Abstract
Using the human cDNA sequence corresponding to guanine deaminase, the Escherichia coli genome was scanned using the Basic Local Alignment Search Tool (BLAST), and a corresponding 439-residue open reading frame of unknown function was identified as having 36% identity to the human protein. The putative gene was amplified, subcloned into the pMAL-c2 vector, expressed, purified, and characterized enzymatically. The 50.2-kDa protein catalyzed the conversion of guanine to xanthine, having a Km of 15 μM with guanine and a kcat of 3.2 s−1. The bacterial enzyme shares a nine-residue heavy metal binding site with human guanine deaminase, PG[FL]VDTHIH, and was found to contain approximately 1 mol of zinc per mol of subunit of protein. The E. coli guanine deaminase locus is 3′ from an open reading frame which shows homology to a bacterial purine base permease.
Guanine deaminase (EC 3.5.4.3) is an aminohydrolase responsible for the conversion of guanine to xanthine and ammonia. This reaction is one of two, the other being guanylate reductase, which removes the guanine base from the pool of guanine-containing metabolites. As such, these enzymes may under certain circumstances play a role in the regulation of cellular GTP and the guanylate nucleotide pool. The levels of GMP reductase increase with increasing concentrations of guanine in cultures of Escherichia coli and Salmonella enterica serovar Typhimurium (3, 12), thereby allowing guanylate to serve as a substrate for the synthesis of adenine nucleotides. Conversely, the synthesis of the enzymes IMP dehydrogenase and GMP synthetase (guaA and guaB), which convert IMP, the terminal product of the de novo purine pathway, to GMP, are both repressed when wild-type cultures of E. coli are supplemented with guanine (9, 12). Guanine and hypoxanthine have subsequently been shown to be corepressors for purR (10, 14), which regulates transcription of the operons required for de novo purine synthesis and the synthesis of AMP and GMP from IMP (11, 16).
The human cDNA corresponding to guanine deaminase (GenBank AF095286) was recently cloned, sequenced, and demonstrated to be part of a family of amino- and amidohydrolases that share a heavy metal binding motif associated with zinc or manganese (15). In the present work, we have identified a putative E. coli gene corresponding to the human guanine deaminase cDNA using the Basic Local Alignment Search Tool (BLAST) (1). Expression and kinetic analyses have verified the identity of the corresponding bacterial gene to be a previously identified open reading frame of unassigned function.
Strategy for the identification of E. coli guanine deaminase.
BLAST analysis of the E. coli genome (accession no., U00096) for homology to the protein sequence corresponding to the human cDNA for guanine deaminase revealed a homologous open reading frame of 1,317 nucleotides. This functionally unassigned gene at map position 65.2 min encompasses nucleotides 3023787 to 3025106 of the E. coli genome. The putative gene encodes a 439-residue protein having a predicted molecular mass of 50,244 Da that is similar to the human gene product for guanine deaminase of 51,040 Da (15).
Amplification, expression, purification, and characterization of the putative E. coli guanine deaminase.
The putative E. coli guanine deaminase sequence was amplified by PCR using primers ED1 (5′GGGGAATTCATGATGTCAGGAGAACACA) and ED2 (5′TGGATGTTAAAGCTTTATTA) based on the E. coli sequence. PCR was performed with 5 min of denaturation at 95°C prior to the first cycle, followed by 1 min of annealing (52°C), 1 min of polymerization (72°C), and 1 min of denaturation (94°C) for 30 cycles using vent DNA polymerase (New England Biolabs) and DNA prepared from E. coli strain MG1655 (CGSC strain no. 6300, provided by M. Berlyn, the E. coli Genetic Stock Center, Yale University). The 1.3-kb PCR product was digested with EcoRI and HindIII and cloned into the multiple cloning site of pMAL-c2 (New England Biolabs) and subsequently transformed into DH5-α cells. The sequence of the amplified gene was identical with that of the E. coli genome. The expressed maltose fusion protein was purified on an amylose affinity column according to the supplier's instructions, cleaved with factor Xa, and passed a second time through the affinity column. The cleaved protein was then purified to homogeneity on a MonoQ (Pharmacia) column eluted at 1 ml/min by loading in 20 mM bis-TrisHCl–20 mM NaCl (pH 6.2) (buffer A) and washing for 15 min. The protein was then eluted by forming a linear gradient between buffer A and buffer B (buffer A with 600 mM NaCl). The expression and purification steps were monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. As a consequence of the site of subcloning into pMAL, the cleaved recombinant product contained an extra four residues at the N terminus, LSEF. The purified protein had an apparent molecular mass of 50 kDa, in agreement with the calculated mass.
Kinetic characterization of the expressed protein and heavy metal association.
Verification of the function of the expressed gene product was obtained by examination of its catalytic potential with guanine. Guanine deaminase activity was assayed spectrophotometrically by following the conversion of guanine to xanthine in a coupled reaction at 512 nm at 37°C as previously described (15). The reaction mixture consisted of 1 mM 2,4,6-tribromo-3-hydroxybenzoic acid, 0.1 mM 4-amino-antipyrene, 0.025 U of xanthine oxidase/ml, 0.00325 U of uricase/ml, and 0.002 U of peroxidase/ml, as adapted (8). Optimal activity was obtained with 3 mM MnCl2. Adenine deaminase activity was similarly assayed by following the conversion of adenine to hypoxanthine. Initial rates in kinetic experiments were analyzed by a weighted nonlinear least-squares curve fitting program (4). The purified protein catalyzed the conversion of guanine to xanthine having a Km of 15.4 ± 1.8 μM and a Vmax of 3,795 ± 135 nmol/min per mg of protein and a kcat of 3.18 ± 0.12 s−1. The Km is similar to that described for the mouse erythrocyte (23 μM) and human recombinant (10 μM) enzymes, respectively (15).
Inspection of the primary amino acid sequence of E. coli guanine deaminase revealed it to contain a nine-residue motif, PG[F]VDTHIH, that is also found in human and mouse guanine deaminase, PG[L]VDTHIH (15). This HisXHis-containing motif is found in other amino- and amidohydrolases that have been shown to be ligated to the heavy metal ion zinc. For example, Bacillus subtilis adenine deaminase shares the homologous zinc site (13). The zinc and manganese content of recombinant E. coli guanine deaminase was determined by graphite furnace atomic absorption (Galbraith Laboratories, Knoxville, Tenn.). These analyses revealed there to be approximately 1 atom of zinc (0.69 atom of zinc versus less than 0.1 atom of manganese) per monomer guanine deaminase. Exposure of bacterial guanine deaminase to the heavy metal chelator 1,10-phenanthroline, 7.5 mM at 37°C, resulted in a time-dependent decrease in activity of 85% in 90 min, consistent with chelation of the heavy metal atom. Removal of phenanthroline by ultrafiltration using the BioMax 10,000-Mr-cutoff filter (Millipore), followed by the addition of either Zn++ or Mn++, resulted in partial restoration of activity.
Comparison of human and E. coli guanine deaminase primary sequence and evidence for guanine deaminase in other microorganisms.
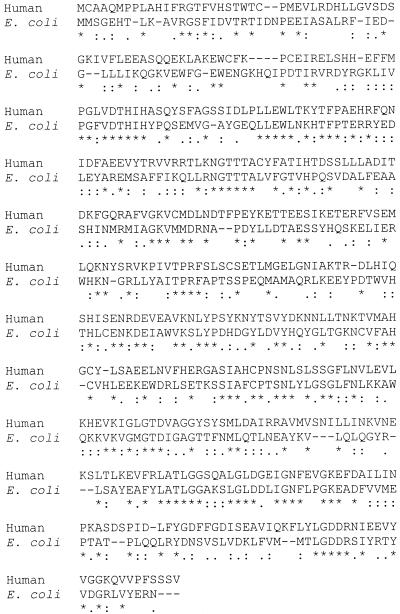
Comparison of the human and E. coli protein sequences using the CLUSTAL program (6) is shown in Fig. 1. The proteins are 36% identical and share several conserved regions, including the HisXHis sequence near the N-terminal region. The E. coli guanine deaminase protein sequence was used in a BLAST scan of the microbial genome databases. Several gene products showing significant alignment and identity with the E. coli sequence were identified in other organisms (Table 1) as putative loci for guanine deaminase.
FIG. 1.
E. coli and human guanine deaminase sequence alignment. Shown is a Clustal W alignment of sequences, with symbols defined as follows: ∗, identical or conserved residues; :, conserved substitutions; ., semiconserved substitutions.
TABLE 1.
Sequences producing significant alignment with E. coli guanine deaminasea
| Source/accession no. | Microbial organism | Identity | Positives | Gaps |
|---|---|---|---|---|
| PAGP | Pseudomonas aeruginosa | 244/422 (57) | 319/422 (74) | 2/422 |
| TIGR | Caulobacter crescentus | 224/425 (52) | 291/425 (67) | 1/425 |
| TIGR | Deinococcus radiodurans | 170/437 (38) | 247/437 (55) | 8/437 |
| GTC | Clostridium acetobutylicum | 140/436 (32) | 230/436 (52) | 13/436 |
| AE 000657 | Aquifex aeolicus | 107/372 (28) | 176/372 (46) | 15/372 |
| TIGR | Enterococcus faecalis | 86/351 (24) | 165/351 (46) | 13/351 |
| AE 000666 | Methanobacterium thermautotrophicum | 105/336 (31) | 169/336 (50) | 11/336 |
| L77117 | Methanococcus jannaschii | 99/352 (28) | 171/352 (48) | 16/352 |
| AE 000782 | Archaeoglobus fulgidus | 110/349 (31) | 156/349 (44) | 14/349 |
Sequences producing significant alignment with E. coli guanine deaminase were obtained using the TBLASTN search tool (1). Preliminary sequence data was obtained from The Institute for Genomic Research website at http://www.tigr.org, TIGR, which also contained sequence data from the Pseudomonas Genome Project (PAGP) and Genome Therapeutics Corporation. Percent identity and similarity as positives are shown in parentheses.
The E. coli guanine deaminase gene is located 3′ to a gene identified as a putative transport protein (Wisconsin E. coli K-12 database). There is evidence for purine base permeases (2, 7), and BLAST analysis shows that this upstream open reading frame shares significant alignment with a xanthine-specific purine permease in B. subtilis (5). Thus a purine base permease, possibly having specificity for guanine, and the gene encoding guanine deaminase are found in proximity within the E. coli genome. Further genetic and molecular analyses are now possible with regard to the regulation of purine base transport and guanine metabolism as a consequence of the present study.
Acknowledgments
This work was supported by the Medical Research Council of Canada, grant MT-6376.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson C E, Hornick D L, Gots J S. Genetic separation of purine transport from phosphoribosyltransferase activity in Salmonella typhimurium. J Gen Microbiol. 1980;121:357–364. doi: 10.1099/00221287-121-2-357. [DOI] [PubMed] [Google Scholar]
- 3.Benson C E, Gots J S. Regulation of GMP reductase in Salmonella typhimurium. Biochim Biophys Acta. 1975;403:47–57. doi: 10.1016/0005-2744(75)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.Brooks S P. A simple computer program with statistical tests for the analysis of enzyme kinetics. BioTechniques. 1992;13:906–911. [PubMed] [Google Scholar]
- 5.Christiansen L C, Schou S, Nygaard P, Saxild H H. Xanthine metabolism in Bacillus subtilis: characterization of the xpt-pbuX operon and evidence for purine- and nitrogen-controlled expression of genes involved in xanthine salvage and catabolism. J Bacteriol. 1997;179:2540–2550. doi: 10.1128/jb.179.8.2540-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins O, Thompson J, Gibson T, Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties with matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Housset P, Nagy M. Study of the role of purine phosphoribosyl-transferases in the uptake of adenine and guanine by Schizosaccharomyces pombe cells. Eur J Biochem. 1977;73:99–105. doi: 10.1111/j.1432-1033.1977.tb11295.x. [DOI] [PubMed] [Google Scholar]
- 8.Le Tissier P R, Peters J, Skidmore C D. Development of an assay method for purine catabolic enzymes in the mouse and its adaptation for use on an autoanalyzer. Anal Biochem. 1994;222:168–175. doi: 10.1006/abio.1994.1469. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R K, Drabble W T. Dual control of the gua operon of Escherichia coli K12 by adenine and guanine nucleotides. J Gen Microbiol. 1981;123:27–37. doi: 10.1099/00221287-123-1-27. [DOI] [PubMed] [Google Scholar]
- 10.Meng L M, Nygaard P. Identification of hypoxanthine and guanine as the corepressors for the purine regulon genes of Escherichia coli. Mol Microbiol. 1990;4:2187–2191. doi: 10.1111/j.1365-2958.1990.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 11.Meng L M, Kilstrup M, Nygaard P. Autoregulation of PurR repressor synthesis and involvement of purR in the regulation of purB, purC, purL, purMN, and guaBA expression in Escherichia coli. Eur J Biochem. 1990;187:373–379. doi: 10.1111/j.1432-1033.1990.tb15314.x. [DOI] [PubMed] [Google Scholar]
- 12.Nijkamp H J J, de Haan P G. Genetic and biochemical studies of the guanosine 5′-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967;145:31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- 13.Nygaard P, Duckert P, Saxild H H. Role of adenine deaminase in purine salvage and nitrogen metabolism and characterization of the ade gene in Bacillus subtilis. J Bacteriol. 1996;178:846–853. doi: 10.1128/jb.178.3.846-853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfes R J, Zalkin H. Purification of the Escherichia coli purine regulon repressor and identification of corepressors. J Bacteriol. 1990;172:5637–5642. doi: 10.1128/jb.172.10.5637-5642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan G, Bin J C, McKay D J, Snyder F F. Cloning and characterization of human guanine deaminase. Purification and partial amino acid sequence of the mouse protein. J Biol Chem. 1999;274:8175–8180. doi: 10.1074/jbc.274.12.8175. [DOI] [PubMed] [Google Scholar]
- 16.Zalkin H, Nygaard P. Biosynthesis of purine nucleotides. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reynikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 561–579. [Google Scholar]