Abstract
Background
Rotavirus is the leading cause of severe dehydrating gastroenteritis among children younger than 5 years in low-income and middle-income countries. Two vaccines—Rotavac and Rotasiil—are used in routine immunisation in India. The safety and immunogenicity of these vaccines administered in a mixed regimen is not documented. We therefore aimed to compare the safety and seroresponse of recipients of a mixed regimen versus a single regimen.
Methods
We did a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial at two sites in India. We recruited healthy infants aged 6–8 weeks. Infants with systemic disorders, weight-for-height Z scores of less than minus three SDs, or a history of persistent diarrhoea were excluded. Eligible infants were randomly allocated to six groups in equal numbers to receive either the single vaccine regimen (ie, Rotavac–Rotavac–Rotavac [group 1] or Rotasiil–Rotasiil–Rotasiil [group 2]) or the mixed vaccine regimen (ie, Rotavac–Rotasiil–Rotavac [group 3], Rotasiil–Rotavac–Rotasiil [group 4], Rotavac–Rotasiil–Rotasiil [group 5], or Rotasiil–Rotavac–Rotavac [group 6]). Randomisation was done using an online software by site in blocks of at least 12. The primary outcome was seroresponse to rotavirus vaccine, measured using rotavirus-specific serum IgA antibodies 4 weeks after the third dose. The seroresponse rates were compared between recipients of the four mixed vaccine regimens (consisting of various combinations of Rotavac and Rotasiil) with recipients of the single vaccine regimens (consisting of Rotavac or Rotasiil only for all three doses). The non-inferiority margin was set at 10%. Safety follow-ups were done for the duration of study participation. This trial was registered with the Clinical Trials Registry India, number CTRI/2018/08/015317.
Findings
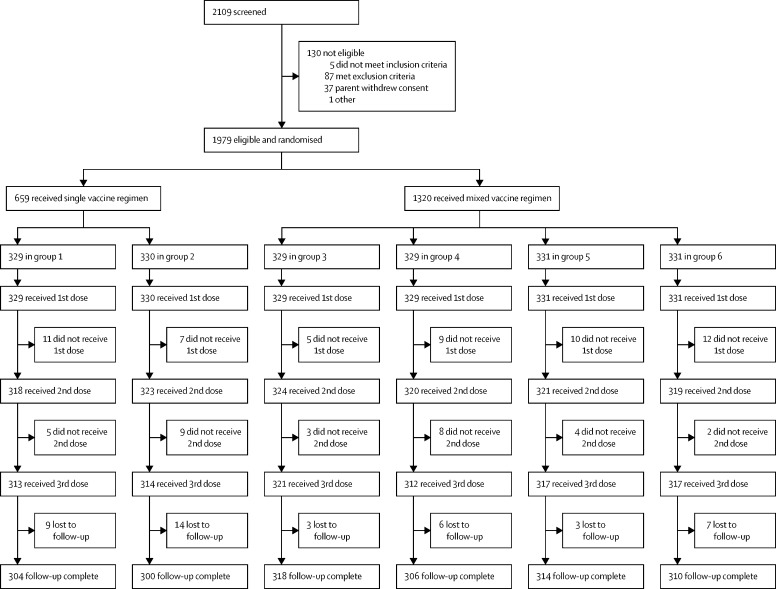
Between March 25, 2019, and Jan 15, 2020, a total of 1979 eligible infants were randomly assigned to receive a single vaccine regimen (n=659; 329 in group 1 and 330 in group 2) or a mixed vaccine regimen (n=1320; 329 each in groups 3 and 4, and 331 each in groups 5 and 6). All eligible participants received the first dose, 1925 (97·3%) of 1979 received the second dose, and 1894 (95·7%) received all three doses of vaccine. 1852 (93·6%) of 1979 participants completed the follow-up. The immunogenicity analysis consisted of 1839 infants (1238 [67·3%] in the mixed vaccine regimen and 601 [32·7%] in the single vaccine regimen; 13 samples were insufficient in quantity) who completed vaccination and provided post-vaccination sera. The seroresponse rate in the mixed vaccine regimen group (33·5% [95% CI 30·9–36·2]) was non-inferior compared with the single vaccine regimen group (29·6% [26·1–33·4]); the seroresponse rate difference was 3·9% (95% CI −0·7 to 8·3). The proportion of participants with any type of solicited adverse events was 90·9% (95% CI 88·4–93·0) in the single vaccine regimen group and 91·1% (89·5–92·6) in the mixed vaccine regimen group. No vaccine-related serious adverse events or intussusception were reported during the study.
Interpretation
Rotavac and Rotasiil can be safely used in an interchangeable manner for routine immunisation since the seroresponse was non-inferior in the mixed vaccine regimen compared with the single vaccine regimen. These results allow for flexibility in administering the vaccines, helping to overcome vaccine shortages and supply chain issues, and targeting migrant populations easily.
Funding
Ministry of Health and Family Welfare, Government of India.
Translation
For the Hindi translation of the abstract see Supplementary Materials section.
Introduction
Rotavirus is the leading cause of diarrhoeal disease in children younger than 5 years and is responsible for substantial morbidity and mortality in low-income and middle-income countries.1 Rotavirus gastroenteritis poses a major public health challenge because of dehydration due to diarrhoea and vomiting, delayed health seeking by the caregivers of affected children, and the paucity of primary health-care facilities in the vicinity of many community settings. In settings with no rotavirus vaccination, children are almost universally infected by 3 years of age. Children in low-income and middle-income countries are far more likely to be infected earlier in life than those in high-income countries and, because access to urgent care can be limited or unavailable in rural, impoverished settings, these children are more likely to develop severe disease and die.2 In India, the Indian Rotavirus Surveillance Network estimated that annually, of 11·37 million children with rotavirus gastroenteritis, 3·27 million (28·8%) visit outpatient facilities of which 872 000 (26·7%) are admitted to hospital, culminating in 10·37 billion Indian rupees in direct costs.3 In 2013, an estimated 47 100 rotavirus deaths occurred in India, which represented 22% of all rotavirus deaths that occurred globally.4 In children younger than 2 years, rotavirus, either alone or with other infective microorganisms, was implicated in 19% of cases with diarrhoea.5
Research in context.
Evidence before this study
A structured search of PubMed, MEDLINE, EMBASE, and Web of Science was conducted on Oct 4, 2021, without time restrictions, using terms such as “rotavirus”, “vaccines”, “interchangeability”, or “mixed regimen” and related terms, combined using appropriate operators to maximise outputs. Additionally, a search of the grey literature was conducted on Oct 4, 2021, without time restriction, particularly covering programme documents, presentations, and reports that were not peer reviewed, using the authors' knowledge of existing resources covering rotavirus vaccination strategies. No evidence exists for the interchangeable use of Rotavac and Rotasiil. Programmatic data are available from several countries, which allow interchangeable use of other rotavirus vaccine products. These documents were identified through a search of the grey literature. One formal clinical trial examined the use of two other rotavirus products in an interchangeable fashion. Evidence from programmatic implementation and this clinical trial agrees on the safety of such mixed regimens. However, formal clinical trials evaluating this hypothesis is sparse.
Added value of this study
The immune response evoked by the mixed regimens of rotavirus vaccines was seen to be non-inferior to that evoked by a single rotavirus vaccine product (either Rotavac or Rotasiil); the observed difference in seroresponse rates was 3·9% (95% CI −0·7 to 8·3). This finding provides evidence to support the safety and immunogenicity of interchangeably administered regimens of Rotavac and Rotasiil. This finding also supports the policy stance of the Indian Government, which has deemed these products to be interchangeable.
Implications of all the available evidence
The findings of this study, taken in context with the existing evidence, show that mixed regimens of Rotavac and Rotasiil can be delivered safely. This finding will enable the scaling-up of vaccination coverage not only in India but also in countries where these products are licensed for use in infants. By increasing the availability of vaccines, current gaps in supply chain issues can be overcome, thus providing a safe and immunogenic option to vaccinate more children across the world.
Currently, two locally manufactured rotavirus vaccines are available in India under the Universal Immunisation Programme: Rotavac (Bharat Biotech, India) and Rotasiil (Serum Institute India, India) which are proven safe, immunogenic, and efficacious.6, 7, 8, 9 Rotavac is a monovalent, liquid frozen vaccine containing live rotavirus 116E strain (G9 P[11]) prepared in Vero cells; the strain being a naturally occurring reassortant containing one bovine and ten human rotavirus genes. Rotasiil is a pentavalent vaccine, based on the bovine rotavirus UK-Compton strain as the backbone; VP7 encoding gene in this formulation generates five G-types (G1, G2, G3, G4, and G9). Both vaccines are live attenuated, delivered orally in a three-dose schedule at 6, 10, and 14 weeks of age along with other childhood vaccines including the oral polio vaccine.10 The risk of severe adverse events, such as intussusception, was low with both vaccines.11
According to the operational guidelines for the introduction of rotavirus vaccines in the Universal Immunisation Programme, Rotavac was approved for use in ten states and Rotasiil was approved for use in one state, with subsequent scale-up to other states as well. The approval of two different vaccine products raises the possibility of infants receiving a mixed regimen of the two vaccines, especially if the parents travel across Indian states or in case of supply chain issues and vaccine stockouts. The safety and immunogenicity of these antigenically different products administered in a mixed regimen is not documented. In this study, we therefore aimed to assess the safety profile and seroresponse in recipients of the mixed regimen of rotavirus vaccines Rotavac and Rotasiil compared with those receiving three doses of Rotavac or Rotasiil only.
Methods
Study design and participants
We did a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial at two sites (Pune in Maharashtra and Kolkata in West Bengal) in India, representing different demographic, sociocultural, and climatic conditions, and both having a high burden of diarrhoeal diseases and intense transmission of rotavirus infection. We recruited healthy infants at the age of 6–8 weeks. Participants were screened to ascertain their eligibility for enrolment in the trial. Infants with systemic disorders, weight-for-height Z scores of less than minus three SDs, or a history of persistent diarrhoea were excluded.
All participants' parents or carers provided video-recorded informed consent for their children to participate. The protocol was approved by the Institution Ethics Committee of the Indian Council of Medical Research (ICMR)–National Institute of Cholera and Enteric Diseases (A-1/2018-EC), KEM Hospital Research Centre (KEMHRC/MHS/ECI2071), ICMR–National Institute of Epidemiology ([NIE] NIE/IHEC/201805-3), and the Christian Medical College, Vellore (IRB-A12-25.07.2018).
Randomisation and masking
Healthy infants aged 6–8 weeks were randomly assigned by site in blocks of at least 12 to ensure the balance between treatment regimens. We randomly assigned participants in equal numbers to each of the six groups: three doses of Rotavac (group 1), three doses of Rotasiil (group 2), Rotavac–Rotasiil–Rotavac (group 3), Rotasiil–Rotavac–Rotasiil (group 4), Rotavac–Rotasiil–Rotasiil (group 5), or Rotasiil–Rotavac–Rotavac (group 6). The randomisation code comprised sequential numbers unique to everyone. This code was hidden with a scratchable opaque cover to ensure allocation concealment. Participants and investigators were not masked to the study interventions, as the vaccine products were already approved for use in the market. A randomisation list was prepared using an online software by an individual not involved in the trial. Before randomisation, the participant was assigned a screening identification number, which was used for randomisation; and after randomisation, a participant identification number ID was assigned to each participant. Thereafter, the participant identification number was used for all further visits.
Procedures
Demographic surveys were undertaken to identify mothers who had just given birth or were likely to give birth during the study period. Families were also contacted at primary health-care facilities and outpost clinics by field health workers or through visits to the household to explain the objective of the study. An information sheet with details of the study, duly approved by the Institutional Ethics Committee, was provided to the families. Study field staff discussed the information provided to the families. Following discussion, if the family was interested in participating, an appointment was set up at the local clinic where the study physician counselled the parent.
Eligible infants were randomly assigned to receive three oral doses of 0·5 mL of the rotavirus vaccines (ie, Rotavac or Rotasiil) at an interval of 3–5 weeks at 6, 10, and 14 weeks of age. Vaccine doses were administered with other routine vaccines as per the Universal Immunisation Programme. The single vaccine regimen consisted of Rotavac–Rotavac–Rotavac (group 1) or Rotasiil–Rotasiil–Rotasiil (group 2) whereas the mixed vaccine regimen consisted of Rotavac–Rotasiil–Rotavac (group 3), Rotasiil–Rotavac–Rotasiil (group 4), Rotavac–Rotasiil–Rotasiil (group 5), or Rotasiil–Rotavac–Rotavac (group 6).
The day of the child's first study vaccination was designated as study day 0. Within 48 h of randomisation, the child was administered the first dose. About 2 mL blood sample was collected at the first visit immediately before the first dose and 28 days after the third dose to check for immunogenicity. A thorough medical examination was done before and 30 min after vaccination. During each visit, a study case record form was used to record information about the vaccines received, date, and time. The total study duration for each participant was 4 months.
Mothers were provided with a post-immunisation diary card and a thermometer to record the health status of the child and any adverse events if any. The study staff trained the mothers to fill the post-immunisation diary card and correctly use the thermometer. Each mother and child enrolled in the study was connected to a member of study staff, and they were provided with the contact information of the investigators as well as the designated field staff to communicate any concerns. Complete records of vaccine procurement and inventory were maintained at each study site.
Participants who missed a scheduled visit were identified at the end of each day by the data management team, and the study staff contacted them immediately to promptly reschedule their visit. Community health workers made home visits to evaluate the health status of the participating children on days 2 and 6 after vaccination. They reviewed and collected the post-immunisation diary cards from the parents and submitted them to the study site for review and data entry. If any solicited reaction persisted on day 7 or beyond after each vaccination, surveillance was continued and recorded on the post-immunisation diary card until the symptom was resolved. At all times between first vaccination and until 28 days after the third vaccination, unsolicited adverse events or serious adverse events, or both, as well as concomitant medications were reported and recorded. Adverse events were managed in accordance with good medical practices by the clinical team that assessed and treated or referred the participant for medical care as appropriate. Parents were asked to promptly contact the staff in the event of any illness occurring in between vaccinations and during the 4-week follow-up after the third dose. All adverse events were monitored until resolution.
Interim contacts and visits to the clinic, occurring in between regularly scheduled follow-up visits, were done from time-to-time at the request of the parents or when deemed necessary by the community health workers in consultation with the study investigators. All interim contacts and visits were documented in study records. All parents who withdrew early from the study for any reason were encouraged to complete the end of study assessments as well as the scheduled Universal Immunisation Programme vaccines. Individuals whose participation in the study was terminated by the investigator remained eligible for care at the site until the 28th day following the scheduled third dose of vaccination.
Rotavirus-specific IgA was detected in plasma samples by an antibody-sandwich enzyme immunoassay, which has been documented previously.12, 13 Briefly, 96 well plates (Costar, Corning) coated with rabbit hyper-immune serum to rotavirus were incubated with purified cell culture lysates (WC3) or mock-infected MA104 cells. Serial dilutions of standard pool of human serum and test sera were added followed by biotinylated rabbit anti-human IgA (Jackson ImmunoResearch Laboratories, West Grove, PA), and absorbance was read at 492 nm. Background corrected optical density values from sample wells were compared with the standard curve and IgA titre was determined based on derived units of IgA arbitrarily assigned to the standard curve. Seropositivity was defined as an anti-rotavirus IgA concentration of 20 IU/mL or more.
Outcomes
The primary outcome measure was anti-rotavirus seroresponse rates in the study participants. Seroresponse in the study was defined as a four-fold increase of rotavirus-specific serum IgA concentration 4 weeks after the administration of the third dose of rotavirus vaccine compared with the baseline value for participants with a serum IgA concentration of 20 IU/mL or more at pre-vaccinated state. When serum IgA concentration was less than 20 IU/mL at pre-vaccinated state, the seropositive cutoff for rotavirus-specific IgA was considered at 20 IU/mL or more irrespective of baseline value.14
The secondary outcome measures were solicited post-vaccination reactions, vaccine-related serious adverse events, and unsolicited vaccine-related adverse events at predefined timepoints (ie, immediately after vaccination, one week after vaccination, and at any time thereafter as reported by caregivers). The rates of adverse events and vaccine-related severe adverse events in the four mixed vaccine regimen groups were compared with those observed in the two single vaccine regimen groups.
Statistical analysis
This study was planned as a non-inferiority trial in which the mixed vaccine regimen was compared against the single vaccine regimen in terms of their ability to elicit adequate immune response. We hypothesised that the seroresponse rate measured at 1 month after three doses of a mixed regimen (Rotavac and Rotasiil) is not inferior to the seroresponse rate 1 month after three doses of a single regimen (Rotavac or Rotasiil only) by more than −10%. Some studies have reported that the seroresponse to rotavirus vaccine varies depending on the population targeted. Seroresponse rates as high as 90% have been observed after the first dose of rotavirus vaccine in rigorously screened healthy participants, under ideal conditions such as withholding of breastfeeding and oral polio vaccine administration.7 Other published evidence has shown more modest seroresponse in more pragmatic settings that are closer to real-life conditions. Under such conditions, seroresponse rates of 40% and 73% have been observed.8, 9 All three seroresponse rates were taken into consideration for the computation of the sample size and establishing the analysis plan for the study. The study endorsed the sample size for the seroresponse of 40%, as it represented the highest estimate and was based on assumptions that were more likely to be mirrored by the conditions in which the study was done. Considering seroresponse rates in both single (ie, standard) and mixed vaccine regimens as 40%, with a non-inferiority margin of 10%, α error of 5%, and power of 80%, we required 297 participants in each group. Considering attrition rate as 10%, it was proposed that 330 participants be recruited in each group resulting in the total sample size to be 1980 participants.
The rates of rotavirus IgA seroresponse were compared between recipients of the four mixed vaccine regimen groups with those seen in participants who received a single vaccine regimen. We estimated the geometric mean titres of IgA antibodies against rotavirus along with 95% CIs in the single and mixed vaccine regimen groups.
The collected data was entered into an electronic data entry portal (RedCap) developed by ICMR–NIE, Chennai. The immunogenicity analysis was done using both the per-protocol and intention-to-treat approaches. Statistical analyses were done using IBM SPSS Statistics (versions 21). The study site Investigators were responsible for continuous close safety monitoring of all study participants, and for alerting the protocol team when concerns arose. An independent Data and Safety Monitoring Board, which consisted of nationally reputed experts in the field of vaccine and infectious diseases and biostatistics, was constituted to monitor the study. The laboratory assays were done at Christian Medical College, Vellore, and data management and analysis were done by ICMR–NIE. DiagnoSearch, Pune, was engaged as the contract research organisation for monitoring the trial.
This trial was registered with the Clinical Trials Registry India, number CTRI/2018/08/015317.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between March 25, 2019, and Jan 15, 2020, a total of 2109 participants were screened; of whom, 130 were deemed to be ineligible. Each site enrolled almost equal number of participants. The 1979 eligible participants were randomly assigned to receive a single vaccine regimen (n=659; 329 in group 1 and 330 in group 2) or a mixed vaccine regimen (n=1320; 329 in groups 3 and 4, and 331 in groups 5 and 6; figure ). Of the 659 participants who received a single vaccine regimen at baseline, 326 (49·5%) were male, mean age was 47·5 days (SD 4·0), mean weight was 4·2 kg (0·6), and mean height was 54·2 cm (2·4). Of the 1320 participants who received a mixed vaccine regimen at baseline, 670 (50·8%) were male, mean age was 47·2 days (SD 3·9), mean weight was 4·2 kg (0·6), and mean height was 54·2 cm (2·4; table 1 ). The baseline characteristics of the participants in each vaccine group are summarised in the appendix 2 (p 1).
Figure.
Trial profile
Group 1 consisted of Rotavac–Rotavac–Rotavac. Group 2 consisted of Rotasiil–Rotasiil–Rotasiil. Group 3 consisted of Rotavac–Rotasiil–Rotavac. Group 4 consisted of Rotasiil–Rotavac–Rotasiil. Group 5 consisted of Rotavac–Rotasiil–Rotasiil. Group 6 consisted of Rotasiil–Rotavac–Rotavac.
Table 1.
Baseline characteristics
| Single vaccine regimen (n=659) | Mixed vaccine regimen (n=1320) | ||
|---|---|---|---|
| Age (days) | 47·5 (4·0) | 47·2 (3·9) | |
| Sex | |||
| Male | 326 (49·5%) | 670 (50·8%) | |
| Female | 333 (50·5%) | 650 (49·2%) | |
| Weight (kg) | 4·2 (0·6) | 4·2 (0·6) | |
| Height (cm) | 54·2 (2·4) | 54·2 (2·4) | |
Data are mean (SD) or n (%).
All eligible participants received the first dose, 1925 (97·3%) of 1979 received the second dose, and 1894 (95·7%) received all three doses of vaccine as per schedule. 1852 (93·6%) of 1979 participants completed the follow-up. The immunogenicity analysis consisted of 1839 infants (1238 [67·3%] in the mixed vaccine regimen and 601 [32·7%] in the single vaccine regimen; 13 samples were insufficient in quantity) who completed vaccination and provided post-vaccination sera. The seroresponse rate was 33·5% (95% CI 30·9–36·2) for the mixed vaccine regimen and 29·6% (26·1–33·4) for the single vaccine regimen. The absolute difference in seroresponse rates between the mixed and single vaccine regimen was 3·9% (95% CI −0·7 to 8·3; table 2 ). The lower limit of the 95% Newcombe-Wilson CI for the difference was higher than the 10% non-inferiority margin, suggesting that the mixed vaccine regimen is not inferior to the single vaccine regimen.
Table 2.
Seroresponse rate in the single and mixed vaccine regimen groups
| Single vaccine regimen | Mixed vaccine regimen | Seroresponse rate difference (95% CI) | ||
|---|---|---|---|---|
| Number of participants | 601 | 1238 | .. | |
| Number of participants with seroresponse* | 178 | 415 | .. | |
| Seroresponse rate (95% CI) | 29·6% (26·1–33·4) | 33·5% (30·9–36·2) | 3·9% (−0·7 to 8·3) | |
| Four-fold increase in IgA antibodies vs baseline titre of ≥20 IU/mL | ||||
| Number of participants | 18 | 28 | .. | |
| Percentage (95% CI) | 3·0% (1·8–4·6) | 2·3% (1·5–3·2) | .. | |
| IgA antibody titre of ≥20 IU/mL vs titre <20 IU/mL at baseline | ||||
| Number of participants | 160 | 387 | .. | |
| Percentage (95% CI) | 26·6% (23·2–30·3) | 31·3% (28·7–33·9) | .. | |
Seroresponse was defined as a four-fold increase in IgA antibody titres after the third vaccine dose when baseline titre was 20 IU/mL or more, or an IgA antibody titre of 20 IU/mL or more after the third vaccine dose when baseline titre was less than 20 IU/mL.
The highest seroresponse was observed in group 5 (ie, Rotavac–Rotasiil–Rotasiil; 38·2% [95% CI 33·0–43·7]) followed by group 2 (ie, Rotasiil–Rotasiil–Rotasiil; 35·2% [30·0–40·8]). In the single vaccine regimen group, the seroresponse rate was higher among individuals who received Rotasiil (ie, group 2; 35·2% [95% CI 30·0–40·8]) than among those who received Rotavac (ie, group 1; 24·1% [19·5–29·1]; appendix 2 p 2). The geometric mean titre of IgA antibodies after three vaccine doses was 13·9 IU/mL (95% CI 12·2–14·8) in the single vaccine regimen and 16·7 IU/mL (15·3–18·3) in the mixed vaccine regimen (appendix 2 p 3). Among individuals with seroresponse, the geometric mean titre of IgA antibodies after three vaccine doses was 76·2 (95% CI 64·5–90·1) in the single vaccine regimen and 78·8 (71·3–87·1) in the mixed vaccine regimen.
In terms of safety, two (0·1%) of 1979 participants (one each in groups 2 and 3) reported immediate adverse events following immunisation that were classified to be of mild severity. The immediate adverse events were non-serious and the participants recovered without any complications. The proportion of participants with any type or at least one type of solicited adverse events was 90·9% (599 of 659; 95% CI 88·4–93·0) in the single vaccine regimen group and 91·1% (1203 of 1320; 89·5–92·6) in the mixed vaccine regimen group (table 3 ). Fever was the most frequently reported adverse event (86·6% [571 of 659; 95% CI 83·8–89·1] in the single vaccine regimen and 86·7% [1145 of 1320; 84·8–88·5] in the mixed vaccine regimen), followed by irritability (75·9% [500 of 659; 72·4–80·0] in the single vaccine regimen and 75·0% [990 of 1320; 72·3–77·3] in the mixed vaccine regimen). The other commonly reported solicited adverse events were decreased appetite and decreased activity level, vomiting, and diarrhoea. The incidence of participants having at least one unsolicited adverse event was 37·9% (250 of 659; 95% CI 34·2–41·8) in the single vaccine regimen and 38·7% (511 of 1320; 36·1–41·4) in the mixed vaccine regimen. Most solicited and unsolicited adverse events in the single vaccine regimen (3268 [86·1%] of 3794) and mixed vaccine regimes (6666 [86·0%] of 7750) were mild in nature (table 4 ). The proportion of solicited adverse events in different vaccine groups is provided in the appendix 2 (p 4).
Table 3.
Distribution of solicited and unsolicited adverse events within 7 days after vaccination (all three doses combined)
|
Single vaccine regimen (n=659) |
Mixed vaccine regimen (n=1320) |
||||||
|---|---|---|---|---|---|---|---|
| Number of participants | Percentage (95% CI) | Number of events | Number of participants | Percentage (95% CI) | Number of events | ||
| Any solicited events | 599 | 90·9% (88·4–93·0) | 3292 | 1203 | 91·1% (89·5–92·6) | 6718 | |
| Diarrhoea | 65 | 9·9% (7·7–12·4) | 73 | 109 | 8·3% (6·8–9·9) | 118 | |
| Vomiting | 77 | 11·7% (9·3–14·4) | 91 | 161 | 12·2% (10·5–14·1) | 198 | |
| Fever | 571 | 86·6% (83·8–89·1) | 1207 | 1145 | 86·7% (84·8–88·5) | 2399 | |
| Decreased activity level | 273 | 41·4% (37·6–45·3) | 453 | 557 | 42·2% (39·5–44·9) | 958 | |
| Decreased appetite | 287 | 43·6% (39·7–47·4) | 475 | 584 | 44·2% (41·5–47·0) | 993 | |
| Irritability | 500 | 75·9% (72·4–80·0) | 936 | 990 | 75·0% (72·3–77·3) | 1932 | |
| Loose motion | 51 | 7·7% (5·8–10·1) | 57 | 105 | 8·0% (6·6–9·5) | 120 | |
| Any unsolicited events | 250 | 37·9% (34·2–41·8) | 502 | 511 | 38·7% (36·1–41·4) | 1032 | |
Table 4.
Severity of solicited and unsolicited adverse events in the single and mixed vaccine regimens
| Single vaccine regimen (n=659) | Mixed vaccine regimen (n=1320) | |
|---|---|---|
| Total number of adverse events | 3794 | 7750 |
| Mild | 3268 (86·1%) | 6666 (86·0%) |
| Moderate | 517 (13·6%) | 1061 (13·7%) |
| Severe | 8 (0·2%) | 23 (0·3%) |
| Life threatening | 1 (<0·1%) | 0 |
Data are n or n (%).
A total of 35 serious adverse events were reported in both vaccine regimens, all of which were classified as severe except for three events: one was life threatening and two were moderate in severity. Incidence of serious adverse events in the single vaccine regimen (2·1% [14 of 659; 95% CI 1·2–3·5]) was similar to the mixed vaccine regimen (1·6% [21 of 1320; 1·0–2·4]; table 5 ).
Table 5.
Serious adverse events among study participants in the single and mixed vaccine regimens
|
Single vaccine regimen (n=659) |
Mixed vaccine regimen (n=1320) |
||||||
|---|---|---|---|---|---|---|---|
| Number of participants | Percentage (95% CI) | Number of events | Number of participants | Percentage (95% CI) | Number of events | ||
| At least one serious adverse event | 14 | 2·1% (1·2–3·5) | 14 | 21 | 1·6% (1·0–2·4) | 21 | |
| Any severity | 14 | 2·1% (1·2–3·5) | 14 | 21 | 1·6% (1·0–2·4) | 21 | |
| Life threatening | 1 | 0·2% (0·0–0·8) | 1 | 0 | 0·0% (0·0–0·3) | 0 | |
| Severe | 12 | 1·8% (0·9–3·2) | 12 | 20 | 1·5% (0·9–2·3) | 20 | |
| Moderate | 1 | 0·2% (0·0–0·8) | 1 | 1 | 0·1% (0·0–0·4) | 1 | |
| Mild | 0 | 0·0% (0·0–0·6) | 0 | 0 | 0·0% (0·0–0·3) | 0 | |
| At least one related serious adverse event | 1 | 0·2% (0·0–0·8) | 1 | 0 | 0·0% (0·0–0·3) | 0 | |
| At least one serious adverse event resulting in death | 1 | 0·2% (0·0–0·8) | 1 | 0 | 0·0% (0·0–0·3) | 0 | |
| At least one serious adverse event resulting in hospitalisation | 13 | 2·0% (1·1–3·4) | 13 | 21 | 1·6% (1·0–2·4) | 21 | |
| At least one serious adverse event leading to study discontinuation | 0 | 0·0% (0·0–0·6) | 0 | 0 | 0·0% (0·0–0·3) | 0 | |
On causality assessment, 34 serious adverse events were classified as not related to the rotavirus vaccines, whereas one case who required hospitalisation due to acute diarrhoea was related to the rotavirus vaccine. One case of sudden infant death syndrome was reported in group 1 and was assumed to be notrelated to the vaccine. No cases of intussusception were noted during the study period. All the serious adverse events met the regulatory requirement of an expedited review and were reported to the relevant regulatory authority.
Discussion
Rotavirus vaccines have been shown to be safe and effective in preventing deaths from rotavirus gastroenteritis infection. In low-income and middle-income countries, including India, issues such as cost, purchase, and supply of the vaccine stocks have always been point of concern.15 The administration of vaccines from a single manufacturer for each dose in a specific dosing regimen might not be possible for similar logistic reasons. Most of the vaccines requires a minimum of three doses to ensure adequate immunogenic response, and no guarantee exists that the shortage will not occur after one or two doses. Therefore, understanding the effects of using these vaccines interchangeably is essential.16 Our study shows that the Rotavac and Rotasiil rotavirus vaccines can be used interchangeably for routine immunisation.
Rotavirus vaccines have been administered to millions of children worldwide.17, 18 Challenge of adult volunteers with wild type rotavirus strains and trials of live rotavirus vaccines have shown a correlation between pre-existing rotavirus antibodies, particularly IgA, and viral infectivity; correspondingly, serum responses after live virus challenge resulting in infection include a substantial increase in antibody concentrations, IgA in particular.19 IgA antibodies are known to not cross the placenta and therefore are not present in pre-immune serum unless natural infection has occurred. Accordingly, the induction of an IgA seroresponse is the tool of choice to assess the immunogenicity of any rotavirus vaccines.
Although seroresponse is not considered as a direct proxy for efficacy, seroresponse does demonstrate that the vaccines given in an interchangeable manner are able to induce a robust immune response.20, 21 Though the information about seroresponse is of great interest, documentation of the safety of switching vaccines from one manufacturer to another is also essential. Considering the importance of rotavirus vaccines in averting very severe disease, which is responsible for a high burden of child morbidity and mortality, the Indian Ministry of Health and Family Welfare recommended that preference should be given to vaccination with the same product; however, in case of interstate migration or shortage of vaccines, an interchangeable regimen might be considered.22 Although the policy approval for using rotavirus vaccines interchangeably is in place, our study provides the first evidence for the interchangeability between Rotavac and Rotasiil in terms of safety and non-inferior seroresponse. These findings are in agreement with interchangeability studies done earlier using different vaccine products, which were documented to be both safe and non-inferior with respect to immunogenicity (measured by serum IgA and neutralising antibody titres) to their corresponding single vaccine standard schedules.23
In our current study, the incidence of solicited adverse events was similar across the study groups. Fever was most frequently reported, followed by irritability. Most events were mild-to-moderate in nature. No unexpected reactions were reported nor any difference between the four mixed vaccine groups versus the two single vaccine groups in terms of adverse events and serious adverse events, which was similar to the study done in 2019 in which fever was the most reported adverse event (65%).24
Immune responses against oral vaccines in low-income and middle-income countries have been documented to be lower than that observed in children from high-income countries. Multiple causes, such as presence of vertically transmitted antibodies, higher prevalence of breastfeeding, and a high burden of diseases affecting gut health and microbiota, such as enteric pathogens, malnutrition, and environmental enteropathy have been hypothesised to affect such disparities.25 Thus, seroresponse to rotavirus vaccines might provide an incomplete proxy measure for protection against very severe disease.
Our study has certain limitations. First, about 6·5% of the participants dropped out from the study; however, this percentage was lower than our assumed 10% dropout rate. Second, we used seroresponse at 4 weeks after three doses of vaccine as a surrogate for vaccine efficacy. We did not follow-up the participants to examine the persistence of immune response. Since the mixed schedules induced seroresponse rates similar to the single vaccine regimens, we do not expect the persistence of antibodies raised against a mixed schedule to be significantly different from the single product regimens. The study focused on immunogenicity as a surrogate measure for vaccine efficacy and did not evaluate clinical endpoints. The study was done in an unblinded manner, but the laboratory testing of immune responses were blinded and done by an institute not associated with the field implementation of the trial, thus limiting bias in computing the immunogenicity results. Lastly, some naturally occurring rotavirus infections could have influenced immunogenicity results; however, since the study was randomised, this effect would most likely have happened across all study groups, including the single vaccine product recipients.
In conclusion, this study establishes that heterologous regimens of Rotavac and Rotasiil in various combinations generate a non-inferior seroresponse in recipients compared with a homologous regimen of either vaccine product. The occurrence of adverse events and serious adverse events are also similar between the different groups. More research is needed to understand the long-term immune effects and protection status afforded by such mixed vaccine regimens, since the current analysis provides satisfactory safety and non-inferior seroresponse profiles in the short term. Additionally, this study provides evidence to support scaling-up of these vaccine products, both of which are prequalified by WHO and approved for use at the national level. The evidence from this study supports the policy stance to consider Rotavac and Rotasiil as interchangeable products.
For the online randomisation list used see http://www.randomization.com
Data sharing
The data for this Article will be made available after publication on request from any qualified researchers or academicians. The data include analysed deidentified participant data, data dictionary, study protocol, statistical analysis plan, and informed consent form, among other data. The data will be shared for 2 years after publication on receipt of request at drshantadutta@gmail.com.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Ministry of Health and Family Welfare through the Indian Council of Medical Research, New Delhi (T.13011/38/2011-Imm). We thank the Indian Council of Medical Research expert panel for providing valuable inputs in designing the study protocol. We also thank the parents of the children and the project staff for their participation in the study.
Contributors
SK, PC, and SD were involved in the design of the protocol and study design, including conceptualisation, methodology, funding acquisition, and resources. SK, PC, RG, SS, AB, and AK did the data curation, investigation, project administration, supervision, and validation. SB and RKN did the laboratory analysis. MM and VKK did the data analysis. The manuscript was drafted by SK and RG and edited by SD, PC, AB, SB, and MM. All authors read and approved the final manuscript. SD, SK, and MM had direct access to data.
Supplementary Materials
References
- 1.Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172:958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez WJ, Kim HW, Brandt CD, et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J. 1987;6:170–176. doi: 10.1097/00006454-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 3.John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK, Kang G. Rotavirus gastroenteritis in India, 2011–2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32(suppl 1):A5–A9. doi: 10.1016/j.vaccine.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization–Coordinated Global Rotavirus Surveillance Network Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(suppl 2):S96–105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 5.Panda S, Deb AK, Chawla-Sarkar M, et al. Factors associated with diarrhoea in young children and incidence of symptomatic rotavirus infection in rural West Bengal, India. Epidemiol Infect. 2014;142:1848–1858. doi: 10.1017/S0950268814000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni PS, Desai S, Tewari T, et al. A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35:6228–6237. doi: 10.1016/j.vaccine.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari N, Sharma P, Taneja S, et al. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–429. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari N, Sharma P, Glass RI, et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine. 2006;24:5817–5823. doi: 10.1016/j.vaccine.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandola TR, Taneja S, Goyal N, et al. ROTAVAC does not interfere with the immune response to childhood vaccines in Indian infants: a randomized placebo controlled trial. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John J, Kawade A, Rongsen-Chandola T, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine. 2014;32(suppl 1):A104–A109. doi: 10.1016/j.vaccine.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Ward RL, Bernstein DI, Smith VE, et al. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis. 2004;189:2290–2293. doi: 10.1086/421248. [DOI] [PubMed] [Google Scholar]
- 13.Paul A, Babji S, Sowmyanarayanan TV, et al. Human and bovine rotavirus strain antigens for evaluation of immunogenicity in a randomized, double-blind, placebo-controlled trial of a single dose live attenuated tetravalent, bovine-human-reassortant, oral rotavirus vaccine in Indian adults. Vaccine. 2014;32:3094–3100. doi: 10.1016/j.vaccine.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019;2019 doi: 10.1002/14651858.CD008521.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravish HS, Sudarshan MK, Madhusudana SN, et al. Assessing safety and immunogenicity of post-exposure prophylaxis following interchangeability of rabies vaccines in humans. Hum Vaccin Immunother. 2014;10:1354–1358. doi: 10.4161/hv.28064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg DP, Feldman S. Vaccine interchangeability. Clin Pediatr. 2003;42:93–99. doi: 10.1177/000992280304200201. [DOI] [PubMed] [Google Scholar]
- 17.Glass RI, Parashar UD, Bresee JS, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 18.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis. 2014;59:1291–1301. doi: 10.1093/cid/ciu564. [DOI] [PubMed] [Google Scholar]
- 19.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Velázquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182:1602–1609. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 21.Ward RL. Mechanisms of protection against rotavirus in humans and mice. J Infect Dis. 1996;174(suppl 1):S51–S58. doi: 10.1093/infdis/174.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health and Family Welfare. Immunization Division Operational guidelines for introduction of rotavirus vaccine in the Universal Immunization Program. 2019. http://www.nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Operational_Guidelines_for_Introduction_of_Rotavac_in_UIP.pdf
- 23.Libster R, McNeal M, Walter EB, et al. Safety and immunogenicity of sequential rotavirus vaccine schedules. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawade A, Babji S, Kamath V, et al. Immunogenicity and lot-to-lot consistency of a ready to use liquid bovine-human reassortant pentavalent rotavirus vaccine (ROTASIIL–Liquid) in Indian infants. Vaccine. 2019;37:2554–2560. doi: 10.1016/j.vaccine.2019.03.067. [DOI] [PubMed] [Google Scholar]
- 25.Parker EP, Ramani S, Lopman BA, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 2018;13:97–118. doi: 10.2217/fmb-2017-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this Article will be made available after publication on request from any qualified researchers or academicians. The data include analysed deidentified participant data, data dictionary, study protocol, statistical analysis plan, and informed consent form, among other data. The data will be shared for 2 years after publication on receipt of request at drshantadutta@gmail.com.