama S, Jantanapornchai N, Yan C, Rakchart S. Efficacy and safety of using noninsulated microneedle radiofrequency alone versus in combination with polynucleotides for the treatment of melasma: a pilot study. Dermatol Ther (Heidelb). 2022;12(6):1325–1336. doi: 10.1007/s13555-022-00728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park BJ, Jung YJ, Ro YS, Chang SE, Kim JE. Therapeutic effects of new pulsed-type microneedling radiofrequency for refractory facial pigmentary disorders. Dermatol Surg. 2022;48(3):327–333. doi: 10.1097/DSS.0000000000003367. [DOI] [PubMed] [Google Scholar]
- 117.Jung JW, Kim WO, Jung HR, Kim SA, Ryoo YW. A face-split study to evaluate the effects of microneedle radiofrequency with Q-switched Nd:YAG laser for the treatment of melasma. Ann Dermatol. 2019;31(2):133–138. doi: 10.5021/ad.2019.31.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khalili M, Amiri R, Iranmanesh B, Zartab H, Aflatoonian M. Safety and efficacy of mesotherapy in the treatment of melasma: a review article. J Cosmet Dermatol. 2022;21(1):118–129. doi: 10.1111/jocd.14644. [DOI] [PubMed] [Google Scholar]
- 119.Sarkar R, Gupta M. Platelet-rich plasma in melasma-a systematic review. Dermatol Surg. 2022;48(1):131–134. doi: 10.1097/DSS.0000000000003266. [DOI] [PubMed] [Google Scholar]
- 120.Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3(1):11–20. doi: 10.1016/j.ijwd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Micek I, Pawlaczyk M, Kroma A, Seraszek-Jaros A, Urbanska M, Gornowicz-Porowska J. Treatment of melasma with a low-fluence 1064 nm Q-switched Nd:YAG laser: laser toning in Caucasian women. Lasers Surg Med. 2022;54(3):366–373. doi: 10.1002/lsm.23474. [DOI] [PubMed] [Google Scholar]
- 122.Brown AS, Hussain M, Goldberg DJ. Treatment of melasma with low fluence, large spot size, 1064-nm Q-switched neodymium-doped yttrium aluminum garnet (Nd:YAG) laser for the treatment of melasma in Fitzpatrick skin types II–IV. J Cosmet Laser Ther. 2011;13(6):280–282. doi: 10.3109/14764172.2011.630084. [DOI] [PubMed] [Google Scholar]
- 123.Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10(7):40–42. [PMC free article] [PubMed] [Google Scholar]
- 124.Vachiramon V, Iamsumang W, Triyangkulsri K. Q-switched double frequency Nd:YAG 532-nm nanosecond laser vs. double frequency Nd:YAG 532-nm picosecond laser for the treatment of solar lentigines in Asians. Lasers Med Sci. 2018;33(9):1941–7. [DOI] [PubMed]
- 125.Lee MC, Lin YF, Hu S, Huang YL, Chang SL, Cheng CY, et al. A split-face study: comparison of picosecond alexandrite laser and Q-switched Nd:YAG laser in the treatment of melasma in Asians. Lasers Med Sci. 2018;33(8):1733–1738. doi: 10.1007/s10103-018-2529-2. [DOI] [PubMed] [Google Scholar]
- 126.Wanitphakdeedecha R, Manuskiatti W, Siriphukpong S, Chen TM. Treatment of melasma using variable square pulse Er:YAG laser resurfacing. Dermatol Surg. 2009;35(3):475–81; discussion 81–2. [DOI] [PubMed]
- 127.Wanitphakdeedecha R, Sy-Alvarado F, Patthamalai P, Techapichetvanich T, Eimpunth S, Manuskiatti W. The efficacy in treatment of facial melasma with thulium 1927-nm fractional laser-assisted topical tranexamic acid delivery: a split-face, double-blind, randomized controlled pilot study. Lasers Med Sci. 2020;35(9):2015–2021. doi: 10.1007/s10103-020-03045-8. [DOI] [PubMed] [Google Scholar]
- 128.Passeron T. Long-lasting effect of vascular targeted therapy of melasma. J Am Acad Dermatol. 2013;69(3):e141–e142. doi: 10.1016/j.jaad.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 129.Kunachak S, Leelaudomlipi P, Wongwaisayawan S. Dermabrasion: a curative treatment for melasma. Aesthetic Plast Surg. 2001;25(2):114–117. doi: 10.1007/s002660010107. [DOI] [PubMed] [Google Scholar]
- 130.Abdel-Motaleb AA, Bakr RM. Microdermabrasion assisted delivery of glycolic acid 70% peel for the treatment of melasma in dark-skinned patients. Dermatol Ther. 2021;34(4):e15025. doi: 10.1111/dth.15025. [DOI] [PubMed] [Google Scholar]


